EP0881279A2 - Process for making granules containing cationic surfactant - Google Patents
Process for making granules containing cationic surfactant Download PDFInfo
- Publication number
- EP0881279A2 EP0881279A2 EP98108983A EP98108983A EP0881279A2 EP 0881279 A2 EP0881279 A2 EP 0881279A2 EP 98108983 A EP98108983 A EP 98108983A EP 98108983 A EP98108983 A EP 98108983A EP 0881279 A2 EP0881279 A2 EP 0881279A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- granules
- weight
- granulation
- cationic surfactant
- cationic surfactants
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/0034—Fixed on a solid conventional detergent ingredient
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/62—Quaternary ammonium compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/06—Powder; Flakes; Free-flowing mixtures; Sheets
Definitions
- the invention is in the field of the production of cationic surfactant-containing granules such as for example in special detergents and cleaning agents, but also in Fabric softeners are used.
- Cationic surfactants are used in special detergents and cleaning agents, but especially in Laundry post-treatment agents such as fabric softeners are used to achieve the appropriate agents treated textiles a soft, fluffy feel and to give pleasant wearing properties. This also makes it easier on the textiles applied cationic surfactant ironing and leads to an antistatic effect that in turn, the wearing properties improved.
- the cationic surfactants are usually in the form of dispersions liquid to pasty agent incorporated.
- German published patent application DE 32 43 983 (Degussa AG) describes a free-flowing plasticizer concentrate which consists of one or more cationic surfactants which, if appropriate in a mixture with one or more solubilizers, are applied to synthetic silica.
- the agents are prepared by mixing a melt or a solution of the cationic surfactant in isopropanol with the precipitated and spray-dried silica.
- Powdery fabric softening agents which contain cationic surface-active substances adsorbed on highly absorbent silica are also described in German Offenlegungsschrift DE 34 02 437 (REWO Chemische Werke GmbH).
- the cationic surfactants are applied as a melt or solution in isopropanol to the silica in the mixer and then granulated under shear.
- German patent specification DE 43 08 794 (Henkel KGaA) describes special solid cationic surfactant preparation forms which are obtained by alkylation of triethanolamine esters in the presence of suitable dispersants (in particular fatty alcohols).
- suitable dispersants in particular fatty alcohols.
- the products obtained in this way have a flake-like structure and low bulk densities.
- the first two methods have the disadvantage that the cationic surfactant is in the form its isopropanol solution or as a melt on an insoluble carrier is applied.
- the isopropanol has to be used again later be removed by evaporation, since alcohols have the powder properties of Change detergent compositions negatively.
- it is a Energy required to melt the cationic surfactant.
- silica in large amounts also leads to the use in detergents and fabric softeners to form residues in the treated textiles.
- the disadvantage of all prior art processes is that they are products deliver that are only poorly soluble or not soluble without residues. The latter Process also delivers products with an undesirable low Bulk density.
- the object of the invention was to provide a method for manufacturing to provide cationic surfactant-containing granules which the process and Overcomes product disadvantages.
- the invention further relates to a process for the preparation of cationic surfactants Granules, in which a solid carrier in a mixer under low Energy input with a cationic surfactant and then with the addition of Water or an aqueous solution of one or more granulating aids under high Granular energy input.
- the cationic surfactant can be added directly to the mixer in its delivery form, or in the form of a liquid to pasty cationic surfactant preparation form on the solid Carriers are injected.
- Such forms of cationic surfactants can be for example by mixing commercially available cationic surfactants with auxiliaries such as Manufacture non-ionic surfactants, polyethylene glycols or polyols. Even lower ones Alcohols such as ethanol and isopropanol can be used, the amount of such lower alcohols in the liquid cationic surfactant preparation form from the reasons mentioned above should be less than 10% by weight.
- the cationic active substance can also by the procedure according to the invention can be introduced directly into the process, without first being melted or in solution to be brought.
- Solid cationic surfactants are simply added and act on the carrier in the course of the granulation; Forms of cationic surfactants other states of matter are added, poured in or depending on the viscosity atomized. All are solid carriers to which the cationic surfactant is applied.
- Carriers or builders usually used in detergents and cleaning agents suitable, carriers from the group of alkali metal sulfates, carbonates, silicates, citrates, aluminum silicates and citric acid are preferred.
- cellulose and Starch and its derivatives are natural polysaccharides such as cellulose and Starch and its derivatives. These include, in particular, cellulose and starch itself, however also cellulose and starch derivatives, which are made from cellulose by polymer-analogous reactions or starch are available. Such chemically modified celluloses or starches include, for example, products from esterifications or etherifications in which Hydroxy hydrogen atoms have been substituted. But also celluloses or starches, in which the hydroxyl groups against functional groups that do not have a Oxygen atoms are bound, have been replaced, can be used as a carrier material. In the group of these derivatives includes, for example, alkali celluloses, Carboxymethyl cellulose (CMC) and starch (CMS), cellulose and starch esters and - ether and aminocelluloses and starches.
- CMC Carboxymethyl cellulose
- CMS starch
- the amount of solid carrier in the resulting granules 50 to 80 wt .-% of the weight of the resulting granules.
- Cationic surfactants used for the process according to the invention are all customary surface-active substances into consideration, with cationic surfactants with fabric softening Effect are clearly preferred.
- the granules are produced by placing the solid support in a mixer and then applied the cationic surfactant with low energy input. Then one or more granulation aids in the form of an aqueous Solution added and the mixture wet granulated with high energy input.
- Low energy input in the sense of the method according to the invention means slow-running mixing tools or the omission of the use of additional chopping units.
- This low energy input is further characterized in that the agitator shaft of the mixer does not swirl the mix, but rather a kind Haufwerksmischen "is carried out. In which the mix in the mixer is raised in the direction of rotation.
- high energy input means a rapid rotation of the mixing tools or the use of additional comminution units.
- particles from the bed of material are whirled into the free mixing space and the Mixing fluidized more and more, in this state the particles fill the mixer in the form of a cloud, the so-called mechanically generated fluidized bed ".
- the product is finally hurled onto the mixer wall in the form of a mixing material ring, which rotates there.
- the process according to the invention can be carried out in both high-intensity and slow-running mixers, the high-intensity mixers being operated slowly in the first process step and the energy input required for the second process step being provided by the slow-moving mixers by means of additional units such as knife rings.
- high-speed mixers are the Lödige® CB 30 recycler, the Schugi® granulator, the Eirich® mixer type R or the Drais® K-TTP 80
- examples of slow-speed mixer granulators are the Drais® KT 160 and the Lödige® KM 300. The latter is often called Lödige ploughshare mixer ".
- aqueous solutions Solutions of carboxymethyl cellulose (CMC), water glass or citric acid are all of the auxiliaries commonly used in wet granulation usable.
- the process according to the invention particularly advantageously uses aqueous solutions Solutions of carboxymethyl cellulose (CMC), water glass or citric acid.
- CMC carboxymethyl cellulose
- the aqueous solutions have a dependency on the particular granulation aid different concentrations of the substances used. This is how CMC for example in 0.2 to 5% by weight aqueous solutions, water glass in the form 10 to 40 wt .-% warmer solutions and citric acid than 5 to 30 wt .-% aqueous Solution used.
- the method according to the invention can also be used as pure water without dissolved ingredients Use granulation aids without losing their advantages.
