US20040142041A1 - Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same - Google Patents
Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same Download PDFInfo
- Publication number
- US20040142041A1 US20040142041A1 US10/731,256 US73125603A US2004142041A1 US 20040142041 A1 US20040142041 A1 US 20040142041A1 US 73125603 A US73125603 A US 73125603A US 2004142041 A1 US2004142041 A1 US 2004142041A1
- Authority
- US
- United States
- Prior art keywords
- particles
- alumina
- particle
- functional
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 95
- 238000000034 method Methods 0.000 title claims description 41
- 235000016709 nutrition Nutrition 0.000 title claims description 18
- 239000002245 particle Substances 0.000 claims abstract description 173
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims abstract description 77
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 51
- 239000003795 chemical substances by application Substances 0.000 claims description 32
- 239000000126 substance Substances 0.000 claims description 27
- 239000000758 substrate Substances 0.000 claims description 20
- 230000036541 health Effects 0.000 claims description 18
- 230000008859 change Effects 0.000 claims description 15
- 230000007613 environmental effect Effects 0.000 claims description 11
- 238000011282 treatment Methods 0.000 claims description 11
- 239000002253 acid Substances 0.000 claims description 9
- 238000012377 drug delivery Methods 0.000 claims description 9
- 210000000416 exudates and transudate Anatomy 0.000 claims description 8
- 239000007788 liquid Substances 0.000 claims description 7
- 125000000217 alkyl group Chemical group 0.000 claims description 6
- 125000003118 aryl group Chemical group 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- 238000013271 transdermal drug delivery Methods 0.000 claims description 4
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 4
- 239000003814 drug Substances 0.000 abstract description 16
- 239000008204 material by function Substances 0.000 abstract description 10
- 239000003205 fragrance Substances 0.000 abstract description 9
- 239000000203 mixture Substances 0.000 abstract description 8
- 239000004599 antimicrobial Substances 0.000 abstract description 5
- 239000002676 xenobiotic agent Substances 0.000 abstract description 5
- 239000003443 antiviral agent Substances 0.000 abstract description 4
- 239000000463 material Substances 0.000 description 50
- 239000002105 nanoparticle Substances 0.000 description 27
- 239000010410 layer Substances 0.000 description 22
- 239000000377 silicon dioxide Substances 0.000 description 20
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 18
- 229930101283 tetracycline Natural products 0.000 description 18
- 239000004098 Tetracycline Substances 0.000 description 17
- 229960002180 tetracycline Drugs 0.000 description 17
- 235000019364 tetracycline Nutrition 0.000 description 17
- 150000003522 tetracyclines Chemical class 0.000 description 17
- 229920000642 polymer Polymers 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 14
- 239000000725 suspension Substances 0.000 description 12
- 239000000243 solution Substances 0.000 description 11
- 230000003115 biocidal effect Effects 0.000 description 10
- 239000008177 pharmaceutical agent Substances 0.000 description 10
- 239000004480 active ingredient Substances 0.000 description 9
- 229960000890 hydrocortisone Drugs 0.000 description 9
- SMQUZDBALVYZAC-UHFFFAOYSA-N salicylaldehyde Chemical compound OC1=CC=CC=C1C=O SMQUZDBALVYZAC-UHFFFAOYSA-N 0.000 description 9
- 238000010521 absorption reaction Methods 0.000 description 8
- 238000002371 ultraviolet--visible spectrum Methods 0.000 description 8
- 230000002378 acidificating effect Effects 0.000 description 7
- 239000003242 anti bacterial agent Substances 0.000 description 7
- -1 carboxy-hydroxy moiety Chemical group 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 235000010323 ascorbic acid Nutrition 0.000 description 6
- 239000011668 ascorbic acid Substances 0.000 description 6
- 229960005070 ascorbic acid Drugs 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 230000037317 transdermal delivery Effects 0.000 description 6
- 239000012790 adhesive layer Substances 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 5
- 0 */N=C(C)/C=C(/C)O.C.C.C.C.C.C.C.C.C.C/C=C\C=O.C=C(O)/C=C(/C)O.C=C(O)C=C(C)C.CC(=O)C(C)N.CC(=O)CC(C)=O.CC(=O)CC(C)O.O=C/C=C\O.O=CCO Chemical compound */N=C(C)/C=C(/C)O.C.C.C.C.C.C.C.C.C.C/C=C\C=O.C=C(O)/C=C(/C)O.C=C(O)C=C(C)C.CC(=O)C(C)N.CC(=O)CC(C)=O.CC(=O)CC(C)O.O=C/C=C\O.O=CCO 0.000 description 4
- 206010017533 Fungal infection Diseases 0.000 description 4
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- ORIHZIZPTZTNCU-YVMONPNESA-N salicylaldoxime Chemical compound O\N=C/C1=CC=CC=C1O ORIHZIZPTZTNCU-YVMONPNESA-N 0.000 description 4
- 230000001960 triggered effect Effects 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 238000001179 sorption measurement Methods 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- NWXMGUDVXFXRIG-WESIUVDSSA-N (4s,4as,5as,6s,12ar)-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide Chemical class C1=CC=C2[C@](O)(C)[C@H]3C[C@H]4[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]4(O)C(=O)C3=C(O)C2=C1O NWXMGUDVXFXRIG-WESIUVDSSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- FXNFHKRTJBSTCS-UHFFFAOYSA-N Baicalein Natural products C=1C(=O)C=2C(O)=C(O)C(O)=CC=2OC=1C1=CC=CC=C1 FXNFHKRTJBSTCS-UHFFFAOYSA-N 0.000 description 2
- 230000002745 absorbent Effects 0.000 description 2
- 239000002250 absorbent Substances 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 235000010357 aspartame Nutrition 0.000 description 2
- UDFLTIRFTXWNJO-UHFFFAOYSA-N baicalein Chemical compound O1C2=CC(=O)C(O)=C(O)C2=C(O)C=C1C1=CC=CC=C1 UDFLTIRFTXWNJO-UHFFFAOYSA-N 0.000 description 2
- 229940015301 baicalein Drugs 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 239000011162 core material Substances 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000002417 nutraceutical Substances 0.000 description 2
- 239000010453 quartz Substances 0.000 description 2
- 210000000813 small intestine Anatomy 0.000 description 2
- 239000012258 stirred mixture Substances 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 210000001215 vagina Anatomy 0.000 description 2
- 238000000733 zeta-potential measurement Methods 0.000 description 2
- WLEKZZMZTPAGDD-ZSESPEEFSA-N (2s,3s,4s,5r,6s)-6-(5,6-dihydroxy-4-oxo-2-phenylchromen-7-yl)oxy-3,4,5-trihydroxyoxane-2-carboxylic acid;hydrate Chemical compound O.O1[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OC(C(=C1O)O)=CC2=C1C(=O)C=C(C=1C=CC=CC=1)O2 WLEKZZMZTPAGDD-ZSESPEEFSA-N 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- LHZYYYOMMZFCQW-UHFFFAOYSA-N CC1=CC2C=C3CC4OCOC5=C4/C(=C(/O)C4=C5C(=O)C5=C(C4)C(C(=O)O)CCC5O)C3=C(O)C2C(=O)N1N.NC(=O)C1=C(O)C=CC=C1.O=C(O)C1=C(OC(=O)C2=C(O)C=CC=C2)C=CC=C1.[H]N(C(=O)C1=C(O)C=CC=C1)C1=CC=CC=C1.[H]N(C(C)=O)C(=O)C1=C(O)C=CC=C1 Chemical compound CC1=CC2C=C3CC4OCOC5=C4/C(=C(/O)C4=C5C(=O)C5=C(C4)C(C(=O)O)CCC5O)C3=C(O)C2C(=O)N1N.NC(=O)C1=C(O)C=CC=C1.O=C(O)C1=C(OC(=O)C2=C(O)C=CC=C2)C=CC=C1.[H]N(C(=O)C1=C(O)C=CC=C1)C1=CC=CC=C1.[H]N(C(C)=O)C(=O)C1=C(O)C=CC=C1 LHZYYYOMMZFCQW-UHFFFAOYSA-N 0.000 description 1
- KNBSPPYPEJKVFU-XDJYKUFASA-N CN(C)C1C(O)=C(C(N)=O)C(=O)[C@@]2(O)C(O)=C3C(=O)C4=C(O)C=CC=C4[C@@](C)(O)C3CC12.[H][C@@]12CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])[C@@H](O)C[C@@]1(C)[C@@]2([H])CC[C@]1(O)C(=O)CO Chemical compound CN(C)C1C(O)=C(C(N)=O)C(=O)[C@@]2(O)C(O)=C3C(=O)C4=C(O)C=CC=C4[C@@](C)(O)C3CC12.[H][C@@]12CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])[C@@H](O)C[C@@]1(C)[C@@]2([H])CC[C@]1(O)C(=O)CO KNBSPPYPEJKVFU-XDJYKUFASA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 1
- 208000034423 Delivery Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- GPDJGLOROGNHJD-UHFFFAOYSA-N O=C1CC(C2=CC=CC=C2)OC2=C1C(O)=C(O)C(O)=C2 Chemical compound O=C1CC(C2=CC=CC=C2)OC2=C1C(O)=C(O)C(O)=C2 GPDJGLOROGNHJD-UHFFFAOYSA-N 0.000 description 1
- UVNUGBQJLDGZKE-UHFFFAOYSA-N O=C1CC(C2=CC=CC=C2)OC2=C1C(O)=C(O)C(OC1OC(C(=O)O)C(O)C(O)C1O)=C2 Chemical compound O=C1CC(C2=CC=CC=C2)OC2=C1C(O)=C(O)C(OC1OC(C(=O)O)C(O)C(O)C1O)=C2 UVNUGBQJLDGZKE-UHFFFAOYSA-N 0.000 description 1
- CUOKHACJLGPRHD-UHFFFAOYSA-N O=C1OC(CO)C(O)C1O Chemical compound O=C1OC(CO)C(O)C1O CUOKHACJLGPRHD-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 206010046914 Vaginal infection Diseases 0.000 description 1
- 229930003268 Vitamin C Natural products 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000000862 absorption spectrum Methods 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 210000004404 adrenal cortex Anatomy 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 150000001408 amides Chemical group 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 235000019846 buffering salt Nutrition 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 238000003851 corona treatment Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
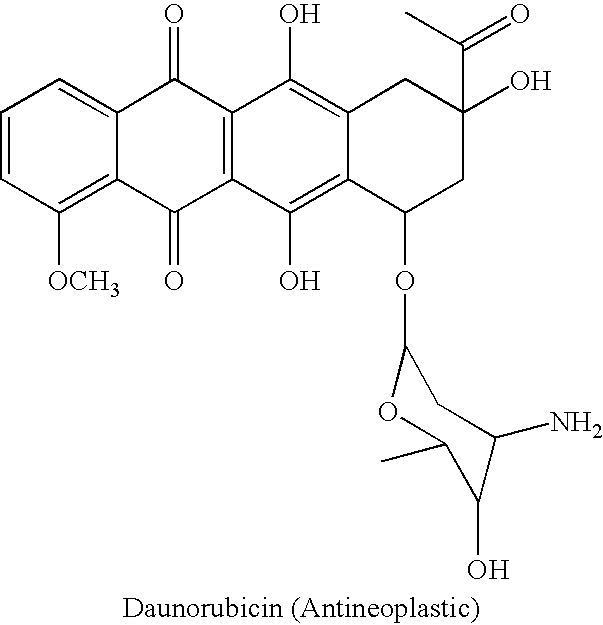
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 239000000416 hydrocolloid Substances 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 230000003116 impacting effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910052809 inorganic oxide Inorganic materials 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 239000004750 melt-blown nonwoven Substances 0.000 description 1
- 230000037353 metabolic pathway Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 235000021436 nutraceutical agent Nutrition 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920000307 polymer substrate Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 239000003352 sequestering agent Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 238000006557 surface reaction Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000000870 ultraviolet spectroscopy Methods 0.000 description 1
- 230000009677 vaginal delivery Effects 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 235000019154 vitamin C Nutrition 0.000 description 1
- 239000011718 vitamin C Substances 0.000 description 1
- 239000002351 wastewater Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L27/00—Spices; Flavouring agents or condiments; Artificial sweetening agents; Table salts; Dietetic salt substitutes; Preparation or treatment thereof
- A23L27/30—Artificial sweetening agents
- A23L27/31—Artificial sweetening agents containing amino acids, nucleotides, peptides or derivatives
- A23L27/32—Artificial sweetening agents containing amino acids, nucleotides, peptides or derivatives containing dipeptides or derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L27/00—Spices; Flavouring agents or condiments; Artificial sweetening agents; Table salts; Dietetic salt substitutes; Preparation or treatment thereof
- A23L27/70—Fixation, conservation, or encapsulation of flavouring agents
- A23L27/77—Use of inorganic solid carriers, e.g. silica
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23P—SHAPING OR WORKING OF FOODSTUFFS, NOT FULLY COVERED BY A SINGLE OTHER SUBCLASS
- A23P10/00—Shaping or working of foodstuffs characterised by the products
- A23P10/30—Encapsulation of particles, e.g. foodstuff additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23P—SHAPING OR WORKING OF FOODSTUFFS, NOT FULLY COVERED BY A SINGLE OTHER SUBCLASS
- A23P20/00—Coating of foodstuffs; Coatings therefor; Making laminated, multi-layered, stuffed or hollow foodstuffs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid, pantothenic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/365—Lactones
- A61K31/375—Ascorbic acid, i.e. vitamin C; Salts thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/57—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/65—Tetracyclines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6923—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being an inorganic particle, e.g. ceramic particles, silica particles, ferrite or synsorb
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/20—Ingredients acting on or related to the structure
- A23V2200/25—Nanoparticles, nanostructures
Definitions
- a delivery system generally refers to a system that aids or otherwise facilitates the delivery of a functional material to a desired location.
- the functional material can be any material that acts upon a substrate or otherwise provides a benefit once delivered to the desired location.
- Examples of functional materials that may benefit from the use of a delivery system include pharmaceuticals that are intended to be ingested, transferred transdermally, or subcutaneously injected into a human or animal patient's body, vitamins and nutrients (nutritional materials), and various other and numerous additives that can similarly be introduced into the body of a patient.
- the present invention is generally directed to a delivery system for various functional materials.
- the functional materials can be, for instance, health-related compounds/materials such as pharmaceuticals, anti-microbial agents, anti-viral agents, antibiotics, xenobiotics, nutriceutical agents (nutritional materials), signal agents, combinations of such, and the like.
- the functional materials are adsorbed onto alumina that is contained in or on a particle, and desirably a nanoparticle. Nanoparticles are particularly desirable for the large surface area they offer and the potential exposure of the functional agent to body tissue.
- the resulting carrier particles can then be used as is or can be combined with a vehicle, such as a liquid vehicle, to deliver the functional material to a desired location within or on a patient's body.
- a vehicle such as a liquid vehicle
- the particles making up the delivery system of the present invention can be incorporated into a liquid vehicle and either ingested, applied subcutaneously, or applied topically to the skin of a patient using any conventional application means.
- the pharmaceutical may then be selectively released from the carrier particle (such as an alumina, silica, or alumina coated silica particle) so as to release the pharmaceutical at a targeted/desirable body location, or at a desirable moment.
- such selective release can be accomplished by exposure of the particle to a change in environmental condition, such as a pH change.
- a change in environmental condition such as a pH change.
- such selective release may be accomplished by exposure to an alkaline environment.
- such selective release may be accomplished by exposure to an acidic environment.
- such selective release may be the result of exposure of the carrier particle to particular chemical stimuli.
- a method for applying a health related compound utilizes a health-related compound coated particle, and selectively releasing the compound upon exposure of the particle to either a change in environmental condition, or upon exposure to a chemical stimulus.
- the present invention is directed to a particle containing alumina. At least a portion of the alumina contained by the particle is present on a surface of the particle. A functional compound is bonded to the alumina on the surface of the particle. The functional compound prior to bonding with the alumina contains a moiety comprising one or more of:
- each of the above moieties can include further R groups attached to the carbon chain shown above.
- any such R group can appear in association with the above moieties as long as the R group does not interfere with the bonding of the moiety to an alumina particle.
- the above moieties have been found to form a bond with alumina in constructing the compositions of the present invention.
- the functional compounds can then in one embodiment, be selectively released in either a basic or acidic environmental condition.
- the functional compounds can be released in the basic/alkaline environment of a vagina experiencing a yeast infection.
- the functional compounds can be released in the basic environment of the small intestine so as to treat an infection, after passing through the acidic environment of the stomach.
- a functional compound may be released as a result of environmental stimuli as an alert or in conjunction with the completion of the delivery of a pharmaceutical material so as to provide indication of such delivery or the success of such treatment.
- Such indicator or signal may be in the form of a dye or fragrance.
- such signal may be the result of a functional material contained on a first type of particle, and such coated particle may be included with additional particles of a different variety, that contain health related compounds.
- the functional material may be released in response to a particular chemical stimuli, which is intentionally applied to the site of the carrier particles.
- a method of utilizing a triggerably releasable delivery system in the treatment of a patient's body includes the steps of providing at least one type of particle selected from alumina particles, alumina covered particles, and silica particles; adsorbing at least one functional compound to the surface of the particle or particles to form at least a partially coated particle or particles; exposing the at least partially coated particle or particles to a patient's body such as by ingestion, injection, transdermal transfer or transmucosal transfer; and exposing the particle or particles to an environmental or chemical condition whereby the health related compound is released from the surface of the particle to the patient's body.
- FIG. 1 illustrates an exploded perspective view of a transdermal drug delivery device in accordance with the invention.
- FIG. 2 illustrates a cross-sectional view of the transdermal delivery device of FIG. 1.
- the present invention is directed to a triggerable delivery system for functional compounds and methods of using the same.
- Functional compounds can be any pharmaceutical and/or nutritionally suitable substance that can provide a benefit to a location on or within a patient's body once delivered.
- patient refers to both human and non-human patients.
- functional materials are health related compounds such as pharmaceutical or nutritional materials.
- the delivery system is generally directed to the construction of a particle containing alumina and use of such particle to selectively deliver functional compounds contained on the particle upon the occurrence/exposure of a triggering mechanism.
- the particle acts as a carrier for a functional compound.
- the alumina contained within the particle provides a bonding site on the surface of the particle for a functional compound.
- the functional compound (the pharmaceutical, nutritional material, or other health related material cited herein) becomes adsorbed onto the surface of the alumina.
- the resulting particle can then be used to deliver the functional compound to a particular location within, or on a body.
- the particles can be used as is, for instance, or can be combined with a liquid, gel or other vehicle which may facilitate delivery of the particles depending upon the particular application. Such liquid and gel vehicles are known to those skilled in the art.
- the particles and/or vehicle can also be used in conjunction with a drug delivery apparatus, such as a modified bandage or modified tampon. Such a bandage or tampon would be modified to include either the particles themselves or a vehicle containing the particles.
- a functional equivalent to one of the above moieties refers to functional materials that include similar reactive groups as shown above, but which are not positioned on a molecule exactly as shown above and yet will still bond with alumina in a similar manner.
- the above moieties may form a relatively strong bond to an alumina surface. Without wishing to be bound by theory, it is believed that the above moieties form a bidentate ligand bonding system with alumina surfaces. For instance, it is believed that alumina forms a covalent bond and a coordinate bond with the above moieties. Further, it is believed that a surface reaction occurs causing the functional compound to remain on the surface of the particle (unless triggerably released) and form a coating thereon.
- the functional material can cover the entire resulting particle or can be located at particular locations on the particle. Further, it should be understood that the particles of the present invention can contain more than one functional compound so as to deliver multiple treatments to address either a patient's multiple symptoms or a patient's multiple conditions.
- particles made according to the present invention can have a zeta potential of greater than 20 mV, particularly greater than 30 mV, and, in some embodiments, greater than 40 mV.
- the particles are well suited for being affixed to substrates that carry a negative surface charge through coulombic attraction.
- the bond of the particle in some applications can be relatively permanent and substantive. Consequently, the delivery system of the present invention can be used to affix functional compounds to various substrates without the use of chemical binders or other attachment structures.
- the alumina particle reacted with the functional compound can contain various other ingredients.
- the particle can contain any material that does not adversely interfere with the ability of the functional material to bond to alumina.
- at least a portion of the alumina contained by the particle should be present on the surface of the particle so that the alumina is available for adsorbing the functional compound.
- any suitable pharmaceutical and/or nutritional functional compound containing one of the above moieties, a tautomer thereof, or a functional equivalent thereof may be used in accordance with the present invention.
- functional compounds include pharmaceuticals, and xenobiotics. Xenobiotics is a general term used to describe any chemical interacting with an organism that does not occur in the normal metabolic pathways of that organism.
- Other functional compounds can include therapeutic agents, nutriceutical (nutritional )agents, anti-viral agents, anti-microbial agents, and the like.
