US20030191439A1 - Disposable device for transferring an active liquid into a body cavity - Google Patents
Disposable device for transferring an active liquid into a body cavity Download PDFInfo
- Publication number
- US20030191439A1 US20030191439A1 US10/400,446 US40044603A US2003191439A1 US 20030191439 A1 US20030191439 A1 US 20030191439A1 US 40044603 A US40044603 A US 40044603A US 2003191439 A1 US2003191439 A1 US 2003191439A1
- Authority
- US
- United States
- Prior art keywords
- liquid
- body cavity
- vagina
- source
- expandable element
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/20—Tampons, e.g. catamenial tampons; Accessories therefor
- A61F13/2022—Tampons, e.g. catamenial tampons; Accessories therefor characterised by the shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/20—Tampons, e.g. catamenial tampons; Accessories therefor
- A61F13/2051—Tampons, e.g. catamenial tampons; Accessories therefor characterised by the material or the structure of the inner absorbing core
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/20—Tampons, e.g. catamenial tampons; Accessories therefor
- A61F13/2074—Tampons, e.g. catamenial tampons; Accessories therefor impregnated with hydrophobic, hydrophilic, skin enhancers, medicinal etc. substances
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S604/00—Surgery
- Y10S604/904—Tampons
Definitions
- the present invention relates to the transfer or circulation of an active liquid into or in a body cavity of the human body or of an animal, particularly in contact with the mucous membrane of said cavity.
- body cavity is to be understood as meaning any bodily cavity, particularly an elongate one, which can be accessed externally for various purposes, particularly clinical, therapeutic, prophylactic or diagnostic, but also for cosmetic or personal hygiene purposes.
- a cavity mention may be made of the woman's vagina extending from the vulva to the neck of the uterus, into which an active liquid is to be transferred or in which an active liquid is to be circulated, in contact with the mucous membranes or tissues of said cavity.
- active liquid is to be understood as meaning any liquid or fluid for treatment, either locally via a topical route, or systemically and in general comprising a liquid or fluid phase in which a treatment agent is distributed in solution or in suspension.
- This treatment agent is chosen from the group consisting of wetting agents, agents for solubilizing and fluidizing particularly a bodily fluid or liquid present in the body cavity, therapeutic, prophylactic and diagnostic agents, cosmetic or personal hygiene agents, antiseptics, bactericides, fungicides and spermicides (in the case of the woman's vagina cavity for example).
- a subject of the present invention is a disposable device allowing better availability of the active principle or principles in contact with the mucous membrane of the wall of the body cavity, particularly when the treatment agent is therapeutic agent.
- Another object of the invention is a device which ensures an almost total absence of external flow of the active liquid and/or of the bodily fluid or of any liquid present in the body cavity in question, throughout the time that said device is present or held in this cavity, simply by constriction thereof.
- the disposable device has sufficient intrinsic consistency that it can be inserted into the body cavity by pushing, and comprises:
- a central element at the source of at least part of the active liquid in that it comprises a substrate which is relatively solid at ambient temperature and outside the body cavity, and becomes relatively liquid or fluid inside this same cavity and at body temperature so that within this cavity the central element liquefies at least partially to generate at least part of the liquid phase of the active liquid,
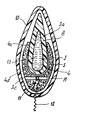
- FIG. 1 depicts a disposable device according to the invention, in position in a body cavity, and just after it has been inserted into the latter by pushing, this being according to a first embodiment of the invention;
- FIG. 1 is an axial section through a device according to the invention, the latter having symmetry of revolution about an axis;
- FIGS. 2 to 4 respectively depict three other embodiments of the present invention, still in axial section;
- FIG. 5 depicts another embodiment of the present invention, still in axial section.
- a disposable device is referenced by the numerical reference 1 .
- it allows an active liquid as previously defined to be dispensed or released into a body cavity denoted by the numerical reference 2 .
- This device has an intrinsic and overall consistency which is relatively firm but soft and stiff or strong enough to allow it to be inserted into the cavity by pushing, without altering the structure of said device, particularly without dissociating its various components and/or rupturing any liquid-containing capsule that may be contained inside said device (cf. embodiment according to FIG. 4).
- the device according to the invention essentially comprises two components assembled together permanently, namely:
- a peripheral element 4 arranged around the central element 3 comprising a pad 5 designed to absorb the active liquid which has circulated or been dispensed into the body cavity, by expanding and thus coming to press in a relatively sealed manner against the mucous membrane of the body cavity (cf. shape depicted in dotted line in FIG. 1).
- the central element 3 comprises a substrate, for example based on gelatin, which is relatively solid at ambient temperature and outside the body cavity and which becomes relatively liquid inside the body cavity and at body temperature so that within this cavity the central element liquefies at least partially to generate at least part of the liquid phase of the active liquid.
- the gelatin may be replaced by any other substrate with the same properties, for example a lipid.
- the substrate of the central element which, by liquefaction within the body cavity, and therefore within the human body or the body of the animal, makes it possible to obtain all or part of the liquid phase of the active liquid.
- an agent for treating the body cavity as previously described is distributed discretely in the solid substrate of the central element, this being so regardless of the physical form or nature of said treatment agent outside the substrate, for example an oily, solid or liquid physical form, or whether it is hydrophobic or hydrophilic by nature.
- This discrete distribution of the treatment agent may be achieved by incorporating this agent into the previously defined substrate in the liquid phase when the disposable device according to the invention is being manufactured.
- the central element 3 is a kind of molded part, molded by the solidification of the liquid substrate, for example the gelatin.
- the pad 5 is a sleeve also molded or shaped from a material capable of absorbing a liquid or a fluid as it expands.
- This material is, for example, a foam of a biocompatible plastic material or alternatively a fibrous material (for example of the cellulose type), opened (for example folded) around the central element 3 .
- the central element 3 and the peripheral element 4 are thus tailored to one another so that they fit together by simple axial pushing of the central element toward the peripheral element or vice versa.
- the peripheral element 4 has a hole 4 a , or bore, particularly a blind hole as shown in FIG. 1, the shape and size of which are tailored correspondingly to receive the body 3 a of the central element 3 .