- the water or the aqueous solutions of the granulation aids are used in amounts of 1 to 5, preferably from 2 to 4% by weight, based on the weight of the resulting granules, added in the second stage of the process.
- the granulated particles that arise in the process according to the invention can subjected to customary post-treatment steps for detergents and cleaning agents will.
- drying of the granules in the fluidized bed is Connection to the granulation preferred.
- powdering, rounding or a different surface treatment can be applied directly to the granulation or to a connect any drying
- the cationic surfactant-containing granules according to the invention contain 5 to 40% by weight of one or several textile softening agents of the above formulas I, II or III, 50 up to 90% by weight of a solid carrier from the group of alkali metal sulfates, carbonates, silicates, citrates and aluminum silicates and citric acid and 0 to 5, preferably 0.1 to 5 wt .-% of one or more granulation aids, each based on the Weight of the granulate.
- Granules containing cationic surfactants are preferred in which the Cationic surfactant component a) is a quaternized triethanolamine ester.
- Preferred Granules containing cationic surfactants contain sodium sulfate as solid carrier component b).
- Granulation aids c) are preferably one or more substances selected from the Group of cellulose derivatives, water glasses or organic salts used.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
- Glanulating (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
Die Erfindung liegt auf dem Gebiet der Herstellung kationtensidhaltiger Granulate, wie sie beispielsweise in speziellen Wasch- und Reinigungsmitteln, aber auch in Weichspülern eingesetzt werden.The invention is in the field of the production of cationic surfactant-containing granules such as for example in special detergents and cleaning agents, but also in Fabric softeners are used.
Kationtenside werden in speziellen Wasch- und Reinigungsmitteln, insbesondere aber in Wäschenachbehandlungsmitteln wie Weichspülern eingesetzt, um den mit den entsprechenden Mitteln behandelten Textilien einen weichen, flauschigen Griff und angenehme Trageeigenschaften zu verleihen. Zusätzlich erleichtert das auf die Textilien aufgebrachte Kationtensid das Bügeln und führt zu einem antistatischen Effekt, der wiederum die Trageeigenschaften verbessert.Cationic surfactants are used in special detergents and cleaning agents, but especially in Laundry post-treatment agents such as fabric softeners are used to achieve the appropriate agents treated textiles a soft, fluffy feel and to give pleasant wearing properties. This also makes it easier on the textiles applied cationic surfactant ironing and leads to an antistatic effect that in turn, the wearing properties improved.
Üblicherweise werden die Kationtenside dabei in Form von Dispersionen in die zumeist flüssigen bis pastösen Mittel eingearbeitet. Feste Zubereitungsformen, die als Aktivsubstanz nur Kationtensid und keine anderen Tenside enthalten, sind dabei vergleichsweise selten.The cationic surfactants are usually in the form of dispersions liquid to pasty agent incorporated. Solid preparation forms, which as Active substance containing only cationic surfactant and no other surfactants are included comparatively rare.
Dennoch hat es an Versuchen nicht gefehlt, Kationtenside auch in Form fester lagerstabiler Zubereitungsformen herzustellen, um so optional auch feste Waschmittelzusammen-setzungen oder Weichspüler herstellen zu können.Nevertheless, there was no shortage of tests, cationic surfactants also in the form of more solid ones to prepare storage-stable preparation forms, so optionally also solid ones To be able to produce detergent compositions or fabric softener.
So beschreibt die deutsche Offenlegungsschrift DE 32 43 983 (Degussa AG) ein rieselfähiges Weichmacherkonzentrat, das aus einem oder mehreren Kationtensiden besteht, die -gegebenenfalls in Mischung mit einem oder mehreren Lösungsvermittlernauf synthetische Kieselsäure aufgebracht werden. Die Herstellung der Mittel erfolgt dabei durch Mischen einer Schmelze oder einer Lösung des Kationtensids in Isopropanol mit der gefällten und sprühgetrockneten Kieselsäure.For example, German published patent application DE 32 43 983 (Degussa AG) describes a free-flowing plasticizer concentrate which consists of one or more cationic surfactants which, if appropriate in a mixture with one or more solubilizers, are applied to synthetic silica. The agents are prepared by mixing a melt or a solution of the cationic surfactant in isopropanol with the precipitated and spray-dried silica.
Pulverförmige Wäscheweichspülmittel, die kationische oberflächenaktive Substanzen -adsorbiert auf hochsaugfähiger Kieselsäure- enthalten, sind auch in der deutschen Offenlegungsschrift DE 34 02 437 (REWO Chemische Werke GmbH) beschrieben. Hierbei werden die Kationtenside als Schmelze oder Lösung in Isopropanol auf die im Mischer vorgelegte Kieselsäure aufgegeben und dann unter Scherung granuliert.Powdery fabric softening agents which contain cationic surface-active substances adsorbed on highly absorbent silica are also described in German Offenlegungsschrift DE 34 02 437 (REWO Chemische Werke GmbH). Here, the cationic surfactants are applied as a melt or solution in isopropanol to the silica in the mixer and then granulated under shear.
Spezielle feste Kationtensid-Zubereitungsformen, die durch Alkylierung von Triethanolaminestern in Gegenwart von geeigneten Dispergatoren (insbesondere Fettalkoholen) gewonnen werden, beschreibt die deutsche Patentschrift DE 43 08 794 (Henkel KGaA). Die auf diese Weise erhaltenen Produkte haben eine flockenähnliche Struktur und geringe Schüttgewichte.The German patent specification DE 43 08 794 (Henkel KGaA) describes special solid cationic surfactant preparation forms which are obtained by alkylation of triethanolamine esters in the presence of suitable dispersants (in particular fatty alcohols). The products obtained in this way have a flake-like structure and low bulk densities.
Die beiden erstgenannten Verfahren besitzen den Nachteil, daß das Kationtensid in Form seiner isopropanolischen Lösung oder als Schmelze auf einen unlöslichen Träger aufgebracht wird. Im ersten Fall sind umfangreiche Arbeitsschutz- und Sicherheitsmaßnahmen erforderlich, und zusätzlich muß das Isopropanol später wieder durch Verdampfüng entfernt werden, da Alkohole die Pulvereigenschaften von Waschmittelzusammensetzungen negativ verändern. Im zweiten Falle ist ein Energieaufwand zum Aufschmelzen des Kationtensids erforderlich. Der Einsatz von Kieselsäure in hohen Mengen führt darüber hinaus bei der Verwendung in Wasch- und Weichspülmitteln zu Rückstandsbildungen bei den behandelten Textilien. Allen Verfahren des Standes der Technik ist der Nachteil gemeinsam, daß sie Produkte liefern, die nur schlecht bzw. nicht rückstandsfrei löslich sind. Das letzgenannte Verfahren liefert darüber hinaus Produkte mit einem unerwünschten niedrigem Schüttgewicht. The first two methods have the disadvantage that the cationic surfactant is in the form its isopropanol solution or as a melt on an insoluble carrier is applied. In the first case there are extensive occupational safety and health Safety measures are required, and additionally the isopropanol has to be used again later be removed by evaporation, since alcohols have the powder properties of Change detergent compositions negatively. In the second case it is a Energy required to melt the cationic surfactant. The use of silica in large amounts also leads to the use in detergents and fabric softeners to form residues in the treated textiles. The disadvantage of all prior art processes is that they are products deliver that are only poorly soluble or not soluble without residues. The latter Process also delivers products with an undesirable low Bulk density.