- the terms “functional compound or functional agent” shall be taken to include “health -related compounds” which shall encompass pharmaceuticals, nutritional compounds, xenobiotics, anti-microbial agents, anti-viral agents, therapeutic agents and signal agents.
- a method used to prepare alumina nanoparticles having functional compounds bonded to the surface included the following steps.
- the functional compound was dissolved in water with stirring. To this stirred solution was slowly added the alumina nanoparticles and the resulting mixture stirred for about 5 to 10 minutes to allow the functional compound to bond to the surface of the nanoparticle.
- the UV-VIS spectrum of the water solution was obtained by taking an aliquot of the stirred mixture and placing it in a quartz cell. The UV-VIS spectra were obtained using a UV-VIS spectrophotometer Model UV- 1601 (Shimadzu Corporation) with water as a reference. Zeta Potential and particle size measurements were determined using a ZetaPals Instrument (Brookhaven Instrument Company, Holtsville, N.Y.).
- a method used to release the bonded functional compound utilizing a pH trigger included the following steps.
- the alumina nanoparticle having the functional agent bonded to the surface was placed in an aqueous solution (suspension) with stirring.
- dilute sodium hydroxide (0.1N) dropwise was slowly added to this stirred suspension.
- An aliquot of this suspension was taken and the UV-VIS spectrum measured.
- the bonded functional agent's Lambda max peak can be observed to decrease with the free functional agent's Lambda max peak observed to appear and increase.
- SNOWTEX-AK was initially used in a 50 ml portion of 20% wt/wt suspension.
- the physical parameters of the SNOWTEX-AK nanoparticles are as follows: SNOWTEX-AK-size :62 nm and Zeta Potential of +36 mV.
- additional pharmaceutical agents were evaluated for their propensity to bind strongly to alumina particles. They included the following agents described in Table 1, and which demonstrated the noted shift.
- Still additional pharmaceutical agents which may be used in conjunction with this invention include the following materials.
- the pharmaceutical agent was released as observed by a second red shift of the UV-VIS Lambda Maxima.
- the alkaline agent dilute sodium hydroxide (0.1 N)
- 0.1 N dilute sodium hydroxide
- the tetracycline was released from the alumina surface when the suspension of modified nanoparticles was altered to pH ⁇ fraction (9/10) ⁇ or greater.
- the noted shifts correspond to the absorption maximum of the free pharmaceutical agents.
- a method used to release the bonded functional agent from the silica surface using a pH trigger included the following steps.
- the silica nanoparticle having the functional agent bonded to the surface was placed in aqueous solution (suspension) with stirring.
- dilute hydrochloric acid (0.1N) dropwise was slowly added to this stirred suspension.
- An aliquot of this suspension was taken and the UV-VIS spectrum measured.
- the bonded functional agent's Lambda max peak can be observed to decrease with the free functional agent's Lambda max peak observed to appear and increase.
- the red shift is characteristic of the binding of the aryl aldehyde functionality to the silica surface.
- dilute acid hydrochloric acid
- the aldehyde was released and the fragrance returned.
- the UV-VIS absorption also underwent a blue shift to return to that of the starting aldehyde.
- Such chemistry may be used in conjunction with a pharmaceutical to be released upon the change of an environmental condition to indicate/signal that the pharmaceutical material has been delivered.
- signal agent may be adsorbed onto a silica particle.
- a pharmaceutical compound may be separately adsorbed onto an alumina particle.
- the particles may be combined and jointly used within a delivery vehicle or as part of a modified drug delivery device. The functional agents then would be triggered upon the occurrence of separate chemical events.
- nanoparticle delivery systems may be employed to carry the pharmaceutical agent through the stomach (having an acidic environment) and then release the agents into the small intestine (having a basic/alkaline environment).
- nanoparticle delivery systems may be used as part of a treatment on a tampon for vaginal infections.
- a medicated tampon may include a bound antibiotic (“bound” meaning the functional compound adsorbed to the surface of nanoparticles which are themselves attached through charge attraction to a tampon substrate).
- such nanoparticle delivery systems may be used as an application to a topical bandage. Upon a change in condition or application of a pH changing chemistry, functional materials contained on carrier nanoparticles on the bandage can be selectively released into or onto a wound site.
- the particle acts as a delivery vehicle for delivering the functional compound to a desired location.
- the functional compounds may be easier to handle, may be more stable, or may have other improved properties depending upon the application.
- the resulting particle structure can be incorporated into various other mediums.
- the particle structure can be incorporated into liquid vehicles, can be formed into capsules, can be combined with gels, pastes, other solid materials, and the like, depending on the end-use application.
- the particles of the present invention include a surface layer that contains one or more functional compounds.
- the coating on the particle can be continuous or discontinuous.
- the particle itself is believed to be amorphous.
- compositions made according to the present invention have been found to be well suited to being applied to substrates made from synthetic polymers, such as thermoplastic polymers.
- substrates can include, for instance, woven and non-woven materials made from a polyolefin polymer such as polypropylene or polyethylene, polyester, and the like.
- polyolefin polymer such as polypropylene or polyethylene, polyester, and the like.
- various problems have been experienced in trying to affix materials to these types of materials. These materials can be particularly effective as drug delivery substrates for delivery through the skin of a patient.
- the particles of the present invention can be affixed to these materials (as a result of differences in Zeta potential) without the use of chemical binders or complex chemical constructions.
- substrates made from synthetic polymers can undergo a pretreatment process for increasing the negative surface charge.
- pretreatment processes include subjecting the substrate to a corona treatment or to an electret treatment.
- An electret treatment for instance, is disclosed in U.S. Pat. No. 5,964,926 to Cohen, which is incorporated herein by reference in its entirety.
- Such pretreatments have been found not only to increase the negative surface charge of polymeric materials, but also assist in wetting out the polymer and enhancing surface adhesion between the polymer and the particles of the present invention.
- FIG. 1 depicts an exploded perspective view of a transdermal drug delivery device in accordance with the invention.
- FIG. 2 depicts a cross-sectional view of the transdermal delivery device of FIG. 1.
- the transdermal delivery device 70 is designed to deliver a functional agent/compound, either drugs, medicaments, or other treatments, across the skin of a patient's body.
- the delivery device includes an adhesive layer 72 , for affixing the device (patch) to the skin of the patient.
- the adhesive layer may include a removable protective liner, to protect the adhesive layer during nonuse and also to reduce the likelihood of loss of active ingredient.
- the medicaments may be targeted to narrower areas of skin, depending upon the ability of each polymer component to allow the passage of the functional compound.
- the polymer layer is essentially the skin contacting layer, through which the active ingredient passes after the device is applied to the skin of a consumer.
- the device further includes a backing layer 76 , which includes a raised portion 78 , for housing the functional compound/active ingredient.
- the active ingredient is allowed to pass through the polymer layer 74 / 80 but desirably does not pass through the backing layer 76 .
- the polymer material of the present invention may be utilized as the material for forming a polymer layer in the patch, in order to provide the ability to pass functional compounds to the skin of a user.
- Such polymer layer may be for example a film (such as a selectively permeable or apertured film) or nonwoven sheet (such as a spunbond or meltblown, or a combination of such).
- Such polymer layer may also be in the form of a hydrogel-type material.
- such drug enclosure 82 may in fact be comprised of an absorbent sheet material, such as a nonwoven, that is designed to either retain exudates from a wound site, or to both retain exudates, and also to release moisture or select medicaments that are stored within the absorbent sheet material, upon a change in condition, such as appearance of moisture, body exudates or a change in pH.
- a nonwoven web may be, for instance either a spunbond or meltblown nonwoven web, or a combination of such.
- the depicted wound dressing/transdermal delivery device can function as either a hydrogel or hydrocolloid.
- the bound pharmaceutical or nutritional chemistry could be used with or without triggerable release.
- some of the bound chemistry in a multiple chemistry particle system could triggerably releasable, while other bound chemistry could be intentionally retained on the carrier particles.
- the bound chemistry could perform its advantageous function while still being attached to the carrier particles, for ease of removal or to lower the potential toxicity of the functional agent/compound.
- An example of such usage would be using a bound salicylaldoxime to remove heavy metals from the body or waste water without the loss of or exposure to the free complexing agent.
- tetracycline could function as an antibiotic while still being bound on a particle. This could allow the antibiotic to function in the stomach and intestines without crossing over into the bloodstream of a patient (because of the size of the particle). This control of the antibiotic release could assist with lowering the risk of sensitization of patients who are allergic to such medications.
Abstract
A triggerable delivery system for various functional compounds is disclosed. The delivery system incorporates a carrier composition containing alumina particles, silica particles or alumina coated particles. Various functional materials containing particular moieties may be adsorbed onto the particles and used as desired. The functional compounds can be, for instance, pharmaceuticals, xenobiotics, anti-microbial agents, anti-viral agents, fragrances, and the like.
Description
- This application is a Continuation in Part and claims priority to U.S. patent application Ser. No. 10/325,474 filed on Dec. 20, 2002 in the names of Jason Lye and Gavin MacDonald (and referenced by attorney docket number 18, 113). U.S. patent application Ser. No. 10/325,474 is incorporated by reference herein it its entirety.
- This invention relates to delivery systems for pharmaceutical materials. More specifically, this invention relates to delivery systems and methods of delivering various pharmaceutical materials into or onto a patient's body.
- A delivery system generally refers to a system that aids or otherwise facilitates the delivery of a functional material to a desired location. The functional material can be any material that acts upon a substrate or otherwise provides a benefit once delivered to the desired location. Examples of functional materials that may benefit from the use of a delivery system include pharmaceuticals that are intended to be ingested, transferred transdermally, or subcutaneously injected into a human or animal patient's body, vitamins and nutrients (nutritional materials), and various other and numerous additives that can similarly be introduced into the body of a patient.
- Even in view of recent advances in the art of delivery systems, further improvements in delivery systems for pharmaceutical and nutritional functional materials are still needed. For example, a need currently exists for a delivery system that can bind to various functional materials that does not incorporate relatively expensive chemical formulations or that does not require any complex process steps for incorporating a functional material into the delivery system. With respect to pharmaceutical and nutritional materials, a need also exists in the art for a delivery system for such materials that is capable of affixing the pharmaceutical or other health-related compounds to the delivery system, but will readily release such pharmaceutical materials or other health-related compounds upon the occurrence of a selected event or trigger. A need also exists for a method for selectively triggering the release of a pharmaceutical material or other health-related compound where and when it is needed. It is to such needs that the current invention is directed.
- The present invention is generally directed to a delivery system for various functional materials. The functional materials can be, for instance, health-related compounds/materials such as pharmaceuticals, anti-microbial agents, anti-viral agents, antibiotics, xenobiotics, nutriceutical agents (nutritional materials), signal agents, combinations of such, and the like. In accordance with one embodiment of the present invention, the functional materials are adsorbed onto alumina that is contained in or on a particle, and desirably a nanoparticle. Nanoparticles are particularly desirable for the large surface area they offer and the potential exposure of the functional agent to body tissue. The resulting carrier particles can then be used as is or can be combined with a vehicle, such as a liquid vehicle, to deliver the functional material to a desired location within or on a patient's body. For example, when the functional material is a pharmaceutical, the particles making up the delivery system of the present invention, can be incorporated into a liquid vehicle and either ingested, applied subcutaneously, or applied topically to the skin of a patient using any conventional application means. The pharmaceutical may then be selectively released from the carrier particle (such as an alumina, silica, or alumina coated silica particle) so as to release the pharmaceutical at a targeted/desirable body location, or at a desirable moment. In one embodiment, such selective release can be accomplished by exposure of the particle to a change in environmental condition, such as a pH change. For example, such selective release may be accomplished by exposure to an alkaline environment. Alternatively, such selective release may be accomplished by exposure to an acidic environment. Still further, such selective release may be the result of exposure of the carrier particle to particular chemical stimuli. In an alternative embodiment of the invention, a method for applying a health related compound utilizes a health-related compound coated particle, and selectively releasing the compound upon exposure of the particle to either a change in environmental condition, or upon exposure to a chemical stimulus.
- Thus, in one embodiment, the present invention is directed to a particle containing alumina. At least a portion of the alumina contained by the particle is present on a surface of the particle. A functional compound is bonded to the alumina on the surface of the particle. The functional compound prior to bonding with the alumina contains a moiety comprising one or more of:
- a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group.
- The above moieties can be present as is on a functional compound. Alternatively, however, each of the above moieties can include further R groups attached to the carbon chain shown above. In general, any such R group can appear in association with the above moieties as long as the R group does not interfere with the bonding of the moiety to an alumina particle. The above moieties have been found to form a bond with alumina in constructing the compositions of the present invention.
- The functional compounds can then in one embodiment, be selectively released in either a basic or acidic environmental condition. For instance, in one specific embodiment of the invention, the functional compounds can be released in the basic/alkaline environment of a vagina experiencing a yeast infection. In a second embodiment, the functional compounds can be released in the basic environment of the small intestine so as to treat an infection, after passing through the acidic environment of the stomach. In still a further alternative embodiment, a functional compound may be released as a result of environmental stimuli as an alert or in conjunction with the completion of the delivery of a pharmaceutical material so as to provide indication of such delivery or the success of such treatment. Such indicator or signal may be in the form of a dye or fragrance.
- In still a further alternative embodiment, such signal may be the result of a functional material contained on a first type of particle, and such coated particle may be included with additional particles of a different variety, that contain health related compounds. In still a further alternative embodiment, the functional material may be released in response to a particular chemical stimuli, which is intentionally applied to the site of the carrier particles. In still a further alternative embodiment, a method of utilizing a triggerably releasable delivery system in the treatment of a patient's body includes the steps of providing at least one type of particle selected from alumina particles, alumina covered particles, and silica particles; adsorbing at least one functional compound to the surface of the particle or particles to form at least a partially coated particle or particles; exposing the at least partially coated particle or particles to a patient's body such as by ingestion, injection, transdermal transfer or transmucosal transfer; and exposing the particle or particles to an environmental or chemical condition whereby the health related compound is released from the surface of the particle to the patient's body.
- Other features and aspects of the present invention are discussed in greater detail below.
- FIG. 1 illustrates an exploded perspective view of a transdermal drug delivery device in accordance with the invention.
- FIG. 2 illustrates a cross-sectional view of the transdermal delivery device of FIG. 1.
- In general, the present invention is directed to a triggerable delivery system for functional compounds and methods of using the same. Functional compounds can be any pharmaceutical and/or nutritionally suitable substance that can provide a benefit to a location on or within a patient's body once delivered. For the purposes of this application, it should be understood that the term “patient” refers to both human and non-human patients. Desirably, such functional materials are health related compounds such as pharmaceutical or nutritional materials.
- In accordance with one embodiment of the present invention, the delivery system is generally directed to the construction of a particle containing alumina and use of such particle to selectively deliver functional compounds contained on the particle upon the occurrence/exposure of a triggering mechanism. The particle acts as a carrier for a functional compound.
- Specifically, the alumina contained within the particle provides a bonding site on the surface of the particle for a functional compound. The functional compound (the pharmaceutical, nutritional material, or other health related material cited herein) becomes adsorbed onto the surface of the alumina. Once the functional compound is bonded to the alumina, the resulting particle can then be used to deliver the functional compound to a particular location within, or on a body. The particles can be used as is, for instance, or can be combined with a liquid, gel or other vehicle which may facilitate delivery of the particles depending upon the particular application. Such liquid and gel vehicles are known to those skilled in the art. The particles and/or vehicle can also be used in conjunction with a drug delivery apparatus, such as a modified bandage or modified tampon. Such a bandage or tampon would be modified to include either the particles themselves or a vehicle containing the particles.
-
- a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group. As used herein, a functional equivalent to one of the above moieties refers to functional materials that include similar reactive groups as shown above, but which are not positioned on a molecule exactly as shown above and yet will still bond with alumina in a similar manner.
- Referring to the moieties shown above, moiety (1) may be considered a carboxy-hydroxy moiety. Moiety (2) may be considered a hyrdoxy-hydroxy moiety, while moiety (3) may be considered a carboxy-carboxy moiety. Moieties (4) and (5), on the other hand, can be considered vinylalogous amide moieties. In moieties (4) and (5) above, the amine groups can be primary amines, secondary amines, or tertiary amines. Moieties (6) and (7) may be considered hydroxyl carbonyl moieties. Moiety (8) may be considered a carboxy amine. Moieties such as (8) may be found in amino acids. Moiety (9) may be considered a hydroxy imine. In general, any suitable functional compound containing one of the above moieties or a functional equivalent thereof may be used in accordance with the present invention. Further, it should be understood that various additional R groups may be included with the above moieties as long as the R groups do not interfere with the bond that is formed with alumina.
- The above moieties may form a relatively strong bond to an alumina surface. Without wishing to be bound by theory, it is believed that the above moieties form a bidentate ligand bonding system with alumina surfaces. For instance, it is believed that alumina forms a covalent bond and a coordinate bond with the above moieties. Further, it is believed that a surface reaction occurs causing the functional compound to remain on the surface of the particle (unless triggerably released) and form a coating thereon. The functional material can cover the entire resulting particle or can be located at particular locations on the particle. Further, it should be understood that the particles of the present invention can contain more than one functional compound so as to deliver multiple treatments to address either a patient's multiple symptoms or a patient's multiple conditions.
- Of particular advantage, in many embodiments, it has also been discovered that a functional compound can be bonded to alumina without significantly impacting the positive surface charge of alumina, which can be measured as zeta potential. The term “zeta potential” is used herein to mean without limitation, a potential gradient that arises across an interface. This term especially refers to the potential gradient that arises across the interface between the Stern layer in contact with the particle of the present invention and the diffuse layer surrounding the particle. Zeta potential measurements can be taken using, for instance, a Zetapals instrument which is available from the Brookhaven Instrument Corporation of Holtsville, N.Y. For example, zeta potential measurements can be conducted by adding one to three drops of a sample into a cuvet containing 1 mM KCl solution, and using the instrument's default functions preset for aqueous solutions.
- Thus, once alumina is bonded to the functional material, the resulting molecule continues to maintain a relatively strong positive charge. For instance, particles made according to the present invention can have a zeta potential of greater than 20 mV, particularly greater than 30 mV, and, in some embodiments, greater than 40 mV. By remaining positively charged, the particles are well suited for being affixed to substrates that carry a negative surface charge through coulombic attraction. Depending upon the difference in charge between the particle of the present invention and the surface of a substrate, the bond of the particle in some applications can be relatively permanent and substantive. Consequently, the delivery system of the present invention can be used to affix functional compounds to various substrates without the use of chemical binders or other attachment structures. As an example, the carrier particle (delivery system) can include along its surface a pharmaceutical functional compound, and yet the particle may still retain sufficient positive charge, to allow it to be attached to a negatively charged bandage or other topically contacting substrate layer. Then upon the occurrence of a specific chemical or environmental stimuli, the functional material contained on the particle can be selectively released to the body of a patient, but the carrier particles will remain affixed to the bandage or other charged surface.
- Various different particles and compositions can be used in the present invention. For instance, alumina or silica particles may be used, depending upon the functional compound and the trigger for releasing it. Silica particles are available under the designation SNOWTEX-C through from Nissan Chemical America (Houston, Tex.). Various different particles and compositions that contain alumina can be used in the present invention. For example, in one embodiment, the functional material is combined with an alumina sol. Many different types of alumina sols are commercially available with varying particle size. Of particular advantage, alumina sols can be prepared that carry a relatively strong positive surface charge or zeta potential. In this embodiment, the particle that is reacted with the functional compound contains primarily and in some embodiments exclusively alumina. Examples of alumina particle materials, include Aluminasol-100, and Aluminasol-200, available from Nissan Chemical America (Houston, Tex.).
- In other embodiments, however, the alumina particle reacted with the functional compound can contain various other ingredients. In general, the particle can contain any material that does not adversely interfere with the ability of the functional material to bond to alumina. In this regard, at least a portion of the alumina contained by the particle should be present on the surface of the particle so that the alumina is available for adsorbing the functional compound.
- In one particular embodiment of the present invention, the particle can contain a core material coated with alumina. The alumina can form a continuous coating over the particle or a discontinuous coating. The core material can be, for instance, an inorganic oxide, such as silica. For example, in one embodiment, sols can be used that contain silica nanoparticles that have an alumina surface coating. Such sols are currently commercially available, for instance, from Nissan Chemical America of Houston, Tex. The silica is coated with alumina to provide stability to the sols over certain pH ranges. In fact, alumina coated silica sols may have greater stability in some applications of the present invention in comparison to alumina sols. A specific example of alumina particle materials with silica cores, include Snowtex-AK, available from Nissan Chemical America, Houston, Tex.) and Ludox Cl from Grace Davison, Columbia, Md.
