EP0092220A2 - Fluoresent lamp for stimulating a uniform plant growth - Google Patents
Fluoresent lamp for stimulating a uniform plant growth Download PDFInfo
- Publication number
- EP0092220A2 EP0092220A2 EP83103743A EP83103743A EP0092220A2 EP 0092220 A2 EP0092220 A2 EP 0092220A2 EP 83103743 A EP83103743 A EP 83103743A EP 83103743 A EP83103743 A EP 83103743A EP 0092220 A2 EP0092220 A2 EP 0092220A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- magnesium
- activated
- fluorescent lamp
- phosphor
- emission maximum
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01J—ELECTRIC DISCHARGE TUBES OR DISCHARGE LAMPS
- H01J61/00—Gas-discharge or vapour-discharge lamps
- H01J61/02—Details
- H01J61/38—Devices for influencing the colour or wavelength of the light
- H01J61/42—Devices for influencing the colour or wavelength of the light by transforming the wavelength of the light by luminescence
- H01J61/44—Devices characterised by the luminescent material
Definitions
- the invention relates to a fluorescent lamp for stimulating balanced plant growth, in which the phosphor mixture applied to the inner wall of the lamp bulb contains three components, the first component being a manganese-activated magnesium fluorogermanate with an emission maximum at 660 nm and the second component being a tin-activated strontium-magnesium Orthophosphate with an emission maximum at 626 nm is used.
- the proportion of the magnesium fluorogermanate phosphor with its dark red emission is preferably from 10% to 30% by weight To reduce 20 wt .-%.
- the material price of the phosphor mixture used could be reduced to a third of the original price, but in order to achieve a defined color location of the mixture consisting of a total of four phosphors, the most exact adherence to all manufacturing tolerances is necessary. The reason is that a mixture consisting of four phosphor components is not a sufficient criterion for determining the color location. Although it is possible to set the color locale here using various mixing ratios, the radiation properties of the lamp then shift.
- binders e.g. dilute solutions of a highly viscous nitrocellulose in butyl acetate are used.
- the effort to prevent environmental damage with such solvents is also quite considerable.
- the object of the invention is to provide a phosphor mixture which is suitable for plant irradiation and which is inexpensive to produce and easy to process and for which the environmentally friendly, inexpensive water sludge can be used.
- the effect of the characteristic wavelength ranges that are essential for plant physiology should be as close as possible to that of natural sunlight.
- the fluorescent lamp for stimulating balanced plant growth with the features mentioned in the preamble of the main claim is characterized according to the invention in that the third component is a barium magnesium aluminate activated with divalent europium with an emission maximum at 447 nm.
- the magnesium fluorogermanate activated with manganese is only present in an amount of 10 to 25% by weight, preferably 20% by weight, of the total weight of the phosphor mixture. With more than 25% by weight, the price advantage would no longer be sufficiently attractive, while with less than 10% by weight, certain colors would be rendered inadequately.
- the cheap, tin-activated strontium-magnesium orthophosphate is 55 to 80% by weight, preferably 65% by weight, and the barium-magnesium aluminate activated with divalent europium is 10 to 20% by weight, preferably containing 15% by weight of the total weight of the phosphor mixture.
- the phosphor mixture according to the invention is about two thirds cheaper than the mixture previously used.
- the new phosphor mixture is also easy to manufacture and well suited for processing in mass production.
- the target color location is a clear criterion for the phosphor composition.
- the standard color value share of the preferred mixture is 0.339 for x and 0.247 for y; the lamp thus emits Although in a purple hue that is common in plant lighting, in which plants and ornamental fish have a particularly attractive appearance, the color locus is shifted by approx. 30 SWE (threshold value units) towards the achromatic point. This makes it possible to use such a fluorescent lamp as room lighting in greenhouses and corresponding sales rooms.
- the germanate component is used only to a small extent, the transition from the previously used phosphor suspension with organic binder to the cheaper, environmentally friendly water slurry is possible.
- the new type of phosphor and the simultaneous use of lamp bulbs with a bulb diameter reduced to 26 mm approximately 10% of the electrical energy costs can be saved with an approximately doubled luminous flux.
- the resulting material savings reduce the end costs for the phosphor to approximately a quarter of the original costs.
- FIGS. 1a to 1d show the different courses of the plant physiological action functions in accordance with DIN 5031.
- Photosynthesis. Fig. 1a
- the chlorophyll synthesis Fig. 1b
- the phototropism Fig. 1c
- the photomorphogenesis Fig. 1d
- Fig. 1d shows a narrow induction maximum (curve A) at 660 nm and a somewhat wider reversion maximum (curve B) at approx. 730 nm.
- the green spectral range 510 nm to 610 nm
- the plants show only a lower photosynthetic effectiveness and a weak morphogenetic activity.