Die Aufgabe der Erfindung lag nun darin, ein Verfahren zur Herstellung kationtensidhaltiger Granulate bereitzustellen, das die genannten Verfahrens- und Produktnachteile überwindet.The object of the invention was to provide a method for manufacturing to provide cationic surfactant-containing granules which the process and Overcomes product disadvantages.
Gegenstand der Erfindung ist ein festes, kationtensidhaltiges Granulat, das
Gegenstand der Erfindung ist weiterhin ein Verfahren zur Herstellung kationtensidhaltiger Granulate, bei dem man einen festen Träger in einem Mischer unter niedrigem Energieeintrag mit einem Kationtensid beaufschlagt und anschließend unter Zugabe von Wasser oder einer wäßrigen Lösung eines oder mehrerer Granulierhilfsmittel unter hohem Energieeintrag granuliert.The invention further relates to a process for the preparation of cationic surfactants Granules, in which a solid carrier in a mixer under low Energy input with a cationic surfactant and then with the addition of Water or an aqueous solution of one or more granulating aids under high Granular energy input.
Das Kationtensid kann dabei in seiner Lieferform direkt in den Mischer gegeben werden, oder in Form einer flüssigen bis pastösen Kationtensid-Zubereitungsform auf den festen Träger aufgedüst werden. Solche Kationtensid-Zubereitungsformen lassen sich beispielsweise durch Mischen handelsüblicher Kationtenside mit Hilfsstoffen wie nichtionischen Tensiden, Polyethylenglycolen oder Polyolen herstellen. Auch niedere Alkohole wie Ethanol und Isopropanol können eingesetzt werden, wobei die Menge an solchen niederen Alkoholen in der flüssigen Kationtensid-Zubereitungsform aus den obengenannten Gründen unter 10 Gew.-% liegen sollte. The cationic surfactant can be added directly to the mixer in its delivery form, or in the form of a liquid to pasty cationic surfactant preparation form on the solid Carriers are injected. Such forms of cationic surfactants can be for example by mixing commercially available cationic surfactants with auxiliaries such as Manufacture non-ionic surfactants, polyethylene glycols or polyols. Even lower ones Alcohols such as ethanol and isopropanol can be used, the amount of such lower alcohols in the liquid cationic surfactant preparation form from the reasons mentioned above should be less than 10% by weight.
Durch die erfindungsgemäße Verfahrensweise kann die kationische Aktivsubstanz auch direkt in das Verfahren eingebracht werden, ohne vorher aufgeschmolzen oder in Lösung gebracht werden zu müssen. Hierbei werden feste Kationtenside einfach zugegeben und beaufschlagen den Trägerstoff im Zuge der Granulation; Kationtensid-Lieferungsformen anderer Aggregatzustände werden je nach Viskosität zugegeben, eingegossen oder verdüst. Als feste Träger, auf die das Kationtensid aufgebracht wird, sind sämtliche üblicherweise in Wasch- und Reinigungsmitteln eingesetzten Träger- oder Gerüststoffe geeignet, wobei Träger aus der Gruppe der Alkalimetallsulfate, -carbonate, -silikate, -citrate, -aluminiumsilikate und Citronensäure bevorzugt sind. Besonders bevorzugt sind wasserlösliche Trägerstoffe und hierunter insbesondere das Natriumsulfat.The cationic active substance can also by the procedure according to the invention can be introduced directly into the process, without first being melted or in solution to be brought. Solid cationic surfactants are simply added and act on the carrier in the course of the granulation; Forms of cationic surfactants other states of matter are added, poured in or depending on the viscosity atomized. All are solid carriers to which the cationic surfactant is applied Carriers or builders usually used in detergents and cleaning agents suitable, carriers from the group of alkali metal sulfates, carbonates, silicates, citrates, aluminum silicates and citric acid are preferred. Are particularly preferred water-soluble carriers and in particular the sodium sulfate.
Weitere bevorzugte Trägermaterialien sind natürliche Polysaccharide wie Cellulose und Stärke sowie ihre Derivate. Hierzu zählen insbesondere Cellulose und Stärke selbst, aber auch Cellulose- und Stärke-Derivate, die durch polymeranaloge Reaktionen aus Cellulose bzw. Stärke erhältlich sind. Solche chemisch modifizierten Cellulosen bzw. Stärken umfassen dabei beispielsweise Produkte aus Veresterungen bzw. Veretherungen, in denen Hydroxy-Wasserstoffatome substituiert wurden. Aber auch Cellulosen bzw. Stärken, in denen die Hydroxy-Gruppen gegen funktionelle Gruppen, die nicht über ein Sauerstoffatom gebunden sind, ersetzt wurden, lassen sich als Trägermaterial einsetzen. In die Gruppe dieser Derivate fallen beispielsweise Alkalicellulosen, Carboxymethylcellulose (CMC) und -stärke (CMS), Cellulose- und Stärkeester und - ether sowie Aminocellulosen und -stärken.Other preferred carrier materials are natural polysaccharides such as cellulose and Starch and its derivatives. These include, in particular, cellulose and starch itself, however also cellulose and starch derivatives, which are made from cellulose by polymer-analogous reactions or starch are available. Such chemically modified celluloses or starches include, for example, products from esterifications or etherifications in which Hydroxy hydrogen atoms have been substituted. But also celluloses or starches, in which the hydroxyl groups against functional groups that do not have a Oxygen atoms are bound, have been replaced, can be used as a carrier material. In the group of these derivatives includes, for example, alkali celluloses, Carboxymethyl cellulose (CMC) and starch (CMS), cellulose and starch esters and - ether and aminocelluloses and starches.
Als besonders bevorzugtes Trägermaterial aus dieser Gruppe von Wasch- und Reinigungsmittel-Inhaltsstoffen wird Stärke eingesetzt.As a particularly preferred carrier material from this group of washing and Starch is used in detergent ingredients.
Vorzugsweise macht die Menge des festen Trägerstoffs in den entstehenden Granulaten 50 bis 80 Gew.-% des Gewichts des entstehenden Granulats aus. Preferably the amount of solid carrier in the resulting granules 50 to 80 wt .-% of the weight of the resulting granules.
Als Kationtenside kommen für das erfindungsgemäße Verfahren alle üblichen oberflächenaktiven Stoffe in Betracht, wobei Kationtenside mit textilweichmachender Wirkung deutlich bevorzugt sind.Cationic surfactants used for the process according to the invention are all customary surface-active substances into consideration, with cationic surfactants with fabric softening Effect are clearly preferred.