- As described above, any suitable pharmaceutical and/or nutritional functional compound containing one of the above moieties, a tautomer thereof, or a functional equivalent thereof may be used in accordance with the present invention. Examples of functional compounds include pharmaceuticals, and xenobiotics. Xenobiotics is a general term used to describe any chemical interacting with an organism that does not occur in the normal metabolic pathways of that organism. Other functional compounds can include therapeutic agents, nutriceutical (nutritional )agents, anti-viral agents, anti-microbial agents, and the like. For the purposes of this application, the terms “functional compound or functional agent” shall be taken to include “health -related compounds” which shall encompass pharmaceuticals, nutritional compounds, xenobiotics, anti-microbial agents, anti-viral agents, therapeutic agents and signal agents.
- One example of a therapeutic agent that may be used in the present invention is hydrocortisone. Hydrocortisone is a natural anti-inflammatory hormone of the glucocorticoid family of hormones produced by the adrenal cortex. Examples of nutritional compounds include ascorbic acid and aspartame. In one particular embodiment, the functional compound may be a pharmaceutical/anti-microbial agent such as an antibiotic. An example of such an antibiotic may include tetracycline. Tetracycline is an antibiotic substance produced by Streptomyces spp. Hydrocortisone and tetracycline structural formulas are provided below:
-
- As can be seen by the above structural formula, tetracycline is an antibacterial agent that contains a carbonyl-hydroxy functionality, capable of bonding with alumina in accordance with the present invention. Tetracycline is a series of isomers of cyclomycin.
- In still a further alternative embodiment, a signal agent, such as a fragrance, may be used by itself or in conjunction with a health related compound on a variety of particle types to both treat a condition, and also to provide an indication to the patient of the effectiveness of such treatment or the occurrence of a particular event. As an example, a fragrance may be adsorbed to one type of particle and an antibiotic may be adsorbed to a second type of particle. The particles can be delivered to an infected site simultaneously. If the infected site is alkaline, it will prompt the release of the antibiotic. Upon removal of the infection, and the return to a more normal acidic environment, the fragrance may be released, thereby providing an indication of the effective treatment of the infection. In a further example, the signal can be used to generate an indication of a particular event, such as the release of body fluids or exudates as in a bandage or personal care product, such as a feminine care product or child care diaper product.
- A method used to prepare alumina nanoparticles having functional compounds bonded to the surface included the following steps.
- The functional compound was dissolved in water with stirring. To this stirred solution was slowly added the alumina nanoparticles and the resulting mixture stirred for about 5 to 10 minutes to allow the functional compound to bond to the surface of the nanoparticle. The UV-VIS spectrum of the water solution was obtained by taking an aliquot of the stirred mixture and placing it in a quartz cell. The UV-VIS spectra were obtained using a UV-VIS spectrophotometer Model UV- 1601 (Shimadzu Corporation) with water as a reference. Zeta Potential and particle size measurements were determined using a ZetaPals Instrument (Brookhaven Instrument Company, Holtsville, N.Y.).
- A method used to release the bonded functional compound utilizing a pH trigger included the following steps. The alumina nanoparticle having the functional agent bonded to the surface was placed in an aqueous solution (suspension) with stirring. To this stirred suspension was slowly added dilute sodium hydroxide (0.1N) dropwise and the pH was subsequently measured. An aliquot of this suspension was taken and the UV-VIS spectrum measured. In this manner, the bonded functional agent's Lambda max peak can be observed to decrease with the free functional agent's Lambda max peak observed to appear and increase.
- In a first example of the adsorption of pharmaceutical materials onto the surface of a carrier nanoparticle, the UV-visible absorbance spectrum of Tetracycline was initially measured using a UV-visible spectrophotometer (Perkin-Elmer UV-Visible spectrophotometer.) Tetracycline was found to absorb at 357 nm in water. In particular, 10 mg tetracycline was in 50 ml water. When 5.0 ml SNOWTEX AK suspension 20% wt/wt (a sol containing silica particles that had an alumina surface coating, as obtained from Nissan Chemical America of Houston, Tex.) was added, with stirring, to the tetracycline solution. An aliquot was removed and the UV-VIS spectrum of the solution recorded. A bathochromic shift occurred to give an absorbance of 365 nm, suggesting that the tetracycline had adsorbed onto the alumina surface of SNOWTEX-AK particles. SNOWTEX-AK was initially used in a 50 ml portion of 20% wt/wt suspension. The physical parameters of the SNOWTEX-AK nanoparticles are as follows: SNOWTEX-AK-size :62 nm and Zeta Potential of +36 mV.In further Examples, additional pharmaceutical agents were evaluated for their propensity to bind strongly to alumina particles. They included the following agents described in Table 1, and which demonstrated the noted shift. These agents are considered antineoplastic for use as drugs that kill or stop the spread of cancer cells. Baicalein has been studied for its antiproliferation effect of human T-lymphoid leukemia cells.
TABLE 1 UV-VIS ABSORPTION (nm) SAMPLE FREE AGENT SN-AK/AGENT Baicalin Hydrate 278 and 322 295 and 388 Baicalein 320 348 Daunorubicin 472 480 -
- In a similar manner to the previous systems, examples of nutraceutical agents with the desired functional moieties were evaluated for their propensity to bind to alumina particles. Examples of such compounds were ascorbic acid (Vitamin C) and phenylalanine (sweetener found in Equal®). The structural equations for these materials and their ability to bind to such particles was demonstrated as can be seen in Table 2 which follows:
TABLE 2 SAMPLE UV-VIS ABSORPTION (nm) Ascorbic Acid in water 266 Ascorbic Acid/SN-AK 260 Phenylalanine in water 230 Phenylalanine/SN-AK 224* - It should be noted here that a shift in the absorption maximum was observed on addition of SNOWTEX-AK to the ascorbic acid solution, however a blue shift was observed (hypsochromic). This shift was due to binding, as no shift was observed when dilute acid was added to a separate solution of ascorbic acid. In a similar way, a blue shift (hypsochromic shift) was also observed with the phenylalanine binding to SNOWTEX-AK.
- In a further set of examples, pharmaceutical materials were adsorbed to carrier alumina particles and then selectively released from the carrier particles. In particular, separate 50 ml Solutions of Tetracycline and hydrocortisone agents (0.01 g) in water were prepared to which the alumina nanoparticle (SNOWTEX-AK) suspension (5 ml of 20% wt/wt) were added. A bathochromic shift (red shift) in the UV-VIS Lambda maxima was again observed, indicating strong binding of these pharmaceutical agents to the surface of the alumina particle. The following Table 3 shows the shift in the UV-VIS spectra recorded. Once the pharmaceutical agents had been bound to particles, they were selectively released by a controlled pH trigger mechanism. Thus, by changing the pH of the modified nanoparticle suspension to high pH values, the pharmaceutical agent was released as observed by a second red shift of the UV-VIS Lambda Maxima. In particular, the alkaline agent, dilute sodium hydroxide (0.1 N), was added in 0.5 ml amounts to the samples. The tetracycline was released from the alumina surface when the suspension of modified nanoparticles was altered to pH {fraction (9/10)} or greater. The noted shifts correspond to the absorption maximum of the free pharmaceutical agents.
TABLE 3 SAMPLE UV-VIS ABSORPTION (nm) Hydrocortisone in water 241 Hydrocortisone/SN-AK 234 Hydrocortisone/SN-AK with Base 244 Hydrocortisone with base 244 Tetracycline in water 357 Tetracycline/SN-AK 365 Tetracyclin/SN-AK with base 385 Tetracycline with base 385 - Therefore, these two examples of pharmaceutical agents demonstrate the capability of selectively releasing pharmaceutical agents from the carrier particles. By the use of a “pH trigger” the functional compounds can be released in a controlled manner when needed. It should be noted that such triggering of the delivery system may be accomplished through environmental changes such as infection which results in pH changes, taking advantage of inherent differences in pH depending on body locations, and the intentional act of introducing chemistries such as pH altering materials to the delivery systems to trigger the release of functional compounds. Chemistries that may be introduced to a delivery system include bicarbonates, carbonates and buffering salts which would result in a pH change on becoming wet with water or biological fluid. In yet another example, the delivery system would be incorporated into a tampon. Normal healthy vaginal fluid is acidic, typically in the 3-5 pH range. However, when infected with a yeast infection or other microbial infection, the pH changes to the basic range. This swing in pH would trigger the release of medication or buffering agents to restore the healthy pH of the vaginal fluid and flora.
- Silica Particle Binding and Release:
- The following examples illustrate the use of silica nanoparticles (as opposed to alumina particles) and the bonding of signal functional agents to the surface of the particles. The pH triggered release for silica coated particles is activated by adding acid and lowering the pH to the environment of the silica particles. Dilute acid is used in these examples.
- A method used to prepare silica nanoparticles having functional agents bonded to the surface included the following steps. The functional agent was dissolved into water with stirring. To this stirred solution was slowly added the silica nanoparticles and the resulting mixture stirred for about 5 to 10 minutes to allow the functional agent to bond to the surface of the nanoparticles. The UV-VIS spectrum of the water solution was obtained by taking an aliquot of the stirred mixture and placing it in a quartz cell. The UV-VIS spectra were obtained using the UV-VIS spectrophotometer Model UV-1601 with water as a reference. Zeta Potential and particle size measurements were determined using a ZetaPals Instrument (Brookhaven Instrument Company, Holtsville, N.Y.).
- A method used to release the bonded functional agent from the silica surface using a pH trigger included the following steps. The silica nanoparticle having the functional agent bonded to the surface was placed in aqueous solution (suspension) with stirring. To this stirred suspension was slowly added dilute hydrochloric acid (0.1N) dropwise and the pH measured. An aliquot of this suspension was taken and the UV-VIS spectrum measured. In this manner, the bonded functional agent's Lambda max peak can be observed to decrease with the free functional agent's Lambda max peak observed to appear and increase.
- In a similar fashion, the binding of active fragrance compounds to silica nanoparticles (SNOWTEX C, Nissan Chemicals America, Houston, Tex.) was demonstrated. Accordingly, to a solution (0.01 g of salicyclaldehyde in 50 ml of water) of salicylaldehyde (used in the perfume industry as a base fragrance) was added a dilute suspension (3 ml of 2% wt/wt) of silica nanoparticles (Snowtex C, Nissan Chemicals America, Houston Tex.) with stirring. The UV-VIS absorption of the salicylaldehyde underwent a red shift in its lambda max (see Table 4 below) and the characteristic fragrance disappeared. The red shift is characteristic of the binding of the aryl aldehyde functionality to the silica surface. Upon addition of dilute acid (hydrochloric acid), the aldehyde was released and the fragrance returned. The UV-VIS absorption also underwent a blue shift to return to that of the starting aldehyde. Such chemistry may be used in conjunction with a pharmaceutical to be released upon the change of an environmental condition to indicate/signal that the pharmaceutical material has been delivered. For instance, such signal agent may be adsorbed onto a silica particle. A pharmaceutical compound may be separately adsorbed onto an alumina particle. The particles may be combined and jointly used within a delivery vehicle or as part of a modified drug delivery device. The functional agents then would be triggered upon the occurrence of separate chemical events.
- In a similar manner, salicylaldoxime a metal sequestering agent, was also found to bind to the silica particle surface and undergo a pH triggered release. The structural formulas and exemplary data are illustrated in the following Table 4.
TABLE 4 UV-VIS Absorption (nm) SAMPLE After Addition of Silica After Addition of Acid Salicylaldehyde 382 327 327 nm Salicylaldoxime 350 340 303 nm - Additionally, a titration study using UV-VIS spectroscopy was carried out to determine the pH at which all of the salicylaldehyde was released. This was found to be at pH 6. In a further alternative embodiment, such nanoparticle delivery systems may be employed to carry the pharmaceutical agent through the stomach (having an acidic environment) and then release the agents into the small intestine (having a basic/alkaline environment). In still another alternative embodiment, such nanoparticle delivery systems may be used as part of a treatment on a tampon for vaginal infections. For instance, a medicated tampon may include a bound antibiotic (“bound” meaning the functional compound adsorbed to the surface of nanoparticles which are themselves attached through charge attraction to a tampon substrate). When the pH of a patient's vagina turns alkaline as a result of a yeast infection, the tampon would be triggered to release the bound antibiotic to control the yeast infection, thereby resulting in the pH returning to the normal acidic environment. In still a further alternative embodiment, such nanoparticle delivery systems may be used as an application to a topical bandage. Upon a change in condition or application of a pH changing chemistry, functional materials contained on carrier nanoparticles on the bandage can be selectively released into or onto a wound site.
- Once any of the above-mentioned functional compounds are bound to the alumina or silica particle (as the case may be), the particle acts as a delivery vehicle for delivering the functional compound to a desired location. Once bound to the particle, the functional compounds may be easier to handle, may be more stable, or may have other improved properties depending upon the application. Further, the resulting particle structure can be incorporated into various other mediums. For instance, the particle structure can be incorporated into liquid vehicles, can be formed into capsules, can be combined with gels, pastes, other solid materials, and the like, depending on the end-use application.
- The particles formed according to the present invention and including the functional compound, can be present in various forms, shapes, and sizes depending upon the desired result. For instance, the particles can be of any shape, for example, a sphere, a crystal, a rod, a disk, a tube, or a string of particles. The size of the particle can also vary dramatically. For instance, in one embodiment, the particles can have an average dimension of less than about 1 mm, particularly less than about 500 microns, and more particularly less than about 100 microns. In other embodiments, however, even smaller sizes may be desired. For instance, the particles can have an average diameter of less than about 1,000 nm, and particularly less than about 500 nm. As used herein, the average dimension of a particle refers to the average length, width, height, or diameter of a particle.
- As described above, the particles of the present invention include a surface layer that contains one or more functional compounds. The coating on the particle can be continuous or discontinuous. The particle itself is believed to be amorphous.
- In one particular embodiment, compositions made according to the present invention have been found to be well suited to being applied to substrates made from synthetic polymers, such as thermoplastic polymers. Such substrates can include, for instance, woven and non-woven materials made from a polyolefin polymer such as polypropylene or polyethylene, polyester, and the like. In the past, various problems have been experienced in trying to affix materials to these types of materials. These materials can be particularly effective as drug delivery substrates for delivery through the skin of a patient. The particles of the present invention can be affixed to these materials (as a result of differences in Zeta potential) without the use of chemical binders or complex chemical constructions.
- Although not needed, in some embodiments it may be desirable to pre-treat or post-treat the polymer substrates which may further serve to affix the particles to the materials. For instance, substrates made from synthetic polymers can undergo a pretreatment process for increasing the negative surface charge. For example, such pretreatment processes include subjecting the substrate to a corona treatment or to an electret treatment. An electret treatment, for instance, is disclosed in U.S. Pat. No. 5,964,926 to Cohen, which is incorporated herein by reference in its entirety. Such pretreatments have been found not only to increase the negative surface charge of polymeric materials, but also assist in wetting out the polymer and enhancing surface adhesion between the polymer and the particles of the present invention.
- In addition to pretreatment processes, substrates contacted with the particles of the present invention can also undergo various post treatment processes which further serve to affix the particles to the substrate. For example, in one embodiment, the treated substrate can be subjected to radio frequency radiation or to microwave radiation. Alumina is known to adsorb radio frequency radiation and microwave radiation causing the particles to heat. Once heated, it is believed that the particles become further embedded into the polymeric substrate. Further, the particles can be heated without also heating the substrate to higher than desired temperatures.
- Following being affixed to such substrates, upon exposure to a change in condition (such as pH) the functional compounds would be released from the substrate, but the particles would be left behind.
- In a specific embodiment, carrier particles (and desirably nanoparticles, that is particles having sizes of less than about 1 micron in size, more desirably between about 5 nm and 500 nm in size, and even more desirably, between about 10 nm-200 nm in size) including pharmaceutical compounds, can be applied to a topical bandage by various application methods. The application methods may include a gel, a water suspension, a dry coating or a powder placed between the layers of the bandage. if the particles are included in a vehicle for ease of application. The bandage can then be dried, if appropriate, whereby the charges of the particles would maintain them in close association with the bandage substrate. In this regard, FIG. 1 depicts an exploded perspective view of a transdermal drug delivery device in accordance with the invention. FIG. 2 depicts a cross-sectional view of the transdermal delivery device of FIG. 1. The
transdermal delivery device 70 is designed to deliver a functional agent/compound, either drugs, medicaments, or other treatments, across the skin of a patient's body. The delivery device includes anadhesive layer 72, for affixing the device (patch) to the skin of the patient. The adhesive layer may include a removable protective liner, to protect the adhesive layer during nonuse and also to reduce the likelihood of loss of active ingredient. The adhesive layer may cover the entire lower surface of the transdermal delivery device, or only a peripheral portion of the lower surface, so as not to interfere with the passage of active ingredients across the skin of the consumer. The active ingredient (the coated particle) can be stored in achamber 82 or in thepolymer layer 74. If the active ingredient is stored in a chamber, 82, thepolymer layer 74 separates the active ingredient from the adhesive layer. If the active ingredient is in the polymer layer, no chamber is necessary.Such polymer layer 74 may be a single component layer, or alternatively, may comprise two materials (as shown) such as 74 and 80. If the polymer layer is made from two or more distinct polymer components, the medicaments may be targeted to narrower areas of skin, depending upon the ability of each polymer component to allow the passage of the functional compound. The polymer layer is essentially the skin contacting layer, through which the active ingredient passes after the device is applied to the skin of a consumer. The device further includes abacking layer 76, which includes a raisedportion 78, for housing the functional compound/active ingredient. The active ingredient is allowed to pass through thepolymer layer 74/80 but desirably does not pass through thebacking layer 76. The polymer material of the present invention may be utilized as the material for forming a polymer layer in the patch, in order to provide the ability to pass functional compounds to the skin of a user. Such polymer layer may be for example a film (such as a selectively permeable or apertured film) or nonwoven sheet (such as a spunbond or meltblown, or a combination of such). Such polymer layer may also be in the form of a hydrogel-type material. - While FIGS. 1 and 2 provide one example of a transdermal delivery device/bandage, in accordance with the invention, it should be appreciated that numerous variations are contemplated to be within the scope of the invention. For instance, each of the described layers may be constructed of one or more layers for more defined/targeted or efficient health-related compound application. Further, in the case of a bandage,
such drug enclosure 82, and separate polymer layer, may in fact be comprised of an absorbent sheet material, such as a nonwoven, that is designed to either retain exudates from a wound site, or to both retain exudates, and also to release moisture or select medicaments that are stored within the absorbent sheet material, upon a change in condition, such as appearance of moisture, body exudates or a change in pH. Such nonwoven web, may be, for instance either a spunbond or meltblown nonwoven web, or a combination of such. In such a fashion, the depicted wound dressing/transdermal delivery device can function as either a hydrogel or hydrocolloid. Such a dressing could act to donate moisture, absorb exudates, to release functional compound, or a combination of such. Similar polymeric layers can also be part of transmucosal and vaginal delivery devices (tampons), as previously described. Examples of tampon structures are described in U.S. Pat. No. 6,177,608, which is hereby incorporated by reference in its entirety. - It should be recognized that the bound pharmaceutical or nutritional chemistry could be used with or without triggerable release. Alternatively, some of the bound chemistry in a multiple chemistry particle system could triggerably releasable, while other bound chemistry could be intentionally retained on the carrier particles. In this fashion, the bound chemistry could perform its advantageous function while still being attached to the carrier particles, for ease of removal or to lower the potential toxicity of the functional agent/compound. An example of such usage would be using a bound salicylaldoxime to remove heavy metals from the body or waste water without the loss of or exposure to the free complexing agent.
- In another example, tetracycline could function as an antibiotic while still being bound on a particle. This could allow the antibiotic to function in the stomach and intestines without crossing over into the bloodstream of a patient (because of the size of the particle). This control of the antibiotic release could assist with lowering the risk of sensitization of patients who are allergic to such medications.
- These and other modifications and variations to the present invention may be practiced by those of ordinary skill in the art, without departing from the spirit and scope of the present invention, which is more particularly set forth in the appended claims. In addition, it should be understood that aspects of the various embodiments may be interchanged both in whole or in part. Furthermore, those of ordinary skill in the art will appreciate that the foregoing description is by way of example only, and is not intended to limit the invention so further described in such appended claims.
Claims (27)
1. A method of utilizing a triggerably releasable delivery system in the treatment of a patient's body comprising:
a) providing at least one type of particle selected from alumina particles, alumina covered particles, and silica particles;
b) adsorbing at least one functional compound to the surface of the particle or particles to form at least a partially coated particle or particles;
c) placing the at least partially coated particle or particles in a position adjacent or within a patient's body;
d) exposing the particle or particles to an environmental or chemical condition whereby the functional compound is released from the surface of the particle to the patient's body.
2. The method of claim 1 wherein the environmental or chemical condition is selected from the group consisting of a chemical trigger, a change in pH, introduction of the particle to moisture or body exudates.