- a fluorescent lamp used for plant irradiation should therefore also have a maximum with its total emission, at least in the maxima specified above of the plant physiological reaction curves.
- certain plants thrive not only with the radiation components in the ranges from approx. 400 nm to approx. 500 nm (blue) and approx. 600 nm to approx. 700 nm (red), but also beyond also a certain proportion of daylight, in particular its green-yellow radiation component in the range of approximately 550 nm to 580 nm for the synthesis of enzymes and and other substances.
- FIGS. 2 and 3 show the respective emission spectrum of a fluorescent lamp coated with a conventional and with a phosphor mixture according to the invention.
- the emission spectrum in FIG. 2 originates from a 40 W fluorescent lamp with a bulb diameter of 38 mm, the phosphor mixture of which is approx. 55% by weight from the blue-emitting calcium tungstate and approx. 45% by weight from the expensive red-emitting magnesium fluorogermanate activated with manganese.
- the phosphor mixture is dissolved in a dilute solution of a highly viscous nitrocellulose in butyl acetate. After Beschlämmung the lamp bulb is eworkst in a baking oven of g, wherein the organic constituents of the suspension volatilize.
- the emission spectrum of FIG. 3 comes from a 36 W fluorescent lamp with a bulb diameter of 26 mm.
- the phosphor mixture contains only 20% by weight of the expensive, red-emitting, manganese-activated magnesium fluorogermanate.
- the resultant lack of red in the mixture was made up by adding 65% by weight of the much cheaper, red-orange emitting, tin-activated strontium-magnesium-orthophosphate phosphor.
- the fluorescent lamp obtains the necessary proportion of blue by adding 15% by weight of a barium-magnesium-aluminate phosphor which has been activated with divalent europium to the phosphor mixture.
- the spectrum also shows the balance of the blue and red emissions.
- the green-yellow radiation component (550 nm - 580 nm) was clear Lich raised. In addition to promoting the synthesis of enzymes, this also causes a shift in the color locus towards the achromatic point, which in turn improves color rendering and enables the use of radiation lamps in greenhouses and their sales rooms.
- the fluorescent lamp coated with the phosphor mixture according to the invention is manufactured in a known manner like conventional fluorescent lamps, but the phosphor mixture was dissolved in the much more environmentally friendly water instead of in an organic solvent.
- the radiation proportions of the phosphor mixture according to the invention standardized to chlorophyll synthesis are significantly more favorable compared to a mixture of a calcium tungstate and a magnesium fluorogermanate activated with manganese used in the past if the sunlight is used as a reference source.
- the value of the metamorphosis at 730 nm could be tripled compared to the fluorescent lamps previously used for plant irradiation and thus much closer to sunlight.
- the higher value of photosynthesis also causes an increased production of the biomass.
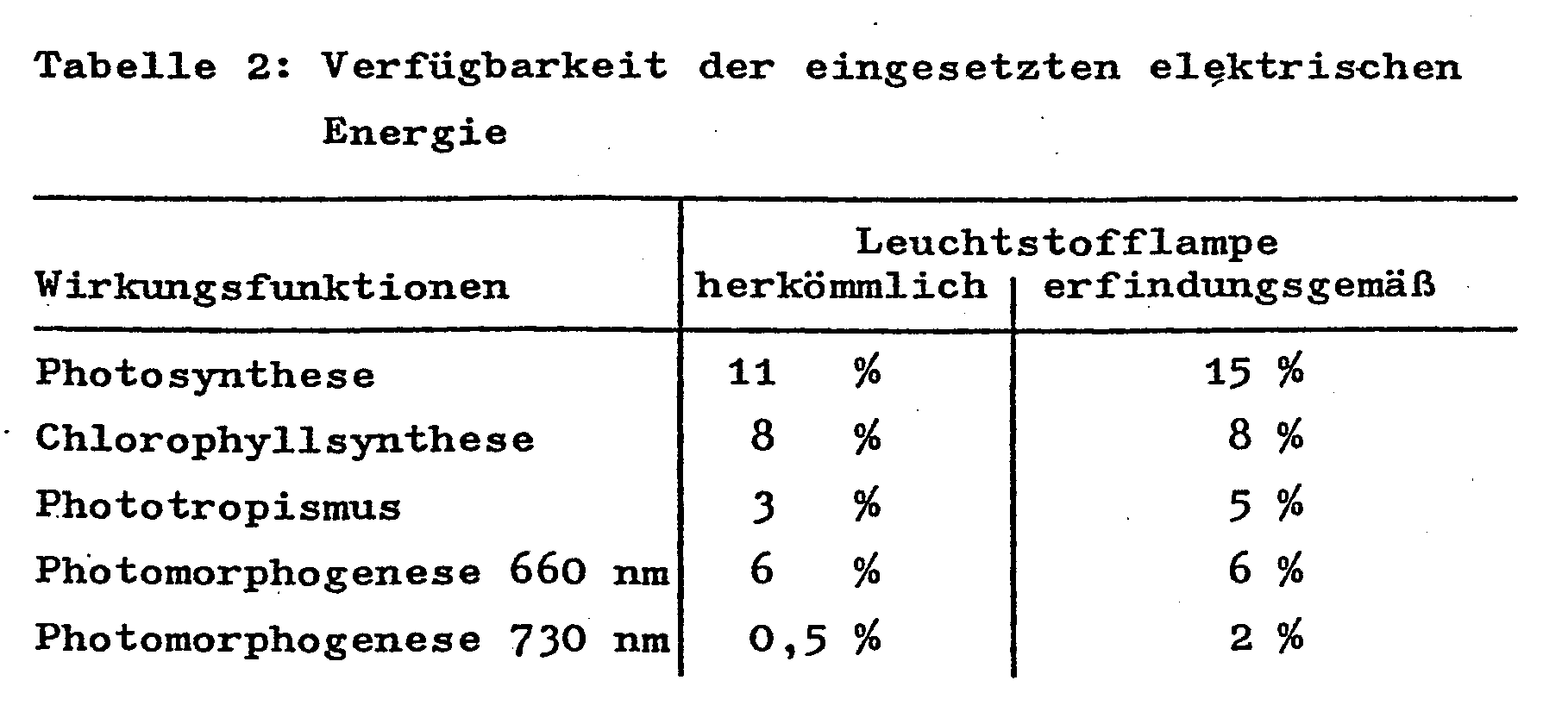
- Table 2 shows what percentage of the electrical power consumed by the lamp relates to the individual radiation components available to the plant.
Abstract
Description
Die Erfindung betrifft eine Leuchtstofflampe zur Anregung eines ausgeglichenen Pflanzenwachstums, bei der die auf die Innenwand des Lampenkolbens aufgetragene Leuchtstoffmischung drei Komponenten enthält, wobei als erste Komponente ein manganaktiviertes Magnesium-Fluorogermanat mit einem Emissionsmaximum bei 660 nm und als zweite Komponente ein zinnaktiviertes Strontium-Magnesium-Orthophosphat mit einem Emissionsmaximum bei 626 nm verwendet wird.The invention relates to a fluorescent lamp for stimulating balanced plant growth, in which the phosphor mixture applied to the inner wall of the lamp bulb contains three components, the first component being a manganese-activated magnesium fluorogermanate with an emission maximum at 660 nm and the second component being a tin-activated strontium-magnesium Orthophosphate with an emission maximum at 626 nm is used.