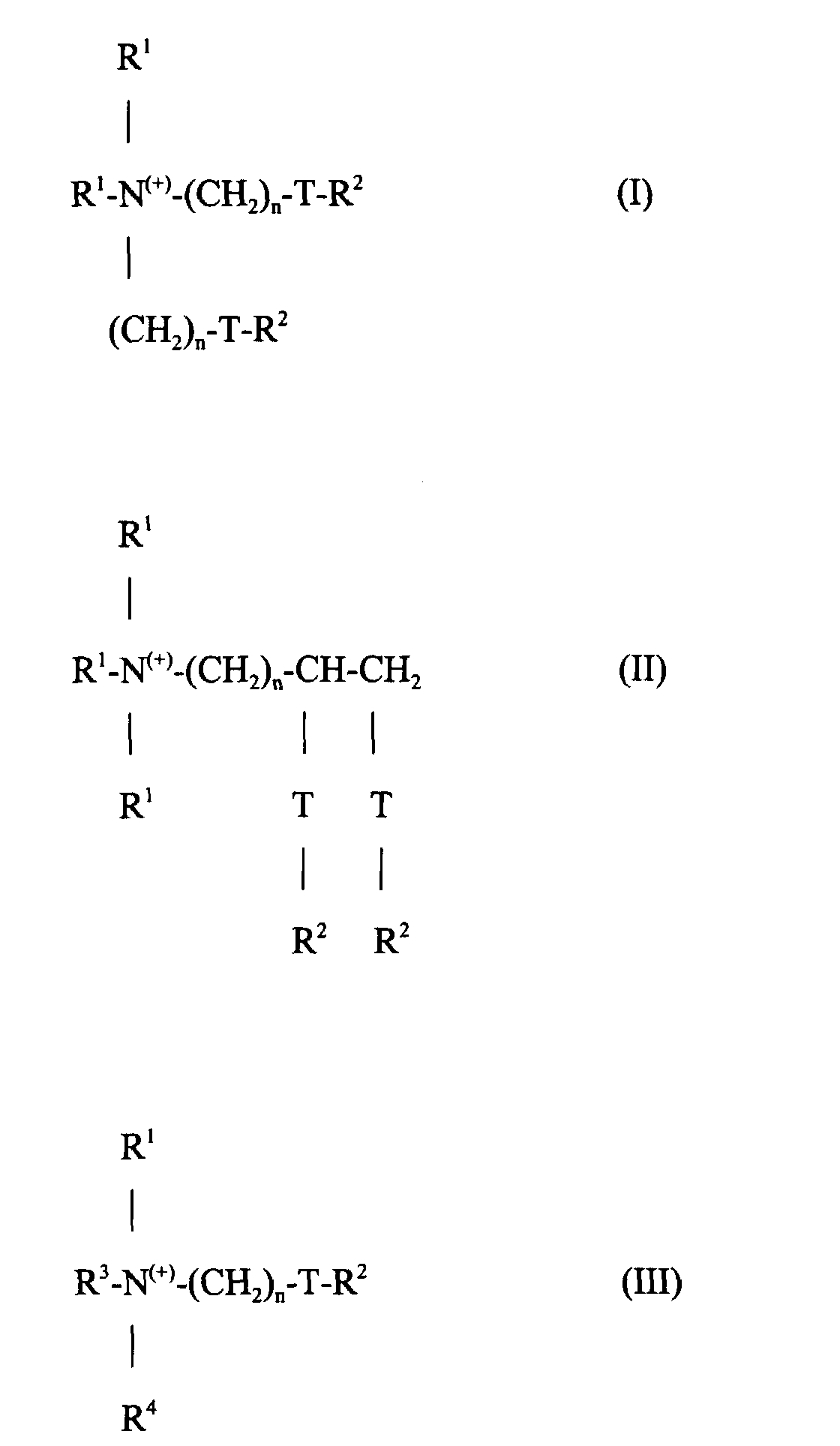
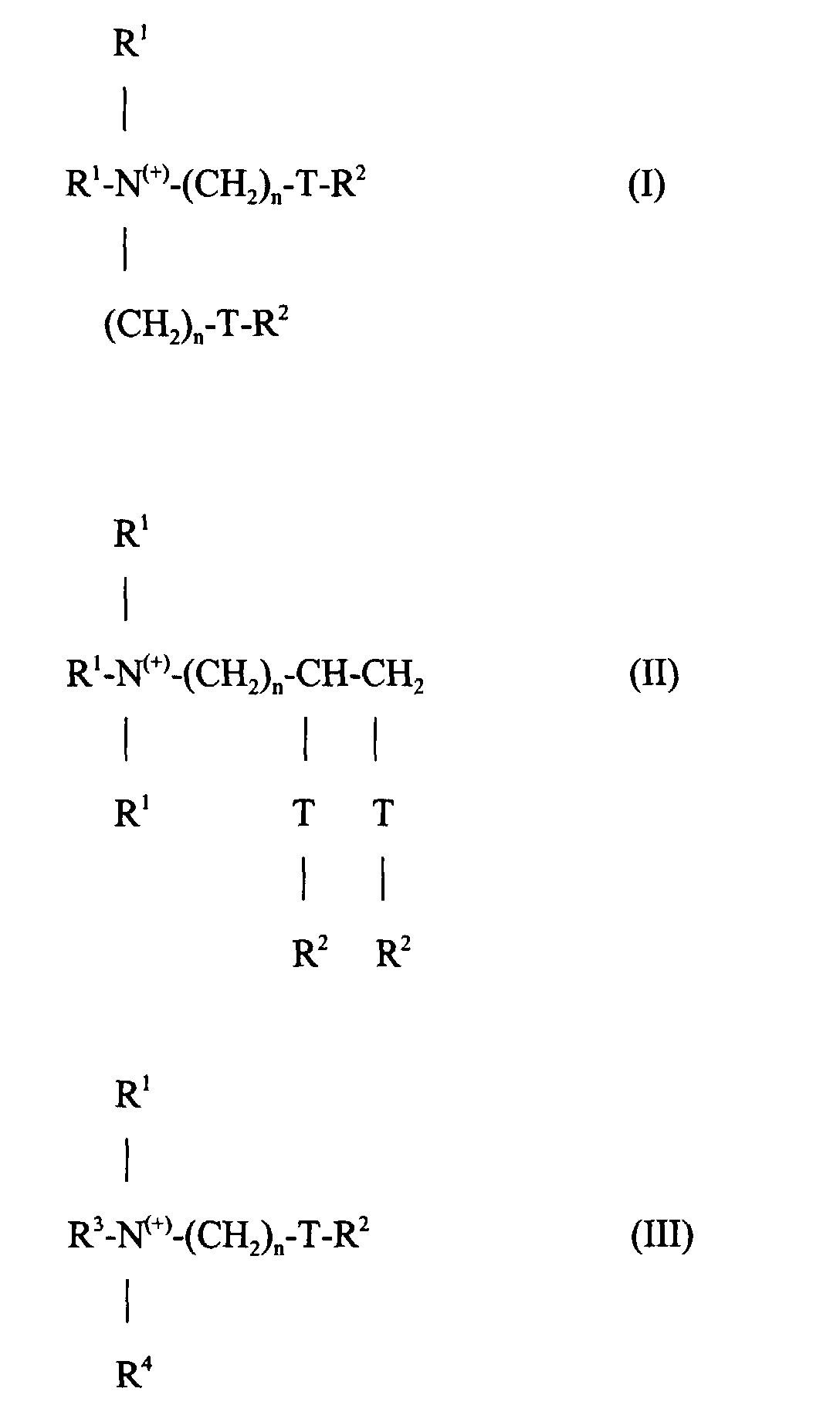
Die Erfindungsgemäßen Mittel enthalten als kationische Aktivsubstanzen mit textilweichmachender Wirkung 5 bis 40 Gew.-% eines oder mehrerer kationischer, textilweichmachender Mittel der Formeln I, II oder III: worin jede Gruppe R1 unabhängig voneinander ausgewählt ist aus C1-6-Alkyl-, -Alkenyl- oder -Hydroxyalkylgruppen; jede Gruppe R2 unabhängig voneinander ausgewählt ist aus C8-28-Alkyl- oder -Alkenylgruppen; R3 = R1 oder (CH2)n-T-R2; R4 = R1 oder R2 oder (CH2)n-T-R2; T = -CH2-, -O-CO-oder -CO-O- und n eine ganze Zahl von 0 bis 5 ist.The agents according to the invention contain, as cationic active substances with a fabric softening effect, 5 to 40% by weight of one or more cationic fabric softening agents of the formulas I, II or III: wherein each R 1 group is independently selected from C 1-6 alkyl, alkenyl or hydroxyalkyl groups; each R 2 group is independently selected from C 8-28 alkyl or alkenyl groups; R 3 = R 1 or (CH 2 ) n -TR 2 ; R 4 = R 1 or R 2 or (CH 2 ) n -TR 2 ; T = -CH 2 -, -O-CO-or -CO-O- and n is an integer from 0 to 5.