- the head 3 b of the central element 3 of convex external shape, for example spherical, has a collar 6 while the peripheral element 4 has a neck 7 , the shape and size of which are tailored to definitively retain the collar 6 , by axially pushing the central element 3 and peripheral element 4 together.
- the neck 7 has an undercut accommodating the collar 6 directed inward, and also of convex external shape, for example spherical.
- the foot 3 c of the central element 3 has a bulging shape, while the peripheral element 4 has a correspondingly flared part 4 d , the shape and size of which are tailored to retain the foot 3 c of the central element 3 .
- the absorbent material of the pad 5 can expand and/or is spongy and consists, for example, of filaments, capillary fibers, or natural fibers (cellulose or viscose for example), etc. As mentioned earlier, it is the liquefaction or melting of the substrate of the central element 3 which, generating at least part of the liquid phase of the active liquid within the body cavity 2 , then allows the pad 5 to expand by collecting said liquid therein, this being the case at body temperature and inside the body cavity 2 .
- the pad 5 is bored for example with four radial passages 11 in a cross configuration, located below the hole 4 a , opening onto the exterior face of the pad 5 and possibly communicating with the bottom of the hole 4 a.
- the collar 6 extends toward the foot 3 c of the central element 3 in the form of a skirt 6 a capping the part 4 b of the peripheral element 4 away from the neck 7 .
- a string 12 attached to the peripheral element 4 allows the disposable device 1 to be extracted from the body cavity 2 after it has treated the latter, that is to say after the substrate of the central element 3 has liquefied.
- a closed temporary protective case 10 encases the central element 3 and peripheral element 4 when these elements are assembled, as described earlier.
- This casing consists, for example, of a substrate with the properties described above, for example based on gelatin. What this means is that the casing is made of a material which is relatively solid at ambient temperature and outside the body cavity so that within this cavity and at the body temperature, this material melts and thus constitutes another part of the liquid phase of the active liquid previously defined.
- this casing 10 may consist of a hard and solid excipient, for example sucrose, as used in a galenic form to obtain sugar-coated pills, and capable of dissolving or breaking up in contact with any bodily liquid present in the cavity 2 .
- the central element 3 is a capsule, the wall 8 of which consists of the previously defined substrate and the interior of which contains another liquid 15 , active or otherwise, the wall 8 being closed and sealed at ambient temperature and outside the body.
- the liquid thus contained in the capsule makes it possible to increase the amount of liquid phase of the active liquid.
- This liquid 15 may have any appropriate form, for example oily, and may contain all or part of a treatment agent as previously described, in solution or in suspension in said liquid.
- the casing 10 depicted in FIG. 4 may be omitted, while the wall 8 forms an appendage 13 that can be broken off at its opposite end of the pad 5 , which allows the user to open the capsule just before inserting the device into the body cavity and to benefit from a small amount of the liquid 15 to moisten the exterior of said device at the time of inserting it into said cavity.
- a device according to the invention prior to its insertion into the body cavity 2 , comprises a distal part 1 a , via which it is inserted into the body cavity 2 , and a proximal part 1 b , the largest transverse diameter of which is smaller than the largest transverse diameter of the distal part 1 a . This allows the device to be held temporarily in the cavity before the active liquid takes over by swelling the pad 5 .
- This configuration also makes it possible to limit or eliminate any contact between the mucous membrane of the body cavity 2 and the absorbent material when the pad 5 is in the dry state at the time of insertion of the device 1 ; as such contact could not only give rise to a not insignificant amount of friction but may also be a source of discomfort or pain to the user.
- it is only the distal part 1 a , covered with the relatively slippery collar 7 , which slides in contact with the mucous membrane of the body cavity as the device is being inserted.
- a disposable device as previously described affords the following essential advantages:
Abstract
A disposable device for transferring an active liquid into a body cavity has sufficient intrinsic consistency for being inserted by being thrust into the cavity. The device includes a central element, that is the source of at least part of the active liquid. The central element is preferably formed from a capsule having a wall that forms a detachable appendage. A peripheral element is arranged around the central element. The peripheral element may be a pad arranged to collect and absorb active liquid delivered into the body cavity by expanding and thereby being urged to press sealingly against mucous membrane of the body cavity.
Description
- This application is a continuation of U.S. patent application Ser. No. 09/830,426, which is the National Stage of International Application No. PCT/FR00/00245, filed Feb. 2, 2000. The entire disclosure of the prior application is hereby incorporated by reference herein in its entirety.
- The present invention relates to the transfer or circulation of an active liquid into or in a body cavity of the human body or of an animal, particularly in contact with the mucous membrane of said cavity.
- The term “body cavity” is to be understood as meaning any bodily cavity, particularly an elongate one, which can be accessed externally for various purposes, particularly clinical, therapeutic, prophylactic or diagnostic, but also for cosmetic or personal hygiene purposes. By way of an example of such a cavity, mention may be made of the woman's vagina extending from the vulva to the neck of the uterus, into which an active liquid is to be transferred or in which an active liquid is to be circulated, in contact with the mucous membranes or tissues of said cavity.
- In consequence, the term “active liquid” is to be understood as meaning any liquid or fluid for treatment, either locally via a topical route, or systemically and in general comprising a liquid or fluid phase in which a treatment agent is distributed in solution or in suspension. This treatment agent is chosen from the group consisting of wetting agents, agents for solubilizing and fluidizing particularly a bodily fluid or liquid present in the body cavity, therapeutic, prophylactic and diagnostic agents, cosmetic or personal hygiene agents, antiseptics, bactericides, fungicides and spermicides (in the case of the woman's vagina cavity for example).
- A subject of the present invention is a disposable device allowing better availability of the active principle or principles in contact with the mucous membrane of the wall of the body cavity, particularly when the treatment agent is therapeutic agent.
- Another object of the invention is a device which ensures an almost total absence of external flow of the active liquid and/or of the bodily fluid or of any liquid present in the body cavity in question, throughout the time that said device is present or held in this cavity, simply by constriction thereof.
- To this end, according to the invention, the disposable device has sufficient intrinsic consistency that it can be inserted into the body cavity by pushing, and comprises:
- a central element at the source of at least part of the active liquid, in that it comprises a substrate which is relatively solid at ambient temperature and outside the body cavity, and becomes relatively liquid or fluid inside this same cavity and at body temperature so that within this cavity the central element liquefies at least partially to generate at least part of the liquid phase of the active liquid,
- a peripheral element arranged around the central element, in the solid state, comprising a pad designed to collect and absorb the active liquid which has circulated into the body cavity by expanding and thus coming to press in a sealed manner against the mucous membrane of the body cavity.
- The present invention is now described by way of nonlimiting example with reference to the schematic appended drawing in which:
- FIG. 1 depicts a disposable device according to the invention, in position in a body cavity, and just after it has been inserted into the latter by pushing, this being according to a first embodiment of the invention; FIG. 1 is an axial section through a device according to the invention, the latter having symmetry of revolution about an axis;
- FIGS. 2 to 4 respectively depict three other embodiments of the present invention, still in axial section;
- FIG. 5 depicts another embodiment of the present invention, still in axial section.
- According to FIG. 1, a disposable device according to the invention is referenced by the
numerical reference 1. In general, it allows an active liquid as previously defined to be dispensed or released into a body cavity denoted by thenumerical reference 2. This device has an intrinsic and overall consistency which is relatively firm but soft and stiff or strong enough to allow it to be inserted into the cavity by pushing, without altering the structure of said device, particularly without dissociating its various components and/or rupturing any liquid-containing capsule that may be contained inside said device (cf. embodiment according to FIG. 4). - The device according to the invention essentially comprises two components assembled together permanently, namely:
- a
central element 3 at the source of at least part of the active liquid as previously defined; - and a peripheral element 4 arranged around the
central element 3, comprising apad 5 designed to absorb the active liquid which has circulated or been dispensed into the body cavity, by expanding and thus coming to press in a relatively sealed manner against the mucous membrane of the body cavity (cf. shape depicted in dotted line in FIG. 1). - The
central element 3 comprises a substrate, for example based on gelatin, which is relatively solid at ambient temperature and outside the body cavity and which becomes relatively liquid inside the body cavity and at body temperature so that within this cavity the central element liquefies at least partially to generate at least part of the liquid phase of the active liquid. - The gelatin may be replaced by any other substrate with the same properties, for example a lipid.
- In consequence, according to one essential feature of the present invention, it is therefore the substrate of the central element which, by liquefaction within the body cavity, and therefore within the human body or the body of the animal, makes it possible to obtain all or part of the liquid phase of the active liquid.
- As a preference, an agent for treating the body cavity as previously described is distributed discretely in the solid substrate of the central element, this being so regardless of the physical form or nature of said treatment agent outside the substrate, for example an oily, solid or liquid physical form, or whether it is hydrophobic or hydrophilic by nature. This discrete distribution of the treatment agent may be achieved by incorporating this agent into the previously defined substrate in the liquid phase when the disposable device according to the invention is being manufactured.
- As clearly shown in FIG. 1, the
central element 3 is a kind of molded part, molded by the solidification of the liquid substrate, for example the gelatin. And thepad 5 is a sleeve also molded or shaped from a material capable of absorbing a liquid or a fluid as it expands. This material is, for example, a foam of a biocompatible plastic material or alternatively a fibrous material (for example of the cellulose type), opened (for example folded) around thecentral element 3. Thecentral element 3 and the peripheral element 4 are thus tailored to one another so that they fit together by simple axial pushing of the central element toward the peripheral element or vice versa. - The peripheral element 4 has a
hole 4 a, or bore, particularly a blind hole as shown in FIG. 1, the shape and size of which are tailored correspondingly to receive thebody 3 a of thecentral element 3. The head 3 b of thecentral element 3, of convex external shape, for example spherical, has acollar 6 while the peripheral element 4 has aneck 7, the shape and size of which are tailored to definitively retain thecollar 6, by axially pushing thecentral element 3 and peripheral element 4 together. As shown in FIG. 1, theneck 7 has an undercut accommodating thecollar 6 directed inward, and also of convex external shape, for example spherical. Thefoot 3 c of thecentral element 3 has a bulging shape, while the peripheral element 4 has a correspondinglyflared part 4 d, the shape and size of which are tailored to retain thefoot 3 c of thecentral element 3. - The absorbent material of the
pad 5 can expand and/or is spongy and consists, for example, of filaments, capillary fibers, or natural fibers (cellulose or viscose for example), etc. As mentioned earlier, it is the liquefaction or melting of the substrate of thecentral element 3 which, generating at least part of the liquid phase of the active liquid within thebody cavity 2, then allows thepad 5 to expand by collecting said liquid therein, this being the case at body temperature and inside thebody cavity 2. - In order possibly to recycle the active liquid in contact with the
body cavity 2, thepad 5 is bored for example with fourradial passages 11 in a cross configuration, located below thehole 4 a, opening onto the exterior face of thepad 5 and possibly communicating with the bottom of thehole 4 a. - According to FIG. 2, the
collar 6 extends toward thefoot 3 c of thecentral element 3 in the form of a skirt 6 a capping thepart 4 b of the peripheral element 4 away from theneck 7. - Moreover, still according to FIG. 2, a
string 12 attached to the peripheral element 4 allows thedisposable device 1 to be extracted from thebody cavity 2 after it has treated the latter, that is to say after the substrate of thecentral element 3 has liquefied. - According to FIG. 3, a closed temporary
protective case 10 encases thecentral element 3 and peripheral element 4 when these elements are assembled, as described earlier. This casing consists, for example, of a substrate with the properties described above, for example based on gelatin. What this means is that the casing is made of a material which is relatively solid at ambient temperature and outside the body cavity so that within this cavity and at the body temperature, this material melts and thus constitutes another part of the liquid phase of the active liquid previously defined. However, thiscasing 10 may consist of a hard and solid excipient, for example sucrose, as used in a galenic form to obtain sugar-coated pills, and capable of dissolving or breaking up in contact with any bodily liquid present in thecavity 2. - According to FIG. 4, the
central element 3 is a capsule, thewall 8 of which consists of the previously defined substrate and the interior of which contains anotherliquid 15, active or otherwise, thewall 8 being closed and sealed at ambient temperature and outside the body. The liquid thus contained in the capsule makes it possible to increase the amount of liquid phase of the active liquid. Thisliquid 15 may have any appropriate form, for example oily, and may contain all or part of a treatment agent as previously described, in solution or in suspension in said liquid. - As depicted in FIG. 5, the
casing 10 depicted in FIG. 4 may be omitted, while thewall 8 forms anappendage 13 that can be broken off at its opposite end of thepad 5, which allows the user to open the capsule just before inserting the device into the body cavity and to benefit from a small amount of theliquid 15 to moisten the exterior of said device at the time of inserting it into said cavity. - According to FIGS. 1 to 4, a device according to the invention, prior to its insertion into the
body cavity 2, comprises a distal part 1 a, via which it is inserted into thebody cavity 2, and a proximal part 1 b, the largest transverse diameter of which is smaller than the largest transverse diameter of the distal part 1 a. This allows the device to be held temporarily in the cavity before the active liquid takes over by swelling thepad 5. - This configuration also makes it possible to limit or eliminate any contact between the mucous membrane of the
body cavity 2 and the absorbent material when thepad 5 is in the dry state at the time of insertion of thedevice 1; as such contact could not only give rise to a not insignificant amount of friction but may also be a source of discomfort or pain to the user. On the other hand, by virtue of the configuration adopted, it is only the distal part 1 a, covered with the relativelyslippery collar 7, which slides in contact with the mucous membrane of the body cavity as the device is being inserted. - A disposable device as previously described affords the following essential advantages:
- the absorption of the treatment agent or agents by the mucous membrane of the body cavity is more complete and rapid;
- the incorporation of this treatment agent into the substrate of the central element makes it possible to have a precise and uniform amount of treatment agent which is also protected by the substrate, this keeping it away from any oxidation or hydrolysis, for example;
- the mucous membrane of the cavity and the active liquid are brought fully into contact with each other.
Claims (26)
1. A disposable device, for transferring an active liquid into a body cavity, having sufficient intrinsic consistency to allow it to be inserted into said cavity by pushing, said device comprising:
a central element comprising a source of at least part of the active liquid;
a peripheral element arranged around the central element, comprising a pad designed to collect and absorb active liquid dispensed into the body cavity by expanding and thus coming to press in a sealed manner against mucous membrane of the body cavity;
wherein the central element is a capsule comprising a wall, and the interior of said capsule contains said active liquid, said wall being closed and sealed with respect to said active liquid, at ambient temperature and outside the body cavity; and
said wall forms an appendage that can be removed to open said source of liquid just before the device is inserted into the body cavity.
2. The device according to claim 1 , wherein the active liquid comprises a treatment agent selected from the group consisting of wetting agents, agents for solubilizing and fluidizing a bodily fluid or liquid present in the body cavity, therapeutic agents, prophylactic agents, diagnostic agents, cosmetic agents, personal hygiene agents, antiseptics, bactericides, fungicides and spermicides.
3. The device according to claim 1 , wherein the peripheral element has a hole, the shape and size of said hole being tailored to receive a body of the central element.
4. The device according to claim 1 , wherein the central element has a foot with a bulging shape, while the peripheral element has a flared part, the shape and size of said flared part being tailored to retain the foot of the central element.
5. The device according to claim 3 , wherein the pad is bored with at least one radial passage opening onto an exterior face of said pad, and communicating with said hole.
6. The device according to claim 1 , wherein the appendage is removable by breaking off the appendage from the wall.
7. A device for transferring a liquid into a body cavity, comprising:
an expandable element that is expandable upon absorption of liquid, said expandable element having a distal end and a proximal end;
a source of liquid at least partially radially surrounded by said expandable element, said source of liquid comprising a wall that is closed and sealed while said device is outside said body cavity, and said wall encloses a substance that is liquid in ambient temperature; and
said wall forms an appendage that can be removed to open said source of liquid just before the device is inserted into the body cavity.
8. A device according to claim 7 , wherein said source of liquid extends distally beyond a distal end of said expandable element.
9. A device according to claim 7 , wherein said source of liquid extends from within a proximal half of said expandable element through a distal half of said expandable element.
10. A device according to claim 7 , wherein said wall comprises a substance that is relatively solid in ambient temperature and atmosphere, but that can at least partially enter a liquid state when in a body cavity.
11. A device according to claim 10 , wherein said substance at least partially melts and/or dissolves under conditions in said body cavity.
12. The device according to claim 7 , wherein the appendage is removable by breaking off the appendage from the wall.
13. A device according to claim 7 , wherein said expandable element comprises a foam of a biocompatible plastic material.
14. The device according to claim 7 , further comprising a material that at least partially covers said distal end of said expandable element and said source of liquid, said material comprising a substance that is relatively solid in ambient temperature and atmosphere, but that can at least partially enter a liquid state when in a body cavity.
15. The device according to claim 14 , wherein said material at least partially melts and/or dissolves under conditions in said body cavity.
16. A device according to claim 14 , wherein said material covers an exposed portion of said central element.
17. A device according to claim 7 , wherein said substance is a liquid that comprises a local treatment agent.
18. A device according to claim 7 , wherein said substance is a liquid that comprises a systemic treatment agent.
19. A device according to claim 7 , wherein said substance is a liquid that comprises at least one member selected from the group consisting of antiseptics, bactericides, spermicides and fungicides.
20. A device for transferring a liquid into a body cavity, comprising:
an expandable element that is expandable upon absorption of liquid, said expandable element having a distal end and a proximal end and comprising a foam of a biocompatible plastic material;
a source of liquid at least partially radially surrounded by said expandable element and extending from within a proximal half of said expandable element through and beyond a distal half of said expandable element, said source of liquid comprising a wall that is closed and sealed while said device is outside said body cavity, and said wall enclosing a substance that is liquid in ambient temperature and forming an appendage that can be removed to open said source of liquid; and
a material that at least partially covers said distal end of said expandable element and said source of liquid, said material comprising a substance that is relatively solid in ambient temperature and atmosphere, but that can at least partially enter a liquid state when in a body cavity.
21. A method of transferring a liquid into a vagina, comprising removing the appendage from a device according to claim 1 , and inserting said device into and in contact with walls of said vagina, allowing said liquid to be released from said source of liquid in said vagina, and withdrawing said device from said vagina.
22. A method according to claim 21 , wherein a proximal end of said expandable element expands to press in a sealed manner against said walls of said vagina upon absorbing said liquid, to seal against external flow of the liquid from the vagina.
23. A method of transferring a liquid into a vagina, comprising removing the appendage from a device according to claim 7 , and inserting said device into and in contact with walls of said vagina,)allowing said liquid to be released from said source of liquid in said vagina, and withdrawing said device from said vagina.
24. A method according to claim 23 , wherein said proximal end of said expandable element expands to press in a sealed manner against said walls of said vagina upon absorbing said liquid, to seal against external flow of the liquid from the vagina.
25. A method of transferring a liquid into a vagina, comprising removing the appendage from a device according to claim 20 , and inserting said device into and in contact with walls of said vagina, allowing said liquid to be released from said source of liquid in said vagina, and withdrawing said device from said vagina.
26. A method according to claim 25 , wherein said proximal end of said expandable element expands to press in a sealed manner against said walls of said vagina upon absorbing said liquid, to seal against external flow of the liquid from the vagina.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/400,446 US20030191439A1 (en) | 1999-02-03 | 2003-03-28 | Disposable device for transferring an active liquid into a body cavity |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9901419A FR2788987B1 (en) | 1999-02-03 | 1999-02-03 | SINGLE-USE DEVICE FOR TRANSFERRING AN ACTIVE LIQUID IN AN INTRACORPOREAL CAVITY |
| FR99/01419 | 1999-02-03 | ||
| US09/830,426 US6558362B1 (en) | 1999-02-03 | 2000-02-02 | Disposable device for transferring an active liquid into a body cavity |
| US10/400,446 US20030191439A1 (en) | 1999-02-03 | 2003-03-28 | Disposable device for transferring an active liquid into a body cavity |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/830,426 Division US6558362B1 (en) | 1999-02-03 | 2000-02-02 | Disposable device for transferring an active liquid into a body cavity |
| PCT/FR2000/000245 Division WO2000045887A1 (en) | 1999-02-03 | 2000-02-02 | Disposable device for transferring an active liquid into a body cavity |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030191439A1 true US20030191439A1 (en) | 2003-10-09 |
Family
ID=9541702
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/830,426 Expired - Fee Related US6558362B1 (en) | 1999-02-03 | 2000-02-02 | Disposable device for transferring an active liquid into a body cavity |
| US10/400,446 Abandoned US20030191439A1 (en) | 1999-02-03 | 2003-03-28 | Disposable device for transferring an active liquid into a body cavity |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/830,426 Expired - Fee Related US6558362B1 (en) | 1999-02-03 | 2000-02-02 | Disposable device for transferring an active liquid into a body cavity |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US6558362B1 (en) |
| EP (1) | EP1150737A1 (en) |
| JP (1) | JP2002536082A (en) |
| CN (1) | CN1338957A (en) |
| AU (1) | AU763780B2 (en) |
| BR (1) | BR0007981A (en) |
| CA (1) | CA2361491A1 (en) |
| FR (1) | FR2788987B1 (en) |
| HK (1) | HK1043948A1 (en) |
| NO (1) | NO20013793L (en) |
| WO (1) | WO2000045887A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070255232A1 (en) * | 2001-10-16 | 2007-11-01 | Bernard Chaffringeon | Disposable device and method for transferring an active liquid into a body cavity |
| US20070293837A1 (en) * | 2006-06-16 | 2007-12-20 | Sokal David C | Vaginal drug delivery system and method |
| US20080083942A1 (en) * | 2006-09-28 | 2008-04-10 | Esin Terzioglu | Single-Poly Non-Volatile Memory Cell |
| US20080132868A1 (en) * | 2006-11-08 | 2008-06-05 | Playtex Products, Inc. | Tampon pledget for increased bypass leakage protection |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1992385A3 (en) * | 2001-10-16 | 2011-03-09 | Bernard Chaffringeon | A disposable device for transferring and/or circulating an active liquid into and/or inside an intracorporeal cavity |
| FR2830748B1 (en) * | 2001-10-16 | 2004-01-30 | Bernard Chaffringeon | SINGLE USE DEVICE FOR TRANSFERRING AND CIRCULATING AN ACTIVE LIQUID IN AN INTRACORPOREAL CAVITY |
| US6932805B2 (en) * | 2002-03-18 | 2005-08-23 | The Procter & Gamble Company | Shaped tampon |
| US8247642B2 (en) * | 2004-05-14 | 2012-08-21 | Mcneil-Ppc, Inc. | Fluid management device with fluid transport element for use within a body |
| US8480833B2 (en) * | 2004-05-14 | 2013-07-09 | Mcneil-Ppc, Inc. | Intravaginal device with fluid transport plates and methods of making |
| US8653322B2 (en) * | 2004-05-14 | 2014-02-18 | Mcneil-Ppc, Inc. | Intravaginal device with fluid transport plates |
| JP5117186B2 (en) * | 2004-05-14 | 2013-01-09 | ジョンソン・アンド・ジョンソン・コンシューマー・カンパニーズ・インコーポレイテッド | Intravaginal device with fluid transfer element |
| US20050256485A1 (en) | 2004-05-14 | 2005-11-17 | Samuel Carasso | Method of using intravaginal device with fluid transport plates |
| US7618403B2 (en) | 2004-05-14 | 2009-11-17 | Mcneil-Ppc, Inc. | Fluid management device with fluid transport element for use within a body |
| US20050256484A1 (en) * | 2004-05-14 | 2005-11-17 | Chase David J | Method of using an intravaginal device with fluid transport plates |
| US20050277904A1 (en) * | 2004-05-14 | 2005-12-15 | Chase David J | Tampon with flexible panels |
| US7845380B2 (en) * | 2004-05-14 | 2010-12-07 | Mcneil-Ppc, Inc. | Intravaginal device with fluid transport plates |
| US8864640B2 (en) * | 2004-05-14 | 2014-10-21 | Mcneil-Ppc, Inc. | Methods of packaging intravaginal device |
| EP1700588A1 (en) * | 2005-03-09 | 2006-09-13 | Björn Andersch | Tampon |
| US7993667B2 (en) * | 2005-03-25 | 2011-08-09 | Kimberly-Clark Worldwide, Inc. | Methods of manufacturing a medicated tampon assembly |
| US7744556B2 (en) * | 2005-03-25 | 2010-06-29 | Kimberly-Clark Worldwide, Inc. | Delivery tube assembly for an applicator |
| US7527614B2 (en) * | 2005-03-25 | 2009-05-05 | Kimberly-Clark Worldwide, Inc. | Protective tube for a medicated tampon |
| US7919453B2 (en) * | 2005-03-25 | 2011-04-05 | Kimberly-Clark Worldwide, Inc. | Dosage cap assembly for an applicator |
| US9295289B2 (en) * | 2005-04-01 | 2016-03-29 | Leslie Jane James | Waist-fastening, hip-encompassing apparel with at least one concealed storage compartment |
| US7708726B2 (en) * | 2005-04-28 | 2010-05-04 | Kimberly-Clark Worldwide, Inc. | Dosage form cap for an applicator |
| US20070141118A1 (en) * | 2005-12-15 | 2007-06-21 | Damico Joyce A | Layered dosage form for a medicated tampon assembly |
| US20070156077A1 (en) * | 2005-12-30 | 2007-07-05 | Pfister Margaret M | Personal hygiene system and methods |
| US7824383B2 (en) * | 2006-06-16 | 2010-11-02 | Family Health International | Vaginal drug delivery system and method |
| FR3052655B1 (en) | 2016-06-17 | 2018-07-13 | Laboratoires Innothera | DEVICE FOR MEASURING A MEASUREMENT |
| US10799399B1 (en) * | 2018-05-09 | 2020-10-13 | Sonya D. Whack | Time-released tampon applicator |
| US20200323699A1 (en) * | 2019-04-11 | 2020-10-15 | Herphoric, LLC | Medication Delivery Device and Method |
| CA3207593A1 (en) | 2019-08-15 | 2021-02-15 | Better Made Hemp, LLC | Absorbent article |
Citations (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US706778A (en) * | 1901-12-03 | 1902-08-12 | Edmund Morse Pond | Medicated tampon. |
| US812769A (en) * | 1904-07-19 | 1906-02-13 | Edmund M Pond | Medicated tampon. |
| US1561020A (en) * | 1922-07-25 | 1925-11-10 | Arthur H Smith | Frectal tampon |
| US1575123A (en) * | 1922-08-01 | 1926-03-02 | Martocci-Pisculli Leon | Medical appliance |
| US1732413A (en) * | 1927-05-16 | 1929-10-22 | Motteram Etta | Medical pessary |
| US1887526A (en) * | 1931-11-02 | 1932-11-15 | Joseph M Spielberg | Medical tampon |
| US2493416A (en) * | 1947-07-17 | 1950-01-03 | A Gazzoni & Co Soc | Odontalgic device |
| US3512527A (en) * | 1966-10-17 | 1970-05-19 | Marguerite Desoye | Dressing,in particular for natural cavities of the human body |
| US3515138A (en) * | 1967-07-05 | 1970-06-02 | Josef Hochstrasser | Process and apparatus for treating elongated deformable articles |
| US3519364A (en) * | 1968-02-02 | 1970-07-07 | Andrew Truhan | Applicator |
| US3521637A (en) * | 1967-11-28 | 1970-07-28 | Nelson J Waterbury | Tampon or similar sanitary napkin containing vitamin a |
| US3559646A (en) * | 1968-08-16 | 1971-02-02 | Joseph Mullan | Tampon |
| US3865108A (en) * | 1971-05-17 | 1975-02-11 | Ortho Pharma Corp | Expandable drug delivery device |
| US3902493A (en) * | 1974-05-13 | 1975-09-02 | Procter & Gamble | Medicated catamenial tampon |
| US3918452A (en) * | 1974-08-01 | 1975-11-11 | Edward Cornfeld | Tampons impregnated with contraceptive compositions |
| US4136680A (en) * | 1976-06-04 | 1979-01-30 | Transmed Corp. | Self-contained apparatus for collection and maintenance of medical specimen and methods of using same |
| US4232673A (en) * | 1975-08-11 | 1980-11-11 | Louis Bucalo | Method for collecting and processing body fluids |
| US4257427A (en) * | 1973-02-05 | 1981-03-24 | Louis Bucalo | Method for collecting body fluids |
| US4286596A (en) * | 1978-02-27 | 1981-09-01 | Herbert Rubinstein | Tampon containing a liquid medicant |
| US4317454A (en) * | 1977-08-04 | 1982-03-02 | Louis Bucalo | Methods and devices for obtaining specimens and for signalling when the specimen has been collected |
| US4318405A (en) * | 1980-07-24 | 1982-03-09 | Sneider Vincent R | Tampon and drug delivery device |
| US4324262A (en) * | 1979-01-02 | 1982-04-13 | University Of Virginia Alumni Patents Foundation | Aspirating culture catheter and method of use |
| US4335720A (en) * | 1980-04-09 | 1982-06-22 | Glassman Jacob A | Catamenial tampon with hollow core |
| US4582717A (en) * | 1982-02-06 | 1986-04-15 | Bayer Aktiengesellschaft | Process for production of vaginal tampons containing pharmaceutical active compound |
| US4858624A (en) * | 1988-02-24 | 1989-08-22 | Vance Products Incorporated D/B/A/ Cook Urological Incorporated And Cook Ob/Gyn | Device and method for intravaginal, barrier-type prevention of conception and infection |
| US5273521A (en) * | 1992-08-26 | 1993-12-28 | Peiler Frances K | Tampon applicator for delivery of a medicament |
| US5282789A (en) * | 1992-09-15 | 1994-02-01 | Niemand Industries, Inc. | Disposable medicine applicator |
| US5299581A (en) * | 1990-07-05 | 1994-04-05 | Donnell John T | Intravaginal device |
| US5357977A (en) * | 1993-04-23 | 1994-10-25 | St. Mary's Hospital And Medical Center, Inc. | Cytological sampling method and device |
| US5542914A (en) * | 1993-02-12 | 1996-08-06 | Kimberly-Clark Corporation | Encapsulated tampon with an applicator |
| US5810745A (en) * | 1994-09-19 | 1998-09-22 | Chaffringeon; Bernard | Single-use body fluid analysis device |
| US5823954A (en) * | 1994-09-22 | 1998-10-20 | Chaffringeon; Bernard | Single-use device for detecting or analyzing a body fluid |
| US5830199A (en) * | 1994-03-29 | 1998-11-03 | Chaffringeon; Bernard | Disposable device for recovery, and if appropriate analysis, of a body fluid |
| US5840055A (en) * | 1994-11-21 | 1998-11-24 | Bernard Chaffringeon | Disposable device for transfer of an active liquid into an intracorporeal cavity |
| US6059735A (en) * | 1995-11-20 | 2000-05-09 | Bernard Chaffringeon | Portable device for extemporaneous analysis of a body-fluid |
| US6068899A (en) * | 1997-03-17 | 2000-05-30 | The Proctor & Gamble Company | Tampon packaging system |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA827582B (en) * | 1981-11-06 | 1983-08-31 | Ansell Inc | Spermicidal condom |
| EP0093740A1 (en) | 1981-11-17 | 1983-11-16 | Portland Surgical Products Pty. Ltd. | Catheter-type sampling device |
| DE3629994A1 (en) * | 1986-09-03 | 1988-03-17 | Weissenbacher Ernst Rainer Pro | Device for administration of medicaments in body cavities or on body surfaces |
| GB2254002B (en) * | 1991-01-16 | 1995-03-22 | Controlled Therapeutics | Retrievable pessary |
-
1999
- 1999-02-03 FR FR9901419A patent/FR2788987B1/en not_active Expired - Fee Related
-
2000
- 2000-02-02 EP EP00901703A patent/EP1150737A1/en not_active Withdrawn
- 2000-02-02 US US09/830,426 patent/US6558362B1/en not_active Expired - Fee Related
- 2000-02-02 BR BR0007981-2A patent/BR0007981A/en not_active Application Discontinuation
- 2000-02-02 AU AU23019/00A patent/AU763780B2/en not_active Ceased
- 2000-02-02 JP JP2000597006A patent/JP2002536082A/en active Pending
- 2000-02-02 WO PCT/FR2000/000245 patent/WO2000045887A1/en not_active Application Discontinuation
- 2000-02-02 CN CN00803445A patent/CN1338957A/en active Pending
- 2000-02-02 CA CA002361491A patent/CA2361491A1/en not_active Abandoned
-
2001
- 2001-08-02 NO NO20013793A patent/NO20013793L/en unknown
-
2002
- 2002-05-06 HK HK02103429.3A patent/HK1043948A1/en unknown
-
2003
- 2003-03-28 US US10/400,446 patent/US20030191439A1/en not_active Abandoned
Patent Citations (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US706778A (en) * | 1901-12-03 | 1902-08-12 | Edmund Morse Pond | Medicated tampon. |
| US812769A (en) * | 1904-07-19 | 1906-02-13 | Edmund M Pond | Medicated tampon. |
| US1561020A (en) * | 1922-07-25 | 1925-11-10 | Arthur H Smith | Frectal tampon |
| US1575123A (en) * | 1922-08-01 | 1926-03-02 | Martocci-Pisculli Leon | Medical appliance |
| US1732413A (en) * | 1927-05-16 | 1929-10-22 | Motteram Etta | Medical pessary |
| US1887526A (en) * | 1931-11-02 | 1932-11-15 | Joseph M Spielberg | Medical tampon |
| US2493416A (en) * | 1947-07-17 | 1950-01-03 | A Gazzoni & Co Soc | Odontalgic device |
| US3512527A (en) * | 1966-10-17 | 1970-05-19 | Marguerite Desoye | Dressing,in particular for natural cavities of the human body |
| US3515138A (en) * | 1967-07-05 | 1970-06-02 | Josef Hochstrasser | Process and apparatus for treating elongated deformable articles |
| US3521637A (en) * | 1967-11-28 | 1970-07-28 | Nelson J Waterbury | Tampon or similar sanitary napkin containing vitamin a |
| US3519364A (en) * | 1968-02-02 | 1970-07-07 | Andrew Truhan | Applicator |
| US3559646A (en) * | 1968-08-16 | 1971-02-02 | Joseph Mullan | Tampon |
| US3865108A (en) * | 1971-05-17 | 1975-02-11 | Ortho Pharma Corp | Expandable drug delivery device |
| US4257427A (en) * | 1973-02-05 | 1981-03-24 | Louis Bucalo | Method for collecting body fluids |
| US3902493A (en) * | 1974-05-13 | 1975-09-02 | Procter & Gamble | Medicated catamenial tampon |
| US3918452A (en) * | 1974-08-01 | 1975-11-11 | Edward Cornfeld | Tampons impregnated with contraceptive compositions |
| US4232673A (en) * | 1975-08-11 | 1980-11-11 | Louis Bucalo | Method for collecting and processing body fluids |
| US4136680A (en) * | 1976-06-04 | 1979-01-30 | Transmed Corp. | Self-contained apparatus for collection and maintenance of medical specimen and methods of using same |
| US4317454A (en) * | 1977-08-04 | 1982-03-02 | Louis Bucalo | Methods and devices for obtaining specimens and for signalling when the specimen has been collected |
| US4286596A (en) * | 1978-02-27 | 1981-09-01 | Herbert Rubinstein | Tampon containing a liquid medicant |
| US4324262A (en) * | 1979-01-02 | 1982-04-13 | University Of Virginia Alumni Patents Foundation | Aspirating culture catheter and method of use |
| US4335720A (en) * | 1980-04-09 | 1982-06-22 | Glassman Jacob A | Catamenial tampon with hollow core |
| US4318405A (en) * | 1980-07-24 | 1982-03-09 | Sneider Vincent R | Tampon and drug delivery device |
| US4582717A (en) * | 1982-02-06 | 1986-04-15 | Bayer Aktiengesellschaft | Process for production of vaginal tampons containing pharmaceutical active compound |
| US4858624A (en) * | 1988-02-24 | 1989-08-22 | Vance Products Incorporated D/B/A/ Cook Urological Incorporated And Cook Ob/Gyn | Device and method for intravaginal, barrier-type prevention of conception and infection |
| US5299581A (en) * | 1990-07-05 | 1994-04-05 | Donnell John T | Intravaginal device |
| US5273521A (en) * | 1992-08-26 | 1993-12-28 | Peiler Frances K | Tampon applicator for delivery of a medicament |
| US5282789A (en) * | 1992-09-15 | 1994-02-01 | Niemand Industries, Inc. | Disposable medicine applicator |
| US5542914A (en) * | 1993-02-12 | 1996-08-06 | Kimberly-Clark Corporation | Encapsulated tampon with an applicator |
| US5357977A (en) * | 1993-04-23 | 1994-10-25 | St. Mary's Hospital And Medical Center, Inc. | Cytological sampling method and device |
| US5830199A (en) * | 1994-03-29 | 1998-11-03 | Chaffringeon; Bernard | Disposable device for recovery, and if appropriate analysis, of a body fluid |
| US5810745A (en) * | 1994-09-19 | 1998-09-22 | Chaffringeon; Bernard | Single-use body fluid analysis device |
| US5823954A (en) * | 1994-09-22 | 1998-10-20 | Chaffringeon; Bernard | Single-use device for detecting or analyzing a body fluid |
| US5840055A (en) * | 1994-11-21 | 1998-11-24 | Bernard Chaffringeon | Disposable device for transfer of an active liquid into an intracorporeal cavity |
| US6059735A (en) * | 1995-11-20 | 2000-05-09 | Bernard Chaffringeon | Portable device for extemporaneous analysis of a body-fluid |
| US6068899A (en) * | 1997-03-17 | 2000-05-30 | The Proctor & Gamble Company | Tampon packaging system |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070255232A1 (en) * | 2001-10-16 | 2007-11-01 | Bernard Chaffringeon | Disposable device and method for transferring an active liquid into a body cavity |
| US20070293837A1 (en) * | 2006-06-16 | 2007-12-20 | Sokal David C | Vaginal drug delivery system and method |
| US8137327B2 (en) | 2006-06-16 | 2012-03-20 | Family Health International | Vaginal drug delivery system and method |
| US20080083942A1 (en) * | 2006-09-28 | 2008-04-10 | Esin Terzioglu | Single-Poly Non-Volatile Memory Cell |
| US7440311B2 (en) * | 2006-09-28 | 2008-10-21 | Novelics, Llc | Single-poly non-volatile memory cell |
| US20080132868A1 (en) * | 2006-11-08 | 2008-06-05 | Playtex Products, Inc. | Tampon pledget for increased bypass leakage protection |
| US7867209B2 (en) | 2006-11-08 | 2011-01-11 | Playtex Products, Inc. | Tampon pledget for increased bypass leakage protection |
Also Published As
| Publication number | Publication date |
|---|---|
| AU763780B2 (en) | 2003-07-31 |
| NO20013793D0 (en) | 2001-08-02 |
| EP1150737A1 (en) | 2001-11-07 |
| US6558362B1 (en) | 2003-05-06 |
| FR2788987B1 (en) | 2001-05-04 |
| CA2361491A1 (en) | 2000-08-10 |
| FR2788987A1 (en) | 2000-08-04 |
| BR0007981A (en) | 2001-11-06 |
| AU2301900A (en) | 2000-08-25 |
| NO20013793L (en) | 2001-09-26 |
| CN1338957A (en) | 2002-03-06 |
| JP2002536082A (en) | 2002-10-29 |
| HK1043948A1 (en) | 2002-10-04 |
| WO2000045887A1 (en) | 2000-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6558362B1 (en) | Disposable device for transferring an active liquid into a body cavity | |
| US5299581A (en) | Intravaginal device | |
| US4286596A (en) | Tampon containing a liquid medicant | |
| US20070255232A1 (en) | Disposable device and method for transferring an active liquid into a body cavity | |
| CA1231602A (en) | Devices for insertion into a body cavity of an animal and/or applicators therefor | |
| US4553965A (en) | Fluid-expansible contraceptive tampon and applicator | |
| US5840055A (en) | Disposable device for transfer of an active liquid into an intracorporeal cavity | |
| US4271835A (en) | Fluid-expansible contraceptive tampon and applicator | |
| JPH06245957A (en) | Capsuled tampon for menses with applicator and without applicator | |
| US7112184B2 (en) | Disposable vaginal cleaning device | |
| MXPA97003691A (en) | Disposable device for the transfer of an active liquid to an intracorous cavity | |
| US4200101A (en) | Catamenial tampon | |
| US2110962A (en) | Medical device | |
| US11382799B2 (en) | Tampon with intravaginal cannabinoid delivery device | |
| US7060057B2 (en) | Comfortable tampon | |
| USRE21943E (en) | Medical device | |
| EP0071599A1 (en) | Fluid expansible contraceptive tampon and applicator | |
| MXPA01007831A (en) | Disposable device for transferring an active liquid into a body cavity | |
| JP2001520538A (en) | Transurethral device | |
| GB2064326A (en) | Tampons | |
| WO2004087026A1 (en) | Method for obtaining a disposable device for transferring and/or circulating an active liquid into and/or inside an intracorporal cavity | |
| EP1455886B1 (en) | A disposable devce for transferring and/or circulating an active liquid into and/or inside an intracorporeal cavity | |
| CA2070426C (en) | Feminine hygiene device | |
| CA1171603A (en) | Fluid expansible contraceptive tampon and applicator | |
| AU2002350987A1 (en) | A disposable device for transferring and/or circulating an active liquid into and/or inside an intracorporeal cavity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO PAY ISSUE FEE |