Für Leuchtstofflampen zum Zweck der Pflanzen-, Blüten-und Zierfischbestrahlung sind bisher verschiedene Leuchtstoffmischungen vorgeschlagen worden, deren Emission auf die unterschiedliche Absorption der Pflanzen bei den einzelnen Wellenlängen, insbesondere des sichtbaren Spektralbereiches abgestimmt ist. In der Praxis werden hierzu vielfach Leuchtstoffmischungen verwendet, die sich etwa zu 55 Gew.-% aus einem Calcium-Wolframat und zu 45 Gew.-% aus einem mit Mangan aktivierten Magnesium-Fluorogermanat zusammensetzen. Das Magnesium-Fluorogermanat ist jedoch sehr teuer, weshalb schon seit langem andere Leuchtstoffmischungen ohne bzw. mit einem wesentlich reduzierten Anteil dieser teuren Komponente gesucht werden, ohne daß die spektralen Emissionseigenschaften der Leuchtstofflampe negativ beeinflußt werden. So ist z.B. aus der US-PS 4 055 781 bekannt, den Anteil des Magnesium-Fluorogermanat-Leuchtstoffes mit seiner dunkelroten Emission auf 10 Gew.-% bis 30 Gew.-%, vorzugsweise 20 Gew.-% zu reduzieren. Zur Erhaltung der Energien in den für die Pflanze wesentlichen Spektralbereichen wurde es jedoch erforderlich, der Leuchtstoffmischung neben dem Magnesium-Fluorogermanat drei weitere Leuchtstoffkomponenten mit unterschiedlichen Emissionseigenschaften hinzuzufügen. Zwar konnte mit Hilfe dieser Maßnahmen der Materialpreis der verwendeten Leuchtstoffmischung auf ein Drittel des ursprünglichen Preises gesenkt werden, doch ist zur Erzielung eines definierten Farbortes der aus insgesamt vier Leuchtstoffen bestehenden Mischung die genaueste Einhaltung sämtlicher Fertigungstoleranzen erforderlich. Der Grund besteht darin, daß eine aus vier Leuchtstoffkomponenten bestehende Mischung kein ausreichendes Kriterium für eine Farbortbestimmung darstellt. Zwar ist die Farborteinstellung hierbei durch verschiedene Mischungsverhältnisse möglich, jedoch verschieben sich dann die Strahlungseigenschaften der Lampe.Various fluorescent mixtures have been proposed for fluorescent lamps for the purpose of irradiation of plants, flowers and ornamental fish, the emission of which is matched to the different absorption of the plants at the individual wavelengths, in particular of the visible spectral range. In practice, phosphor mixtures are often used for this purpose, which are composed of approximately 55% by weight of a calcium tungstate and 45% by weight of a magnesium fluorogermanate activated with manganese. However, the magnesium fluorogermanate is very expensive, which is why other fluorescent mixtures without or with a significantly reduced proportion of this expensive component have long been sought, without the spectral emission properties of the fluorescent lamp being adversely affected. For example, it is known from US Pat. No. 4,055,781 that the proportion of the magnesium fluorogermanate phosphor with its dark red emission is preferably from 10% to 30% by weight To reduce 20 wt .-%. In order to maintain the energies in the spectral ranges essential for the plant, however, it was necessary to add three further phosphor components with different emission properties to the phosphor mixture in addition to the magnesium fluorogermanate. With the help of these measures, the material price of the phosphor mixture used could be reduced to a third of the original price, but in order to achieve a defined color location of the mixture consisting of a total of four phosphors, the most exact adherence to all manufacturing tolerances is necessary. The reason is that a mixture consisting of four phosphor components is not a sufficient criterion for determining the color location. Although it is possible to set the color locale here using various mixing ratios, the radiation properties of the lamp then shift.