3. The method of claim 1 wherein multiple types of particles are coated with functional compounds.
4. The method of claim 1 wherein the particles contain alumina, at least a portion of the alumina being present on a surface of the particles; and the functional compound prior to adsorbing with the alumina particle containing a moiety comprising:
or a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group.
5. A method of utilizing a triggerable delivery system comprising:
providing a plurality of particles, the particles containing alumina, at least a portion of the alumina being present on a surface of the particles; and
bonding to the alumina on the surface of the particles a functional compound, the functional compound prior to bonding with the alumina containing a moiety comprising:
or a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group;
introducing the particles to a body;
exposing the particles to a change in pH such that the functional compound is released from the alumina.
6. The method of claim 5 wherein the particles are introduced to a body via a vehicle.
7. The method of claim 6 wherein the vehicle is selected from a liquid or a gel.
8. The method of claim 5 wherein the particles are affixed to a substrate for application either to the skin of a body or into a body cavity.
9. The method of claim 8 wherein the particles are affixed to a transdermal drug delivery device.
10. The method of claim 5 wherein the functional agent is selected from either a pharmaceutical or nutritional compound.
11. The method of claim 5 wherein the pH is changed from an acid to an alkaline pH.
12. The method of claim 11 wherein the pH is changed to a pH of between 9 to 10.
13. The method of claim 5 wherein the pH is changed from an alkaline to an acid pH.
14. The method of claim 5 , wherein the functional compounds include a chemical signal and either a pharmaceutical or nutritional compound.
15. A method of utilizing a triggerable delivery system comprising:
providing a plurality of particles, the particles containing alumina, at least a portion of the alumina being present on a surface of the particles; and
bonding to the alumina on the surface of the particles a functional compound, the functional compound prior to bonding with the alumina containing a moiety comprising:
or a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group;
introducing the particles into a drug delivery device;
contacting the drug delivery device with a patient's body;
exposing the particles in the drug delivery device to a change in pH such that the functional compound is released from the alumina.
16. The method of claim 15 wherein the particles are introduced into a drug delivery device via a vehicle.
17. The method of claim 16 wherein the vehicle is selected from a liquid or a gel.
18. The method of claim 15 wherein the particles are affixed to the drug delivery device for application either to the skin of a body or into a body cavity.
19. The method of claim 18 wherein the particles are affixed to a transdermal drug delivery device.
20. The method of claim 15 wherein the functional agent is selected from either a pharmaceutical or nutritional compound.
21. The method of claim 15 wherein the pH is changed from an acid to an alkaline pH.
22. The method of claim 21 wherein the pH is changed to a pH of between 9 to 10.
23. The method of claim 15 wherein the pH is changed from an alkaline to an acid pH.
24. The method of claim 15 , wherein the functional compounds include a chemical signal and either a pharmaceutical or nutritional compound.
25. A triggerable delivery system comprising:
a particle; and
a health-related compound adsorbed to the surface of said particle, said health-related compound capable of being released from said particle upon either exposure to a change in pH, moisture, chemical stimuli, or body exudates.
26. The triggerable delivery system of claim 25 wherein the particle contains alumina, at least a portion of the alumina being present on a surface of the particle; and
the health related compound, prior to being adsorbed with the alumina on the surface of the particle containing a moiety comprising:
or a tautomer thereof, or a functional equivalent thereof and wherein R and R′ comprise independently hydrogen, an alkyl group, or an aryl group.
27. A drug delivery device including a triggerable delivery system, said triggerable delivery system comprising a particle; and
a health-related compound adsorbed to the surface of said particle, said health-related compound capable of being released from said particle upon either exposure to a change in pH, moisture, chemical stimuli, or body exudates.
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/731,256 US20040142041A1 (en) | 2002-12-20 | 2003-12-09 | Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same |
| MXPA05005954A MXPA05005954A (en) | 2002-12-20 | 2003-12-11 | Compositions comprising particles containing alumina with compounds bound to the alumina surface, delivery systems and methods of preparation thereof. |
| PCT/US2003/039737 WO2004060378A2 (en) | 2002-12-20 | 2003-12-11 | Compositions comprising particles containing alumina with compounds bound to the alumina surface, delivery systems and methods of preparation thereof |
| JP2005508584A JP2006518773A (en) | 2002-12-20 | 2003-12-11 | Compound comprising alumina and a compound confined to the alumina surface, system and method for supplying the same |
| AU2003297051A AU2003297051A1 (en) | 2002-12-20 | 2003-12-11 | Compositions comprising particles containing alumina with compounds bound to the alumina surface, delivery systems and methods of preparation thereof |
| KR1020057010066A KR101012584B1 (en) | 2002-12-20 | 2003-12-11 | Composition Comprising Particles Containing Alumina With Compounds Bound To The Alumina Surface, Delivery Systems And Methods Of Preparation Thereof |
| EP03814766A EP1589978A2 (en) | 2002-12-20 | 2003-12-11 | Delivery system for functional compounds |
| TW092135502A TWI287034B (en) | 2002-12-20 | 2003-12-16 | Delivery system for functional compounds |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/325,474 US7666410B2 (en) | 2002-12-20 | 2002-12-20 | Delivery system for functional compounds |
| US10/731,256 US20040142041A1 (en) | 2002-12-20 | 2003-12-09 | Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/325,474 Continuation-In-Part US7666410B2 (en) | 2002-12-20 | 2002-12-20 | Delivery system for functional compounds |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040142041A1 true US20040142041A1 (en) | 2004-07-22 |
Family
ID=32593779
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/325,474 Expired - Fee Related US7666410B2 (en) | 2002-12-20 | 2002-12-20 | Delivery system for functional compounds |
| US10/731,256 Abandoned US20040142041A1 (en) | 2002-12-20 | 2003-12-09 | Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same |
| US12/710,413 Expired - Fee Related US8277801B2 (en) | 2002-12-20 | 2010-02-23 | Delivery system for functional compounds |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/325,474 Expired - Fee Related US7666410B2 (en) | 2002-12-20 | 2002-12-20 | Delivery system for functional compounds |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/710,413 Expired - Fee Related US8277801B2 (en) | 2002-12-20 | 2010-02-23 | Delivery system for functional compounds |
Country Status (2)
| Country | Link |
|---|---|
| US (3) | US7666410B2 (en) |
| CN (1) | CN1777431A (en) |
Cited By (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060009099A1 (en) * | 2004-07-12 | 2006-01-12 | Closure Medical Corporation | Adhesive-containing wound closure device and method |
| WO2007011612A3 (en) * | 2005-07-15 | 2007-04-26 | Tyco Healthcare | Wound dressing and methods of making and using the same |
| US20080063718A1 (en) * | 2006-09-08 | 2008-03-13 | Kimberly-Clark Worldwide, Inc. | Delivery Systems For Delivering Functional Compounds to Substrates and Processes of Using the Same |
| US20080179562A1 (en) * | 2007-01-30 | 2008-07-31 | Kimberly-Clark Worldwide, Inc. | Substrate containing a deodorizing ink |
| US20090076542A1 (en) * | 2004-02-18 | 2009-03-19 | Jerry Jonn | Adhesive-Containing Wound Closure Device And Method |
| US20090147905A1 (en) * | 2007-12-05 | 2009-06-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for initiating thermonuclear fusion |
| US20090158936A1 (en) * | 2007-12-21 | 2009-06-25 | Kimberly-Clark Worldwide, Inc. | Gas treatment system |
| US20090162258A1 (en) * | 2007-12-21 | 2009-06-25 | Kimberly-Clark Worldwide, Inc. | Liquid treatment system |
| US20090166177A1 (en) * | 2007-12-28 | 2009-07-02 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing emulsions |
| US20090168591A1 (en) * | 2007-12-28 | 2009-07-02 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for particle dispersion into formulations |
| US7673516B2 (en) | 2006-12-28 | 2010-03-09 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment system |
| US7703698B2 (en) | 2006-09-08 | 2010-04-27 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment chamber and continuous flow mixing system |
| US7712353B2 (en) | 2006-12-28 | 2010-05-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment system |
| US20100215754A1 (en) * | 2002-12-20 | 2010-08-26 | Kimberly-Clark Worldwide, Inc. | Delivery System for Functional Compounds |
| US7785674B2 (en) | 2007-07-12 | 2010-08-31 | Kimberly-Clark Worldwide, Inc. | Delivery systems for delivering functional compounds to substrates and processes of using the same |
| US7947184B2 (en) | 2007-07-12 | 2011-05-24 | Kimberly-Clark Worldwide, Inc. | Treatment chamber for separating compounds from aqueous effluent |
| US7977103B2 (en) | 2006-04-20 | 2011-07-12 | Kimberly-Clark Worldwide, Inc. | Method for detecting the onset of ovulation |
| US7998322B2 (en) | 2007-07-12 | 2011-08-16 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber having electrode properties |
| US8034286B2 (en) | 2006-09-08 | 2011-10-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment system for separating compounds from aqueous effluent |
| US8057573B2 (en) | 2007-12-28 | 2011-11-15 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for increasing the shelf life of formulations |
| US8163388B2 (en) | 2008-12-15 | 2012-04-24 | Kimberly-Clark Worldwide, Inc. | Compositions comprising metal-modified silica nanoparticles |
| US8215822B2 (en) | 2007-12-28 | 2012-07-10 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing antimicrobial formulations |
| US8221328B2 (en) | 2003-10-16 | 2012-07-17 | Kimberly-Clark Worldwide, Inc. | Visual indicating device for bad breath |
| US8632613B2 (en) | 2007-12-27 | 2014-01-21 | Kimberly-Clark Worldwide, Inc. | Process for applying one or more treatment agents to a textile web |
| US8685178B2 (en) | 2008-12-15 | 2014-04-01 | Kimberly-Clark Worldwide, Inc. | Methods of preparing metal-modified silica nanoparticles |
| US9239036B2 (en) | 2006-09-08 | 2016-01-19 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment and delivery system and process |
| USD824525S1 (en) | 2014-09-25 | 2018-07-31 | Ethicon Llc | Release paper for wound treament devices |
| US10470934B2 (en) | 2016-09-29 | 2019-11-12 | Ethicon, Inc. | Methods and devices for skin closure |
| US10470935B2 (en) | 2017-03-23 | 2019-11-12 | Ethicon, Inc. | Skin closure systems and devices of improved flexibility and stretchability for bendable joints |
| US10687986B2 (en) | 2016-09-29 | 2020-06-23 | Ethicon, Inc. | Methods and devices for skin closure |
| US10792024B2 (en) | 2016-09-28 | 2020-10-06 | Ethicon, Inc. | Scaffolds with channels for joining layers of tissue at discrete points |
| US10835495B2 (en) | 2012-11-14 | 2020-11-17 | W. R. Grace & Co.-Conn. | Compositions containing a biologically active material and a non-ordered inorganic oxide material and methods of making and using the same |
| USD907217S1 (en) | 2016-09-29 | 2021-01-05 | Ethicon, Inc. | Release paper for wound treatment devices |
| US10993708B2 (en) | 2018-07-31 | 2021-05-04 | Ethicon, Inc. | Skin closure devices with interrupted closure |
| US11504446B2 (en) | 2017-04-25 | 2022-11-22 | Ethicon, Inc. | Skin closure devices with self-forming exudate drainage channels |
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7582277B2 (en) | 2002-04-19 | 2009-09-01 | Saint-Gobain Ceramics & Plastics, Inc. | Seeded boehmite particulate material and methods for forming same |
| US20050124745A1 (en) | 2002-04-19 | 2005-06-09 | Saint-Gobain Ceramics & Plastics, Inc. | Flame retardant composites |
| CN100386370C (en) * | 2002-04-19 | 2008-05-07 | 圣戈本陶瓷及塑料股份有限公司 | Novel boehmite particles and polymer materials incorporating same |
| US20050227000A1 (en) * | 2004-04-13 | 2005-10-13 | Saint-Gobain Ceramics & Plastics, Inc. | Surface coating solution |
| US8409618B2 (en) | 2002-12-20 | 2013-04-02 | Kimberly-Clark Worldwide, Inc. | Odor-reducing quinone compounds |
| US7488520B2 (en) | 2003-10-16 | 2009-02-10 | Kimberly-Clark Worldwide, Inc. | High surface area material blends for odor reduction, articles utilizing such blends and methods of using same |
| US20060104895A1 (en) | 2004-11-18 | 2006-05-18 | Saint-Gobain Ceramics & Plastics, Inc. | Transitional alumina particulate materials having controlled morphology and processing for forming same |
| US7280441B2 (en) * | 2004-11-30 | 2007-10-09 | Kimberly-Clark Worldwide, Inc. | Visual indicator chronograph and the use of the same |
| EP1817367A2 (en) * | 2004-12-01 | 2007-08-15 | Saint-Gobain Ceramics & Plastics, Inc. | Rubber formulation and methods for manufacturing same |
| US7338516B2 (en) * | 2004-12-23 | 2008-03-04 | Kimberly-Clark Worldwide, Inc. | Method for applying an exothermic coating to a substrate |
| US7763061B2 (en) * | 2004-12-23 | 2010-07-27 | Kimberly-Clark Worldwide, Inc. | Thermal coverings |
| US7816285B2 (en) | 2004-12-23 | 2010-10-19 | Kimberly-Clark Worldwide, Inc. | Patterned application of activated carbon ink |
| US20060223052A1 (en) * | 2005-03-30 | 2006-10-05 | Kimberly-Clark Worldwide, Inc. | Technique for detecting microorganisms |
| US7829181B2 (en) * | 2005-08-31 | 2010-11-09 | Kimberly-Clark Worldwide, Inc. | Solvatochromic visual indicator and the use of the same |
| US7479324B2 (en) * | 2005-11-08 | 2009-01-20 | Saint-Gobain Ceramics & Plastics, Inc. | Pigments comprising alumina hydrate and a dye, and polymer composites formed thereof |
| US20080145268A1 (en) * | 2006-12-15 | 2008-06-19 | Martin Stephanie M | Deodorizing container that includes an anthraquinone ink |
| US20080145269A1 (en) * | 2006-12-15 | 2008-06-19 | Martin Stephanie M | Deodorizing container that includes a modified nanoparticle ink |
| US8066956B2 (en) * | 2006-12-15 | 2011-11-29 | Kimberly-Clark Worldwide, Inc. | Delivery of an odor control agent through the use of a presaturated wipe |
| WO2009085870A2 (en) * | 2007-12-19 | 2009-07-09 | Saint-Gobain Ceramics & Plastics, Inc. | Aggregates of alumina hydrates |
| US9421504B2 (en) * | 2007-12-28 | 2016-08-23 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing emulsions |
| US20120034156A1 (en) * | 2010-08-03 | 2012-02-09 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Artificial cells |
| US8034363B2 (en) * | 2008-12-11 | 2011-10-11 | Advanced Technologies And Regenerative Medicine, Llc. | Sustained release systems of ascorbic acid phosphate |
| US8460768B2 (en) * | 2008-12-17 | 2013-06-11 | Saint-Gobain Ceramics & Plastics, Inc. | Applications of shaped nano alumina hydrate in inkjet paper |
| WO2014133526A1 (en) * | 2013-02-28 | 2014-09-04 | Empire Technology Development Llc | Colored pigment particles for electrophoretic displays |
| CN105307509B (en) * | 2013-06-26 | 2020-01-03 | 弗门尼舍有限公司 | Coated flavor powder |
| US10384156B2 (en) | 2014-09-12 | 2019-08-20 | Hollingsworth & Vose Company | Filter media comprising fibers including charged particles |
| US11840797B1 (en) | 2014-11-26 | 2023-12-12 | Microban Products Company | Textile formulation and product with odor control |
| CN111902150B (en) * | 2017-10-20 | 2024-04-02 | 乔治亚大学研究基金公司 | Surface and coating compositions having anti-fouling, antithrombotic and antibacterial properties and methods of making the same |
| CN110590388B (en) * | 2019-10-25 | 2022-07-01 | 中国人民解放军国防科技大学 | Preparation method of low-cost and high-efficiency alumina fiber reinforced alumina composite material |
| CA3192184A1 (en) * | 2020-08-19 | 2022-02-24 | Cytosorbents Corporation | Therapeutic and cosmetic wound treatment |
| CN113520913B (en) * | 2021-07-30 | 2022-10-21 | 绍兴孚原生物科技有限公司 | Hair dye containing silica nanoparticles loaded with dihydroxyindole carboxylic acid |
Citations (97)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2593146A (en) * | 1945-10-19 | 1952-04-15 | Sutcliffe Speakman & Company L | Laminated paper containing activated carbon |
| US3381688A (en) * | 1963-08-12 | 1968-05-07 | Kendall & Co | Absorbent pads with silica gel layer for use as surgical receptacles |
| US3494821A (en) * | 1967-01-06 | 1970-02-10 | Du Pont | Patterned nonwoven fabric of hydraulically entangled textile fibers and reinforcing fibers |
| US3502763A (en) * | 1962-02-03 | 1970-03-24 | Freudenberg Carl Kg | Process of producing non-woven fabric fleece |
| US3502538A (en) * | 1964-08-17 | 1970-03-24 | Du Pont | Bonded nonwoven sheets with a defined distribution of bond strengths |
| US3507269A (en) * | 1965-04-26 | 1970-04-21 | Homer H Berry | Clinical diagnostic device for halitosis |
| US3794497A (en) * | 1969-10-24 | 1974-02-26 | Ici Ltd | Photographic element comprising an olefinically unsaturated monomer and a photo-labile organo cobalt compound |
| US3802817A (en) * | 1969-10-01 | 1974-04-09 | Asahi Chemical Ind | Apparatus for producing non-woven fleeces |
| US4006030A (en) * | 1972-11-21 | 1977-02-01 | Nissan Chemical Industries, Ltd. | Method of preventing deterioration of inorganic substrate surface |
| US4078029A (en) * | 1976-09-23 | 1978-03-07 | Nissan Chemical Industries, Ltd. | Process for preparing mold |
| US4313820A (en) * | 1980-02-28 | 1982-02-02 | Phillips Petroleum Co. | Hydrodesulfurization of organic sulfur compounds and hydrogen sulfide removal with incompletely sulfided zinc titanate materials |
| US4375448A (en) * | 1979-12-21 | 1983-03-01 | Kimberly-Clark Corporation | Method of forming a web of air-laid dry fibers |
| US4451388A (en) * | 1981-11-02 | 1984-05-29 | Nalco Chemical Company | Preparation of aluminum oxide coated silica sols using ultrafiltration |