Die Granulate werden hergestellt, indem man den festen Träger in einem Mischer vorlegt und dann unter niedrigem Energieeintrag mit dem Kationtensid beaufschlagt. Anschließend werden ein oder mehrere Granulierhilfsmittel in Form einer wäßrigen Lösung zugegeben und die Mischung unter hohem Energieeintrag naßgranuliert.The granules are produced by placing the solid support in a mixer and then applied the cationic surfactant with low energy input. Then one or more granulation aids in the form of an aqueous Solution added and the mixture wet granulated with high energy input.
Niedriger Energieeintrag im Sinne des erfindungsgemäßen Verfahrens bedeutet
langsamlaufende Mischwerkzeuge bzw. der Verzicht auf den Einsatz zusätzlicher
Zerhack-Aggregate. Dieser niedrige Energieeintrag ist weiterhin dadurch gekennzeichnet,
daß die Rührwelle des Mischers das Mischgut nicht verwirbelt, sondern vielmehr eine Art
Haufwerksmischen" durchgeführt wird. bei dem das Mischgut im Mischer in
Drehrichtung angehoben wird. Hoher Energieeintrag bedeutet im Sinne des
erfindungsgemäßen Verfahrens eine schnelle Drehung der Mischwerkzeuge bzw. der
Einsatz zusätzlicher Zerkleinerungsaggregate. Hierbei werden vermehrt Teilchen aus dem
Gutbett in den freien Mischraum hineingewirbelt und die Mischung mehr und mehr
fluidisiert. In diesem Zustand füllen die Teilchen den Mischer in Form einer Wolke, des
sogenannten
Das erfindungsgemäße Verfahren kann sowohl in Hochintensitäts- als auch in
langsamlaufenden Mischern durchgeführt werden, wobei die Hochintensitätsmischer in
der ersten Verfahrensstufe langsamlaufend betrieben werden und der für die zweite
Verfahrensstufe benötigte Energieeintrag bei den langsamlaufenden Mischern durch
Zusatzaggregate wie beispielsweise Messerkränze erbracht wird. Beispiele für
schnellaufende Mischer sind der Lödige® CB 30 Recycler, der Schugi® Granulator, der
Eirich® -Mischer Typ R oder der Drais® K-TTP 80, Beispiele für langsamlaufende
Mischgranulatoren sind der Drais® K-T 160 sowie der Lödige® KM 300. Letzterer wird
oftmals als
Als Granulierhilfsmittel, die in der zweiten Verfahrensstufe in Form wäßriger Lösungen zugegeben werden, sind alle üblicherweise bei Naßgranulationen verwendeten Hilfsstoffe einesetzbar. Mit besonderem Vorteil verwendet das erfindungsgemäße Verfahren wäßrige Lösungen von Carboxymethylcellulose (CMC), Wasserglas oder Citronensäure. In Abhängigkeit vom jeweiligen Granulationshilfsmittel weisen die wäßrigen Lösungen unterschiedliche Konzentrationen an den eingesetzten Stoffen auf. So wird CMC beispielsweise in 0,2 bis 5 Gew.-%-igen wäßrigen Lösungen, Wasserglas in Form 10 bis 40 Gew.-%iger wäriger Lösungen und Citronensäure als 5 bis 30 Gew.-%ige wäßrige Lösung eingesetzt.As a granulation aid in the second stage of the process in the form of aqueous solutions are added are all of the auxiliaries commonly used in wet granulation usable. The process according to the invention particularly advantageously uses aqueous solutions Solutions of carboxymethyl cellulose (CMC), water glass or citric acid. In The aqueous solutions have a dependency on the particular granulation aid different concentrations of the substances used. This is how CMC for example in 0.2 to 5% by weight aqueous solutions, water glass in the form 10 to 40 wt .-% warmer solutions and citric acid than 5 to 30 wt .-% aqueous Solution used.
Das erfindungsgemäße Verfahren kann auch reines Wasser ohne gelöste Inhaltsstoffe als Granulierhilfsmittel verwenden, ohne dabei seine Vorteile einzubüßen. Das Wasser oder die wäßrigen Lösungen der Granulierhilfsmittel werden in Mengen von 1 bis 5, vorzugsweise von 2 bis 4 Gew.-%, bezogen auf das Gewicht des entstehenden Granulats, in der zweiten Verfahrensstufe zugegeben.The method according to the invention can also be used as pure water without dissolved ingredients Use granulation aids without losing their advantages. The water or the aqueous solutions of the granulation aids are used in amounts of 1 to 5, preferably from 2 to 4% by weight, based on the weight of the resulting granules, added in the second stage of the process.
Die granulierten Teilchen, die im erfindungsgemäßen Verfahren entstehen, können den bei Wasch- und Reinigungsmitteln üblichen Nachbehandlungsschritten unterzogen werden. So ist beispielsweise eine Trocknung des Granulats in der Wirbelschicht im Anschluß an die Granulierung bevorzugt. Auch eine Abpuderung, Verrundung oder andersartige Oberflächenbehandlung kann sich direkt an die Granulation oder an eine eventuelle Trocknung anschließenThe granulated particles that arise in the process according to the invention can subjected to customary post-treatment steps for detergents and cleaning agents will. For example, drying of the granules in the fluidized bed is Connection to the granulation preferred. Also powdering, rounding or a different surface treatment can be applied directly to the granulation or to a connect any drying
Die erfindungsgemäßen kationtensidhaltigen Granulate enthalten 5 bis 40 Gew.-% eines oder mehrerer, textilweichmachender Mittel der obengenannten Formeln I, II oder III, 50 bis 90 Gew.-% eines festens Trägers aus der Gruppe der Alkalimetallsulfate, -carbonate, - silikate, -citrate und Aluminiumsilikate und Citronensäure sowie 0 bis 5, vorzugsweise 0,1 bis 5 Gew.-% eines oder mehrerer Granulierhilfsmittel, jeweils bezogen auf das Gewicht des Granulats.The cationic surfactant-containing granules according to the invention contain 5 to 40% by weight of one or several textile softening agents of the above formulas I, II or III, 50 up to 90% by weight of a solid carrier from the group of alkali metal sulfates, carbonates, silicates, citrates and aluminum silicates and citric acid and 0 to 5, preferably 0.1 to 5 wt .-% of one or more granulation aids, each based on the Weight of the granulate.