Ein weiterer Nachteil der in der Praxis verwendeten, aus Calcium-Wolframat und Magnesium-Fluorogermanat bestehenden Leuchtstoffmischung liegt in der Notwendigkeit, die Beschlämmung der Lampenkolben mit einer einen organischen Binder enthaltenden Suspension vornehmen zu müssen. Als Binder werden z.B. verdünnte Lösungen einer hochviskosen Nitrozellulose in Butylacetat verwendet. Außer einer bei der Verarbeitung von mit diesen organischen Bindern aufbereiteten Leuchtstoffsuspensionen auftretenden Geruchsbelästigung ist auch der Aufwand zur Verhütung von Umweltschäden mit solchen Lösungsmitteln recht beträchtlich.Another disadvantage of the phosphor mixture used in practice, consisting of calcium tungstate and magnesium fluorogermanate, is the necessity to have to coat the lamp bulbs with a suspension containing an organic binder. As binders e.g. dilute solutions of a highly viscous nitrocellulose in butyl acetate are used. In addition to an odor nuisance that occurs during the processing of phosphor suspensions prepared with these organic binders, the effort to prevent environmental damage with such solvents is also quite considerable.
Aufgabe der Erfindung ist es, eine für Pflanzenbestrahlung geeignete Leuchtstoffmischung zu-schaffen, die billig in der Herstellung sowie leicht verarbeitbar ist und für die die umweltfreundliche, kostengünstige Wasserbeschlämmung angewendet werden kann. Die für die pflanzenphysiologischen wesentlichen charakteristischen Wellenlängenbereiche sollten außerdem in ihrer Wirkung möglichst dem des natürlichen Sonnenlichts angeglichen werden.The object of the invention is to provide a phosphor mixture which is suitable for plant irradiation and which is inexpensive to produce and easy to process and for which the environmentally friendly, inexpensive water sludge can be used. In addition, the effect of the characteristic wavelength ranges that are essential for plant physiology should be as close as possible to that of natural sunlight.
Die Leuchtstofflampe zur Anregung eines ausgeglichenen Pflanzenwachstums mit den im Oberbegriff des Hauptanspruchs genannten Merkmalen ist erfindungsgemäß dadurch gekennzeichnet, daß die dritte Komponente ein mit zweiwertigem Europium aktiviertes Barium-Magnesium-Aluminat mit einem Emissionsmaximum bei 447 nm ist. Das mit Mangan aktivierte Magnesium-Fluorogermanat ist dabei nur zu 10 bis 25 Gew.-%, vorzugsweise mit 20 Gew.-% vom Gesamtgewicht der Leuchtstoffmischung enthalten. Bei mehr als 25 Gew.-% würde der Preisvorteil nicht mehr ausreichend attraktiv ausfallen, während bei weniger als 10 Gew.-% bestimmte Farben visuell unzureichend wiedergegeben werden. Das billige, mit Zinn aktivierte Strontium-Magnesium-Orthophosphat ist mit 55 bis 80 Gew.-%, vorzugsweise mit 65 Gew.-% und das mit zweiwertigem Europium aktivierte Barium-Magnesium-Aluminat ist mit 10 bis 20 Gew.-%, vorzugsweise mit 15 Gew.-% an dem Gesamtgewicht der Leuchtstoffmischung enthalten.The fluorescent lamp for stimulating balanced plant growth with the features mentioned in the preamble of the main claim is characterized according to the invention in that the third component is a barium magnesium aluminate activated with divalent europium with an emission maximum at 447 nm. The magnesium fluorogermanate activated with manganese is only present in an amount of 10 to 25% by weight, preferably 20% by weight, of the total weight of the phosphor mixture. With more than 25% by weight, the price advantage would no longer be sufficiently attractive, while with less than 10% by weight, certain colors would be rendered inadequately. The cheap, tin-activated strontium-magnesium orthophosphate is 55 to 80% by weight, preferably 65% by weight, and the barium-magnesium aluminate activated with divalent europium is 10 to 20% by weight, preferably containing 15% by weight of the total weight of the phosphor mixture.
Die erfindungsgemäße Leuchtstoffmischung ist um ca. zwei Drittel billiger als die bisher verwendete Mischung. Die neue Leuchtstoffmischung ist darüber hinaus leicht herstellbar und für die Verarbeitung in einer Massenfertigung gut geeignet. Durch die Verwendung von nur drei Komponenten ist der Zielfarbort ein eindeutiges Kriterium für die Leuchtstoffzusammensetzung. Der Normfarbwertanteil der Vorzugsmischung liegt für x bei 0.339 und für y bei 0.247; die Lampe emittiert somit zwar in einem bei Pflanzenbeleuchtung üblichen violetten Farbton, bei dem Pflanzen und Zierfische ein besonders ansehnliches Aussehen zeigen, doch ist der Farbort um ca. 30 SWE (Schwellenwerteinheiten) in Richtung auf den Unbunt-Punkt verschoben. Dadurch wird es möglich, eine solche Leuchtstofflampe auch als Raumbeleuchtung in Gewächshäusern und entsprechenden Verkaufsräumen einzusetzen.The phosphor mixture according to the invention is about two thirds cheaper than the mixture previously used. The new phosphor mixture is also easy to manufacture and well suited for processing in mass production. By using only three components, the target color location is a clear criterion for the phosphor composition. The standard color value share of the preferred mixture is 0.339 for x and 0.247 for y; the lamp thus emits Although in a purple hue that is common in plant lighting, in which plants and ornamental fish have a particularly attractive appearance, the color locus is shifted by approx. 30 SWE (threshold value units) towards the achromatic point. This makes it possible to use such a fluorescent lamp as room lighting in greenhouses and corresponding sales rooms.
Aufgrund der nur noch zu einem geringen Anteil verwendeten Germanat-Komponente ist der Übergang von der bisher verwendeten Leuchtstoffsuspension mit organischem Binder auf die billigere, umweltfreundliche Wasserbeschlämmung möglich. Mit dem neuartigen Leuchtstoff und gleichzeitiger Verwendung von Lampenkolben mit einem auf 26 mm reduzierten Kolbendurchmesser lassen sich bei einem etwa verdoppelten Lichtstrom zusätzlich ca. 10 % der elektrischen Energiekosten einsparen. Außerdem werden mit der dadurch verbundenen Materialeinsparung die Endkosten für den Leuchtstoff auf ca. ein Viertel der Ursprungskosten reduziert.Because the germanate component is used only to a small extent, the transition from the previously used phosphor suspension with organic binder to the cheaper, environmentally friendly water slurry is possible. With the new type of phosphor and the simultaneous use of lamp bulbs with a bulb diameter reduced to 26 mm, approximately 10% of the electrical energy costs can be saved with an approximately doubled luminous flux. In addition, the resulting material savings reduce the end costs for the phosphor to approximately a quarter of the original costs.
Die Erfindung wird nachstehend anhand der Figuren näher erläutert:
- Figur 1a zeigt die relative spektrale Wirkungsfunktion s (λ) sy, rel der Photosynthese.
- Figur 1b zeigt die relative spektrale Wirkungsfunktion s (λ)ch, rel der Chlorophyllsynthese.
- Figur 1c zeigt die relative spektrale Wirkungsfunktion s (λ) tr, rel des Phototropismus.
- Figur 1d zeigt die relative spektrale Wirkungsfunktion s (λ) mo, rel der Photomorphogenese.
- Figur 2 zeigt das Emissionsspektrum einer Leuchtstofflampe, die mit einer herkömmlichen Leuchtstoffmischung beschlämmt wurde.
- Figur 3 zeigt das Emissionsspektrum einer Leuchtstofflampe, die mit der erfindungsgemäßen Leuchtstoffmischung beschlämmt wurde.
- FIG. 1a shows the relative spectral function s (λ) sy, rel of photosynthesis.
- Figure 1b shows the relative spectral function s (λ) ch, rel of chlorophyll synthesis.
- Figure 1c shows the relative spectral function s (λ) tr, rel of the phototropism.
- FIG. 1d shows the relative spectral function s (λ) mo, rel of the photomorphogenesis.
- Figure 2 shows the emission spectrum of a fluorescent lamp, which was slurried with a conventional phosphor mixture.
- FIG. 3 shows the emission spectrum of a fluorescent lamp which was slurried with the phosphor mixture according to the invention.
In den Figuren 1a bis 1d sind entsprechend DIN 5031 die unterschiedlichen Verläufe der pflanzenphysiologischen Wirkungsfunktionen dargestellt. Die Photosynthese . (Fig. 1a) und die Chlorophyllsynthese (Fig. 1b) zeigen jeweils Maxima bei 445 nm sowie 660 nm. Der Phototropismus (Fig. 1c) zeigt einen breiten Kurvenverlauf mit Maxima bei 440 nm und 470 nm und die Photomorphogenese (Fig. 1d) zeigt ein schmales Induktionsmaximum (Kurve A) bei 660 nm sowie ein etwas breiteres Reversionsmaximum (Kurve B) bei ca. 730 nm. Im grünen Spektralbereich (510 nm bis 610 nm) zeigen die Pflanzen nur eine geringere photosynthetische Effektivität und eine schwache morphogenetische Aktivität. Strahlung der Wellenlängen oberhalb 1000 nm, sofern die Pflanze diese überhaupt absorbiert, wird ohne Störung der biochemischen Prozesse von der Pflanze in Wärme umgewandelt. Eine für die Pflanzenbestrahlung verwendete Leuchtstofflampe soll mit ihrer Gesamtemission deshalb zumindest in den oben angegebenen Maxima der pflanzenphysiologischen Reaktionskurven ebenfalls ein Maximum aufweisen. Bei der Pflanzenaufzucht hat sich aber auch gezeigt, daß bestimmte Pflanzen nicht nur mit den Strahlungsanteilen in den Bereichen ca. 400 nm bis ca. 500 nm (blau) sowie ca. 600 nm bis ca. 700 nm (rot) gedeihen, sondern darüber hinaus auch einen gewissen Anteil an Tageslicht, insbesondere dessen grün-gelben Strahlungsanteil im Bereich ca. 550 nm bis 580 nm für die Synthese von Enzymen und und anderen Stoffen benötigen.FIGS. 1a to 1d show the different courses of the plant physiological action functions in accordance with DIN 5031. Photosynthesis. (Fig. 1a) and the chlorophyll synthesis (Fig. 1b) each show maxima at 445 nm and 660 nm. The phototropism (Fig. 1c) shows a broad curve with maxima at 440 nm and 470 nm and the photomorphogenesis (Fig. 1d) shows a narrow induction maximum (curve A) at 660 nm and a somewhat wider reversion maximum (curve B) at approx. 730 nm. In the green spectral range (510 nm to 610 nm) the plants show only a lower photosynthetic effectiveness and a weak morphogenetic activity. Radiation of wavelengths above 1000 nm, if the plant absorbs it at all, is converted into heat by the plant without disturbing the biochemical processes. A fluorescent lamp used for plant irradiation should therefore also have a maximum with its total emission, at least in the maxima specified above of the plant physiological reaction curves. In plant breeding, however, it has also been shown that certain plants thrive not only with the radiation components in the ranges from approx. 400 nm to approx. 500 nm (blue) and approx. 600 nm to approx. 700 nm (red), but also beyond also a certain proportion of daylight, in particular its green-yellow radiation component in the range of approximately 550 nm to 580 nm for the synthesis of enzymes and and other substances.
In den Figuren 2 und 3 ist das jeweilige Emissionsspektrum einer mit einer herkömmlichen und einer erfindungsgemäßen Leuchtstoffmischung beschichteten Leuchtstofflampe vergleichend dargestellt. Das Emissionsspektrum der Figur 2 stammt dabei von einer 40-W-Leuchtstofflampe mit einem Kolbendurchmesser von 38 mm, wobei deren Leuchtstoffmischung zu ca. 55 Gew.-% aus dem blau emittierenden Calciumwolframat und zu ca. 45 Gew.-% aus dem teuren, rot emittierenden, mit Mangan aktivierten Magnesium-Fluorogermanat besteht. Die Leuchtstoffmischung ist in einer verdünnten Lösung einer hochviskosen Nitrozellulose in Butylacetat gelöst. Nach erfolgter Beschlämmung wird der Lampenkolben in einem Ausheizofen ausgeheizt, wobei sich die organischen Bestandteile der Suspension verflüchtigen.FIGS. 2 and 3 show the respective emission spectrum of a fluorescent lamp coated with a conventional and with a phosphor mixture according to the invention. The emission spectrum in FIG. 2 originates from a 40 W fluorescent lamp with a bulb diameter of 38 mm, the phosphor mixture of which is approx. 55% by weight from the blue-emitting calcium tungstate and approx. 45% by weight from the expensive red-emitting magnesium fluorogermanate activated with manganese. The phosphor mixture is dissolved in a dilute solution of a highly viscous nitrocellulose in butyl acetate. After Beschlämmung the lamp bulb is eheizt in a baking oven of g, wherein the organic constituents of the suspension volatilize.