| US4494629A (en) * | 1981-08-12 | 1985-01-22 | Raeburn John L | Lowering device and method |
| US4494278A (en) * | 1977-11-08 | 1985-01-22 | Karl Kristian Kobs Kroyer | Apparatus for the production of a fibrous web |
| US4517308A (en) * | 1981-09-04 | 1985-05-14 | Collo Gmbh | Method of producing a sorptive body, particularly for eliminating odors, air freshening, etc. and the resultant product |
| US4575556A (en) * | 1982-11-08 | 1986-03-11 | Medi-Physics, Inc. | Bifunctional chelating agents |
| US4640810A (en) * | 1984-06-12 | 1987-02-03 | Scan Web Of North America, Inc. | System for producing an air laid web |
| US4643801A (en) * | 1986-02-24 | 1987-02-17 | Nalco Chemical Company | Papermaking aid |
| US4655757A (en) * | 1984-04-23 | 1987-04-07 | Kimberly-Clark Corporation | Selective layering of superabsorbents in meltblown substrates |
| US4725415A (en) * | 1986-06-02 | 1988-02-16 | Phillips Petroleum Company | Selective removal of hydrogen sulfide over zinc titanate and alumina |
| US4734324A (en) * | 1987-03-27 | 1988-03-29 | Hercules Incorporated | Heat sealable microporous polypropylene films |
| USRE32649E (en) * | 1985-06-18 | 1988-04-19 | The Procter & Gamble Company | Hydrogel-forming polymer compositions for use in absorbent structures |
| US4798603A (en) * | 1987-10-16 | 1989-01-17 | Kimberly-Clark Corporation | Absorbent article having a hydrophobic transport layer |
| US4802473A (en) * | 1983-11-07 | 1989-02-07 | Tecnol, Inc. | Face mask with ear loops |
| US4818464A (en) * | 1984-08-30 | 1989-04-04 | Kimberly-Clark Corporation | Extrusion process using a central air jet |
| US4823404A (en) * | 1988-06-10 | 1989-04-25 | Kimberly-Clark Corporation | Two piece protective garment |
| US4823803A (en) * | 1987-07-31 | 1989-04-25 | Winners Japan Company Limited | Halitosis detector device |
| US4904304A (en) * | 1986-12-29 | 1990-02-27 | Nissan Chemical Industries Ltd. | Chemical grout for ground injection and method for accretion |
| US4988505A (en) * | 1988-09-16 | 1991-01-29 | Nissan Chemical Industries, Ltd. | Deodorizer |
| US5000746A (en) * | 1987-08-11 | 1991-03-19 | Friedrichsfeld Gmbh Keramik- Und Kunststoffwerke | Wound covering having connected discrete elements |
| US5100702A (en) * | 1990-02-22 | 1992-03-31 | Nissan Chemical Industries, Ltd. | Thin platinum film-forming composition |
| US5100581A (en) * | 1990-02-22 | 1992-03-31 | Nissan Chemical Industries Ltd. | Method of preparing high-purity aqueous silica sol |
| US5108739A (en) * | 1986-08-25 | 1992-04-28 | Titan Kogyo Kabushiki Kaisha | White colored deodorizer and process for producing the same |
| US5178931A (en) * | 1990-11-26 | 1993-01-12 | Kimberly-Clark Corporation | Three-layer nonwoven laminiferous structure |
| US5183656A (en) * | 1990-08-03 | 1993-02-02 | Mitsubishi Denki Kabushiki Kaisha | Deodorant and product in which the deodorant is used |
| US5188885A (en) * | 1989-09-08 | 1993-02-23 | Kimberly-Clark Corporation | Nonwoven fabric laminates |
| US5196177A (en) * | 1990-01-17 | 1993-03-23 | Nissan Chemical Industries, Ltd. | Production of stable aqueous silica sol |
| US5204111A (en) * | 1989-04-07 | 1993-04-20 | L'oreal | Process for the preparation of alginate capsules, apparatus for producing said capsules and cosmetic compositions containing said capsules |
| US5204429A (en) * | 1987-08-07 | 1993-04-20 | Hoechst Aktiengesellschaft | Process for the preparation of an olefin polymer |
| US5209998A (en) * | 1991-11-25 | 1993-05-11 | Xerox Corporation | Colored silica particles |
| US5284703A (en) * | 1990-12-21 | 1994-02-08 | Kimberly-Clark Corporation | High pulp content nonwoven composite fabric |
| US5292868A (en) * | 1989-05-26 | 1994-03-08 | Akzo N.V. | Chelating agents for attaching metal ions to proteins |
| US5294717A (en) * | 1991-10-24 | 1994-03-15 | Spyros Theodoropulos | Bifunctional chelating agents, their chelates and process of preparation |
| US5300365A (en) * | 1990-09-28 | 1994-04-05 | Himont Incorporated | Olefin polymer films |
| US5314855A (en) * | 1992-11-09 | 1994-05-24 | Akzo N.V. | Adsorbent compositions and methods of manufacture |
| US5382400A (en) * | 1992-08-21 | 1995-01-17 | Kimberly-Clark Corporation | Nonwoven multicomponent polymeric fabric and method for making same |
| US5383450A (en) * | 1987-10-02 | 1995-01-24 | Tcnl Technologies, Inc. | Liquid shield visor for a surgical mask |
| US5397667A (en) * | 1994-04-28 | 1995-03-14 | Xerox Corporation | Toner with metallized silica particles |
| US5407442A (en) * | 1990-02-12 | 1995-04-18 | Karapasha; Nancy | Carbon-containing odor controlling compositions |
| US5407600A (en) * | 1991-07-23 | 1995-04-18 | Nissan Chemical Industries, Ltd. | Stable aqueous alumina sol and method for preparing the same |
| US5420090A (en) * | 1991-01-18 | 1995-05-30 | The Dow Chemical Company | Silica supported transition metal catalysts |
| US5480636A (en) * | 1992-06-03 | 1996-01-02 | Ishihara Sangyo Kaisha, Ltd. | Titanium oxide particles and method of scavenging noxious materials |
| US5486356A (en) * | 1991-05-30 | 1996-01-23 | Fuji Photo Film Co., Ltd. | Deodorant composition combining transition metal oxide or alloy with catalytic metal on carrier |
| US5591797A (en) * | 1993-10-25 | 1997-01-07 | Wacker-Chemie Gmbh | Transition metal-containing hydrophobic silica |
| US5597575A (en) * | 1994-06-06 | 1997-01-28 | Breitbarth; Richard | Composition for stimulating and inducing hair growth |
| US5597512A (en) * | 1993-10-15 | 1997-01-28 | Nissan Chemical Industries, Ltd. | Method for preparing elongated-shaped silica sol |
| US5616315A (en) * | 1994-10-13 | 1997-04-01 | Gillette Canada Inc. | Particles including degradable material and anti-microbial agent |
| US5733272A (en) * | 1993-03-31 | 1998-03-31 | The Procter & Gamble Company | Absorbent articles for odor control with positive scent signal |
| US5747003A (en) * | 1995-03-22 | 1998-05-05 | Ppg Industries, Inc. | Amorphous precipitated silica abrasive |
| US5855788A (en) * | 1996-02-07 | 1999-01-05 | Kimberly-Clark Worldwide, Inc. | Chemically charged-modified filter for removing particles from a liquid and method thereof |
| US5858503A (en) * | 1995-10-26 | 1999-01-12 | Kimberly-Clark Worldwide, Inc. | Method of applying chemical charge modifiers to a substrate and article thereof |
| US5861144A (en) * | 1997-06-09 | 1999-01-19 | The Procter & Gamble Company | Perfumed compositions for reducing body odors and excess moisture |
| US5871872A (en) * | 1997-05-30 | 1999-02-16 | Shipley Company, Ll.C. | Dye incorporated pigments and products made from same |
| US5874067A (en) * | 1996-10-24 | 1999-02-23 | The Procter & Gamble Company | Methods for controlling environmental odors on the body |
| US5880309A (en) * | 1997-01-28 | 1999-03-09 | Nissan Chemical Industries, Ltd. | Phenylphosphonic acid derivative and production process therefor |
| US5880176A (en) * | 1994-10-21 | 1999-03-09 | Hitachi Maxell, Ltd. | Fluorescent marking composition and fluorescent mark formed by said composition |
| US5882638A (en) * | 1996-10-24 | 1999-03-16 | The Proctor & Gamble Company | Methods using uncomplexed cyclodextrin solutions for controlling environmental odors |
| US5885599A (en) * | 1996-10-28 | 1999-03-23 | The Procter & Gamble Company | Methods and compositions for reducing body odors and excess moisture |
| US5897541A (en) * | 1994-09-30 | 1999-04-27 | Kimberly-Clark Worldwide, Inc. | Laminate material and absorbent garment comprising same |
| US5902226A (en) * | 1994-08-05 | 1999-05-11 | Nissan Chemical Industries, Ltd. | Method of preparing a propanol sol of silica |
| US5905101A (en) * | 1995-03-22 | 1999-05-18 | Nissan Motor Co., Ltd. | Ablator compositions |
| US6024786A (en) * | 1997-10-30 | 2000-02-15 | Hewlett-Packard Company | Stable compositions of nano-particulate unmodified pigments and insoluble colorants in aqueous microemulsions, and principle of stability and methods of formation thereof |
| US6045900A (en) * | 1997-09-15 | 2000-04-04 | Kimberly-Clark Worldwide, Inc. | Breathable filled film laminate |
| US6047413A (en) * | 1998-03-31 | 2000-04-11 | Kimberly-Clark Worldwide, Inc. | Conformable backpack for encapsulated chemical protection suit |
| US6060410A (en) * | 1998-04-22 | 2000-05-09 | Gillberg-Laforce; Gunilla Elsa | Coating of a hydrophobic polymer substrate with a nonstoichiometric polyelectrolyte complex |
| US6172173B1 (en) * | 1991-01-18 | 2001-01-09 | The Dow Chemical Company | Silica supported transition metal catalyst |
| US6177608B1 (en) * | 1994-06-30 | 2001-01-23 | Kimberly-Clark Worldwide, Inc. | Tampon |
| US6190814B1 (en) * | 1994-04-28 | 2001-02-20 | Xerox Corporation | Modified silica particles |
| US6193844B1 (en) * | 1995-06-07 | 2001-02-27 | Mclaughlin John R. | Method for making paper using microparticles |
| US6200555B1 (en) * | 1998-03-10 | 2001-03-13 | Mazda Motor Corporation | Deodorant composition, deodorizer and filter each containing the same, and method of deodorization |
| US6210625B1 (en) * | 1996-02-20 | 2001-04-03 | Mikuni Corporation | Method for producing granulated material |
| US6225524B1 (en) * | 1996-06-07 | 2001-05-01 | The Procter & Gamble Company | Absorbent articles having an odor control system consisting of absorbent gelling material and silica |
| US6334988B1 (en) * | 1998-08-21 | 2002-01-01 | The University Of Vermont And State Agricultural College | Mesoporous silicates and method of making same |
| US6344272B1 (en) * | 1997-03-12 | 2002-02-05 | Wm. Marsh Rice University | Metal nanoshells |
| US6344218B1 (en) * | 1998-11-23 | 2002-02-05 | The Procter & Gamble Company | Skin deodorizing and santizing compositions |
| US6358909B1 (en) * | 1996-10-17 | 2002-03-19 | The Clorox Company | Suspoemulsion system for delivery of actives |
| US6358537B1 (en) * | 1998-11-10 | 2002-03-19 | Dainichiseika Color & Chemicals Mfg. Co, Ltd. | Deodorant and antimicrobial dispersions |
| US6361780B1 (en) * | 1998-11-12 | 2002-03-26 | Cardiac Pacemakers, Inc. | Microporous drug delivery system |
| US6369290B1 (en) * | 2000-02-17 | 2002-04-09 | Tyco Healthcare Retail Services Ag | Time release odor control composition for a disposable absorbent article |
| US6376741B1 (en) * | 1996-06-07 | 2002-04-23 | The Procter & Gamble Company | Activated carbon free absorbent articles having a silica and zeolite odor control system |
| US6517199B1 (en) * | 1999-11-12 | 2003-02-11 | Canon Kabushiki Kaisha | Liquid composition, ink set, colored area formation on recording medium, and ink-jet recording apparatus |
| US6531704B2 (en) * | 1998-09-14 | 2003-03-11 | Nanoproducts Corporation | Nanotechnology for engineering the performance of substances |
| US6536890B1 (en) * | 1999-11-12 | 2003-03-25 | Canon Kabushiki Kaisha | Liquid composition as well as ink set, image forming method, image forming apparatus and bleed alleviation method using the same |
| US6548264B1 (en) * | 2000-05-17 | 2003-04-15 | University Of Florida | Coated nanoparticles |
| US6551457B2 (en) * | 2000-09-20 | 2003-04-22 | Akzo Nobel N.V. | Process for the production of paper |
| US6693071B2 (en) * | 2001-01-30 | 2004-02-17 | The Procter & Gamble Company | Rinse aid surface coating compositions for modifying dishware surfaces |
Family Cites Families (295)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US553912A (en) * | 1896-02-04 | Wrought-metal header and means for manufacturing same | ||
| US649800A (en) * | 1899-08-02 | 1900-05-15 | Arthur Bollard | Receptacle for tobacco. |
| US669307A (en) * | 1900-11-23 | 1901-03-05 | American Car & Foundry Co | End sill for railway-cars. |
| FR739214A (en) | 1931-12-17 | 1933-01-06 | Erba A G Fabrik Chemischer Pro | Process for treating textiles and the like |
| US3266973A (en) | 1963-07-25 | 1966-08-16 | Richard P Crowley | Method of preparing adsorbent filter paper containing crystalline zeolite particles, and paper thereof |
| US3615478A (en) | 1966-03-18 | 1971-10-26 | Keuffel & Esser Co | Method of fixing photographic material containing a free radial producing compound |
| US3919437A (en) | 1972-02-22 | 1975-11-11 | Owens Corning Fiberglass Corp | Method for electrostatically impregnating strand |
| GB1453447A (en) | 1972-09-06 | 1976-10-20 | Kimberly Clark Co | Nonwoven thermoplastic fabric |
| US3960494A (en) | 1974-11-11 | 1976-06-01 | Saskatchewan Power Corporation | Colorimetric odorant level test in natural, synthetic and L.P. gas and the like |
| US4038046A (en) * | 1975-12-31 | 1977-07-26 | Norton Company | Coated abrasive bonded with urea-formaldehyde, phenolic resin blends |
| US4172781A (en) | 1977-06-15 | 1979-10-30 | Standard Oil Company (Indiana) | Waste water process for treatment of strong wastes |
| USRE30797E (en) | 1977-11-09 | 1981-11-17 | Scott Paper Company | Associated dye salts and method of forming colored indicia therewith |
| USRE30803E (en) | 1977-11-09 | 1981-11-24 | Scott Paper Company | Colorless recording paper |
| IT1103817B (en) | 1978-06-27 | 1985-10-14 | Guaber Spa | GRANULAR DEODORANT COMPOSITION FOR ASHTRAY |
| JPS5517157A (en) * | 1978-07-24 | 1980-02-06 | Ricoh Co Ltd | Hologram information reproducing method |
| US4325735A (en) | 1979-06-22 | 1982-04-20 | Canon Kabushiki Kaisha | Recording liquid composition |
| DE2952414A1 (en) | 1979-12-27 | 1981-07-02 | Siemens AG, 1000 Berlin und 8000 München | COLORING AND RECORDING AGENTS |
| US4407960A (en) | 1980-06-25 | 1983-10-04 | American Sterilizer Company | Visual chemical indicating composition for monitoring sterilization |
| US4488969A (en) | 1982-02-09 | 1984-12-18 | Amf Incorporated | Fibrous media containing millimicron-sized particulates |
| ZA833317B (en) | 1982-05-14 | 1984-02-29 | Johnson Matthey Plc | Composition compressing inorganic particles |
| US4469746A (en) | 1982-06-01 | 1984-09-04 | The Procter & Gamble Company | Silica coated absorbent fibers |
| JPS5937956A (en) | 1982-08-24 | 1984-03-01 | カネボウ株式会社 | Particle filled fiber structure |
| JPS59133235A (en) | 1983-01-21 | 1984-07-31 | Kanebo Ltd | Zeolite particle-containing polymer and its production |
| US4585484A (en) | 1983-03-24 | 1986-04-29 | Canon Kabushiki Kaisha | Recording liquid |
| US4522203A (en) | 1984-03-09 | 1985-06-11 | Chicopee | Water impervious materials |
| JPS60217900A (en) | 1984-04-13 | 1985-10-31 | Kyowa Medetsukusu Kk | Method for determination of mercapto group-containing compound |
| DE3575571D1 (en) | 1985-01-08 | 1990-03-01 | Ici Plc | WATER-SOLUBLE DYE. |
| DE3503587A1 (en) | 1985-02-02 | 1986-08-07 | Basf Ag, 6700 Ludwigshafen | METHOD FOR PRODUCING A CATALYST CONTAINING COPPER AND SILICON OXIDE |
| US5122418A (en) | 1985-12-09 | 1992-06-16 | Shiseido Company Ltd. | Composite powder and production process |
| JPS62142996U (en) | 1986-02-04 | 1987-09-09 | ||
| GB8616294D0 (en) | 1986-07-03 | 1986-08-13 | Johnson Matthey Plc | Antimicrobial compositions |
| DE3625605A1 (en) | 1986-07-29 | 1988-02-11 | Hoechst Ag | AQUEOUS PIGMENT PREPARATIONS AND THEIR USE |
| GB8624370D0 (en) | 1986-10-10 | 1986-11-12 | Ici Plc | Ink |
| US4957553A (en) | 1986-12-01 | 1990-09-18 | Canon Kabushiki Kaisha | Ink for ink-jet recording and ink-jet recording process employing the same |
| US4783220A (en) | 1986-12-18 | 1988-11-08 | Xerox Corporation | Vesicle ink compositions |
| JPS63162773A (en) | 1986-12-26 | 1988-07-06 | Canon Inc | Ink for ink jet recording and method of ink jet recording by using it |
| EP0282287B2 (en) | 1987-03-10 | 1996-04-24 | Lion Corporation | Deodorizer |
| US5091004A (en) | 1987-09-22 | 1992-02-25 | Dainippon Ink And Chemicals, Inc. | Ink composition |
| AU2554088A (en) | 1987-10-02 | 1989-04-18 | Personal Pet Products Partnership | Absorbent composition, and method of making same |
| US4836851A (en) | 1988-02-11 | 1989-06-06 | Hewlett-Packard Company | Dyes containing polyhydroxyl groups for ink-jet printing inks |
| JPH01319576A (en) | 1988-06-20 | 1989-12-25 | Orient Chem Ind Ltd | Aqueous ink composition |
| AU3669589A (en) | 1988-06-30 | 1990-01-04 | Kimberly-Clark Corporation | Absorbent article containing an anhydrous deodorant |
| JPH0826263B2 (en) | 1988-07-26 | 1996-03-13 | キヤノン株式会社 | Recording liquid and ink jet recording method using the same |
| US5203912A (en) | 1988-08-24 | 1993-04-20 | Imperial Chemical Industries Plc | Anionic dye |
| EP0363139B1 (en) | 1988-10-03 | 1994-12-07 | Seiko Epson Corporation | A recording ink for an ink jet printer |
| EP0369643B1 (en) | 1988-11-02 | 1995-09-06 | Hewlett-Packard Company | Ink-jet printing inks |
| EP0376448B1 (en) | 1988-12-29 | 1993-07-14 | Lion Corporation | Deodorizer composition |
| EP0389015A3 (en) | 1989-03-20 | 1991-08-28 | The Procter & Gamble Company | Absorbent structures with odor control material |
| CA2011670A1 (en) | 1989-03-20 | 1990-09-20 | Diane L. Furio | Absorbent structures with odor control |
| EP0388837B1 (en) | 1989-03-21 | 1996-04-10 | Ciba-Geigy Ag | Initiators for cationically polymerisable materials |
| US4963189A (en) | 1989-08-24 | 1990-10-16 | Hewlett-Packard Company | Waterfast ink formulations with a novel series of anionic dyes containing two or more carboxyl groups |
| US5006862A (en) | 1989-10-27 | 1991-04-09 | Hewlett-Packard Company | Fixation of reactive dyes to paper by ink-jet printing |
| US5169706A (en) | 1990-01-10 | 1992-12-08 | Kimberly-Clark Corporation | Low stress relaxation composite elastic material |
| AU658137B2 (en) | 1990-02-12 | 1995-04-06 | Procter & Gamble Company, The | Carbon-containing odor controlling compositions |
| CA2071962C (en) | 1990-02-12 | 1994-09-20 | Nancy Karapasha | High capacity odor controlling compositions |
| BR9106040A (en) | 1990-02-12 | 1993-01-05 | Procter & Gamble | PROCESS TO REDUCE THE ODOR ASSOCIATED WITH CORPOREAL FLUIDS, MANUFACTURING ARTICLE AND NAPPY, HYGIENIC ABSORBENT OR PANTY COATING |
| US5190581A (en) | 1990-03-06 | 1993-03-02 | Canon Kabushiki Kaisha | Ink, ink-jet recording method, and instrument employing the ink |
| JP3060319B2 (en) | 1990-03-09 | 2000-07-10 | キヤノン株式会社 | Ink, inkjet recording method, recording unit, ink cartridge, and inkjet recording apparatus |
| US5064694A (en) | 1990-06-01 | 1991-11-12 | Dow Corning Corporation | Use of silicone emulsions in the web printing process |
| US5062893A (en) | 1990-06-22 | 1991-11-05 | Hewlett-Packard Company | Ink formulations by mixing anionic waterfast dyes containing two or more carboxyl groups |