Dabei sind kationtensidhaltige Granulate bevorzugt, in denen die Kationtensidkomponente a) ein quaternierter Triethanolaminester ist. Bevorzugte kationtensidhaltige Granulate enthalten als feste Trägerkomponente b) Natriumsulfat. Als Granulierhilfsmittel c) werden bevorzugt einer oder mehrere Stoff, ausgewählt aus der Gruppe der Cellulosederivate, Wassergläser oder organischen Salze eingesetzt. Granules containing cationic surfactants are preferred in which the Cationic surfactant component a) is a quaternized triethanolamine ester. Preferred Granules containing cationic surfactants contain sodium sulfate as solid carrier component b). As Granulation aids c) are preferably one or more substances selected from the Group of cellulose derivatives, water glasses or organic salts used.
Verschiedene Trägerstoffe (siehe Tabelle) wurde in einem Lödige-Mischer vorgelegt und
bei niedrigem Energieeintrag (ohne Zerhacker) unter Zugabe des Kationtensids gemischt.
Anschließend wurden 3 Gew.-% wäßrige Lösungen von Carboxymethylcellulose (1%ig)
bzw. Wasserglas (30%ig) bzw. Citronensäure (25%ig), bezogen auf das Gewicht des
Granulats, zugegeben und die Mischung bei hohem Energieeintrag granuliert. Im
Anschluß an die Granulation erfolgte eine Trocknung der Produkte in der Wirbelschicht
bzw. eine Abpuderung/Verrundung. Als Vergleich wurde versucht, ein Kationtensid-Compound
durch Imprägnierung der betreffenden Trägerstoffe mit Kationtensid in einem
Mischer herzustellen. Die Zusammensetzungen der Mittel nach der Trocknung sind in
Tabelle 1 wiedergegeben, die physikalischen Eigenschaften finden sich in Tabelle 2.
Methyl-N-(2-hydroxyethyl-)-N,N-di(talgacyloxyethyl)ammoniummethosulfat,
95%ig in Isopropanol, Firma Stepan
CMC: Carboxymethylcellulose
Methyl-N- (2-hydroxyethyl -) - N, N-di (tallow acyloxyethyl) ammonium methosulfate, 95% in isopropanol, Stepan company
CMC: carboxymethyl cellulose
Weitere erfindungsgemäße Mittel, deren Zusammensetzung in der Tabelle 3 angegeben
ist, wurden nach der oben beschriebenen Verfahrensweise hergestellt; ihre physikalischen
Eigenschaften finden sich in Tabelle 4.
Methyl-N-(2-hydroxyethyl-)-N,N-di(talgacyloxyethyl)ammoniummethosulfat,
95%ig in Isopropanol, Firma Stepan
Stepantex® VL 90:
Methyl-N-(2-hydroxyethyl-)-N,N-di(talgacyloxyethyl)ammoniummethosulfat,
95%ig in Isopropanol, Firma Stepan
Stepantex® DC 90:
Methyl-N-(2-hydroxyethyl-)-N,N-di(oleyloxyethyl)ammoniummethosulfat, 90%ig
in Isopropanol, Firma Stepan
Dehyquart® AU 48:
Methyl-N-(2-hydroxyethyl-)-N,N-di(acyloxyethyl)ammoniummethosulfat, 90%ig
in Isopropanol, Firma Pulcra
Methyl-N- (2-hydroxyethyl -) - N, N-di (tallow acyloxyethyl) ammonium methosulfate, 95% in isopropanol, Stepan company
Stepantex® VL 90:
Methyl-N- (2-hydroxyethyl -) - N, N-di (tallow acyloxyethyl) ammonium methosulfate, 95% in isopropanol, Stepan company
Stepantex® DC 90:
Methyl-N- (2-hydroxyethyl -) - N, N-di (oleyloxyethyl) ammonium methosulfate, 90% in isopropanol, Stepan company
Dehyquart® AU 48:
Methyl-N- (2-hydroxyethyl) - N, N-di (acyloxyethyl) ammonium methosulfate, 90% in isopropanol, Pulcra company
Claims (11)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19721885 | 1997-05-26 | ||
| DE19721885A DE19721885A1 (en) | 1997-05-26 | 1997-05-26 | Process for the production of granules containing cationic surfactants |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| EP0881279A2 true EP0881279A2 (en) | 1998-12-02 |
| EP0881279A3 EP0881279A3 (en) | 1999-06-16 |
| EP0881279B1 EP0881279B1 (en) | 2003-01-02 |
| EP0881279B2 EP0881279B2 (en) | 2007-04-18 |
Family
ID=7830469
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP98108983A Expired - Lifetime EP0881279B2 (en) | 1997-05-26 | 1998-05-18 | Granules containing cationic surfactant |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0881279B2 (en) |
| AT (1) | ATE230431T1 (en) |
| DE (2) | DE19721885A1 (en) |
| ES (1) | ES2189034T5 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003083025A1 (en) * | 2002-03-28 | 2003-10-09 | Unilever Plc | Solid fabric conditioning compositions |
| WO2003083027A1 (en) * | 2002-03-28 | 2003-10-09 | Unilever Plc | Solid fabric conditioning compositions |
| EP1502942A1 (en) * | 2003-07-29 | 2005-02-02 | Clariant International Ltd. | Solid softener composition |
| EP3645693A4 (en) * | 2017-06-27 | 2021-03-17 | Henkel IP & Holding GmbH | Particulate fragrance enhancers |
| WO2023046690A1 (en) * | 2021-09-22 | 2023-03-30 | Unilever Ip Holdings B.V. | Fabric softening composition |
Citations (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3537893A (en) † | 1967-10-30 | 1970-11-03 | Neckar Chemie Dr Heinrich Kopp | Method of producing surfactant-modified starch |
| US3573091A (en) † | 1967-11-13 | 1971-03-30 | Armour & Co | Method of preparing water-dispersible softener compositions and products thereby |
| US3974125A (en) † | 1974-09-27 | 1976-08-10 | Exxon Research And Engineering Company | Higher dialkyl dimethyl ammonium clay gelling agents for unsaturated polyester compositions |
| US4000077A (en) † | 1972-05-04 | 1976-12-28 | Colgate-Palmolive Company | Enhancement of cationic softener |
| US4126586A (en) † | 1976-02-10 | 1978-11-21 | Lever Brothers Company | Process of spray drying nonionic surfactant-containing detergents also containing a cationic nitrogen compound |
| US4128485A (en) † | 1976-08-16 | 1978-12-05 | Colgate-Palmolive Company | Fabric softening compounds |
| GB2125452A (en) † | 1982-07-05 | 1984-03-07 | Lion Corp | Additive composition for granular detergent |
| EP0111074A2 (en) * | 1982-11-27 | 1984-06-20 | Degussa Aktiengesellschaft | Laundry softening concentrate |
| US4510073A (en) † | 1982-07-05 | 1985-04-09 | Lion Corporation | Method for granulating cationic surfactant |
| EP0149264A1 (en) † | 1983-11-09 | 1985-07-24 | Unilever N.V. | Stable, free-flowing particulate adjuncts for use in detergent compositions |
| US4536316A (en) † | 1983-06-01 | 1985-08-20 | Colgate-Palmolive Co. | Fabric softening composition containing surface modified clay |
| EP0151936A2 (en) * | 1984-01-25 | 1985-08-21 | REWO Chemische Werke GmbH | Washing agent having laundry softening properties and process for its production |
| EP0163910A1 (en) * | 1984-05-04 | 1985-12-11 | Hoechst Aktiengesellschaft | Washing materials containing softening agent |
| EP0175287A2 (en) * | 1984-09-21 | 1986-03-26 | Hoechst Aktiengesellschaft | Use of organophilic modified layered-silicic acid adsorbentsas additives in detergents |
| US4764292A (en) † | 1986-04-11 | 1988-08-16 | Lever Brothers Company | Fabric-softening particles |
| US4786422A (en) † | 1986-10-06 | 1988-11-22 | Colgate-Palmolive Co. | Fabric softening and antistatic particulate wash cycle laundry additive containing cationic/anionic surfactant complex on bentonite |
| EP0340013A2 (en) † | 1988-04-29 | 1989-11-02 | Unilever Plc | Detergent compositions and process for preparing them |