Das Emissionsspektrum der Figur 3 stammt von einer 36-W-Leuchtstofflampe mit einem Kolbendurchmesser von 26 mm. Die Leuchtstoffmischung enthält gemäß der Erfindung nur 20 Gew.-% des teuren, rot emittierenden, mit Mangan aktivierten Magnesium-Fluorogermanat. Der dadurch fehlende Rotanteil der Mischung wurde durch Zugabe von 65 Gew.-% des wesentlich billigeren, rot-orange emittierenden, mit Zinn aktivierten Strontium-Magnesium-Orthophosphat-Leuchtstoffes aufgefüllt. Den erforderlichen Blauanteil erhält die Leuchtstofflampe, indem der Leuchtstoffmischung 15 Gew.-% eines mit zweiwertigem Europium aktivierten Barium-Magnesium-Aluminat-Leuchtstoffes zugegeben sind. Das Spektrum zeigt neben einer Optimierung im blauen Bereich auch die Ausgewogenheit der Blau- und Rotemission. Ebenso konnte - verglichen mit dem Spektrum einer herkömmlichen Leuchtstofflampe - der grün-gelbe Strahlungsanteil (550 nm - 580 nm) deutlich angehoben werden. Hierdurch wird neben einer Förderung der Synthese von Enzymen auch gleichzeitig eine Verschiebung des Farbortes in Richtung auf den Unbunt-Punkt bewirkt, wodurch wiederum die Farbwiedergabe verbessert und die Verwendung der Bestrahlungslampen auch in Gewächshäusern und deren Verkaufsräumen ermöglicht wird. Aus dem Emissionsspektrum ergibt sich in der Normfarbtafel nach DIN 5033 ein Farbort mit den Normfarbwertanteilen x = 0.339 und y = 0.247. Die mit der erfindungsgemäßen Leuchtstoffmischung beschichtete Leuchtstofflampe wird auf bekannte Weise wie herkömmliche Leuchtstofflampen gefertigt, wobei die Leuchtstoffmischung jedoch anstatt in einem organischen Lösungsmittel in dem wesentlich umweltfreundlicheren Wasser gelöst war.The emission spectrum of FIG. 3 comes from a 36 W fluorescent lamp with a bulb diameter of 26 mm. According to the invention, the phosphor mixture contains only 20% by weight of the expensive, red-emitting, manganese-activated magnesium fluorogermanate. The resultant lack of red in the mixture was made up by adding 65% by weight of the much cheaper, red-orange emitting, tin-activated strontium-magnesium-orthophosphate phosphor. The fluorescent lamp obtains the necessary proportion of blue by adding 15% by weight of a barium-magnesium-aluminate phosphor which has been activated with divalent europium to the phosphor mixture. In addition to optimization in the blue area, the spectrum also shows the balance of the blue and red emissions. Likewise - compared to the spectrum of a conventional fluorescent lamp - the green-yellow radiation component (550 nm - 580 nm) was clear Lich raised. In addition to promoting the synthesis of enzymes, this also causes a shift in the color locus towards the achromatic point, which in turn improves color rendering and enables the use of radiation lamps in greenhouses and their sales rooms. The emission spectrum in the standard color chart according to DIN 5033 results in a color locus with the standard color value components x = 0.339 and y = 0.247. The fluorescent lamp coated with the phosphor mixture according to the invention is manufactured in a known manner like conventional fluorescent lamps, but the phosphor mixture was dissolved in the much more environmentally friendly water instead of in an organic solvent.
Wie nachfolgende Tabelle 1 zeigt, liegen die auf die Chlorophyllsynthese normierten Strahlungsanteile der erfindungsgemäßen Leuchtstoffmischung im Vergleich zu einer in der bisherigen Praxis verwendeten Mischung eines Calcium-Wolframats und eines mit Mangan aktivierten Magnesium-Fluorogermanats deutlich günstiger, wenn das Sonnenlicht als Bezugsquelle herangezogen wird. Insbesondere konnte der Wert der Metamorphose bei 730 nm gegenüber den bisher verwendeten Leuchtstofflampen für Pflanzenbestrahlung verdreifacht und damit dem Sonnenlicht sehr viel mehr angenähert werden. Der höhere Wert der Photosynthese bewirkt zusätzlich eine verstärkte Produktion der Biomasse.
Der Tabelle 2 ist zu entnehmen, wieviel Prozent der von der Lampe aufgenommenen elektrischen Leistung auf die einzelnen für die Pflanze verfügbaren Strahlungsanteile entfallen.
Claims (3)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19823214550 DE3214550A1 (en) | 1982-04-20 | 1982-04-20 | FLUORESCENT LAMP FOR EXCITING A BALANCED PLANT GROWTH |
| DE3214550 | 1982-04-20 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0092220A2 true EP0092220A2 (en) | 1983-10-26 |
| EP0092220A3 EP0092220A3 (en) | 1984-03-28 |
| EP0092220B1 EP0092220B1 (en) | 1985-12-04 |
Family
ID=6161384
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83103743A Expired EP0092220B1 (en) | 1982-04-20 | 1983-04-18 | Fluoresent lamp for stimulating a uniform plant growth |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0092220B1 (en) |
| DE (2) | DE3214550A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0188211A1 (en) * | 1985-01-07 | 1986-07-23 | GTE Products Corporation | Fluorescent lamp substantially approximating the ultraviolet spectrum of natural sunlight |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4055781A (en) * | 1974-09-09 | 1977-10-25 | Gte Sylvania Incorporated | Special purpose fluorescent lamp |
-
1982
- 1982-04-20 DE DE19823214550 patent/DE3214550A1/en not_active Withdrawn
-
1983
- 1983-04-18 EP EP83103743A patent/EP0092220B1/en not_active Expired
- 1983-04-18 DE DE8383103743T patent/DE3361390D1/en not_active Expired
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4055781A (en) * | 1974-09-09 | 1977-10-25 | Gte Sylvania Incorporated | Special purpose fluorescent lamp |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0188211A1 (en) * | 1985-01-07 | 1986-07-23 | GTE Products Corporation | Fluorescent lamp substantially approximating the ultraviolet spectrum of natural sunlight |
Also Published As
| Publication number | Publication date |
|---|---|
| DE3214550A1 (en) | 1983-10-20 |
| EP0092220A3 (en) | 1984-03-28 |
| DE3361390D1 (en) | 1986-01-16 |
| EP0092220B1 (en) | 1985-12-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE2339181C2 (en) | Fluorescent lamp for the effective stimulation of plant growth | |
| DE2642704C3 (en) | Fluorescent cover for a fluorescent lamp | |
| DE19521119C2 (en) | Slowly decaying phosphors | |
| DE2446479C3 (en) | Phosphor layer for a low-pressure mercury vapor discharge lamp | |
| DE3047655A1 (en) | FLUORESCENT LAMP | |
| DE2202521C2 (en) | High pressure mercury vapor lamp | |
| DE2726523A1 (en) | FLUORESCENT LAMP | |
| DE2848726C2 (en) | Fluorescent layer for a fluorescent lamp | |
| DE2812120A1 (en) | HIGH PRESSURE MERCURY VAPOR DISCHARGE LAMP | |
| DE4321812A1 (en) | Luminescent substance (phosphor, luminophore) emitting blue light for use in fluorescent lamps, and fluorescent lamp utilising this substance | |
| DE2029303A1 (en) | ||
| DE2837867C2 (en) | Fluorescent cover for a fluorescent lamp | |
| DE1905181B2 (en) | FLUORS LAMP WITH IMPROVED COLOR REPRODUCTION | |
| DE2029302A1 (en) | ||
| EP0092220B1 (en) | Fluoresent lamp for stimulating a uniform plant growth | |
| DE2935711C2 (en) | Fluorescent coating of a fluorescent lamp | |
| DE1132274B (en) | Alkaline earth orthophosphate phosphor and process for its preparation | |
| DE2848725C2 (en) | Alkaline earth borate phosphate phosphor activated with divalent europium | |
| DE1810999C3 (en) | Alkaline earth silicate phosphor | |
| DE1901693A1 (en) | Luminous red fluorescent material for the screens of electron beam tubes | |
| DE3127679C2 (en) | Fluorescent lamp with a mixture of two phosphors | |
| DE2129100C3 (en) | Fluorescent lamp with a single layer of a mixture of phosphors | |
| DE2803448C2 (en) | Fluorescent cover for a fluorescent lamp | |
| DE2239212C3 (en) | Fluorescent lamp | |
| DE1592828C (en) | Process for the production of a phosphor based on alkaline earth phosphates and zinc silicate |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT SE |
|
| 17P | Request for examination filed |
Effective date: 19840505 |
|
| ITF | It: translation for a ep patent filed |
Owner name: JACOBACCI & PERANI S.P.A. |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT SE |
|
| REF | Corresponds to: |
Ref document number: 3361390 Country of ref document: DE Date of ref document: 19860116 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 83103743.7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19970421 Year of fee payment: 15 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980419 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 83103743.7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19990413 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19990422 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000418 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20000418 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20001229 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20020618 Year of fee payment: 20 |