| US5145518A (en) | 1990-06-27 | 1992-09-08 | Xerox Corporation | Inks containing block copolymer micelles |
| US5100470A (en) | 1990-10-25 | 1992-03-31 | Hewlett-Packard Company | Waterfast ink formulations for thermal ink-jet using organic amines |
| EP0483500A1 (en) | 1990-10-31 | 1992-05-06 | Colgate-Palmolive Company | Odor absorbing articles |
| US5145727A (en) | 1990-11-26 | 1992-09-08 | Kimberly-Clark Corporation | Multilayer nonwoven composite structure |
| US5069719A (en) | 1990-12-21 | 1991-12-03 | Orient Chemical Industries, Ltd. | Organic solvent based ink composition |
| US5160535A (en) | 1991-01-11 | 1992-11-03 | Trident, Inc. | Rapidly drying impulse ink jet ink compositions |
| US5230732A (en) | 1991-03-19 | 1993-07-27 | Hewlett-Packard Company | Solid driver for the solid ink jet ink |
| CA2054095A1 (en) | 1991-04-22 | 1992-10-23 | Stephanie R. Majors | Multicomponent odor control device |
| US5382283A (en) | 1991-04-26 | 1995-01-17 | Fuji Xerox Co., Ltd. | Ink containing propylene oxide/ethylene oxide block copolymers for ink jet recording |
| US5221332A (en) | 1991-04-29 | 1993-06-22 | Xerox Corporation | Ink compositions |
| US5156675A (en) | 1991-05-16 | 1992-10-20 | Xerox Corporation | Ink for ink jet printing |
| US5302195A (en) | 1991-05-22 | 1994-04-12 | Xerox Corporation | Ink compositions containing cyclodextrins |
| US5427844A (en) | 1991-06-12 | 1995-06-27 | New Japan Chemical Co., Ltd. | Articles of natural cellulose fibers with improved deodorant properties and process for producing same |
| JP2964018B2 (en) | 1991-06-24 | 1999-10-18 | 株式会社サクラクレパス | Ink composition for marking ethylene oxide sterilization |
| JP3207873B2 (en) | 1991-07-17 | 2001-09-10 | キヤノン株式会社 | Method for producing multi-valued recorded matter and apparatus for producing multi-valued recorded matter |
| US5133803A (en) | 1991-07-29 | 1992-07-28 | Hewlett-Packard Company | High molecular weight colloids which control bleed |
| US5223026A (en) | 1991-07-30 | 1993-06-29 | Xerox Corporation | Ink jet compositions and processes |
| DE4227591A1 (en) | 1992-08-20 | 1994-02-24 | Basf Ag | Use of liquid dye preparations containing a disazo dye in the ink-jet process and disazo dye |
| US5245117A (en) | 1991-09-10 | 1993-09-14 | Withers L Andrew | Personal use syringe dispensing and collecting system |
| US5663224A (en) | 1991-12-03 | 1997-09-02 | Rohm And Haas Company | Process for preparing an aqueous dispersion |
| CA2087911C (en) | 1992-01-24 | 1999-06-29 | Kiyoshi Abe | Spherical granules of porous silica or silicate, process for the production thereof, and applications thereof |
| US5220346A (en) | 1992-02-03 | 1993-06-15 | Xerox Corporation | Printing processes with microwave drying |
| US5269840A (en) | 1992-02-04 | 1993-12-14 | Minnesota Mining And Manufacturing Company | Sol bonded colorant clusters and process for making |
| JP2983742B2 (en) | 1992-02-12 | 1999-11-29 | オリヱント化学工業株式会社 | New trisazo dyes and dye compositions containing them |
| US5451450A (en) | 1992-02-19 | 1995-09-19 | Exxon Chemical Patents Inc. | Elastic articles and a process for their production |
| US5226957A (en) | 1992-03-17 | 1993-07-13 | Hewlett-Packard Company | Solubilization of water-insoluble dyes via microemulsions for bleedless, non-threading, high print quality inks for thermal ink-jet printers |
| EP0565286B1 (en) | 1992-04-10 | 1996-05-29 | Zeneca Limited | Dye compositions and ink-jet printing ink |
| US5344872A (en) | 1993-02-19 | 1994-09-06 | Eastman Chemical Company | Ink compositions containing certain methacrylates |
| US5274025A (en) | 1993-02-19 | 1993-12-28 | Eastman Kodak Company | Ink and coating compositions containing a blend of water-dispersible polyester and hydantoin-formaldehyde resins |
| US5916596A (en) | 1993-02-22 | 1999-06-29 | Vivorx Pharmaceuticals, Inc. | Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof |
| US5441561A (en) | 1993-02-23 | 1995-08-15 | Fuji Xerox Co., Ltd. | Ink-jet recording ink and ink-jet recording methods thereof |
| DK168670B1 (en) | 1993-03-09 | 1994-05-16 | Niro Separation As | Apparatus for distributing fibers |
| JP3221142B2 (en) | 1993-03-22 | 2001-10-22 | ダイソー株式会社 | Loading method of metal fine particles |
| JP3397365B2 (en) | 1993-04-01 | 2003-04-14 | キヤノン株式会社 | Ink, ink manufacturing method, ink jet recording method, recording unit, ink cartridge, and ink jet recording apparatus |
| US6258974B1 (en) | 1993-04-13 | 2001-07-10 | Southwest Research Institute | Metal oxide compositions composites thereof and method |
| DE4318983A1 (en) | 1993-06-08 | 1994-12-15 | Basf Ag | Naphthalocyanines |
| AU676299B2 (en) | 1993-06-28 | 1997-03-06 | Akira Fujishima | Photocatalyst composite and process for producing the same |
| US5681380A (en) | 1995-06-05 | 1997-10-28 | Kimberly-Clark Worldwide, Inc. | Ink for ink jet printers |
| US5472775A (en) | 1993-08-17 | 1995-12-05 | The Dow Chemical Company | Elastic materials and articles therefrom |
| EP0643014B1 (en) | 1993-09-14 | 1998-08-12 | Kuraray Chemical Co., Ltd. | Deodorant comprising metal oxide-carrying activated carbon |
| US5998222A (en) | 1993-11-29 | 1999-12-07 | Utah State University | Reconditioning antibiotic-adulterated milk products |
| US5540916A (en) | 1993-12-15 | 1996-07-30 | Westvaco Corporation | Odor sorbing packaging |
| CA2116081C (en) | 1993-12-17 | 2005-07-26 | Ann Louise Mccormack | Breathable, cloth-like film/nonwoven composite |
| JP3444670B2 (en) | 1993-12-28 | 2003-09-08 | 水澤化学工業株式会社 | Method for producing granular amorphous silica |
| USH1732H (en) | 1994-03-10 | 1998-06-02 | Johnson; Theresa Louise | Absorbent articles containing antibacterial agents in the topsheet for odor control |
| IT1273087B (en) | 1994-03-25 | 1997-07-04 | P & G Spa | ABSORBENT ITEM WITH MATERIAL FOR ODOR CONTROL, RELATED USE AND COMPOSITION |
| US5973025A (en) | 1994-04-12 | 1999-10-26 | Sri International | Aqueous ink compositions containing a binder of a neutralized acidic resin |
| JPH07331141A (en) | 1994-06-03 | 1995-12-19 | Brother Ind Ltd | Recording ink |
| US5484475A (en) | 1994-08-29 | 1996-01-16 | Xerox Corporation | Micellar-based ink compositions |
| US5531817A (en) | 1994-09-01 | 1996-07-02 | Hewlett-Packard Company | Use of high viscosity, meltable gel inks for controlling bleed |
| US5736473A (en) | 1994-09-14 | 1998-04-07 | Kimberly-Clark Corp. | Fibrous composite structure including particulates |
| JPH0892517A (en) | 1994-09-20 | 1996-04-09 | Brother Ind Ltd | Recording ink |
| JP3376183B2 (en) | 1994-09-29 | 2003-02-10 | キヤノン株式会社 | Aqueous ink for ink jet, ink jet recording method and bleed mitigation method |
| US5993527A (en) | 1994-11-17 | 1999-11-30 | Canon Kabushiki Kaisha | Ink-jet color recording process and ink set therefor |
| US5539124A (en) | 1994-12-19 | 1996-07-23 | Occidental Chemical Corporation | Polymerization catalysts based on transition metal complexes with ligands containing pyrrolyl ring |
| ZA9510307B (en) | 1994-12-20 | 1996-06-11 | Kimberly Clark Co | Mechanically compatibilized film/non-woven laminates |
| US6309736B1 (en) | 1994-12-20 | 2001-10-30 | Kimberly-Clark Worldwide, Inc. | Low gauge films and film/nonwoven laminates |
| ZA9510604B (en) | 1994-12-20 | 1996-07-03 | Kimberly Clark Co | Low gauge films and film/nonwoven laminates |
| US5661197A (en) | 1994-12-20 | 1997-08-26 | Bic Corporation | Erasable ink composition containing a polymer-encapsulated colorant derived from monomer containing dissolved colorant |
| US5756561A (en) | 1994-12-21 | 1998-05-26 | Bic Corporation | Erasable ink composition containing a graft-polymerized dye |
| US5852073A (en) | 1994-12-21 | 1998-12-22 | Bic Corporation | Erasable ink composition containing a polymer-encapsulated colorant obtained by polymerizing monomer in the presence of solid colorant particles |
| DE69529966T2 (en) | 1994-12-27 | 2003-09-11 | Seiko Epson Corp | Ink composition and method of making the same |
| US5693126A (en) | 1994-12-27 | 1997-12-02 | Seiko Epson Corporation | Water-base ink composition and process for producing the same |
| US5554775A (en) | 1995-01-17 | 1996-09-10 | Occidental Chemical Corporation | Borabenzene based olefin polymerization catalysts |
| JPH08218015A (en) | 1995-02-14 | 1996-08-27 | Dainippon Ink & Chem Inc | Polymer fine particle for jet ink and jet ink containing the same |
| US5580655A (en) | 1995-03-03 | 1996-12-03 | Dow Corning Corporation | Silica nanoparticles |
| JP2888166B2 (en) | 1995-04-04 | 1999-05-10 | 富士ゼロックス株式会社 | Ink jet recording ink and ink jet recording method |
| JPH0995044A (en) | 1995-04-10 | 1997-04-08 | Canon Inc | Recording paper and ink jet recording using recording paper |
| JP3539054B2 (en) | 1995-04-19 | 2004-06-14 | セイコーエプソン株式会社 | Color ink jet recording ink composition set and recording method |
| ES2093562B1 (en) | 1995-05-26 | 1997-07-01 | Univ Santiago Compostela | STABILIZATION OF COLLOID SYSTEMS THROUGH FORMATION OF LIPIDO-POLISACARIDO IONIC COMPLEXES. |
| PT752245E (en) | 1995-07-05 | 2002-09-30 | Europ Economic Community | BIOCOMPATIBLE AND BIODEGRADABLE NANOPARTICLES FOR THE ABSORPTION AND ADMINISTRATION OF PROTEINACEAL DRUGS |
| US5626655A (en) | 1995-07-11 | 1997-05-06 | Hewlett-Packard Company | Use of co-surfactants to adjust properties of ink-jet inks |
| US5656072A (en) | 1995-07-17 | 1997-08-12 | Brother Kogyo Kabushiki Kaisha | Ink composition process for its preparation and ink-jet recording process |
| US5565022A (en) | 1995-09-14 | 1996-10-15 | Hewlett-Packard Company | Fast drying, bleed-free ink-jet ink compositions |
| US6019827A (en) | 1995-09-14 | 2000-02-01 | Hewlett-Packard Company | Reliability enhancement of microemulsion-based ink-jet inks |
| US5679724A (en) | 1995-09-29 | 1997-10-21 | Xerox Corporation | Submicron particles for ink jet inks |
| US6025412A (en) | 1995-09-29 | 2000-02-15 | Xerox Corporation | Colored particulates for ink jet inks |
| DE19539116A1 (en) | 1995-10-20 | 1997-04-24 | Merck Patent Gmbh | Process for the preparation of inclusion pigments |
| JPH09137091A (en) | 1995-11-16 | 1997-05-27 | Brother Ind Ltd | Water-based magenta ink for recording and ink-jet recording method |
| JPH09208606A (en) | 1995-11-28 | 1997-08-12 | Hitachi Maxell Ltd | Fine particle of polymer and ink composition containing the same |
| US5679138A (en) | 1995-11-30 | 1997-10-21 | Eastman Kodak Company | Ink jet inks containing nanoparticles of organic pigments |
| US5633109A (en) | 1995-12-05 | 1997-05-27 | Xerox Corporation | Ink compositions with liposomes containing photochromic compounds |
| US5626654A (en) | 1995-12-05 | 1997-05-06 | Xerox Corporation | Ink compositions containing liposomes |
| WO1997025076A1 (en) | 1996-01-11 | 1997-07-17 | Isk Biosciences Corporation | Odor control for compositions containing organic sulfur compounds |
| US5944883A (en) | 1996-01-26 | 1999-08-31 | Hitachi Maxell, Ltd. | Ultrafine particle organic pigment color ink and method for producing the same |
| JPH09272832A (en) | 1996-02-07 | 1997-10-21 | Hitachi Maxell Ltd | Black pigment ink and its production |
| US5795985A (en) | 1996-03-05 | 1998-08-18 | Ciba Specialty Chemicals Corporation | Phenyl alkyl ketone substituted by cyclic amine and a process for the preparation thereof |
| JP3257391B2 (en) | 1996-03-18 | 2002-02-18 | 東洋インキ製造株式会社 | Inkjet recording liquid |
| US6254894B1 (en) | 1996-04-05 | 2001-07-03 | Zodiac Pool Care, Inc. | Silver self-regulating water purification compositions and methods |
| AU724452B2 (en) | 1996-06-07 | 2000-09-21 | Procter & Gamble Company, The | Absorbent article having an odour control system of zeolite and silica in close physical proximity |
| US6159649A (en) | 1996-06-13 | 2000-12-12 | Clariant Gmbh | Electrophotographic, resin-containing, electret, or inkjet compositions containing magenta azo pigment and use thereof |
| US5684063A (en) | 1996-06-17 | 1997-11-04 | Xerox Corporation | Ink process |
| US5810917A (en) | 1996-07-08 | 1998-09-22 | Brother Kogyo Kabushiki Kaisha | Water-based recording magenta ink composition and ink-jet recording process |
| JP3106966B2 (en) | 1996-07-17 | 2000-11-06 | 富士ゼロックス株式会社 | Ink jet recording ink and ink jet recording method |
| AU6471696A (en) | 1996-07-22 | 1998-02-10 | Kouki Bussan Yugenkaisha | Novel adsorbent |
| US5769931A (en) | 1996-07-25 | 1998-06-23 | Bic Corporation | Ink composition |
| US5879439A (en) | 1996-08-01 | 1999-03-09 | Ricoh Company, Ltd. | Recording ink composition and recording method using the same |
| JPH1060338A (en) | 1996-08-21 | 1998-03-03 | Fuji Xerox Co Ltd | Ink for ink-jet recording and method of ink jet recording |
| US5972389A (en) | 1996-09-19 | 1999-10-26 | Depomed, Inc. | Gastric-retentive, oral drug dosage forms for the controlled-release of sparingly soluble drugs and insoluble matter |
| EP0929615B1 (en) | 1996-10-01 | 2000-07-19 | Avecia Limited | Aqueous ink compositions |
| US5928419A (en) | 1996-10-07 | 1999-07-27 | Toyo Ink Manufacturing Co., Ltd. | Surface-treated organic pigment and process for the production thereof |
| GB2318122B (en) | 1996-10-11 | 2001-02-07 | Zeneca Ltd | Bisazo ink-jet dyes |
| GB9621269D0 (en) | 1996-10-11 | 1996-11-27 | Zeneca Ltd | Bisazo ink-jet dyes |
| GB9621265D0 (en) | 1996-10-11 | 1996-11-27 | Zeneca Ltd | Bisazo ink-jet dyes |
| GB9621224D0 (en) | 1996-10-11 | 1996-11-27 | Zeneca Ltd | Bisazo ink-jet dyes |
| US5788753A (en) | 1996-10-28 | 1998-08-04 | Hewlett-Packard Company | Polyamines complexed to anionic dyes, thereby forming water-soluble cationic dyes |
| US5785745A (en) | 1996-10-31 | 1998-07-28 | Hewlett-Packard Company | Amphiphilic dyes |
| US5935309A (en) | 1996-10-31 | 1999-08-10 | Hewlett-Packard Company | Ink-jet inks for improved print quality |
| FR2755612B1 (en) | 1996-11-13 | 1998-12-24 | Atochem Elf Sa | SUPERABSORBENT COMPOSITION FOR HYGIENE ARTICLES WHICH DOES NOT DEVELOP INCOMING ODORS |
| US5964926A (en) | 1996-12-06 | 1999-10-12 | Kimberly-Clark Worldwide, Inc. | Gas born particulate filter and method of making |
| JPH10168373A (en) | 1996-12-12 | 1998-06-23 | Fuji Xerox Co Ltd | Ink for ink jet recording and recording using the same |
| AU739247B2 (en) | 1996-12-17 | 2001-10-04 | Procter & Gamble Company, The | Absorbent articles with odor control system |
| US6111163A (en) | 1996-12-27 | 2000-08-29 | Kimberly-Clark Worldwide, Inc. | Elastomeric film and method for making the same |
| US5833744A (en) | 1997-01-13 | 1998-11-10 | Xerox Corporation | Waterfast ink jet inks containing a surfactant |
| US5993856A (en) | 1997-01-24 | 1999-11-30 | Femmepharma | Pharmaceutical preparations and methods for their administration |
| JP3753490B2 (en) | 1997-01-28 | 2006-03-08 | 三菱鉛筆株式会社 | Oil-based ink for ballpoint pens |
| US5980623A (en) | 1997-01-29 | 1999-11-09 | Fuji Xerox Co., Ltd. | Ink set for ink jet recording and ink jet recording method |
| US5788749A (en) | 1997-02-14 | 1998-08-04 | Xerox Corporation | Pigmented ink compositions containing liposomes |
| US5968244A (en) | 1997-02-21 | 1999-10-19 | Minolta Co., Ltd. | Ink for ink-jet recording |
| US5928416A (en) | 1997-03-07 | 1999-07-27 | Xerox Corporation | Dipropylene glycol and countercation activation of dodecylbenzenesulfonate in thermal ink jet inks |
| US5891934A (en) | 1997-03-24 | 1999-04-06 | Hewlett-Packard Company | Waterfast macromolecular chromophores using amphiphiles |
| US5948483A (en) | 1997-03-25 | 1999-09-07 | The Board Of Trustees Of The University Of Illinois | Method and apparatus for producing thin film and nanoparticle deposits |
| US5981623A (en) | 1997-03-27 | 1999-11-09 | Lexmark International, Inc. | Ink jet ink containing wetting agent |
| JPH10291365A (en) | 1997-04-21 | 1998-11-04 | Fuji Xerox Co Ltd | Multicolor ink set and ink jet recording method |
| EP0874026B1 (en) | 1997-04-22 | 2003-08-06 | Ciba SC Holding AG | Method for producing coloured effect pigments |
| US6024785A (en) | 1997-04-23 | 2000-02-15 | Konica Corporation | Ink-jet recording ink and an ink-jet recording method |
| DE69818140T2 (en) | 1997-05-16 | 2004-04-08 | Seiko Epson Corp. | Jet recording inks |
| DE19821665A1 (en) | 1997-05-28 | 1998-12-03 | Basf Ag | Composite pigment with good fastness to colour bleeding |
| US5911816A (en) | 1997-05-29 | 1999-06-15 | Hewlett-Packard Company | Liposomal ink compositions with water-insoluble dyes and pigments |
| DE19722546B4 (en) | 1997-05-30 | 2004-08-26 | J. S. Staedtler Gmbh & Co. Kg | Ink for changing color applications |
| US5817300A (en) | 1997-06-02 | 1998-10-06 | Calwood Chemical Industries, Inc. | Odor reducing compositions |
| US6004625A (en) | 1997-06-16 | 1999-12-21 | Ibick Corporation | Method for adhering particles to an object by supplying air ions |
| US6015454A (en) | 1997-06-17 | 2000-01-18 | Ciba Specialty Chemicals Corporation | Process for printing textile fibre materials in accordance with the ink-jet printing process |
| DE19726043C1 (en) | 1997-06-19 | 1999-03-18 | Pelikan Produktions Ag | Ink for use in inkjet printers |
| JPH1112526A (en) | 1997-06-24 | 1999-01-19 | Mitsubishi Pencil Co Ltd | Dye ink composition for direct liquid-type aqueous ball-point pen |
| US6165440A (en) | 1997-07-09 | 2000-12-26 | Board Of Regents, The University Of Texas System | Radiation and nanoparticles for enhancement of drug delivery in solid tumors |
| US6099627A (en) | 1997-07-28 | 2000-08-08 | Hitachi Maxell, Ltd. | Dispersion ink |
| JPH1161012A (en) | 1997-08-22 | 1999-03-05 | Hitachi Maxell Ltd | Pigment ink and preparation thereof |
| US6238767B1 (en) | 1997-09-15 | 2001-05-29 | Kimberly-Clark Worldwide, Inc. | Laminate having improved barrier properties |
| DK176196B1 (en) | 1997-10-07 | 2007-01-08 | Ejvind Jersie Pedersen | Oral hygiene composition for the treatment of halitosis and the use of a chelate comprising a metal ion moiety and an amino acid moiety as a component of the composition |
| US5891232A (en) | 1997-10-28 | 1999-04-06 | Hewlett-Packard Company | Smearfastness and fast drying times in inks containing macromolecular chromophores |
| DE19749082A1 (en) | 1997-11-06 | 1999-05-12 | Bayer Ag | Ink-jet inks containing nanoscale inorganic pigments |
| WO1999029424A1 (en) | 1997-12-10 | 1999-06-17 | Toto Ltd. | Photocatalyst composition, substance containing photocatalyst, and material functioning as photocatalyst and process for producing the same |
| US6153001A (en) | 1997-12-18 | 2000-11-28 | Fuji Xerox Co., Ltd. | Ink jet recording ink, method for producing the same, and ink jet recording method |