| US4911851A (en) † | 1987-11-06 | 1990-03-27 | The Procter & Gamble Company | Detergent compatible, dryer released fabric softening/antistatic agents |
| US5160454A (en) † | 1983-12-13 | 1992-11-03 | Southern Clay Products, Inc. | Process for manufacturing organoclays having enhanced gelling properties |
| EP0521635A1 (en) † | 1991-06-25 | 1993-01-07 | Unilever Plc | Particulate detergent composition or component |
| EP0523287A1 (en) * | 1991-07-18 | 1993-01-20 | The Procter & Gamble Company | Perfume additives for fabric-softening compositions |
| EP0547722A1 (en) † | 1991-12-18 | 1993-06-23 | Colgate-Palmolive Company | Free-flowing powder fabric softening composition and process for its manufacture |
| WO1995029215A1 (en) † | 1994-04-20 | 1995-11-02 | The Procter & Gamble Company | Process for the manufacture of free-flowing detergent granules |
| EP0690123A2 (en) † | 1994-06-30 | 1996-01-03 | Amway Corporation | Process for increasing liquid surfactant loading in free flowing powder detergents |
| WO1996017042A1 (en) † | 1994-12-02 | 1996-06-06 | The Procter & Gamble Company | Detergent compositions comprising cationic surfactant and process for making the composition |
| EP0753571A1 (en) † | 1995-07-10 | 1997-01-15 | The Procter & Gamble Company | Process for making granular detergent composition |
| US5597794A (en) † | 1990-07-05 | 1997-01-28 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of detergent surfactant granules comprising a recycle step |
| WO1997005221A1 (en) † | 1995-08-02 | 1997-02-13 | The Procter & Gamble Company | Detergent composition |
-
1997
- 1997-05-26 DE DE19721885A patent/DE19721885A1/en not_active Withdrawn
-
1998
- 1998-05-18 AT AT98108983T patent/ATE230431T1/en not_active IP Right Cessation
- 1998-05-18 EP EP98108983A patent/EP0881279B2/en not_active Expired - Lifetime
- 1998-05-18 DE DE59806786T patent/DE59806786D1/en not_active Expired - Lifetime
- 1998-05-18 ES ES98108983T patent/ES2189034T5/en not_active Expired - Lifetime
Patent Citations (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3537893A (en) † | 1967-10-30 | 1970-11-03 | Neckar Chemie Dr Heinrich Kopp | Method of producing surfactant-modified starch |
| US3573091A (en) † | 1967-11-13 | 1971-03-30 | Armour & Co | Method of preparing water-dispersible softener compositions and products thereby |
| US4000077A (en) † | 1972-05-04 | 1976-12-28 | Colgate-Palmolive Company | Enhancement of cationic softener |
| US3974125A (en) † | 1974-09-27 | 1976-08-10 | Exxon Research And Engineering Company | Higher dialkyl dimethyl ammonium clay gelling agents for unsaturated polyester compositions |
| US4126586A (en) † | 1976-02-10 | 1978-11-21 | Lever Brothers Company | Process of spray drying nonionic surfactant-containing detergents also containing a cationic nitrogen compound |
| US4128485A (en) † | 1976-08-16 | 1978-12-05 | Colgate-Palmolive Company | Fabric softening compounds |
| GB2125452A (en) † | 1982-07-05 | 1984-03-07 | Lion Corp | Additive composition for granular detergent |
| US4510073A (en) † | 1982-07-05 | 1985-04-09 | Lion Corporation | Method for granulating cationic surfactant |
| EP0111074A2 (en) * | 1982-11-27 | 1984-06-20 | Degussa Aktiengesellschaft | Laundry softening concentrate |
| US4536316A (en) † | 1983-06-01 | 1985-08-20 | Colgate-Palmolive Co. | Fabric softening composition containing surface modified clay |
| EP0149264A1 (en) † | 1983-11-09 | 1985-07-24 | Unilever N.V. | Stable, free-flowing particulate adjuncts for use in detergent compositions |
| US5160454A (en) † | 1983-12-13 | 1992-11-03 | Southern Clay Products, Inc. | Process for manufacturing organoclays having enhanced gelling properties |
| EP0151936A2 (en) * | 1984-01-25 | 1985-08-21 | REWO Chemische Werke GmbH | Washing agent having laundry softening properties and process for its production |
| EP0163910A1 (en) * | 1984-05-04 | 1985-12-11 | Hoechst Aktiengesellschaft | Washing materials containing softening agent |
| EP0175287A2 (en) * | 1984-09-21 | 1986-03-26 | Hoechst Aktiengesellschaft | Use of organophilic modified layered-silicic acid adsorbentsas additives in detergents |
| US4764292A (en) † | 1986-04-11 | 1988-08-16 | Lever Brothers Company | Fabric-softening particles |
| US4786422A (en) † | 1986-10-06 | 1988-11-22 | Colgate-Palmolive Co. | Fabric softening and antistatic particulate wash cycle laundry additive containing cationic/anionic surfactant complex on bentonite |
| US4911851A (en) † | 1987-11-06 | 1990-03-27 | The Procter & Gamble Company | Detergent compatible, dryer released fabric softening/antistatic agents |
| EP0340013A2 (en) † | 1988-04-29 | 1989-11-02 | Unilever Plc | Detergent compositions and process for preparing them |
| US5597794A (en) † | 1990-07-05 | 1997-01-28 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of detergent surfactant granules comprising a recycle step |
| EP0521635A1 (en) † | 1991-06-25 | 1993-01-07 | Unilever Plc | Particulate detergent composition or component |
| EP0523287A1 (en) * | 1991-07-18 | 1993-01-20 | The Procter & Gamble Company | Perfume additives for fabric-softening compositions |
| EP0547722A1 (en) † | 1991-12-18 | 1993-06-23 | Colgate-Palmolive Company | Free-flowing powder fabric softening composition and process for its manufacture |
| WO1995029215A1 (en) † | 1994-04-20 | 1995-11-02 | The Procter & Gamble Company | Process for the manufacture of free-flowing detergent granules |
| EP0690123A2 (en) † | 1994-06-30 | 1996-01-03 | Amway Corporation | Process for increasing liquid surfactant loading in free flowing powder detergents |
| WO1996017042A1 (en) † | 1994-12-02 | 1996-06-06 | The Procter & Gamble Company | Detergent compositions comprising cationic surfactant and process for making the composition |
| EP0753571A1 (en) † | 1995-07-10 | 1997-01-15 | The Procter & Gamble Company | Process for making granular detergent composition |
| WO1997005221A1 (en) † | 1995-08-02 | 1997-02-13 | The Procter & Gamble Company | Detergent composition |
Non-Patent Citations (2)
| Title |
|---|
| Chemical Abstracts of JP 63254199, JP 63138000, JP 01213476, JP 57010699, JP 59024800. † |
| The manufacture of detergent powder, de Groot, 1995, University of Eindhoven, Netherlands † |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003083025A1 (en) * | 2002-03-28 | 2003-10-09 | Unilever Plc | Solid fabric conditioning compositions |
| WO2003083027A1 (en) * | 2002-03-28 | 2003-10-09 | Unilever Plc | Solid fabric conditioning compositions |
| US6916779B2 (en) | 2002-03-28 | 2005-07-12 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Fabric conditioning compositions |
| US6989361B2 (en) | 2002-03-28 | 2006-01-24 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Solid fabric conditioning compositions |
| CN1306015C (en) * | 2002-03-28 | 2007-03-21 | 荷兰联合利华有限公司 | Solid fabric conditioning compositions |
| EP1502942A1 (en) * | 2003-07-29 | 2005-02-02 | Clariant International Ltd. | Solid softener composition |
| EP3645693A4 (en) * | 2017-06-27 | 2021-03-17 | Henkel IP & Holding GmbH | Particulate fragrance enhancers |
| US11441106B2 (en) | 2017-06-27 | 2022-09-13 | Henkel Ag & Co. Kgaa | Particulate fragrance enhancers |
| WO2023046690A1 (en) * | 2021-09-22 | 2023-03-30 | Unilever Ip Holdings B.V. | Fabric softening composition |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0881279A3 (en) | 1999-06-16 |
| ES2189034T5 (en) | 2007-11-16 |
| DE19721885A1 (en) | 1998-12-03 |
| EP0881279B1 (en) | 2003-01-02 |
| EP0881279B2 (en) | 2007-04-18 |
| DE59806786D1 (en) | 2003-02-06 |
| ES2189034T3 (en) | 2003-07-01 |
| ATE230431T1 (en) | 2003-01-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CH660198A5 (en) | PARTICULATE TEXTILE DETERGENT. | |
| CH657145A5 (en) | PARTICULATE, NON-IONOGENIC DETERGENT COMPOSITION WITH IMPROVED DIRT CLEANING POSSIBILITY. | |
| WO1989012087A1 (en) | Granular adsorbant with improved ease of rinsing | |
| DE69734043T2 (en) | Perfume delivery system | |
| DE3518100A1 (en) | FABRIC SOFTENING POWDERED DETERGENT | |
| DE2843390A1 (en) | DETERGENT COMPOSITION | |
| EP0151936B1 (en) | Washing agent having laundry softening properties and process for its production | |
| EP0881279B1 (en) | Process for making granules containing cationic surfactant | |
| CH633579A5 (en) | Free-flowing granular detergent and cleaning agent of high bulk density containing a fabric softener | |
| EP3368648B1 (en) | Highly-active three-phase heavy-duty detergent cloth and method for the production thereof | |
| WO1992011349A1 (en) | Granulate with encapsulated bleaching activator | |
| EP0986629B2 (en) | Granular detergent | |
| WO1991009927A1 (en) | Granular, brightening detergent additive and process for manufacturing it | |
| AT395170B (en) | ANTISTATIC DETERGENT | |
| EP0473622B1 (en) | Granular, phosphate-free additive for detergents, containing non-ionic tensides | |
| DE69925037T2 (en) | Cationic particles and process for its preparation | |
| WO1999058630A1 (en) | Alkyl sulfate granulates | |
| DE3942066A1 (en) | METHOD FOR PRODUCING A GRANULAR AVIVATING DETERGENT ADDITIVE | |
| EP1347037B1 (en) | Detergent additive with a high nonionic surfactant content and good dissolution properties | |
| DE3643334A1 (en) | Addition for a granular detergent and cleaner | |
| EP0874684B1 (en) | Process for producing a granular additive | |
| AT402407B (en) | TEXTILE-CONDITIONING COMPOSITION FOR WASHING AND METHOD FOR PRODUCING THE SAME | |
| WO1993019151A1 (en) | Granular, phosphate-free additive containing non-ionic surface-active agents for washing and cleaning agents | |
| DE3702173A1 (en) | PARTICULATE DETERGENT AND SOFTENER | |
| DE60016428T2 (en) | PROCESS FOR PREPARING HIGH-BREATHING DETERGENT WASHING MACHINES |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19980518 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE DE ES FR IT NL |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| RIC1 | Information provided on ipc code assigned before grant |
Free format text: 6C 11D 1/62 A, 6C 11D 17/06 B |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COGNIS DEUTSCHLAND GMBH |
|
| AKX | Designation fees paid |
Free format text: AT BE DE ES FR IT NL |
|
| 17Q | First examination report despatched |
Effective date: 20001130 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE DE ES FR IT NL |
|
| REF | Corresponds to: |
Ref document number: 230431 Country of ref document: AT Date of ref document: 20030115 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 59806786 Country of ref document: DE Date of ref document: 20030206 Kind code of ref document: P |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030518 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2189034 Country of ref document: ES Kind code of ref document: T3 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20030729 Year of fee payment: 6 |
|
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBQ | Unpublished change to opponent data |
Free format text: ORIGINAL CODE: EPIDOS OPPO |
|
| PLBQ | Unpublished change to opponent data |
Free format text: ORIGINAL CODE: EPIDOS OPPO |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: UNILEVER N.V. / UNILEVER PLC Effective date: 20030916 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: UNILEVER N.V. / UNILEVER PLC |
|
| 26 | Opposition filed |
Opponent name: THE PROCTER AND GAMBLE COMPANY Effective date: 20031002 Opponent name: UNILEVER N.V. / UNILEVER PLC Effective date: 20030916 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: THE PROCTER AND GAMBLE COMPANY Opponent name: UNILEVER N.V. / UNILEVER PLC |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040531 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| BERE | Be: lapsed |
Owner name: *COGNIS DEUTSCHLAND G.M.B.H. & CO. K.G. Effective date: 20040531 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG Effective date: 20050706 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: COGNIS IP MANAGEMENT GMBH |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: COGNIS IP MANAGEMENT GMBH Effective date: 20050907 |
|
| RTI2 | Title (correction) |
Free format text: GRANULES CONTAINING CATIONIC SURFACTANT |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 20070418 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE DE ES FR IT NL |
|
| NLR2 | Nl: decision of opposition |
Effective date: 20070418 |
|
| NLR3 | Nl: receipt of modified translations in the netherlands language after an opposition procedure | ||
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: DC2A Date of ref document: 20070622 Kind code of ref document: T5 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| NLS | Nl: assignments of ep-patents |
Owner name: COGNIS IP MANAGEMENT GMBH Effective date: 20090507 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20120530 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20120618 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20120522 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20120629 Year of fee payment: 15 Ref country code: DE Payment date: 20120730 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20131201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20131203 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59806786 Country of ref document: DE Effective date: 20131203 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20131201 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130518 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20140131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130531 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20140609 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130519 |