| US5958998A (en) | 1998-02-05 | 1999-09-28 | Xerox Corporation | Ink jet inks |
| EP0972563A1 (en) | 1998-07-15 | 2000-01-19 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.V. | Fabrication of multilayer-coated particles and hollow shells via electrostatic self-assembly of nanocomposite multilayers on decomposable colloidal templates |
| WO1999047253A1 (en) | 1998-03-19 | 1999-09-23 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Fabrication of multilayer-coated particles and hollow shells via electrostatic self-assembly of nanocomposite multilayers on decomposable colloidal templates |
| EP1867325B1 (en) | 1998-03-19 | 2011-09-14 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.V. | Capsules comprising lipids in the coating |
| DE19824947A1 (en) | 1998-06-04 | 1999-12-09 | Degussa | Aqueous dispersions of soot |
| US6699501B1 (en) | 1998-07-15 | 2004-03-02 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften. E.V. | Polyelectrolyte coverings on biological templates |
| US6140390A (en) | 1998-08-31 | 2000-10-31 | Eastman Kodak Company | Melt-fusible inkjet recording elements and inks with improved durability |
| US6147139A (en) | 1998-08-31 | 2000-11-14 | Eastman Kodak Company | Inks containing heat fusible particles and method for use |
| US6491790B1 (en) | 1998-09-10 | 2002-12-10 | Bayer Corporation | Methods for reducing amine odor in paper |
| US6149719A (en) | 1998-10-28 | 2000-11-21 | Hewlett-Packard Company | Light sensitive invisible ink compositions and methods for using the same |
| US6073771A (en) | 1998-11-02 | 2000-06-13 | Lord Corporation | Container for storing sulfur-containing compounds |
| US6428814B1 (en) | 1999-10-08 | 2002-08-06 | Elan Pharma International Ltd. | Bioadhesive nanoparticulate compositions having cationic surface stabilizers |
| DE19852972A1 (en) | 1998-11-17 | 2000-05-18 | Henkel Kgaa | Colorants with transition metal complexes |
| US6277489B1 (en) | 1998-12-04 | 2001-08-21 | The Regents Of The University Of California | Support for high performance affinity chromatography and other uses |
| EP1054730A1 (en) | 1998-12-11 | 2000-11-29 | Mazda Motor Corporation | Composition for use in adsorption treatment, products formed with the same, and a method for producing adsorbent using the same |
| US6264615B1 (en) | 1999-01-21 | 2001-07-24 | Diamond General Development Corporation | Method for diagnosing the presence and extent of halitosis activity |
| JP3417862B2 (en) | 1999-02-02 | 2003-06-16 | 新東工業株式会社 | Silica gel highly loaded with titanium oxide photocatalyst and method for producing the same |
| US6479150B1 (en) | 1999-02-26 | 2002-11-12 | Kimberly-Clark Worldwide, Inc. | Layer materials treated with surfactant-modified hydrophobic odor control agents |
| US6045606A (en) | 1999-03-04 | 2000-04-04 | Westvaco Corporation | Water-based ink jet ink compositions containing carboxylated lignin |
| EP1034800A1 (en) | 1999-03-05 | 2000-09-13 | The Procter & Gamble Company | Articles having an odour control system comprising an oxidising agent and an odour absorbing agent |
| DE19915378A1 (en) | 1999-04-06 | 2000-10-12 | Inst Neue Mat Gemein Gmbh | Household appliances with a catalytic composition |
| AUPQ014699A0 (en) | 1999-05-04 | 1999-05-27 | Access Pharmaceuticals Australia Pty Limited | Amplification of folate-mediated targeting to tumor cells using nanoparticles |
| SE514339C2 (en) | 1999-06-11 | 2001-02-12 | Sca Hygiene Prod Ab | ABSORBING PRODUCTS WITH VISUAL INDICATOR TO DETERMINE THE ACTIVITY OF AN ACTIVE ADDITIVE CONTAINING IN THE ABSORBENT PRODUCT |
| DE60033308D1 (en) | 1999-07-14 | 2007-03-22 | Kaneka Corp | REGENERATED COLLAGEN FIBERS WITH EXCELLENT HEAT RESISTANCE |
| WO2001006054A1 (en) * | 1999-07-19 | 2001-01-25 | Avantgarb, Llc | Nanoparticle-based permanent treatments for textiles |
| DE19939662A1 (en) | 1999-08-20 | 2001-02-22 | Stockhausen Chem Fab Gmbh | Absorbent, crosslinked polymer, used as absorber aqueous liquid, e.g. body fluids, packaging material, plant culture, soil improver or carrier, contains bound or enclosed cyclodextrin (derivative) and silicon-rich zeolite |
| EP1081181A1 (en) | 1999-09-01 | 2001-03-07 | Westvaco Corporation | Method for making odor sorbing packaging material |
| US6460989B1 (en) | 1999-11-12 | 2002-10-08 | Canon Kabushiki Kaisha | Ink set, formation of colored area on recording medium, and ink-jet recording apparatus |
| US6653356B2 (en) | 1999-12-13 | 2003-11-25 | Jonathan Sherman | Nanoparticulate titanium dioxide coatings, and processes for the production and use thereof |
| ES2167201B1 (en) | 2000-01-18 | 2003-10-01 | Univ Oviedo | OPTICAL VISUAL DEVICE FOR HALITOSIS CONTROL. |
| DE60011079T2 (en) | 2000-01-26 | 2005-06-16 | Toray Industries, Inc. | FIBER STRUCTURE WITH DEODORATIVE OR ANTIBACTERIAL PROPERTIES |
| US6358499B2 (en) | 2000-02-18 | 2002-03-19 | Colgate-Palmolive Company | Deodorant with small particle zinc oxide |
| EP1146057A1 (en) | 2000-04-15 | 2001-10-17 | Givaudan SA | Polymeric nanoparticles including olfactive molecules |
| DE10019877A1 (en) | 2000-04-20 | 2001-10-25 | Clariant Gmbh | Detergents and cleaning agents containing bleach-active dendrimer ligands and their metal complexes |
| EP1157672A1 (en) | 2000-05-23 | 2001-11-28 | The Procter & Gamble Company | Liquid and odour absorbent structure for inanimate places such as refrigerators |
| US6575383B2 (en) | 2000-06-12 | 2003-06-10 | Orlandi, Inc. | Prescented and custom scented card insert |
| US7066998B2 (en) | 2000-06-14 | 2006-06-27 | The Procter & Gamble Company | Coatings for modifying hard surfaces and processes for applying the same |
| KR100772772B1 (en) | 2000-06-19 | 2007-11-01 | 킴벌리-클라크 월드와이드, 인크. | Novel Photoinitiators and Applications Therefor |
| US6425530B1 (en) | 2000-06-29 | 2002-07-30 | Dan Coakley | Scented fresh rolls |
| JP2002019268A (en) | 2000-07-03 | 2002-01-23 | Nippon Aerosil Co Ltd | Ultrafine particle ceramic powder aggregate dispersed water for forming ink absorption layer of ink jet recording medium |
| US20020106466A1 (en) | 2000-08-18 | 2002-08-08 | Karlheinz Hausmann | Active amine scavenging film for fresh fish packaging |
| DE60107445T2 (en) | 2000-09-07 | 2005-04-07 | Solutia Inc. | COMPOSITION AND METHOD OF REDUCE ODOR |
| AU2001293108A1 (en) | 2000-09-29 | 2002-04-08 | Salvona L.L.C. | Multi component controlled release system for sanitary paper products |
| US7371456B2 (en) | 2000-10-02 | 2008-05-13 | Kimberly-Clark Worldwide, Inc. | Nanoparticle based inks and methods of making the same |
| DE10051317A1 (en) | 2000-10-17 | 2002-04-18 | Degussa | Catalysis of peroxy compound delignification or bleaching of fibrous materials in aqueous suspension uses transition metal complexes, some of which are novel compounds |
| US6565873B1 (en) | 2000-10-25 | 2003-05-20 | Salvona Llc | Biodegradable bioadhesive controlled release system of nano-particles for oral care products |
| US6589562B1 (en) | 2000-10-25 | 2003-07-08 | Salvona L.L.C. | Multicomponent biodegradable bioadhesive controlled release system for oral care products |
| US6543385B2 (en) | 2000-12-07 | 2003-04-08 | Nestec, Ltd. | Animal litter composition containing silica gel and methods therefor |
| JP2002179509A (en) | 2000-12-12 | 2002-06-26 | Takasago Internatl Corp | Antifugal perfume composition |
| US20030050211A1 (en) | 2000-12-14 | 2003-03-13 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Enzymatic detergent compositions |
| EP1214878A1 (en) | 2000-12-15 | 2002-06-19 | The Procter & Gamble Company | Methods, compositions and articles for control of malodor produced by urea-containing body fluids |
| DE10063092A1 (en) | 2000-12-18 | 2002-06-20 | Henkel Kgaa | Nanoscale materials in hygiene products |
| EP1216675A1 (en) | 2000-12-19 | 2002-06-26 | SCA Hygiene Products AB | Indicator means for detecting faecal matter |
| US6467897B1 (en) | 2001-01-08 | 2002-10-22 | 3M Innovative Properties Company | Energy curable inks and other compositions incorporating surface modified, nanometer-sized particles |
| US6586483B2 (en) | 2001-01-08 | 2003-07-01 | 3M Innovative Properties Company | Foam including surface-modified nanoparticles |
| WO2002064877A2 (en) | 2001-01-30 | 2002-08-22 | The Procter & Gamble Company | Coating compositions for modifying surfaces |
| US6660713B2 (en) | 2001-01-30 | 2003-12-09 | The Procter & Gamble Company | Hydrophobic nanozeolites for malodor control |
| US6726989B2 (en) | 2001-02-09 | 2004-04-27 | Fiber Innovation Technology, Inc. | Fibers including a nanocomposite material |
| US6843835B2 (en) | 2001-03-27 | 2005-01-18 | The Procter & Gamble Company | Air cleaning apparatus and method for cleaning air |
| WO2002084017A1 (en) | 2001-04-12 | 2002-10-24 | Firstex L.L.C. | Functional treatment of textile materials |
| WO2002083297A1 (en) | 2001-04-16 | 2002-10-24 | Ims Llc | Adsorbent materials for treating biodegradable waste and process for their preparation |
| WO2002085386A2 (en) | 2001-04-23 | 2002-10-31 | Nucryst Pharmaceuticals Corp. | Medicament containing a metal such as silver, gold, platinum or palladium as an antimicrobial agent and their use to induce apoptosis in cancerous tissue |
| US6998155B2 (en) | 2001-05-23 | 2006-02-14 | Traptek Llc | Woven materials with incorporated solids and processes for the production thereof |
| WO2002094329A1 (en) | 2001-05-23 | 2002-11-28 | Basf Aktiengesellschaft | Odor control containing absorbent materials |
| US6926862B2 (en) | 2001-06-01 | 2005-08-09 | Kimberly-Clark Worldwide, Inc. | Container, shelf and drawer liners providing absorbency and odor control |
| EP1402105A2 (en) | 2001-06-26 | 2004-03-31 | Traptek LLC | A treated yarn and methods for making same |
| WO2003051278A2 (en) | 2001-07-10 | 2003-06-26 | North Carolina State University | Nanoparticle delivery vehicle |
| US6894085B2 (en) | 2001-09-17 | 2005-05-17 | Cellresin Technologies, Llc | Barrier material with nanosize metal particles |
| EP1298071A1 (en) | 2001-09-26 | 2003-04-02 | Givaudan SA | Odour delivery system for food products |
| US7563457B2 (en) | 2001-10-02 | 2009-07-21 | The Regents Of The University Of California | Nanoparticle assembled hollow spheres |
| WO2003032959A1 (en) | 2001-10-15 | 2003-04-24 | Bosch William H | Nanoparticulate compositions comprising inorganic cores |
| US20030100842A1 (en) | 2001-10-25 | 2003-05-29 | Rosenberg Melvyn Nevo | Method and kit for indicating the level of bad breath |
| GB0126923D0 (en) | 2001-11-09 | 2002-01-02 | Procter & Gamble | Chitosan compositions |
| ATE525095T1 (en) | 2002-01-08 | 2011-10-15 | Bernard Technologies Inc | ANTIMICROBIAL BODY COVERING ARTICLES |
| KR20040102109A (en) | 2002-04-16 | 2004-12-03 | 코스메티카, 인크. | Polymeric odor absorption ingredients for personal care products |
| US7578997B2 (en) | 2002-04-30 | 2009-08-25 | Kimberly-Clark Worldwide, Inc. | Metal ion modified high surface area materials for odor removal and control |
| US7008979B2 (en) | 2002-04-30 | 2006-03-07 | Hydromer, Inc. | Coating composition for multiple hydrophilic applications |
| US7115321B2 (en) | 2002-07-26 | 2006-10-03 | Kimberly-Clark Worldwide, Inc. | Absorbent binder coating |
| US6962714B2 (en) | 2002-08-06 | 2005-11-08 | Ecolab, Inc. | Critical fluid antimicrobial compositions and their use and generation |
| US6780896B2 (en) | 2002-12-20 | 2004-08-24 | Kimberly-Clark Worldwide, Inc. | Stabilized photoinitiators and applications thereof |
| US7666410B2 (en) | 2002-12-20 | 2010-02-23 | Kimberly-Clark Worldwide, Inc. | Delivery system for functional compounds |
| KR101012584B1 (en) | 2002-12-20 | 2011-02-07 | 킴벌리-클라크 월드와이드, 인크. | Composition Comprising Particles Containing Alumina With Compounds Bound To The Alumina Surface, Delivery Systems And Methods Of Preparation Thereof |
| US20040122387A1 (en) | 2002-12-23 | 2004-06-24 | Kimberly-Clark Worldwide, Inc. | Absorbent articles that include a stretchable substrate having odor control properties |
| US7582308B2 (en) | 2002-12-23 | 2009-09-01 | Kimberly-Clark Worldwide, Inc. | Odor control composition |
| US7413550B2 (en) | 2003-10-16 | 2008-08-19 | Kimberly-Clark Worldwide, Inc. | Visual indicating device for bad breath |
| US7488520B2 (en) | 2003-10-16 | 2009-02-10 | Kimberly-Clark Worldwide, Inc. | High surface area material blends for odor reduction, articles utilizing such blends and methods of using same |
| US7141518B2 (en) | 2003-10-16 | 2006-11-28 | Kimberly-Clark Worldwide, Inc. | Durable charged particle coatings and materials |
| US7678367B2 (en) | 2003-10-16 | 2010-03-16 | Kimberly-Clark Worldwide, Inc. | Method for reducing odor using metal-modified particles |
| US7438875B2 (en) | 2003-10-16 | 2008-10-21 | Kimberly-Clark Worldwide, Inc. | Method for reducing odor using metal-modified silica particles |
| US7754197B2 (en) | 2003-10-16 | 2010-07-13 | Kimberly-Clark Worldwide, Inc. | Method for reducing odor using coordinated polydentate compounds |
| US7879350B2 (en) | 2003-10-16 | 2011-02-01 | Kimberly-Clark Worldwide, Inc. | Method for reducing odor using colloidal nanoparticles |
-
2002
- 2002-12-20 US US10/325,474 patent/US7666410B2/en not_active Expired - Fee Related
-
2003
- 2003-12-09 US US10/731,256 patent/US20040142041A1/en not_active Abandoned
- 2003-12-11 CN CNA2003801050879A patent/CN1777431A/en active Pending
-
2010
- 2010-02-23 US US12/710,413 patent/US8277801B2/en not_active Expired - Fee Related
Patent Citations (100)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2593146A (en) * | 1945-10-19 | 1952-04-15 | Sutcliffe Speakman & Company L | Laminated paper containing activated carbon |
| US3502763A (en) * | 1962-02-03 | 1970-03-24 | Freudenberg Carl Kg | Process of producing non-woven fabric fleece |
| US3381688A (en) * | 1963-08-12 | 1968-05-07 | Kendall & Co | Absorbent pads with silica gel layer for use as surgical receptacles |
| US3502538A (en) * | 1964-08-17 | 1970-03-24 | Du Pont | Bonded nonwoven sheets with a defined distribution of bond strengths |
| US3507269A (en) * | 1965-04-26 | 1970-04-21 | Homer H Berry | Clinical diagnostic device for halitosis |
| US3494821A (en) * | 1967-01-06 | 1970-02-10 | Du Pont | Patterned nonwoven fabric of hydraulically entangled textile fibers and reinforcing fibers |
| US3802817A (en) * | 1969-10-01 | 1974-04-09 | Asahi Chemical Ind | Apparatus for producing non-woven fleeces |
| US3794497A (en) * | 1969-10-24 | 1974-02-26 | Ici Ltd | Photographic element comprising an olefinically unsaturated monomer and a photo-labile organo cobalt compound |
| US4006030A (en) * | 1972-11-21 | 1977-02-01 | Nissan Chemical Industries, Ltd. | Method of preventing deterioration of inorganic substrate surface |
| US4078029A (en) * | 1976-09-23 | 1978-03-07 | Nissan Chemical Industries, Ltd. | Process for preparing mold |
| US4494278A (en) * | 1977-11-08 | 1985-01-22 | Karl Kristian Kobs Kroyer | Apparatus for the production of a fibrous web |
| US4375448A (en) * | 1979-12-21 | 1983-03-01 | Kimberly-Clark Corporation | Method of forming a web of air-laid dry fibers |
| US4313820A (en) * | 1980-02-28 | 1982-02-02 | Phillips Petroleum Co. | Hydrodesulfurization of organic sulfur compounds and hydrogen sulfide removal with incompletely sulfided zinc titanate materials |
| US4494629A (en) * | 1981-08-12 | 1985-01-22 | Raeburn John L | Lowering device and method |
| US4517308A (en) * | 1981-09-04 | 1985-05-14 | Collo Gmbh | Method of producing a sorptive body, particularly for eliminating odors, air freshening, etc. and the resultant product |
| US4451388A (en) * | 1981-11-02 | 1984-05-29 | Nalco Chemical Company | Preparation of aluminum oxide coated silica sols using ultrafiltration |
| US4575556A (en) * | 1982-11-08 | 1986-03-11 | Medi-Physics, Inc. | Bifunctional chelating agents |
| US4802473A (en) * | 1983-11-07 | 1989-02-07 | Tecnol, Inc. | Face mask with ear loops |
| US4655757A (en) * | 1984-04-23 | 1987-04-07 | Kimberly-Clark Corporation | Selective layering of superabsorbents in meltblown substrates |
| US4640810A (en) * | 1984-06-12 | 1987-02-03 | Scan Web Of North America, Inc. | System for producing an air laid web |
| US4818464A (en) * | 1984-08-30 | 1989-04-04 | Kimberly-Clark Corporation | Extrusion process using a central air jet |
| USRE32649E (en) * | 1985-06-18 | 1988-04-19 | The Procter & Gamble Company | Hydrogel-forming polymer compositions for use in absorbent structures |
| US4643801A (en) * | 1986-02-24 | 1987-02-17 | Nalco Chemical Company | Papermaking aid |
| US4725415A (en) * | 1986-06-02 | 1988-02-16 | Phillips Petroleum Company | Selective removal of hydrogen sulfide over zinc titanate and alumina |
| US5108739A (en) * | 1986-08-25 | 1992-04-28 | Titan Kogyo Kabushiki Kaisha | White colored deodorizer and process for producing the same |
| US4904304A (en) * | 1986-12-29 | 1990-02-27 | Nissan Chemical Industries Ltd. | Chemical grout for ground injection and method for accretion |
| US4734324A (en) * | 1987-03-27 | 1988-03-29 | Hercules Incorporated | Heat sealable microporous polypropylene films |
| US4823803A (en) * | 1987-07-31 | 1989-04-25 | Winners Japan Company Limited | Halitosis detector device |
| US5204429A (en) * | 1987-08-07 | 1993-04-20 | Hoechst Aktiengesellschaft | Process for the preparation of an olefin polymer |
| US5000746A (en) * | 1987-08-11 | 1991-03-19 | Friedrichsfeld Gmbh Keramik- Und Kunststoffwerke | Wound covering having connected discrete elements |
| US5383450A (en) * | 1987-10-02 | 1995-01-24 | Tcnl Technologies, Inc. | Liquid shield visor for a surgical mask |
| US4798603A (en) * | 1987-10-16 | 1989-01-17 | Kimberly-Clark Corporation | Absorbent article having a hydrophobic transport layer |
| US4823404A (en) * | 1988-06-10 | 1989-04-25 | Kimberly-Clark Corporation | Two piece protective garment |
| US4988505A (en) * | 1988-09-16 | 1991-01-29 | Nissan Chemical Industries, Ltd. | Deodorizer |
| US5204111A (en) * | 1989-04-07 | 1993-04-20 | L'oreal | Process for the preparation of alginate capsules, apparatus for producing said capsules and cosmetic compositions containing said capsules |
| US5488126A (en) * | 1989-05-26 | 1996-01-30 | Akzo Nobel N.V. | Bifunctional chelating agents |
| US5292868A (en) * | 1989-05-26 | 1994-03-08 | Akzo N.V. | Chelating agents for attaching metal ions to proteins |
| US5188885A (en) * | 1989-09-08 | 1993-02-23 | Kimberly-Clark Corporation | Nonwoven fabric laminates |
| US5196177A (en) * | 1990-01-17 | 1993-03-23 | Nissan Chemical Industries, Ltd. | Production of stable aqueous silica sol |
| US5407442A (en) * | 1990-02-12 | 1995-04-18 | Karapasha; Nancy | Carbon-containing odor controlling compositions |
| US5100581A (en) * | 1990-02-22 | 1992-03-31 | Nissan Chemical Industries Ltd. | Method of preparing high-purity aqueous silica sol |
| US5100702A (en) * | 1990-02-22 | 1992-03-31 | Nissan Chemical Industries, Ltd. | Thin platinum film-forming composition |
| US5183656A (en) * | 1990-08-03 | 1993-02-02 | Mitsubishi Denki Kabushiki Kaisha | Deodorant and product in which the deodorant is used |
| US5300365A (en) * | 1990-09-28 | 1994-04-05 | Himont Incorporated | Olefin polymer films |
| US5178931A (en) * | 1990-11-26 | 1993-01-12 | Kimberly-Clark Corporation | Three-layer nonwoven laminiferous structure |
| US5284703A (en) * | 1990-12-21 | 1994-02-08 | Kimberly-Clark Corporation | High pulp content nonwoven composite fabric |
| US5420090A (en) * | 1991-01-18 | 1995-05-30 | The Dow Chemical Company | Silica supported transition metal catalysts |
| US6172173B1 (en) * | 1991-01-18 | 2001-01-09 | The Dow Chemical Company | Silica supported transition metal catalyst |
| US5487938B1 (en) * | 1991-01-18 | 1997-11-18 | Dow Chemical Co | Silica supported transition metal catalyst |
| US5487938A (en) * | 1991-01-18 | 1996-01-30 | The Dow Chemical Company | Silica supported transition metal catalyst |
| US5486356A (en) * | 1991-05-30 | 1996-01-23 | Fuji Photo Film Co., Ltd. | Deodorant composition combining transition metal oxide or alloy with catalytic metal on carrier |
| US5407600A (en) * | 1991-07-23 | 1995-04-18 | Nissan Chemical Industries, Ltd. | Stable aqueous alumina sol and method for preparing the same |
| US5294717A (en) * | 1991-10-24 | 1994-03-15 | Spyros Theodoropulos | Bifunctional chelating agents, their chelates and process of preparation |
| US5209998A (en) * | 1991-11-25 | 1993-05-11 | Xerox Corporation | Colored silica particles |
| US5480636A (en) * | 1992-06-03 | 1996-01-02 | Ishihara Sangyo Kaisha, Ltd. | Titanium oxide particles and method of scavenging noxious materials |
| US5382400A (en) * | 1992-08-21 | 1995-01-17 | Kimberly-Clark Corporation | Nonwoven multicomponent polymeric fabric and method for making same |
| US5314855A (en) * | 1992-11-09 | 1994-05-24 | Akzo N.V. | Adsorbent compositions and methods of manufacture |
| US5733272A (en) * | 1993-03-31 | 1998-03-31 | The Procter & Gamble Company | Absorbent articles for odor control with positive scent signal |
| US5597512A (en) * | 1993-10-15 | 1997-01-28 | Nissan Chemical Industries, Ltd. | Method for preparing elongated-shaped silica sol |
| US5591797A (en) * | 1993-10-25 | 1997-01-07 | Wacker-Chemie Gmbh | Transition metal-containing hydrophobic silica |
| US6190814B1 (en) * | 1994-04-28 | 2001-02-20 | Xerox Corporation | Modified silica particles |
| US5397667A (en) * | 1994-04-28 | 1995-03-14 | Xerox Corporation | Toner with metallized silica particles |
| US5597575A (en) * | 1994-06-06 | 1997-01-28 | Breitbarth; Richard | Composition for stimulating and inducing hair growth |
| US6177608B1 (en) * | 1994-06-30 | 2001-01-23 | Kimberly-Clark Worldwide, Inc. | Tampon |
| US5902226A (en) * | 1994-08-05 | 1999-05-11 | Nissan Chemical Industries, Ltd. | Method of preparing a propanol sol of silica |
| US5897541A (en) * | 1994-09-30 | 1999-04-27 | Kimberly-Clark Worldwide, Inc. | Laminate material and absorbent garment comprising same |
| US5616315A (en) * | 1994-10-13 | 1997-04-01 | Gillette Canada Inc. | Particles including degradable material and anti-microbial agent |
| US5880176A (en) * | 1994-10-21 | 1999-03-09 | Hitachi Maxell, Ltd. | Fluorescent marking composition and fluorescent mark formed by said composition |
| US5905101A (en) * | 1995-03-22 | 1999-05-18 | Nissan Motor Co., Ltd. | Ablator compositions |
| US5747003A (en) * | 1995-03-22 | 1998-05-05 | Ppg Industries, Inc. | Amorphous precipitated silica abrasive |
| US6193844B1 (en) * | 1995-06-07 | 2001-02-27 | Mclaughlin John R. | Method for making paper using microparticles |
| US5858503A (en) * | 1995-10-26 | 1999-01-12 | Kimberly-Clark Worldwide, Inc. | Method of applying chemical charge modifiers to a substrate and article thereof |
| US5855788A (en) * | 1996-02-07 | 1999-01-05 | Kimberly-Clark Worldwide, Inc. | Chemically charged-modified filter for removing particles from a liquid and method thereof |
| US6210625B1 (en) * | 1996-02-20 | 2001-04-03 | Mikuni Corporation | Method for producing granulated material |
| US6225524B1 (en) * | 1996-06-07 | 2001-05-01 | The Procter & Gamble Company | Absorbent articles having an odor control system consisting of absorbent gelling material and silica |
| US6376741B1 (en) * | 1996-06-07 | 2002-04-23 | The Procter & Gamble Company | Activated carbon free absorbent articles having a silica and zeolite odor control system |
| US6358909B1 (en) * | 1996-10-17 | 2002-03-19 | The Clorox Company | Suspoemulsion system for delivery of actives |
| US5882638A (en) * | 1996-10-24 | 1999-03-16 | The Proctor & Gamble Company | Methods using uncomplexed cyclodextrin solutions for controlling environmental odors |
| US5874067A (en) * | 1996-10-24 | 1999-02-23 | The Procter & Gamble Company | Methods for controlling environmental odors on the body |
| US5885599A (en) * | 1996-10-28 | 1999-03-23 | The Procter & Gamble Company | Methods and compositions for reducing body odors and excess moisture |
| US5880309A (en) * | 1997-01-28 | 1999-03-09 | Nissan Chemical Industries, Ltd. | Phenylphosphonic acid derivative and production process therefor |
| US6344272B1 (en) * | 1997-03-12 | 2002-02-05 | Wm. Marsh Rice University | Metal nanoshells |
| US5871872A (en) * | 1997-05-30 | 1999-02-16 | Shipley Company, Ll.C. | Dye incorporated pigments and products made from same |
| US5861144A (en) * | 1997-06-09 | 1999-01-19 | The Procter & Gamble Company | Perfumed compositions for reducing body odors and excess moisture |
| US6045900A (en) * | 1997-09-15 | 2000-04-04 | Kimberly-Clark Worldwide, Inc. | Breathable filled film laminate |
| US6024786A (en) * | 1997-10-30 | 2000-02-15 | Hewlett-Packard Company | Stable compositions of nano-particulate unmodified pigments and insoluble colorants in aqueous microemulsions, and principle of stability and methods of formation thereof |
| US6200555B1 (en) * | 1998-03-10 | 2001-03-13 | Mazda Motor Corporation | Deodorant composition, deodorizer and filter each containing the same, and method of deodorization |
| US6047413A (en) * | 1998-03-31 | 2000-04-11 | Kimberly-Clark Worldwide, Inc. | Conformable backpack for encapsulated chemical protection suit |
| US6060410A (en) * | 1998-04-22 | 2000-05-09 | Gillberg-Laforce; Gunilla Elsa | Coating of a hydrophobic polymer substrate with a nonstoichiometric polyelectrolyte complex |
| US6334988B1 (en) * | 1998-08-21 | 2002-01-01 | The University Of Vermont And State Agricultural College | Mesoporous silicates and method of making same |
| US6531704B2 (en) * | 1998-09-14 | 2003-03-11 | Nanoproducts Corporation | Nanotechnology for engineering the performance of substances |
| US6358537B1 (en) * | 1998-11-10 | 2002-03-19 | Dainichiseika Color & Chemicals Mfg. Co, Ltd. | Deodorant and antimicrobial dispersions |
| US6361780B1 (en) * | 1998-11-12 | 2002-03-26 | Cardiac Pacemakers, Inc. | Microporous drug delivery system |
| US6344218B1 (en) * | 1998-11-23 | 2002-02-05 | The Procter & Gamble Company | Skin deodorizing and santizing compositions |
| US6517199B1 (en) * | 1999-11-12 | 2003-02-11 | Canon Kabushiki Kaisha | Liquid composition, ink set, colored area formation on recording medium, and ink-jet recording apparatus |
| US6536890B1 (en) * | 1999-11-12 | 2003-03-25 | Canon Kabushiki Kaisha | Liquid composition as well as ink set, image forming method, image forming apparatus and bleed alleviation method using the same |
| US6369290B1 (en) * | 2000-02-17 | 2002-04-09 | Tyco Healthcare Retail Services Ag | Time release odor control composition for a disposable absorbent article |
| US6548264B1 (en) * | 2000-05-17 | 2003-04-15 | University Of Florida | Coated nanoparticles |
| US6551457B2 (en) * | 2000-09-20 | 2003-04-22 | Akzo Nobel N.V. | Process for the production of paper |
| US6693071B2 (en) * | 2001-01-30 | 2004-02-17 | The Procter & Gamble Company | Rinse aid surface coating compositions for modifying dishware surfaces |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8277801B2 (en) | 2002-12-20 | 2012-10-02 | Kimberly-Clark Worldwide, Inc. | Delivery system for functional compounds |
| US20100215754A1 (en) * | 2002-12-20 | 2010-08-26 | Kimberly-Clark Worldwide, Inc. | Delivery System for Functional Compounds |
| US8221328B2 (en) | 2003-10-16 | 2012-07-17 | Kimberly-Clark Worldwide, Inc. | Visual indicating device for bad breath |
| US8702618B2 (en) | 2003-10-16 | 2014-04-22 | Kimberly-Clark Worldwide, Inc. | Visual indicating device for bad breath |
| US9655622B2 (en) | 2004-02-18 | 2017-05-23 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US10398802B2 (en) | 2004-02-18 | 2019-09-03 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US11413370B2 (en) | 2004-02-18 | 2022-08-16 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US10434211B2 (en) | 2004-02-18 | 2019-10-08 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US20090076542A1 (en) * | 2004-02-18 | 2009-03-19 | Jerry Jonn | Adhesive-Containing Wound Closure Device And Method |
| US20060009099A1 (en) * | 2004-07-12 | 2006-01-12 | Closure Medical Corporation | Adhesive-containing wound closure device and method |
| US20080255610A1 (en) * | 2004-07-12 | 2008-10-16 | Closure Medical Corporation | Adhesive-Containing Wound Closure Device and Method |
| US9623142B2 (en) | 2004-07-12 | 2017-04-18 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US10398800B2 (en) | 2004-07-12 | 2019-09-03 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| US11446407B2 (en) | 2004-07-12 | 2022-09-20 | Ethicon, Inc. | Adhesive-containing wound closure device and method |
| WO2007011612A3 (en) * | 2005-07-15 | 2007-04-26 | Tyco Healthcare | Wound dressing and methods of making and using the same |
| US7977103B2 (en) | 2006-04-20 | 2011-07-12 | Kimberly-Clark Worldwide, Inc. | Method for detecting the onset of ovulation |
| US9239036B2 (en) | 2006-09-08 | 2016-01-19 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment and delivery system and process |
| US9283188B2 (en) * | 2006-09-08 | 2016-03-15 | Kimberly-Clark Worldwide, Inc. | Delivery systems for delivering functional compounds to substrates and processes of using the same |
| KR101450139B1 (en) * | 2006-09-08 | 2014-10-13 | 킴벌리-클라크 월드와이드, 인크. | Delivery Systems for Delivering Functional Compounds to Substrates and Processes of Using the Same |
| US8034286B2 (en) | 2006-09-08 | 2011-10-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment system for separating compounds from aqueous effluent |
| US20080063718A1 (en) * | 2006-09-08 | 2008-03-13 | Kimberly-Clark Worldwide, Inc. | Delivery Systems For Delivering Functional Compounds to Substrates and Processes of Using the Same |
| US8616759B2 (en) | 2006-09-08 | 2013-12-31 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment system |
| US7703698B2 (en) | 2006-09-08 | 2010-04-27 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment chamber and continuous flow mixing system |
| US7712353B2 (en) | 2006-12-28 | 2010-05-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment system |
| US7673516B2 (en) | 2006-12-28 | 2010-03-09 | Kimberly-Clark Worldwide, Inc. | Ultrasonic liquid treatment system |
| US20080179562A1 (en) * | 2007-01-30 | 2008-07-31 | Kimberly-Clark Worldwide, Inc. | Substrate containing a deodorizing ink |
| US7785674B2 (en) | 2007-07-12 | 2010-08-31 | Kimberly-Clark Worldwide, Inc. | Delivery systems for delivering functional compounds to substrates and processes of using the same |
| US7998322B2 (en) | 2007-07-12 | 2011-08-16 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber having electrode properties |
| US7947184B2 (en) | 2007-07-12 | 2011-05-24 | Kimberly-Clark Worldwide, Inc. | Treatment chamber for separating compounds from aqueous effluent |
| US20090147905A1 (en) * | 2007-12-05 | 2009-06-11 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for initiating thermonuclear fusion |
| US8454889B2 (en) | 2007-12-21 | 2013-06-04 | Kimberly-Clark Worldwide, Inc. | Gas treatment system |
| US20090158936A1 (en) * | 2007-12-21 | 2009-06-25 | Kimberly-Clark Worldwide, Inc. | Gas treatment system |
| US20090162258A1 (en) * | 2007-12-21 | 2009-06-25 | Kimberly-Clark Worldwide, Inc. | Liquid treatment system |
| US8858892B2 (en) | 2007-12-21 | 2014-10-14 | Kimberly-Clark Worldwide, Inc. | Liquid treatment system |
| US8632613B2 (en) | 2007-12-27 | 2014-01-21 | Kimberly-Clark Worldwide, Inc. | Process for applying one or more treatment agents to a textile web |
| US20090166177A1 (en) * | 2007-12-28 | 2009-07-02 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing emulsions |
| US8057573B2 (en) | 2007-12-28 | 2011-11-15 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for increasing the shelf life of formulations |
| US20090168591A1 (en) * | 2007-12-28 | 2009-07-02 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for particle dispersion into formulations |
| US8206024B2 (en) | 2007-12-28 | 2012-06-26 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for particle dispersion into formulations |
| US8143318B2 (en) | 2007-12-28 | 2012-03-27 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing emulsions |
| US8215822B2 (en) | 2007-12-28 | 2012-07-10 | Kimberly-Clark Worldwide, Inc. | Ultrasonic treatment chamber for preparing antimicrobial formulations |
| US8685178B2 (en) | 2008-12-15 | 2014-04-01 | Kimberly-Clark Worldwide, Inc. | Methods of preparing metal-modified silica nanoparticles |
| US8163388B2 (en) | 2008-12-15 | 2012-04-24 | Kimberly-Clark Worldwide, Inc. | Compositions comprising metal-modified silica nanoparticles |
| US10835495B2 (en) | 2012-11-14 | 2020-11-17 | W. R. Grace & Co.-Conn. | Compositions containing a biologically active material and a non-ordered inorganic oxide material and methods of making and using the same |
| USD824525S1 (en) | 2014-09-25 | 2018-07-31 | Ethicon Llc | Release paper for wound treament devices |
| USD854171S1 (en) | 2014-09-25 | 2019-07-16 | Ethicon Llc | Release paper for wound treatment devices |
| US10792024B2 (en) | 2016-09-28 | 2020-10-06 | Ethicon, Inc. | Scaffolds with channels for joining layers of tissue at discrete points |
| US10687986B2 (en) | 2016-09-29 | 2020-06-23 | Ethicon, Inc. | Methods and devices for skin closure |
| USD907217S1 (en) | 2016-09-29 | 2021-01-05 | Ethicon, Inc. | Release paper for wound treatment devices |
| US10470934B2 (en) | 2016-09-29 | 2019-11-12 | Ethicon, Inc. | Methods and devices for skin closure |
| USD979768S1 (en) | 2016-09-29 | 2023-02-28 | Ethicon, Inc. | Release paper for wound treatment devices |
| US11679034B2 (en) | 2016-09-29 | 2023-06-20 | Ethicon, Inc. | Methods and devices for skin closure |
| US10470935B2 (en) | 2017-03-23 | 2019-11-12 | Ethicon, Inc. | Skin closure systems and devices of improved flexibility and stretchability for bendable joints |
| US11883264B2 (en) | 2017-03-23 | 2024-01-30 | Ethicon, Inc. | Skin closure systems and devices of improved flexibility and stretchability for bendable joints |
| US11504446B2 (en) | 2017-04-25 | 2022-11-22 | Ethicon, Inc. | Skin closure devices with self-forming exudate drainage channels |
| US10993708B2 (en) | 2018-07-31 | 2021-05-04 | Ethicon, Inc. | Skin closure devices with interrupted closure |
Also Published As
| Publication number | Publication date |
|---|---|
| US20100215754A1 (en) | 2010-08-26 |
| US8277801B2 (en) | 2012-10-02 |
| US20040120904A1 (en) | 2004-06-24 |
| CN1777431A (en) | 2006-05-24 |
| US7666410B2 (en) | 2010-02-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20040142041A1 (en) | Triggerable delivery system for pharmaceutical and nutritional compounds and methods of utilizing same | |
| US11707549B2 (en) | Adhesive containing microparticles | |
| KR101158711B1 (en) | Cooling topical patch preparation | |
| ES2310942T3 (en) | TOLTERODINE ADMINISTERED BY TRANSDERMIC AS AN ANTI-MUSCARINIC AGENT FOR THE TREATMENT OF HYPERACTIVE BLADDER. | |
| JP5175107B2 (en) | Nitrogen oxide neuropathy treatment device, method and use | |
| US9445996B2 (en) | Extended production of nitric oxide from a microencapsulated nitrite salt and an aqueous acidified gel | |
| Akhtar et al. | Topical delivery of drugs for the effective treatment of fungal infections of skin | |
| US20140369949A1 (en) | Cosmetic Treatment with Nitric Oxide, Device for Performing Said Treatment and Manufacturing Method Therefor | |
| MX2007009691A (en) | Device and method for treatment of dermatomycosis, and in particular onychomycosis. | |
| JP2008530055A5 (en) | ||
| AU2015209466B2 (en) | Abuse and misuse deterrent transdermal systems | |
| JPS63227521A (en) | Skin penetration accelerator composition using sucrose ester | |
| WO2004069169A3 (en) | Localized drug delivery using drug-loaded nanocapsules and implantable device coated with the same | |
| US9585826B2 (en) | Triggerable compositions for two-stage, controlled release of active chemistry | |
| CN100558350C (en) | The improvement transdermal delivery system | |
| KR101012584B1 (en) | Composition Comprising Particles Containing Alumina With Compounds Bound To The Alumina Surface, Delivery Systems And Methods Of Preparation Thereof | |
| WO2008036429A1 (en) | Gallium compositions for the treatment of liver cancer and methods of use | |
| DE60207606T2 (en) | ARTICLES OF WOUND TREATMENT | |
| JP2531179B2 (en) | Poultice | |
| CA3007747A1 (en) | Multifunctional transdermal dressing for wounds | |
| JP2540861B2 (en) | Poultice | |
| Yadav et al. | COLON TARGETED DRUG DELIVERY SYSTEM: DELIVERY APPROACHES, RECENT ADVANCEMENTS AND PATENTS | |
| WO2006134937A1 (en) | Skin preparation for external use | |
| RU96117257A (en) | GALENA FORM DERIVATIVES OF 5-NITROIMIDAZOLE | |
| JPH01131115A (en) | Clebopride percataneous poultice |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: KIMBERLY-CLARK WORLDWIDE, INC., WISCONSIN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:MACDONALD, JOHN G.;LYE, JASON;REEL/FRAME:014783/0131 Effective date: 20031209 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION |