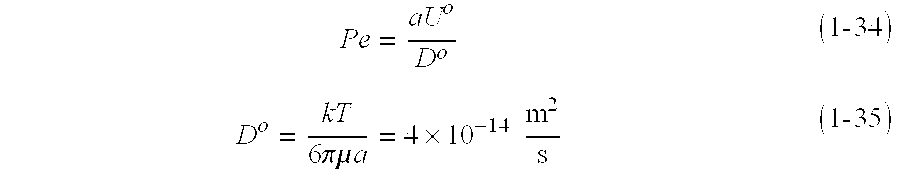US20040166555A1 - Cell sorting apparatus and methods for manipulating cells using the same - Google Patents
Cell sorting apparatus and methods for manipulating cells using the same Download PDFInfo
- Publication number
- US20040166555A1 US20040166555A1 US10/778,831 US77883104A US2004166555A1 US 20040166555 A1 US20040166555 A1 US 20040166555A1 US 77883104 A US77883104 A US 77883104A US 2004166555 A1 US2004166555 A1 US 2004166555A1
- Authority
- US
- United States
- Prior art keywords
- cell
- captured
- well
- cells
- sorting apparatus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034 method Methods 0.000 title claims description 77
- 230000005684 electric field Effects 0.000 claims abstract description 17
- 239000012530 fluid Substances 0.000 claims description 32
- 230000004044 response Effects 0.000 claims description 19
- 230000007246 mechanism Effects 0.000 claims description 14
- 238000003556 assay Methods 0.000 claims description 10
- 230000036755 cellular response Effects 0.000 claims description 10
- 230000003287 optical effect Effects 0.000 claims description 9
- 239000000523 sample Substances 0.000 claims description 9
- 238000001514 detection method Methods 0.000 claims description 7
- 230000001413 cellular effect Effects 0.000 claims description 5
- 239000000758 substrate Substances 0.000 claims description 4
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 3
- 230000027455 binding Effects 0.000 claims description 3
- 229910052791 calcium Inorganic materials 0.000 claims description 3
- 239000011575 calcium Substances 0.000 claims description 3
- 230000003834 intracellular effect Effects 0.000 claims description 3
- 239000003446 ligand Substances 0.000 claims description 3
- 230000002123 temporal effect Effects 0.000 claims description 3
- 108700008625 Reporter Genes Proteins 0.000 claims description 2
- 239000003814 drug Substances 0.000 claims description 2
- 230000003213 activating effect Effects 0.000 claims 3
- 239000003795 chemical substances by application Substances 0.000 claims 2
- 230000008878 coupling Effects 0.000 claims 2
- 238000010168 coupling process Methods 0.000 claims 2
- 238000005859 coupling reaction Methods 0.000 claims 2
- 230000001939 inductive effect Effects 0.000 claims 2
- 230000006916 protein interaction Effects 0.000 claims 2
- 108090000623 proteins and genes Proteins 0.000 claims 2
- 102000004169 proteins and genes Human genes 0.000 claims 2
- 230000004715 cellular signal transduction Effects 0.000 claims 1
- 229940079593 drug Drugs 0.000 claims 1
- 230000006698 induction Effects 0.000 claims 1
- 230000021670 response to stimulus Effects 0.000 claims 1
- 238000004458 analytical method Methods 0.000 abstract description 19
- 238000012544 monitoring process Methods 0.000 abstract description 2
- 210000004027 cell Anatomy 0.000 description 174
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 94
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 62
- 229910052697 platinum Inorganic materials 0.000 description 44
- 239000002245 particle Substances 0.000 description 36
- 238000009835 boiling Methods 0.000 description 34
- 239000011521 glass Substances 0.000 description 32
- 230000006911 nucleation Effects 0.000 description 27
- 238000010899 nucleation Methods 0.000 description 27
- 238000012360 testing method Methods 0.000 description 25
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 22
- 229910052710 silicon Inorganic materials 0.000 description 21
- 239000010703 silicon Substances 0.000 description 21
- 239000011324 bead Substances 0.000 description 17
- 230000008569 process Effects 0.000 description 16
- 239000007788 liquid Substances 0.000 description 12
- 235000012431 wafers Nutrition 0.000 description 11
- 238000010438 heat treatment Methods 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 9
- 238000004364 calculation method Methods 0.000 description 8
- 238000005259 measurement Methods 0.000 description 8
- 229920002120 photoresistant polymer Polymers 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 7
- 230000005484 gravity Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 239000004793 Polystyrene Substances 0.000 description 6
- 238000013461 design Methods 0.000 description 6
- 239000004205 dimethyl polysiloxane Substances 0.000 description 6
- 235000013870 dimethyl polysiloxane Nutrition 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 239000010408 film Substances 0.000 description 6
- 230000004907 flux Effects 0.000 description 6
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 6
- 229920002223 polystyrene Polymers 0.000 description 6
- 239000010409 thin film Substances 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- 238000005054 agglomeration Methods 0.000 description 5
- 230000002776 aggregation Effects 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 238000005381 potential energy Methods 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 238000003491 array Methods 0.000 description 4
- 238000004720 dielectrophoresis Methods 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 230000017525 heat dissipation Effects 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 230000008901 benefit Effects 0.000 description 3
- 239000008364 bulk solution Substances 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000008367 deionised water Substances 0.000 description 3
- 229910021641 deionized water Inorganic materials 0.000 description 3
- 238000005868 electrolysis reaction Methods 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 238000000137 annealing Methods 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000005511 kinetic theory Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000007814 microscopic assay Methods 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000006104 solid solution Substances 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 239000012798 spherical particle Substances 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 244000153158 Ammi visnaga Species 0.000 description 1
- 235000010585 Ammi visnaga Nutrition 0.000 description 1
- 101100269850 Caenorhabditis elegans mask-1 gene Proteins 0.000 description 1
- 206010013710 Drug interaction Diseases 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 108700002232 Immediate-Early Genes Proteins 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- 229920005372 Plexiglas® Polymers 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 101150036080 at gene Proteins 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 238000011325 biochemical measurement Methods 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 230000005587 bubbling Effects 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 230000036978 cell physiology Effects 0.000 description 1
- 230000009134 cell regulation Effects 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 238000007822 cytometric assay Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 238000004880 explosion Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229920005570 flexible polymer Polymers 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 229910021420 polycrystalline silicon Inorganic materials 0.000 description 1
- 229920005591 polysilicon Polymers 0.000 description 1
- 235000019353 potassium silicate Nutrition 0.000 description 1
- 238000010248 power generation Methods 0.000 description 1
- 230000004850 protein–protein interaction Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 238000005309 stochastic process Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 238000009834 vaporization Methods 0.000 description 1
- 230000008016 vaporization Effects 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N15/1456—Electro-optical investigation, e.g. flow cytometers without spatial resolution of the texture or inner structure of the particle, e.g. processing of pulse signals
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N15/1468—Electro-optical investigation, e.g. flow cytometers with spatial resolution of the texture or inner structure of the particle
- G01N15/147—Electro-optical investigation, e.g. flow cytometers with spatial resolution of the texture or inner structure of the particle the analysis being performed on a sample stream
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N15/1484—Electro-optical investigation, e.g. flow cytometers microstructural devices
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/0005—Field flow fractionation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00277—Apparatus
- B01J2219/00279—Features relating to reactor vessels
- B01J2219/00306—Reactor vessels in a multiple arrangement
- B01J2219/00313—Reactor vessels in a multiple arrangement the reactor vessels being formed by arrays of wells in blocks
- B01J2219/00315—Microtiter plates
- B01J2219/00317—Microwell devices, i.e. having large numbers of wells
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00605—Making arrays on substantially continuous surfaces the compounds being directly bound or immobilised to solid supports
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00605—Making arrays on substantially continuous surfaces the compounds being directly bound or immobilised to solid supports
- B01J2219/00612—Making arrays on substantially continuous surfaces the compounds being directly bound or immobilised to solid supports the surface being inorganic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00605—Making arrays on substantially continuous surfaces the compounds being directly bound or immobilised to solid supports
- B01J2219/00614—Delimitation of the attachment areas
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00605—Making arrays on substantially continuous surfaces the compounds being directly bound or immobilised to solid supports
- B01J2219/00632—Introduction of reactive groups to the surface
- B01J2219/00637—Introduction of reactive groups to the surface by coating it with another layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00653—Making arrays on substantially continuous surfaces the compounds being bound to electrodes embedded in or on the solid supports
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00583—Features relative to the processes being carried out
- B01J2219/00603—Making arrays on substantially continuous surfaces
- B01J2219/00659—Two-dimensional arrays
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/0068—Means for controlling the apparatus of the process
- B01J2219/00702—Processes involving means for analysing and characterising the products
- B01J2219/00704—Processes involving means for analysing and characterising the products integrated with the reactor apparatus
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00274—Sequential or parallel reactions; Apparatus and devices for combinatorial chemistry or for making arrays; Chemical library technology
- B01J2219/00718—Type of compounds synthesised
- B01J2219/0072—Organic compounds
- B01J2219/0074—Biological products
- B01J2219/00743—Cells
-
- G01N15/149—
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N15/1404—Fluid conditioning in flow cytometers, e.g. flow cells; Supply; Control of flow
- G01N2015/1415—Control of particle position
Definitions
- This invention relates to cell analysis and sorting devices and methods for manipulating cells using these devices. More particularly, the invention relates to a cell analysis and sorting apparatus that can capture and hold single cells at known locations and then selectively release certain of these cells. A method of manipulating the cells using the cell analysis and sorting apparatus is also provided.
- MEMS micro electromechanical systems
- DEP refers to the action of neutral particles in non-uniform electric fields.
- Neutral polarizable particles experience a force in non-uniform electric fields which propels them toward the electric field maxima or minima, depending on whether the particle is more or less polarizable than the medium it is in.
- an electric field may be produced to stably trap dielectric particles.
- Microfabrication has been utilized to make electrode arrays for cell manipulation since the late 1980s.
- researchers have successfully trapped many different cell types, including mammalian cells, yeast cells, plant cells, and polymeric particles.
- Much work involves manipulating cells by exploiting differences in the dielectric properties of varying cell types to evoke separations, such as separation of viable from non-viable yeast, and enrichment of CD34+ stem cells from bone marrow and peripheral blood stem cells.
- More relevant work on trapping cells in various two- and three-dimensional microfabricated electrode geometries has been shown by several groups.
- trapping arrays of cells with the intention of releasing selected subpopulations of cells has not yet been widely explored.
- DEP can potentially induce large temperature changes, causing not only convection effects but also profoundly affecting cell physiology.
- the present invention provides a cell sorting apparatus that is capable of monitoring over time the behavior of each cell in a large population of cells.
- the cell analysis and sorting apparatus contains individually addressable cell locations. Each location is capable of capturing and holding a single cell, and selectively releasing that cell from that particular location.
- the cells are captured and held in wells, and released using vapor bubbles as a means of cell election.
- the cells are captured, held and released using electric field traps.
- the cell analysis and sorting apparatus has an array of geometric sites for capturing cells traveling along a fluid flow.
- the geometric sites are arranged in a defined pattern across a substrate such that individual sites are known and identifiable.
- Each geometric site is configured and dimensioned to hold a single cell.
- each site contains a release mechanism to selectively release the single cell from that site. Because each site is able to hold only one cell, and each site has a unique address, the apparatus allows the user to know the location of any particular cell that has been captured. Further, each site is independently controllable so that the user is able to arbitrarily capture cells at select locations, and to release cells at various locations across the array.
- the geometric sites are configured as wells. As a fluid of cells is flown across the array of specifically sized wells, cells will fall into the wells and become trapped. Each well is sized and shaped to capture only a single cell, and is configured such that the cell will not escape into the laminar flow of the fluid above the well. The single cell can be held inside the well by gravitational forces. Each well can further be attached via a narrow channel to a chamber located below the well. Within the chamber is a heating element that is able to induce bubble nucleation, the mechanism for releasing the cell from the site. The bubble creates volume expansion inside the chamber which, when filled with fluid, will displace a jet of fluid out of the narrow channel and eject the cell out of the well. Fluid flow above the well will sweep the ejected cell away to be either collected or discarded.
- the geometric sites are formed from a three-dimensional electric field trap.
- Each trap comprises four electrodes arranged in a trapezoidal configuration, where each electrode represents a corner of the trapezoid.
- the electric fields of the electrodes create a potential energy well for capturing a single cell within the center of the trap.
- the cell is ejected out of the site and into the fluid flow around the trap. Ejected cells can then be washed out and collected or discarded.
- an integrated system can be a microfabrication-based dynamic array cytometer ( ⁇ DAC) having as one of its components the cell analysis and sorting apparatus previously described.
- ⁇ DAC microfabrication-based dynamic array cytometer
- the cells can be placed on a cell array chip containing a plurality of cell sites.
- the cells are held in place within the plurality of cell sites in a manner similar to that described above and analyzed, for example, by photometric assay.
- the response of the cells can be measured, with the intensity of the fluorescence reflecting the intensity of the cellular response.
- the cells exhibiting the desired response, or intensity may be selectively released into a cell sorter to be further studied or otherwise selectively processed.
- Such an integrated system would allow researchers to also look at the cell's time response.
- FIGS. 1A, 1B, 1 C, and 1 D show the mechanism by which one embodiment of the present invention uses to capture, hold and release a single cell.
- FIGS. 2A, 2B, and 2 C show a process by which another embodiment of the present invention uses to capture, hold and release a single cell.
- FIGS. 3A and 3B show a top-down view of the cell sorting apparatus of FIG. 2.
- FIG. 4 shows an exploded view of the cell sorting apparatus of FIG. 2.
- FIG. 5 shows an exploded view of yet another embodiment of the present invention in which a cell sorting apparatus is integrated into a fluorescence-detecting system.
- FIG. 6 is the thermodynamic pressure-volume diagram for water.
- FIG. 7A shows a top view of a resistor of the present invention.
- FIG. 7B shows a cross-section of the resistor of FIG. 7A.
- FIG. 8 shows thermal resistances as seen by a heater of the present invention.
- FIGS. 9A and 9B show flow lines for flow over rectangular cavities of different aspect ratios.
- FIG. 10 shows a schematic of forces on a particle in a well.
- FIG. 11A shows a top view of a heater of the present invention.
- FIG. 11B shows a cross-section of the heater of FIG. 11A.
- FIG. 12A shows a side view of a cell well of the present invention.
- FIG. 12B shows a top-down view of the cell well of FIG. 12A.
- FIGS. 13A, 13B, and 13 C shows a top-down view of a silicon processing mask set for use in the present invention.
- FIG. 14 shows a top-down view of a glass processing mask.
- FIG. 15 shows a diagram of a flow system for testing devices of the present invention.
- FIG. 16A shows a top-down view of a flow chamber of the present invention.
- FIG. 16B shows a side view of the flow chamber of FIG. 16A.
- FIG. 17 is a graph of pressure drop vs. flow rate for the flow chamber of FIGS. 16A and 16B.
- FIG. 18A shows a top-down view of the chamber base of flow chamber of FIG. 16A and 16B.
- FIG. 18B shows a side view of the chamber base of FIG. 18A.
- FIG. 18C shows a top-down view of the chamber lid of flow chamber of FIG. 16A and 16B.
- FIG. 18D shows a side view of the chamber lid of FIG. 18C.
- FIGS. 19 A- 19 C show a process of fabricating a glass slide of the present invention.
- FIGS. 20 A- 20 H show a process of fabricating a silicon wafer of the present invention.
- FIGS. 21 A- 21 D show a process of assembling the silicon wafer of FIGS. 20 A- 20 H onto the glass slide of FIGS. 19 A- 19 C.
- FIG. 22 is a graph of temperature v. resistance for platinum resistors of the present invention.
- FIG. 23 shows a configuration for a resistor testing apparatus used in the present invention.
- FIG. 24 is a graph of current v. voltage for the onset of boiling in platinum line resistors of the present invention.
- FIG. 25 is a graph of current v. temperature for the platinum line resistors of FIG. 24.
- FIG. 26 is a graph of temperature v. resistance for a set of annealed platinum line resistors of the present invention.
- FIG. 27 is a graph of temperature v. resistance for a set of annealed platinum line resistors which were heated on a hot plate.
- FIG. 28 is a graph of current v. voltage for a set of annealed platinum line resistors of the present invention.
- FIG. 29 is a graph of current v. temperature for the resistors of FIG. 28.
- FIG. 30 is a graph of current v. voltage for the resistors of FIG. 28 under repeated boiling tests.
- FIGS. 1 A- 1 D illustrate an exemplary system of the present invention.
- a cell site 10 shown in cross-section, contains a well 12 sized and shaped to hold a single cell 18 .
- a narrow channel 14 Connected to the bottom of the well 12 is a narrow channel 14 that opens into a chamber 16 situated below the well.
- the well 12 and narrow channel 14 are etched out of a silicon wafer.
- the silicon wafer is attached to a glass slide on which there is a platinum heater 20 , and the alignment is such that the heater 20 is sealed inside the chamber 16 , which is filled with a fluid such as water.
- the well 12 functions as a capture and hold mechanism. In operation, fluid containing cells is flown over the top of the apparatus, and then the flow is stopped. As shown in FIG. 1A, the cells then settle and gravitational forces will allow one cell 18 to fall into and become trapped within the well 12 . At this point the flow is started again, and the cell in the well is trapped while the cells not in wells are flushed away by convection.
- FIG. 1B shows how the well 12 is dimensioned and configured to hold only one cell 18 within the well 12 at a time. In addition, the well 12 is configured such that the cell 18 will not be swept out of the well due to laminar or fluid flow above.
- Experiments may be performed on the trapped cells, such as by adding a reagant.
- the cells exhibiting the desired characteristics may be selectively released from the wells.
- the operator can apply a voltage to the heating element 20 within the chamber 16 .
- the heating element 20 is then heated to a temperature above the superlimit of the fluid contained within the chamber 16 to initiate vapor bubble nucleation at the surface of the heating element 20 , as seen in FIG. 1C.
- a microbubble 22 is formed inside the chamber, creating a volume displacement.
- the operator can control the size of the microbubble 22 .
- the volume expansion in the chamber will displace a jet of fluid within the chamber 16 out of the narrow channel 14 , ejecting the cell 18 out of the well 12 .
- the released cell 18 can be swept into the fluid flow outside the well 12 , to be later collected or discarded.
- the cell site 30 includes electric field traps.
- FIGS. 2 A- 2 C show, in cross-section, two cell sites on a substrate such as a microfabricated chip 36 .
- Each site includes a plurality of electrodes 32 .
- each cell site 30 contains four electrodes, positioned in a trapezoidal configuration, as seen in FIGS. 3A and 3B.
- the cell site 30 is configured and positioned such that only one cell can be held within the site.
- the electrodes 32 create a non-uniform electric field trap within which a single cell 34 can be held and subsequently released.
- FIG. 4 illustrates how the location and polarity of the electrodes 32 can create an electric field trap for capturing the cell 34 .
- cells in fluid medium flow over the cell sites 30 , as shown in FIG. 2A.
- a potential energy well can be created within each cell site 30 .
- the potential energy well is of sufficient strength to capture a single cell 34 traveling along the fluid flow and to hold the cell 34 within the center of the trap, as seen in FIG. 2B.
- FIG. 2C shows how this in turn removes the potential energy well, releasing the cell 34 back into the fluid flow. The cell 34 can then be collected or discarded.
- the electrodes forming the electric field trap are preferably thin-film poles formed of gold. This creates a three-dimensional electric field trap that is effective in holding a cell against the laminar flow of the fluid surrounding the electrodes.
- the cell sorting apparatus can contain anywhere from a single cell site to an infinite number of cell sites, for sorting mass quantities of cells.
- the embodiments herein are described as holding cells, it is understood that what is meant by cells includes biological cells, cellular fragments, particles, biological molecules, ions, and other biological entities.
- the cell sorting apparatus of the present invention allows the operator to know the location of each cell in the array of cell sites, the operator is able to manipulate the cells and arbitrarily sort the cells based on their characteristic under time-responsive assays.
- One such method contemplates using scanning techniques to observe dynamic responses from cells.
- an integrated cellular analysis system 100 is proposed in which cells are tested using light-emitting assays to determine the cell's response to stimuli over time.
- the integrated system can be a microfabrication-based dynamic array cytometer ( ⁇ DAC).
- ⁇ DAC microfabrication-based dynamic array cytometer
- the tested cells are placed on a cell array chip 110 similar to the cell sorting apparatus above, to be held in place within the plurality of cell sites, such as those described above.
- the response of the cells can be measured, with the intensity of the fluorescence reflecting the intensity of the cellular response.
- the cells exhibiting the desired response, or intensity may be selectively released, to be collected or later discarded.
- Such an integrated system would allow researchers to look at the cell's time response.
- any light-emitting assay in which the cell's response may vary in time is suited for study using this proposed system. It is ideally suited for finding phenotype inhomogeneities in a nominally homogeneous cell population.
- Such a system could be used to investigate time-based cellular responses for which practical assays do not currently exist. Instead of looking at the presence/absence or intensity of a cell's response to stimulus, the researcher can look at its time response. Furthermore, the researcher can gain information about a statistically significant number of cells without the potential of masking important differences as might occur in a bulk experiment. Specific applications may include the study of molecular interactions such as receptor-ligand binding or protein-protein interactions. Signal transduction pathways, such as those involving intracellular calcium, can also be investigated.
- An advantage of the proposed integrated system is that the full time-response of all the cells can be accumulated and then sorting can be performed. This is contrasted with flow cytometry, where each cell is only analyzed at one time-point and sorting must happen concurrently with acquisition. Geneticists can look at gene expression, such as with immediate-early genes, either in response to environmental stimuli or for cell-cycle analysis. Another large application area is drug discovery using reporter-gene based assays. The integrated system can also be used to investigate fundamental biological issues dealing with the kinetics of drug interactions with cells, sorting and analyzing cells that display interesting pharmacodynamic responses. Another application is looking at heterogeneity in gene expression to investigate stochastic processes in cell regulation. Finally, once temporal responses to certain stimuli are determined, the integrated system can be used in a clinical setting to diagnose disease and monitor treatment by looking for abnormal time responses in patients' cells.
- One objective of the present invention is to provide a cell analysis and sorting apparatus which uses hydraulic forces to capture individual cells into addressable locations, and can utilize microbubble actuation to release these individual cells from their locations.
- this apparatus it was necessary to model and understand many physical phenomena, not the least important of which includes the theory behind bubble nucleation on micro-heaters. Further, it was necessary to design a device with the proper dimensions so that single particles, or cells, could be held in wells against a flow. Biological cells were not used in these experiments, as polystyrene microspheres of the same dimensions were thought to be more robust for testing purposes. The fabrication process had to be designed in order to build chips with the desired attributes, and various problems which arose needed to be resolved. Finally, it was necessary to understand the heating of the resistors so that sufficiently high temperatures could be reached.
- bubbles are nucleated by high-energy molecular groups.
- pure liquids have local fluctuations in density, or vapor clusters. These are groups of highly energized molecules which have energies significantly higher than the average energy of molecules in the liquid. These molecules are called activated molecules and their excess energy is called the energy of activation.
- the nucleation process occurs by a stepwise collision process that is reversible, whereby molecules may increase or decrease their energy. When a cluster of activated molecules reaches a critical size, then bubble nucleation can occur.
- FIG. 6 is the thermodynamic pressure-volume diagram for water, which shows a region of stable liquid to the far left, stable vapor to the far right, metastable regions, and an unstable region in the center of the dashed curve.
- the dashed line is called the spinodal, and to the left of the critical point represents the upper limit to the existence of a superheated liquid.
- Equation (1-1) holds true, and within the spinodal, Equation (1-2) applies.
- ⁇ is the specific volume
- R is the gas constant
- a and b are constants.
- n 0 for the van der Waals equation
- n 1 for the Berthelot equation
- n 0.5 for the modified Berthelot equation.
- a and b were computed using Equation (1-3), given the fact that at the critical point, Equations (1-4) and (1-5) are true.
- a kinetic limit of superheat may also be computed using the kinetic theory of the activated molecular clusters.
- the kinetic limit of superheat for water is about 300° C.
- T w is the surface temperature
- T sat is the saturation temperature (100° C. for water)
- ⁇ is the surface tension
- h fg is the latent heat of vaporization
- p v is the vapor density
- r c is the cavity radius.
- the surface temperature necessary to nucleate bubbles in water with a surface that has a 1 ⁇ m cavity radius is about 133° C.
- the temperature to nucleate a bubble is about 432° C., well above the highest thermodynamic water superheat limit of 322° C.
- FIG. 7A and 7B The schematic and boundary conditions for this resistor model are shown in FIG. 7A and 7B.
- the water above the heater was 450 ⁇ m thick, corresponding to the height of the silicon chamber containing the water. It was assumed that the ambient temperature was maintained at the top of the water in the well since above this there was silicon with water at the ambient temperature flowing over the top of it. The bottom of the glass slide was also assumed to be at the ambient temperature since it was contacting a surface at the ambient temperature.
- the resistor was about 10,000 times thinner than the glass slide and had ohmic heating, or power generation equal to I 2 R for the entire volume of the resistor.
- L is the characteristic length for conduction and ⁇ is the thermal diffusivity of the material.
- the characteristic time for conduction through 1 mm of glass was about 2.3 seconds.
- the characteristic time for conduction through 450 ⁇ m of water was 1.38 seconds. Accordingly, for this system the time to reach steady state would be about four times greater than the highest characteristic time, about 9 seconds.
- homogeneous bubble nucleation was likely to occur, which is a molecular process and thus may be assumed to be approximately instantaneous. The time for a bubble to nucleate was therefore far shorter than the 9 seconds necessary for the system to reach steady state, so steady state conditions are unlikely to be achieved before the bubble nucleates.
- the Biot number measures the ratio of internal conduction resistance to external convection resistance. Since the Biot number was much less than unity, the lumped body approximation was used and an assumption was made that the entire resistor was at a uniform temperature.
- FIG. 8 shows the thermal resistances between the resistor and the ambient temperature.
- steady state conditions were used in determining thermal resistances.
- the thermal resistance due to convection through the water was computed. For this case it was assumed there was natural convection since the water above the heater was stagnant, and boiling was not occurring.
- the thermal resistance due to convection was calculated below.
- w is the resistor width (3 ⁇ m) and L is the resistor length (1000 ⁇ m).
- L g is the length of glass through which heat conducts (1 mm)
- k g is the conductivity of glass (0.81 W/mK)
- L is the length of the resistor (1000 ⁇ m)
- w is the width of the resistor (3 ⁇ m)
- L w is the length of water through which heat conducts (450 ⁇ m)
- k w is the conductivity of water (0.67 W/mK).
- K is the thermal conductivity (0.61 W/mK for water and 0.88 W/mK for glass)
- q is the heat flux
- subscript ‘1’ denotes water
- ‘2’ denotes glass
- ⁇ is the thermal diffusivity (1.47 ⁇ 10 ⁇ 7 m/s 2 for water and 4.4 ⁇ 10 ⁇ 7 m/s 2 for glass) and T o is the initial temperature of the body.
- the solution was also used to check the semi-infinite body assumption. For times equal to or less than 1 ms, and a reasonable heat flux such as 2.5 ⁇ 10 7 W/m 2 , the heat penetration depths into the glass and water were less than 100 ⁇ m. The total thickness of the water was 450 ⁇ m and of the glass was 1 mm, so the semi-infinite body assumption held true. The one-dimensional model was sufficient for determining the temperature of the resistor at small times.
- the heat flux necessary to heat the resistor to 305° C. in 1 ms was computed from (1-19) to be 1.32 ⁇ 10 7 W/m 2 .
- the necessary power was about 120 mW.
- micromachined wells must be of the proper dimensions to ensure that particles which settle into them remain held in the wells once a flow above them is initiated.
- the theory of slow viscous flow over cavities has been well characterized and the streamlines for various geometries have been calculated and experimentally verified.
- FIG. 9 shows the flow pattern for laminar flow over a rectangular cavity for two different width to height aspect ratios. From these flow patterns it was seen that there was a separating flow line which penetrates slightly into the cavity. Below this line there were one or two vortices, depending on the aspect ratio of the cavities. A particle below the separating flow line would not be swept out of the cavity by a slow flow in the laminar range, though the vortex may agitate the particle.
- the force of gravity acting on the particle was dependent on the difference in density between the particle and the water, ⁇ .
- the density of water is approximately 1000 kg/m 3
- the density of the polystyrene beads used in the experiments was given by the manufacturer as 1060 kg/m 3 .
- ⁇ is the viscosity of water (1 ⁇ 10 ⁇ 3 kg/ms) and u(y) is the velocity profile as a function of y, the distance from the wall.
- a is the sphere radius (5 ⁇ m for a polystyrene bead)
- ⁇ s is the density of the bead (about 1060 kg/m 3 )
- ⁇ is the density of water (1000 kg/m 3 )
- ⁇ is the viscosity of water.
- Pe aU o D o ( 1 ⁇ - ⁇ 34 )
- D o is the Brownian diffusivity
- k is the Boltzmann's constant (1.38 ⁇ 10 ⁇ 16 erg/cm).
- ⁇ is the particle volume fraction (about 0.01 for this case). Accordingly, the time necessary for all the particles to settle to the bottom of the flow chamber was calculated using the hindered velocity and the chamber height, the maximum distance to be traveled.
- Q is the volume flow rate
- ⁇ P is the pressure drop
- c is the aperture radius ( ⁇ 2.5 or 4 ⁇ m)
- ⁇ is the water viscosity
- the volume flow rate out of the chamber was estimated in a different way. Because water is incompressible, it was assumed in the model that the bubble formation as a volume injection into the chamber resulted in the same volume being ejected from the chamber over the characteristic bubble formation time. For instance, if it took 1 ms to form a 10 ⁇ m diameter bubble, then the resulting volume flow rate out of the chamber was calculated as follows.
- ⁇ is the difference in densities between the water and the polystyrene beads (60 kg/m 3 ). It was seen that as the particle radius increased, the effect of gravity increased. For typical cells, the radius ranges from 5 ⁇ m (red blood cells) to 20 ⁇ m (most other cells) to 100 ⁇ m (embryos and eggs). This device will most likely be used for cells on the order of 5-10 ⁇ m in radius so the above calculation was representative of the expected applications.
- resistive heaters were used.
- the heaters were made of thin-film platinum on standard glass slides.
- the design constraint for this step was the need to keep the current density below the electromigration limit of platinum, while retaining an adequate degree of ohmic heating.
- the electromigration limit is the maximum current density which platinum can endure before the atoms begin to migrate leaving the resistor inoperable.
- R is the resistance ( ⁇ )
- L is the length of the resistor (m)
- t is the film thickness (m)
- w is the width of the resistor (m)
- ⁇ is resistivity of platinum ( ⁇ m).
- I is the current (A) and J is the current density.
- the resistances were chosen to range from 167 ⁇ -1000 ⁇ , yielding maximum powers before electromigration of 70-1166 mW. These powers were chosen to be up to an order of magnitude greater than necessary to avoid reaching the electromigration limit in the operation of the resistors.
- FIG. 11A is a top view of a heater configuration, while FIG. 11B shows a cross-sectional view of the heater and its dimensions. A table of resistor dimensions, maximum currents, and maximum power outputs is also shown in Table 2.
- the lines connecting the contact pads to the heaters were designed to have a far lower resistance than the heaters. This was done to ensure that the lines did not heat up, and that they remained approximately at the ambient temperature.
- the connector line widths were chosen to be 1500 ⁇ m with lengths of 12 mm. The total resistance of each line was about 7.7 ⁇ .
- Photomasks for use in the device fabrication were created using standard mask layout software.
- the mask set for the silicon processing are shown in FIGS. 13 A- 13 C and the glass mask set is shown in FIG. 14A.
- FIG. 13A Three masks were designed for the silicon portion of the device processing.
- One mask was created for the cell wells (FIG. 13A), one for the narrow channels within the wells (FIG. 13B), and one for the large wells (FIG. 13C) etched from the backside of the wafer to enclose the heaters.
- Two masks were made for the fabrication of the platinum heaters on the glass slides.
- One mask (FIG. 14A) was designed to pattern the metal.
- a fluidic system as illustrated in FIG. 15 was designed and assembled.
- a syringe pump 150 was used as the flow source for the bulk fluid, and flow rates ranging from 1 to 100 ⁇ L/min were specified. Beads, cells, or cell stimuli were injected through the sample injection valve 152 .
- a pressure sensor 154 was located before the flow chamber 156 so that the pressure drop across the chamber could be monitored. All fluid was outlet into a waste beaker 158 which could be reused if desired.
- FIGS. 16A and 16B A schematic of the flow chamber 156 is shown in FIGS. 16A and 16B.
- the flow chamber was machined from plexiglass so that it was clear and a microscope was used to observe cell behavior from above the chamber.
- HPLC high-performance liquid chromatography
- fittings were used with tube dimensions of ⁇ fraction (1/16) ⁇ inch outer diameter and 0.020 inch inner diameter.
- the gasket between the slide and the top cover were made from PDMS (poly dimethyl siloxane), a flexible polymer.
- a seal was formed by screwing the top plate down onto the bottom plate.
- Aluminum molds were machined in order to create PDMS gaskets of the proper dimensions. Gaskets were compressed until a hard stop was reached. The stop was provided by the spacers, made of metal shim stock, in order to accurately specify the channel height.
- the aspect ratio of the channel's width to height was greater than 10, allowing the assumption of a parabolic velocity profile-plane Poiseuille flow.
- the height of the flow chamber was 790 ⁇ m (determined by thickness of metal spacer). Flow rates ranged from 1 to 100 ⁇ L/min and corresponded to Reynolds numbers of 0.001-0.1. In this creeping flow regime, the entrance length for fully developed flow was calculated to be negligible. These calculations are shown below.
- X e ⁇ h ⁇ ⁇ Re max 30 2.6 ⁇ ⁇ ⁇ ⁇ ⁇ m ( 1 ⁇ - ⁇ 56 )
- V ⁇ min is the minimum average velocity
- Q min is the minimum volume flow rate (1 ⁇ L/min)
- V min is the maximum average velocity
- Q max is the maximum volume flow rate (100 ⁇ L/min)
- Re is the Reynolds number
- v is the kinematic viscosity of water (1 ⁇ 10 ⁇ 6 m 2 /s)
- X e is the entrance length for fully-developed flow.
- ⁇ is the viscosity of water (1 ⁇ 10 ⁇ 3 kg/ms)
- r is the tube radius (0.254 mm)
- ⁇ x is the tube length (m).
- the pressure drop through the chamber was calculated to be negligible in comparison.
- the flow chamber schematic with dimensions is shown in FIGS. 18 A- 18 D.
- FIGS. 19 A- 19 C The platinum heaters were fabricated on standard 1 ⁇ 3 in glass slides using a lift-off process. The process flow is shown in FIGS. 19 A- 19 C.
- photoresist was spun onto the glass slide, exposed using mask 4 , and developed.
- 100 ⁇ of titanium and 1000 ⁇ of platinum were evaporated onto the slide, as seen in FIG. 19B.
- the titanium served as an adhesion layer between the glass and the platinum.
- the slide was submerged in acetone to dissolve the photoresist and lift away the metal which was deposited on top of the photoresist, as depicted in FIG. 19C. Only the platinum resistors were left on the glass slide.
- Some slides were then annealed in a tube furnace at 600° C. for 1 hour. While not used in this example, it is contemplated that photoresist may be applied manually to the slide to attach the silicon chip to the slide.
- FIGS. 20 A- 20 H Double Side Polished (DSP) four inch diameter silicon wafers were used.
- DSP Double Side Polished
- the first step shown as FIG. 20A 1 ⁇ m of thermal oxide was grown on the wafer.
- the oxide was patterned using mask 1 , FIG. 20B.
- Resist was spun on top of the oxide and patterned using mask 2 .
- the resulting configuration was called a nested mask, shown as FIG. 20C.
- the photoresist mask was used to etch the narrow 5 ⁇ m trenches, then the oxide mask was used to etch the cell wells, as shown in FIGS. 20D and 20E.
- the wafer was turned over and photoresist was deposited and patterned on the back side using mask 3 (FIG. 20F).
- a deep silicon etch was then performed to etch through the wafer and intersect the narrow trenches etched previously (FIG. 20G) to obtain a finished wafer (FIG. 20H).
- a complete device consisted of a silicon chip attached to a glass slide by photoresist, as shown in FIGS. 21C and D.
- the resist provided a water-tight seal so that volume expansion in the bubble wells resulted in a burst of fluid being pushed through the narrow channel and ejecting a cell.
- alignment marks were fabricated on the glass slide and matching holes were etched in the silicon chip.
- the alignment tolerances were sufficiently large (about 2 mm) that the chip could be aligned to the slide by hand using just the naked eye, while still positioning the bubble wells over the platinum heaters.
- Photoresist was painted onto the silicon chip around the bubble wells using a toothpick. Drops of water were deposited into each well using a pipette, then the glass slide was visually aligned from above and stuck down onto the chip. The drops of water served to fill the bubble wells and get pushed through the narrow channel to fill it with water. The device was now ready to be tested in the flow chamber.
- the film thickness was first measured using a profilometer.
- the platinum thickness measurements ranged from about 800-900 ⁇ , so the average value of 850 ⁇ was used in the subsequent calculations.
- the resistance along metal lines wide enough not to be strongly affected by variation of a few microns was measured using a multimeter.
- the lines used for this measurement were measured in an optical microscope to be about 1510 ⁇ m wide.
- the length of the lines was about 8 mm. Knowing the width, thickness, and length of these lines, as well as the measured resistance, the resistivity of the thin film platinum at room temperature was determined.
- the measured resistance was 15 ⁇ , and the computed resistivity was calculated below.
- t is the film thickness (850 ⁇ )
- w is the line width (1513 ⁇ m)
- R is the measured resistance (15 ⁇ )
- L is the length of the line (8 mm).
- This resistivity was more than twice the value for bulk platinum (1 ⁇ 10 ⁇ 7 ⁇ m), but was a reasonable value for thin film platinum. This is because bulk platinum is a crystalline material, whereas thin film platinum is polycrystalline and the grain boundaries significantly increase resistance.
- the main objective for the resistors was that they be able to reach high enough temperatures to boil water.
- the resistors were tested on a probe station using an HP4145b to vary the voltage and measure the resulting current through the resistor.
- a PDMS gasket was place on top of the slide and filled with water. The gasket contained the water and kept it from touching the electrical contacts and probes.
- FIG. 23 is a schematic of this configuration.
- new resistor slides were annealed at 600° C. for 1 hour as the last step in their process. This temperature is higher than operating temperatures are likely to reach, but not so high that major agglomeration will result. Once the anneal was complete, the new resistors were characterized as described above for the first generation resistors.
- the resistivity of the platinum at room temperature was found to be 2.056 ⁇ 10 ⁇ 7 ⁇ m, less than the unannealed resistors that were 2.41 ⁇ 10 ⁇ 7 ⁇ m.
- the resistances were measured using a multimeter, and the line widths were computed as before, as shown in Table 6. TABLE 6 Measured resistances and computed line widths of second generation resistors.
- Computed Design Resistor # L (um) R (Ohms) Line Width (um) (um) Slide 3 1 3000 553 13.12 6 2 2500 481 12.57 6 3 500 146 8.28 3 4 1000 281 8.61 3 5 1000 205 11.80 6 6 2000 409 11.83 6 7 1500 310 11.70 6 8 1000 186 13.00 6
- bubbles were nucleated in radii ranging from 0.3-1.2 ⁇ m. As discussed previously, these cavities were most likely formed during the 600° C. anneal, during which the grooves at the grain boundaries widened creating cavities.
- the second generation resistors were also tested for the repeatability of their boiling temperatures. I-V curves were measured as in the previous section, and then remeasured for the same conditions several times. Between measurements, time was given for the vapor bubbles to dissipate so that the characteristic jump in the I-V curve at boiling could be observed with each measurement. The boiling point was found to be very repeatable, and an example of the results is shown in FIG. 30. This result demonstrated the potential of a control system based on a jump in the I-V curve at the onset of boiling, since the boiling point remained fixed.
- the cell chip was attached to the glass resistor slide as described earlier, and then tested in two ways. First tests were done with stagnant fluid on the device. Then the device was put into the flow chamber for testing. The results of these tests are described below.
Abstract
A cell analysis and sorting apparatus is capable of monitoring over time the behavior of each cell in a large population of cells. The cell analysis and sorting apparatus contains individually addressable cell locations. Each location is capable of capturing and holding a single cell, and selectively releasing that cell from that particular location. In one aspect of the invention, the cells are captured and held in wells, and released using vapor bubbles as a means of cell actuation. In another aspect of the invention, the cells are captured, held and released using electric field traps.
Description
- This application is a continuation of U.S. patent application Ser. No. 09/710,032, filed on Nov. 10, 2000, now U.S. Pat. No. 6,692,952, which claims priority to U.S. Provisional Application No. 60/164,643, filed on Nov. 10, 1999.
- This invention relates to cell analysis and sorting devices and methods for manipulating cells using these devices. More particularly, the invention relates to a cell analysis and sorting apparatus that can capture and hold single cells at known locations and then selectively release certain of these cells. A method of manipulating the cells using the cell analysis and sorting apparatus is also provided.
- Many recent technological advances have enhanced the study of cellular biology and biomechanical engineering, most notably by improving methods and devices for carrying out cellular analysis. For example, in the past decade an explosion in the number of optical probes available for cell analysis has enabled an increase in the amount of information gleaned from microscopic and flow cytometric assays. Microscopic assays allow the researcher to monitor the time-response of a limited number of cells using optical probes. Flow cytometry, on the other hand, uses optical probes for assays on statistically significant quantities of cells for sorting into subpopulations.
- However, these mechanisms alone are insufficient for time-dependent analysis. Microscopic assays can only track a few cells over time, and do not allow the user to track the location of individual cells. With flow cytometry, the user can only observe each cell once, and can only easily sort a cell population into three subpopulations. Flow cytometry techniques fail to provide for analysis of the same cell multiple times, or for arbitrary sorting of subpopulations. These kinds of bulk assay techniques produce mean statistics, but cannot provide the researcher with distribution statistics.
- Advances in microsystems technology have also influenced many applications in the fields of cell biology and biomedical engineering. Scaling down to the micron level allows the use of smaller sample sizes than those used in conventional techniques. Additionally, the smaller size and ability to make large arrays of devices enables multiple processes to be run in parallel.
- Integrated circuits have been fabricated on silicon chips since the 1950s, and as processing techniques improve, the size of transistors continues to shrink. The ability to produce large numbers of complex devices on a single chip sparked interest in fabricating mechanical structures on silicon as well. The range of applications for micro electromechanical systems (MEMS) is enormous. Accelerometers, pressure sensors, and actuators are just a few of the many MEMS devices currently produced. Another application of MEMS is in biology and medicine. Micromachined devices have been made for use in drug-delivery, DNA analysis, diagnostics, and detection of cell properties.
- Manipulation of cells is another application of MEMS. For example, in the early 1990's, Sato et al. described in his paper, which is hereby incorporated by reference, Individual and Mass Operation of Biological Cells using Micromechanical Silicon Devices, Sensors and Actuators, 1990, A21-A23:948-953, the use of pressure differentials to hold cells. Sato et al. microfabricated hydraulic capture chambers that were used to capture plant cells for use in cell fusion experiments. Pressure differentials were applied so that single cells were sucked down to plug an array of holes. Cells could not be individually released from the array, however, because the pressure differential was applied over the whole array, not to individual holes.
- Bousse et al. in his paper, which is hereby incorporated by reference, Micromachined Multichannel Systems for the Measurement of Cellular Metabolism, Sensors and Actuators B, 1994, 20:145-150, described arrays of wells etched into silicon to passively capture cells by gravitational settling. Multiple cells were allowed to settle into each of an array of wells where they were held against flow due to the hydrodynamics resulting from the geometry of the wells. Changes in the pH of the medium surrounding the cells were monitored by sensors in the bottom of the wells, but the wells lacked a cell-release mechanism, and multiple cells were trapped in each well. Another known method of cell capture is dielectrophoresis (DEP). DEP refers to the action of neutral particles in non-uniform electric fields. Neutral polarizable particles experience a force in non-uniform electric fields which propels them toward the electric field maxima or minima, depending on whether the particle is more or less polarizable than the medium it is in. By arranging the electrodes properly, an electric field may be produced to stably trap dielectric particles.
- Microfabrication has been utilized to make electrode arrays for cell manipulation since the late 1980s. Researchers have successfully trapped many different cell types, including mammalian cells, yeast cells, plant cells, and polymeric particles. Much work involves manipulating cells by exploiting differences in the dielectric properties of varying cell types to evoke separations, such as separation of viable from non-viable yeast, and enrichment of CD34+ stem cells from bone marrow and peripheral blood stem cells. More relevant work on trapping cells in various two- and three-dimensional microfabricated electrode geometries has been shown by several groups. However, trapping arrays of cells with the intention of releasing selected subpopulations of cells has not yet been widely explored. Additionally, DEP can potentially induce large temperature changes, causing not only convection effects but also profoundly affecting cell physiology.
- These studies demonstrate that it is possible to trap individual and small numbers of cells in an array on a chip, but without the ability to subsequently manipulate and selectively release individual cells. This inability to select or sort based on a biochemical measurement poses a limitation to the kinds of scientific inquiring that may be of interest.
- The currently available mechanisms for carrying out cell analysis and sorting are thus limited in their applications. There is thus a need for an improved method and apparatus for sorting and releasing large quantities of cells that can easily and efficiently be used. In addition, there is a need for an analysis and sorting device that allows the user to look at each cell multiple times, and to track many cells over time. Finally, there is a need for a cell sorter that lets the user know the cell locations, and to be able to hold and selectively release the cells so that the user can arbitrarily sort based on any aspect of the cells' characteristic during time-responsive assays.
- The present invention provides a cell sorting apparatus that is capable of monitoring over time the behavior of each cell in a large population of cells. The cell analysis and sorting apparatus contains individually addressable cell locations. Each location is capable of capturing and holding a single cell, and selectively releasing that cell from that particular location. In one aspect of the invention, the cells are captured and held in wells, and released using vapor bubbles as a means of cell election. In another aspect of the invention, the cells are captured, held and released using electric field traps.
- According to one aspect of the present invention, the cell analysis and sorting apparatus has an array of geometric sites for capturing cells traveling along a fluid flow. The geometric sites are arranged in a defined pattern across a substrate such that individual sites are known and identifiable. Each geometric site is configured and dimensioned to hold a single cell. Additionally, each site contains a release mechanism to selectively release the single cell from that site. Because each site is able to hold only one cell, and each site has a unique address, the apparatus allows the user to know the location of any particular cell that has been captured. Further, each site is independently controllable so that the user is able to arbitrarily capture cells at select locations, and to release cells at various locations across the array.
- In one embodiment of the present invention, the geometric sites are configured as wells. As a fluid of cells is flown across the array of specifically sized wells, cells will fall into the wells and become trapped. Each well is sized and shaped to capture only a single cell, and is configured such that the cell will not escape into the laminar flow of the fluid above the well. The single cell can be held inside the well by gravitational forces. Each well can further be attached via a narrow channel to a chamber located below the well. Within the chamber is a heating element that is able to induce bubble nucleation, the mechanism for releasing the cell from the site. The bubble creates volume expansion inside the chamber which, when filled with fluid, will displace a jet of fluid out of the narrow channel and eject the cell out of the well. Fluid flow above the well will sweep the ejected cell away to be either collected or discarded.
- In another embodiment of the present invention, the geometric sites are formed from a three-dimensional electric field trap. Each trap comprises four electrodes arranged in a trapezoidal configuration, where each electrode represents a corner of the trapezoid. The electric fields of the electrodes create a potential energy well for capturing a single cell within the center of the trap. By removing the potential energy well of the trap, the cell is ejected out of the site and into the fluid flow around the trap. Ejected cells can then be washed out and collected or discarded.
- In yet another embodiment of the present invention, an integrated system is proposed. The system can be a microfabrication-based dynamic array cytometer (μDAC) having as one of its components the cell analysis and sorting apparatus previously described. To analyze a population of cells, the cells can be placed on a cell array chip containing a plurality of cell sites. The cells are held in place within the plurality of cell sites in a manner similar to that described above and analyzed, for example, by photometric assay. Using an optical system to detect fluorescence, the response of the cells can be measured, with the intensity of the fluorescence reflecting the intensity of the cellular response. Once the experiment is complete, the cells exhibiting the desired response, or intensity, may be selectively released into a cell sorter to be further studied or otherwise selectively processed. Such an integrated system would allow researchers to also look at the cell's time response.
- Further features and advantages of the present invention as well as the structure and operation of various embodiments of the present invention are described in detail below with reference to the accompanying drawings.
- This invention is pointed out with particularity in the appended claims. The above and further advantages of this invention may be better understood by referring to the following description when taken in conjunction with the accompanying drawings, in which:
- FIGS. 1A, 1B, 1C, and 1D show the mechanism by which one embodiment of the present invention uses to capture, hold and release a single cell.
- FIGS. 2A, 2B, and 2C show a process by which another embodiment of the present invention uses to capture, hold and release a single cell.
- FIGS. 3A and 3B show a top-down view of the cell sorting apparatus of FIG. 2.
- FIG. 4 shows an exploded view of the cell sorting apparatus of FIG. 2.
- FIG. 5 shows an exploded view of yet another embodiment of the present invention in which a cell sorting apparatus is integrated into a fluorescence-detecting system.
- FIG. 6 is the thermodynamic pressure-volume diagram for water.
- FIG. 7A shows a top view of a resistor of the present invention.
- FIG. 7B shows a cross-section of the resistor of FIG. 7A.
- FIG. 8 shows thermal resistances as seen by a heater of the present invention.
- FIGS. 9A and 9B show flow lines for flow over rectangular cavities of different aspect ratios.
- FIG. 10 shows a schematic of forces on a particle in a well.
- FIG. 11A shows a top view of a heater of the present invention.
- FIG. 11B shows a cross-section of the heater of FIG. 11A.
- FIG. 12A shows a side view of a cell well of the present invention.
- FIG. 12B shows a top-down view of the cell well of FIG. 12A.
- FIGS. 13A, 13B, and 13C shows a top-down view of a silicon processing mask set for use in the present invention.
- FIG. 14 shows a top-down view of a glass processing mask.
- FIG. 15 shows a diagram of a flow system for testing devices of the present invention.
- FIG. 16A shows a top-down view of a flow chamber of the present invention.
- FIG. 16B shows a side view of the flow chamber of FIG. 16A.
- FIG. 17 is a graph of pressure drop vs. flow rate for the flow chamber of FIGS. 16A and 16B.
- FIG. 18A shows a top-down view of the chamber base of flow chamber of FIG. 16A and 16B.
- FIG. 18B shows a side view of the chamber base of FIG. 18A.
- FIG. 18C shows a top-down view of the chamber lid of flow chamber of FIG. 16A and 16B.
- FIG. 18D shows a side view of the chamber lid of FIG. 18C.
- FIGS. 19A-19C show a process of fabricating a glass slide of the present invention.
- FIGS. 20A-20H show a process of fabricating a silicon wafer of the present invention.
- FIGS. 21A-21D show a process of assembling the silicon wafer of FIGS. 20A-20H onto the glass slide of FIGS. 19A-19C.
- FIG. 22 is a graph of temperature v. resistance for platinum resistors of the present invention.
- FIG. 23 shows a configuration for a resistor testing apparatus used in the present invention.
- FIG. 24 is a graph of current v. voltage for the onset of boiling in platinum line resistors of the present invention.
- FIG. 25 is a graph of current v. temperature for the platinum line resistors of FIG. 24.
- FIG. 26 is a graph of temperature v. resistance for a set of annealed platinum line resistors of the present invention.
- FIG. 27 is a graph of temperature v. resistance for a set of annealed platinum line resistors which were heated on a hot plate.
- FIG. 28 is a graph of current v. voltage for a set of annealed platinum line resistors of the present invention.
- FIG. 29 is a graph of current v. temperature for the resistors of FIG. 28.
- FIG. 30 is a graph of current v. voltage for the resistors of FIG. 28 under repeated boiling tests.
- FIGS. 1A-1D illustrate an exemplary system of the present invention. A
cell site 10, shown in cross-section, contains a well 12 sized and shaped to hold asingle cell 18. Connected to the bottom of the well 12 is anarrow channel 14 that opens into achamber 16 situated below the well. In this particular example, the well 12 andnarrow channel 14 are etched out of a silicon wafer. The silicon wafer is attached to a glass slide on which there is aplatinum heater 20, and the alignment is such that theheater 20 is sealed inside thechamber 16, which is filled with a fluid such as water. - The well 12 functions as a capture and hold mechanism. In operation, fluid containing cells is flown over the top of the apparatus, and then the flow is stopped. As shown in FIG. 1A, the cells then settle and gravitational forces will allow one
cell 18 to fall into and become trapped within thewell 12. At this point the flow is started again, and the cell in the well is trapped while the cells not in wells are flushed away by convection. FIG. 1B shows how the well 12 is dimensioned and configured to hold only onecell 18 within the well 12 at a time. In addition, the well 12 is configured such that thecell 18 will not be swept out of the well due to laminar or fluid flow above. - Experiments may be performed on the trapped cells, such as by adding a reagant. When the experiments are concluded, the cells exhibiting the desired characteristics may be selectively released from the wells. In this example, when it is desired to release
cell 18 from the well 12, the operator can apply a voltage to theheating element 20 within thechamber 16. Theheating element 20 is then heated to a temperature above the superlimit of the fluid contained within thechamber 16 to initiate vapor bubble nucleation at the surface of theheating element 20, as seen in FIG. 1C. In FIG. 1D, amicrobubble 22 is formed inside the chamber, creating a volume displacement. By adjusting the voltage of theheating element 20, the operator can control the size of themicrobubble 22. When themicrobubble 22 is of sufficient size, the volume expansion in the chamber will displace a jet of fluid within thechamber 16 out of thenarrow channel 14, ejecting thecell 18 out of the well 12. The releasedcell 18 can be swept into the fluid flow outside the well 12, to be later collected or discarded. - In another exemplary system of the present invention, the
cell site 30 includes electric field traps. FIGS. 2A-2C show, in cross-section, two cell sites on a substrate such as amicrofabricated chip 36. Each site includes a plurality ofelectrodes 32. Preferably, eachcell site 30 contains four electrodes, positioned in a trapezoidal configuration, as seen in FIGS. 3A and 3B. Thecell site 30 is configured and positioned such that only one cell can be held within the site. Theelectrodes 32 create a non-uniform electric field trap within which asingle cell 34 can be held and subsequently released. FIG. 4 illustrates how the location and polarity of theelectrodes 32 can create an electric field trap for capturing thecell 34. - In use, cells in fluid medium flow over the
cell sites 30, as shown in FIG. 2A. By adjusting the electric field of eachelectrode 32, a potential energy well can be created within eachcell site 30. The potential energy well is of sufficient strength to capture asingle cell 34 traveling along the fluid flow and to hold thecell 34 within the center of the trap, as seen in FIG. 2B. When the operator selects to release acell 34, he can adjust the electric fields of theelectrodes 32 forming the trap. FIG. 2C shows how this in turn removes the potential energy well, releasing thecell 34 back into the fluid flow. Thecell 34 can then be collected or discarded. - The electrodes forming the electric field trap are preferably thin-film poles formed of gold. This creates a three-dimensional electric field trap that is effective in holding a cell against the laminar flow of the fluid surrounding the electrodes. Further, while only one or two cell sites are illustrated, it is understood that the drawings are merely exemplary of the kind of site that can be included in the cell sorting apparatus of the present invention. The cell sorting apparatus can contain anywhere from a single cell site to an infinite number of cell sites, for sorting mass quantities of cells. Moreover, while the embodiments herein are described as holding cells, it is understood that what is meant by cells includes biological cells, cellular fragments, particles, biological molecules, ions, and other biological entities.
- Because the cell sorting apparatus of the present invention allows the operator to know the location of each cell in the array of cell sites, the operator is able to manipulate the cells and arbitrarily sort the cells based on their characteristic under time-responsive assays. One such method contemplates using scanning techniques to observe dynamic responses from cells. As shown in FIG. 5, an integrated
cellular analysis system 100 is proposed in which cells are tested using light-emitting assays to determine the cell's response to stimuli over time. The integrated system can be a microfabrication-based dynamic array cytometer (μDAC). The tested cells are placed on a cell array chip 110 similar to the cell sorting apparatus above, to be held in place within the plurality of cell sites, such as those described above. Using anoptical system 120 to detect fluorescence, the response of the cells can be measured, with the intensity of the fluorescence reflecting the intensity of the cellular response. Once the experiment is complete, the cells exhibiting the desired response, or intensity, may be selectively released, to be collected or later discarded. Such an integrated system would allow researchers to look at the cell's time response. - Any light-emitting assay in which the cell's response may vary in time is suited for study using this proposed system. It is ideally suited for finding phenotype inhomogeneities in a nominally homogeneous cell population. Such a system could be used to investigate time-based cellular responses for which practical assays do not currently exist. Instead of looking at the presence/absence or intensity of a cell's response to stimulus, the researcher can look at its time response. Furthermore, the researcher can gain information about a statistically significant number of cells without the potential of masking important differences as might occur in a bulk experiment. Specific applications may include the study of molecular interactions such as receptor-ligand binding or protein-protein interactions. Signal transduction pathways, such as those involving intracellular calcium, can also be investigated.
- An advantage of the proposed integrated system is that the full time-response of all the cells can be accumulated and then sorting can be performed. This is contrasted with flow cytometry, where each cell is only analyzed at one time-point and sorting must happen concurrently with acquisition. Geneticists can look at gene expression, such as with immediate-early genes, either in response to environmental stimuli or for cell-cycle analysis. Another large application area is drug discovery using reporter-gene based assays. The integrated system can also be used to investigate fundamental biological issues dealing with the kinetics of drug interactions with cells, sorting and analyzing cells that display interesting pharmacodynamic responses. Another application is looking at heterogeneity in gene expression to investigate stochastic processes in cell regulation. Finally, once temporal responses to certain stimuli are determined, the integrated system can be used in a clinical setting to diagnose disease and monitor treatment by looking for abnormal time responses in patients' cells.
- One objective of the present invention is to provide a cell analysis and sorting apparatus which uses hydraulic forces to capture individual cells into addressable locations, and can utilize microbubble actuation to release these individual cells from their locations. In developing this apparatus, it was necessary to model and understand many physical phenomena, not the least important of which includes the theory behind bubble nucleation on micro-heaters. Further, it was necessary to design a device with the proper dimensions so that single particles, or cells, could be held in wells against a flow. Biological cells were not used in these experiments, as polystyrene microspheres of the same dimensions were thought to be more robust for testing purposes. The fabrication process had to be designed in order to build chips with the desired attributes, and various problems which arose needed to be resolved. Finally, it was necessary to understand the heating of the resistors so that sufficiently high temperatures could be reached.
- Under the theory of bubble nucleation, pool boiling takes place when a heater surface is submerged in a pool of liquid. As the heater surface temperature increases and exceeds the saturation temperature of the liquid by an adequate amount, vapor bubbles nucleate on the heater. The layer of fluid directly next to the heater is superheated, and bubbles grow rapidly in this region until they become sufficiently large and depart upwards by a buoyancy force. While rising the bubbles either collapse or continue growing depending on the temperature of the bulk fluid.
- There are two modes of bubble nucleation: homogeneous and heterogeneous. Homogeneous nucleation occurs in a pure liquid, whereas heterogeneous nucleation occurs on a heated surface.
- In a pure liquid containing no foreign objects, bubbles are nucleated by high-energy molecular groups. According to kinetic theory, pure liquids have local fluctuations in density, or vapor clusters. These are groups of highly energized molecules which have energies significantly higher than the average energy of molecules in the liquid. These molecules are called activated molecules and their excess energy is called the energy of activation. The nucleation process occurs by a stepwise collision process that is reversible, whereby molecules may increase or decrease their energy. When a cluster of activated molecules reaches a critical size, then bubble nucleation can occur.
- In order to determine at what temperature water will begin to boil in the homogeneous nucleation regime, it was useful to know the thermodynamic superheat limit of water. FIG. 6 is the thermodynamic pressure-volume diagram for water, which shows a region of stable liquid to the far left, stable vapor to the far right, metastable regions, and an unstable region in the center of the dashed curve. The dashed line is called the spinodal, and to the left of the critical point represents the upper limit to the existence of a superheated liquid. Along this line, Equation (1-1) holds true, and within the spinodal, Equation (1-2) applies.
-
- Where ν is the specific volume, R is the gas constant, and a and b are constants. n=0 for the van der Waals equation, n=1 for the Berthelot equation, and n=0.5 for the modified Berthelot equation. a and b were computed using Equation (1-3), given the fact that at the critical point, Equations (1-4) and (1-5) are true.
- Using the above equations, the thermodynamic superheat limit of water was computed. The results are shown below in Table 1.
Equation of State T/Tcr (Tcr = 647° K.) Superheat Limit (° C.) Van der Waals 0.844 273 Modified Berthelot 0.893 305 Berthelot 0.919 322 - These values represent the temperature above which homogeneous nucleation must begin.
- A kinetic limit of superheat may also be computed using the kinetic theory of the activated molecular clusters. The kinetic limit of superheat for water is about 300° C.
- When liquid is heated in the presence of a solid surface, heterogeneous nucleation usually occurs. In this regime, bubbles typically nucleate in cavities (surface defects) on the heated surface. The degree of superheat necessary to nucleate a bubble in a cavity is inversely dependent on the cavity radius, as shown in Equation (1-6).
- Where T w is the surface temperature, Tsat is the saturation temperature (100° C. for water), σ is the surface tension, hfg is the latent heat of vaporization, pv is the vapor density, and rc is the cavity radius. For example, the surface temperature necessary to nucleate bubbles in water with a surface that has a 1 μm cavity radius is about 133° C. For a 0.1 μm cavity radius the temperature to nucleate a bubble is about 432° C., well above the highest thermodynamic water superheat limit of 322° C.
- Accordingly, for surfaces with cavity sizes well below 1 μm, it is likely that homogeneous nucleation will occur since the liquid will reach the superheat limit before a bubble nucleates in a cavity. Micromachined surfaces tend to have very smooth surfaces. For instance, the platinum resistors are only 3-6 μm wide, and 0.1 μm thick, so it is unlikely that cavities will exist on the surface which are large enough for heterogeneous nucleation to occur. The largest likely nucleation cavity would be the thickness of the resistor, which is 0.1 μm, and results in a boiling temperature for heterogeneous nucleation above the thermodynamic superheat limit as shown above. Thus, it was assumed that homogeneous nucleation was the most likely method of bubble nucleation to occur for the resistors of this invention.
- However, when platinum films are annealed, thermal grooving and agglomeration can take place at the grain boundaries. A groove will develop on the surface of a hot polycrystalline material where a grain boundary meets the surface. As the surface gets hotter, the grooves deepen, initiating holes, and the platinum begins the process of balling up in order to reduce surface area. This process is called agglomeration. The agglomeration rate is insignificant at anneal temperatures below 700° C. However, for a 600° C. anneal of platinum for 1 hour, the onset of agglomeration can cause small voids in the platinum with radii of up to about 0.5 μm. In this case, heterogeneous nucleation would be possible at a temperature of about 166° C.
- Next, it was desirable to predict the electrical current necessary to achieve a certain temperature of the resistor. The schematic and boundary conditions for this resistor model are shown in FIG. 7A and 7B. For the cross-sectional slice through the resistor ( 7B), the water above the heater was 450 μm thick, corresponding to the height of the silicon chamber containing the water. It was assumed that the ambient temperature was maintained at the top of the water in the well since above this there was silicon with water at the ambient temperature flowing over the top of it. The bottom of the glass slide was also assumed to be at the ambient temperature since it was contacting a surface at the ambient temperature. The resistor was about 10,000 times thinner than the glass slide and had ohmic heating, or power generation equal to I2R for the entire volume of the resistor.
-
- Where L is the characteristic length for conduction and α is the thermal diffusivity of the material.
- Using this relation, it was found that the characteristic time for conduction through 1 mm of glass was about 2.3 seconds. Similarly, the characteristic time for conduction through 450 μm of water was 1.38 seconds. Accordingly, for this system the time to reach steady state would be about four times greater than the highest characteristic time, about 9 seconds. As established above, homogeneous bubble nucleation was likely to occur, which is a molecular process and thus may be assumed to be approximately instantaneous. The time for a bubble to nucleate was therefore far shorter than the 9 seconds necessary for the system to reach steady state, so steady state conditions are unlikely to be achieved before the bubble nucleates.
- It was then necessary to determine the dominant modes of heat transfer from the resistor to its surroundings. The purpose of this model was to predict the temperature of the heater for a given current, before the onset of boiling. For this model, heat transfer due to radiation was neglected.
-
- Where t is the platinum resistor thickness (0.1 μm) and k Pt is the thermal conductivity of platinum (71.5 W/mK). It was assumed in this model a heat transfer coefficient of h=5 W/m2K as a high bound for natural convection. The Biot number measures the ratio of internal conduction resistance to external convection resistance. Since the Biot number was much less than unity, the lumped body approximation was used and an assumption was made that the entire resistor was at a uniform temperature.
- FIG. 8 shows the thermal resistances between the resistor and the ambient temperature. For the purpose of this order of magnitude estimate of the heat transfer mechanisms, steady state conditions were used in determining thermal resistances. First, the thermal resistance due to convection through the water was computed. For this case it was assumed there was natural convection since the water above the heater was stagnant, and boiling was not occurring. The thermal resistance due to convection was calculated below.
- Where w is the resistor width (3 μm) and L is the resistor length (1000 μm).
-
-
-
- Where L g is the length of glass through which heat conducts (1 mm), kg is the conductivity of glass (0.81 W/mK), L is the length of the resistor (1000 μm), w is the width of the resistor (3 μm), Lw is the length of water through which heat conducts (450 μm), and kw is the conductivity of water (0.67 W/mK).
- From this it was shown that R glass and Rwater were the dominant thermal resistances for the system. Thus, heat transfer due to convection in the water and conduction through the platinum were negligible.
- An estimate the temperature of the resistor as a function of time for a given current using semi-infinite body theory was then made. For small times (t<1 ms) it was assumed that both the water and glass are semi-infinite bodies with initial temperature T a. At t=0, a constant heat flux (due to the resistor) is applied at the water-glass interface (x=0). The one-dimensional temperature profile was computed using the infinite composite solid solution. The region x>0 is water, x=0 is the resistor, and x<0 is the glass. A one-dimensional model was used for short times since the length of the resistor (L=1000 μm) was much less than the width of the resistor (L=6 μm). The temperature was assumed to be constant along the resistor, and lateral conduction was neglected for small times. This model will break down when the lateral conduction becomes significant, and when the assumption of semi-infinite bodies becomes invalid. The boundary conditions for this problem are given below.
- Where K is the thermal conductivity (0.61 W/mK for water and 0.88 W/mK for glass), q is the heat flux, and the subscript ‘1’ denotes water, and ‘2’ denotes glass.
-
- Where α is the thermal diffusivity (1.47×10 −7 m/s2 for water and 4.4×10−7 m/s2 for glass) and To is the initial temperature of the body.
- The solution was also used to check the semi-infinite body assumption. For times equal to or less than 1 ms, and a reasonable heat flux such as 2.5×10 7 W/m2, the heat penetration depths into the glass and water were less than 100 μm. The total thickness of the water was 450 μm and of the glass was 1 mm, so the semi-infinite body assumption held true. The one-dimensional model was sufficient for determining the temperature of the resistor at small times.
- Using the theory described above, it was possible to predict the power necessary to form a bubble. Since homogeneous bubble nucleation was assumed, the bubbles would form at approximately the superheat limit of water. The value of 305° C. given by the modified Berthelot equation (Table 1) was used. Next, the infinite composite solid solution was used to calculate the temperature of the heater for a given time, say 1 ms. Rearranging equation (1-17) to solve for the heat flux, or power per unit area at position x=0, it was derived:
- For an initial temperature of 20° C., and the other properties given above, the heat flux necessary to heat the resistor to 305° C. in 1 ms was computed from (1-19) to be 1.32×10 7 W/m2. For typical resistor dimensions of w=6 μm and L=1500 μm, the necessary power was about 120 mW.
- The micromachined wells must be of the proper dimensions to ensure that particles which settle into them remain held in the wells once a flow above them is initiated. The theory of slow viscous flow over cavities has been well characterized and the streamlines for various geometries have been calculated and experimentally verified.
- FIG. 9 shows the flow pattern for laminar flow over a rectangular cavity for two different width to height aspect ratios. From these flow patterns it was seen that there was a separating flow line which penetrates slightly into the cavity. Below this line there were one or two vortices, depending on the aspect ratio of the cavities. A particle below the separating flow line would not be swept out of the cavity by a slow flow in the laminar range, though the vortex may agitate the particle.
- An order of magnitude calculation was performed in order to compare the relative sizes of the gravity force pulling a particle down, compared to the viscous shear force pulling a particle out of the well. A diagram of a particle in a well with flow over the top is shown in FIG. 10.
- The force of gravity acting on the particle was dependent on the difference in density between the particle and the water, Δρ. The density of water is approximately 1000 kg/m 3, and the density of the polystyrene beads used in the experiments was given by the manufacturer as 1060 kg/m3. The density of cells ranges from 1050-1100 kg/m3. Accordingly, the force of gravity, Fg was computed as shown:
- Where a is the particle radius (5×10 −6 m), and g is the gravitational constant.
-
- Where μ is the viscosity of water (1×10 −3 kg/ms) and u(y) is the velocity profile as a function of y, the distance from the wall.
-
- Where {overscore (V)} is the average flow velocity, w is the chamber width, and h is the chamber height.
-
-
-
- It was necessary that the ratio of forces be greater than one so that the gravity force was stronger than the viscous force. These numbers were used to aid in determining a range of acceptable operating flow rates.
-
-
-
- Thus, the assumption of low Reynolds number was valid. The Reynolds number is the ratio of inertial effects to viscous forces. For this case, only the highly viscous regime applied and inertial effects were negligible.
-
- Where D o is the Brownian diffusivity, and k is the Boltzmann's constant (1.38×10−16 erg/cm). Thus the Peclet number was sufficiently high for settling to dominate over diffusion.
- The value calculated above for the Stokes velocity is that for an isolated particle; however, in the case at hand there were many beads settling at once. This was taken into account in the calculation of the hindered velocity. A function of the particle volume fraction is multiplied by the Stokes velocity to result in the hindered velocity of particles in the suspension.
-
- Where h is the chamber height (790 μm). This settling time was used as a guideline in experiments.
- A more reasonable assumption for calculating the settling time was that the distance the particles fell is an average of half the chamber height. For this case a settling time of about 83 seconds was obtained.
- For the given pressure increase associated with the bubble formation in the large sealed well, the flow rate out of the channel in the top of the well was calculated. Since the Reynolds number was in the creeping flow regime (Re<1), inertial effects neglected, and the initial, instantaneous flow out of the channel was computed using the steady state equation for flow through a circular aperture at low Reynolds number.
- Where Q is the volume flow rate, ΔP is the pressure drop, c is the aperture radius (˜2.5 or 4 μm), and μ is the water viscosity.
- Since the pressure change due to the bubble formation was not easily calculable, the volume flow rate out of the chamber was estimated in a different way. Because water is incompressible, it was assumed in the model that the bubble formation as a volume injection into the chamber resulted in the same volume being ejected from the chamber over the characteristic bubble formation time. For instance, if it took 1 ms to form a 10 μm diameter bubble, then the resulting volume flow rate out of the chamber was calculated as follows.
-
- Where c is the channel radius (2.5 μm). The force of the fluid jet on the particle was calculated using the Stokes drag force:
- F D=6πμa{overscore (V)}=2.5×10−9 N (1-46)
-
- Where Δρ is the difference in densities between the water and the polystyrene beads (60 kg/m 3). It was seen that as the particle radius increased, the effect of gravity increased. For typical cells, the radius ranges from 5 μm (red blood cells) to 20 μm (most other cells) to 100 μm (embryos and eggs). This device will most likely be used for cells on the order of 5-10 μm in radius so the above calculation was representative of the expected applications.
- A. Resistive Heaters
- In order to heat the water to a sufficiently high temperature for microbubble formation, resistive heaters were used. The heaters were made of thin-film platinum on standard glass slides. In designing the heaters it was necessary first to determine a range of resistances and currents to attain the desired power output. The design constraint for this step was the need to keep the current density below the electromigration limit of platinum, while retaining an adequate degree of ohmic heating. The electromigration limit is the maximum current density which platinum can endure before the atoms begin to migrate leaving the resistor inoperable.
- The electromigration limit of platinum was reported to be J=9×10 6 A/cm2. It was necessary to design the resistors to operate at a current density below this limit.
-
- Where R is the resistance (Ω), L is the length of the resistor (m), t is the film thickness (m), w is the width of the resistor (m), and ρ is resistivity of platinum (Ωm).
-
- Where I is the current (A) and J is the current density.
- Accordingly, as the currents were limited by the electromigration limit, the resistances needed to be sufficiently high to achieve the desired power output. The power output necessary to form a bubble was estimated by using the numbers from Lin et al.'s paper, ‘Microbubble Powered Actuator’, herein incorporated by reference, where microbubbles were formed on a polysilicon line heater. Their resistor was on top of a thin dielectric layer, which was on a silicon wafer. It was reasonable to assume that the heat dissipation of this configuration might well be greater than the heat dissipation of the platinum line resistor fabricated on a glass slide. Also, a liquid with a higher boiling necessary to nucleate bubbles under these conditions was approximately 65 mW.
TABLE 2 Resistor dimensions, resistances, and electromigration limits. Resis- Max Slide Re- Length Width tance Electromigration Power Name sistor (um) (um) (Ohms) Limit (mA) (mW) Slide 11 3000 3 1000 22 467 2 2500 3 833 22 389 3 500 3 167 22 78 4 1000 3 333 22 156 5 1000 4 250 29 207 6 2000 3 667 22 311 7 1500 3 500 22 233 8 1000 5 200 36 259 Slide 21 3000 3,6 483 22 226 2 2500 3,6 400 22 187 3 500 3 167 22 78 4 1000 3,6 150 22 70 5 1000 6 167 43 311 6 2000 3,6 317 22 148 7 1500 3,6 233 22 109 8 1000 3,5 180 22 84 Slide 31 3000 6 625 43 1166 2 2500 6 521 43 972 3 500 3 208 22 97 4 1000 3 417 22 194 5 1000 6 208 43 389 6 2000 6 417 43 778 7 1500 6 313 43 583 8 1000 6 208 43 389 - Using this as a guideline, the resistances were chosen to range from 167 Ω-1000 Ω, yielding maximum powers before electromigration of 70-1166 mW. These powers were chosen to be up to an order of magnitude greater than necessary to avoid reaching the electromigration limit in the operation of the resistors.
- The resistivity of platinum actually varies with temperature and film deposition conditions, but for these calculations it was taken to be 1×10 −7 Ωm. This is the value for bulk platinum, however the resistivity of thin film platinum can vary widely. Heater widths range from 3-6 μm and lengths range from 500-3000 μm. Some heaters were designed to have a narrow region, 100 μm long in the center, which would be hotter than the rest of the resistor. FIG. 11A is a top view of a heater configuration, while FIG. 11B shows a cross-sectional view of the heater and its dimensions. A table of resistor dimensions, maximum currents, and maximum power outputs is also shown in Table 2.
- The lines connecting the contact pads to the heaters were designed to have a far lower resistance than the heaters. This was done to ensure that the lines did not heat up, and that they remained approximately at the ambient temperature. The connector line widths were chosen to be 1500 μm with lengths of 12 mm. The total resistance of each line was about 7.7 Ω.
- B. Wells
- Square wells were micromachined into silicon in order to hold cells. It was necessary to choose a range of dimensions for these wells to allow for tests with different particle sizes and flow rates. The final goal was to have the ability to trap one particle in each of an array of wells.
TABLE 3 Well Dimensions Chip Number Well Dimension (um) Hole Dimension (um) 1 16 5 b 16 8 2 10 5 2b 10 8 3 20 5 3b 20 8 4 30 5 4b 30 8 5 40 5 5b 40 8 6 50 5 6b 50 8 - Side lengths of the wells were chosen to range from 10 μm, corresponding to the smallest test bead size, up to 50 μm. Well sizes ranging from 10-50 μm were chosen. Narrow channel widths of 5 μm and 8 μm were chosen since both these sizes are smaller than the minimum test particle size of 10 μm and it is necessary that particles not be able to settle down into the narrow channel. The table of well dimensions is shown in Table 3. A diagram of the well geometry is shown in FIG. 12A, which shows a side view, while FIG. 12B shows a top-down view.
- Photomasks for use in the device fabrication were created using standard mask layout software. The mask set for the silicon processing are shown in FIGS. 13A-13C and the glass mask set is shown in FIG. 14A.
- Three masks were designed for the silicon portion of the device processing. One mask was created for the cell wells (FIG. 13A), one for the narrow channels within the wells (FIG. 13B), and one for the large wells (FIG. 13C) etched from the backside of the wafer to enclose the heaters. Two masks were made for the fabrication of the platinum heaters on the glass slides. One mask (FIG. 14A) was designed to pattern the metal.
- In order to test the finished devices, a fluidic system as illustrated in FIG. 15 was designed and assembled. A
syringe pump 150 was used as the flow source for the bulk fluid, and flow rates ranging from 1 to 100 μL/min were specified. Beads, cells, or cell stimuli were injected through thesample injection valve 152. Apressure sensor 154 was located before theflow chamber 156 so that the pressure drop across the chamber could be monitored. All fluid was outlet into awaste beaker 158 which could be reused if desired. - A schematic of the
flow chamber 156 is shown in FIGS. 16A and 16B. The flow chamber was machined from plexiglass so that it was clear and a microscope was used to observe cell behavior from above the chamber. HPLC (high-performance liquid chromatography) fittings were used with tube dimensions of {fraction (1/16)} inch outer diameter and 0.020 inch inner diameter. The gasket between the slide and the top cover were made from PDMS (poly dimethyl siloxane), a flexible polymer. A seal was formed by screwing the top plate down onto the bottom plate. Aluminum molds were machined in order to create PDMS gaskets of the proper dimensions. Gaskets were compressed until a hard stop was reached. The stop was provided by the spacers, made of metal shim stock, in order to accurately specify the channel height. The aspect ratio of the channel's width to height was greater than 10, allowing the assumption of a parabolic velocity profile-plane Poiseuille flow. - The height of the flow chamber was 790 μm (determined by thickness of metal spacer). Flow rates ranged from 1 to 100 μL/min and corresponded to Reynolds numbers of 0.001-0.1. In this creeping flow regime, the entrance length for fully developed flow was calculated to be negligible. These calculations are shown below.
- Where {overscore (V)} min is the minimum average velocity, Qmin is the minimum volume flow rate (1 μL/min), Ac is the cross-sectional area of the channel (h=790 μm, w=12 mm), Vmin is the maximum average velocity, Qmax is the maximum volume flow rate (100 μL/min), Re is the Reynolds number, v is the kinematic viscosity of water (1×10−6 m2/s), and Xe is the entrance length for fully-developed flow.
- Electrical connections to the contact pads were made using a probe station. Contact pads were positioned outside of the PDMS gasket and were thus kept outside of the fluid flow.
- In order to ensure the proper flow characteristics of the flow chamber, dye was injected into the flow and the resulting profile was observed. The results were used to discover problems such as blockages in the flow chamber and correct them. When a uniform flow was established, 1 μm diameter beads were injected into the flow and observed under a microscope.
- The pressure drop across the flow chamber was monitored using a pressure transducer. The majority of the pressure drop was caused by the connector tubing, but by comparing the pressure reading to the theoretical value, the presence of bubbles and other blockages to the flow may be detected.
- The pressure versus flow rate plot for the flow chamber is shown in FIG. 17. The theoretical value is plotted with the experimental measurements. When these two values do not match, a blockage in the chamber or tubing is probable.
-
- Where μ is the viscosity of water (1×10 −3 kg/ms), r is the tube radius (0.254 mm), and Δx is the tube length (m). The pressure drop through the chamber was calculated to be negligible in comparison. The flow chamber schematic with dimensions is shown in FIGS. 18A-18D.
- The platinum heaters were fabricated on standard 1×3 in glass slides using a lift-off process. The process flow is shown in FIGS. 19A-19C. In the first step illustrated as FIG. 19A, photoresist was spun onto the glass slide, exposed using
mask 4, and developed. Next, 100 Å of titanium and 1000 Å of platinum were evaporated onto the slide, as seen in FIG. 19B. The titanium served as an adhesion layer between the glass and the platinum. In the following step, the slide was submerged in acetone to dissolve the photoresist and lift away the metal which was deposited on top of the photoresist, as depicted in FIG. 19C. Only the platinum resistors were left on the glass slide. Some slides were then annealed in a tube furnace at 600° C. for 1 hour. While not used in this example, it is contemplated that photoresist may be applied manually to the slide to attach the silicon chip to the slide. - The silicon chip process flow is shown in FIGS. 20A-20H. Double Side Polished (DSP) four inch diameter silicon wafers were used. In the first step shown as FIG. 20A, 1 μm of thermal oxide was grown on the wafer. Next the oxide was patterned using
mask 1, FIG. 20B. Resist was spun on top of the oxide and patterned usingmask 2. The resulting configuration was called a nested mask, shown as FIG. 20C. - First the photoresist mask was used to etch the narrow 5 μm trenches, then the oxide mask was used to etch the cell wells, as shown in FIGS. 20D and 20E. Next the wafer was turned over and photoresist was deposited and patterned on the back side using mask 3 (FIG. 20F). A deep silicon etch was then performed to etch through the wafer and intersect the narrow trenches etched previously (FIG. 20G) to obtain a finished wafer (FIG. 20H).
- A complete device consisted of a silicon chip attached to a glass slide by photoresist, as shown in FIGS. 21C and D. The resist provided a water-tight seal so that volume expansion in the bubble wells resulted in a burst of fluid being pushed through the narrow channel and ejecting a cell.
- To facilitate the assembly process, alignment marks were fabricated on the glass slide and matching holes were etched in the silicon chip. The alignment tolerances were sufficiently large (about 2 mm) that the chip could be aligned to the slide by hand using just the naked eye, while still positioning the bubble wells over the platinum heaters.
- Photoresist was painted onto the silicon chip around the bubble wells using a toothpick. Drops of water were deposited into each well using a pipette, then the glass slide was visually aligned from above and stuck down onto the chip. The drops of water served to fill the bubble wells and get pushed through the narrow channel to fill it with water. The device was now ready to be tested in the flow chamber.
- Next, the resistance of the platinum resistors were studied. The film thickness was first measured using a profilometer. The platinum thickness measurements ranged from about 800-900 Å, so the average value of 850 Å was used in the subsequent calculations. The resistance along metal lines wide enough not to be strongly affected by variation of a few microns was measured using a multimeter. The lines used for this measurement were measured in an optical microscope to be about 1510 μm wide. The length of the lines was about 8 mm. Knowing the width, thickness, and length of these lines, as well as the measured resistance, the resistivity of the thin film platinum at room temperature was determined. The measured resistance was 15 Ω, and the computed resistivity was calculated below.
- Where t is the film thickness (850 Å), w is the line width (1513 μm), R is the measured resistance (15 Ω), and L is the length of the line (8 mm). This resistivity was more than twice the value for bulk platinum (1×10 −7 Ωm), but was a reasonable value for thin film platinum. This is because bulk platinum is a crystalline material, whereas thin film platinum is polycrystalline and the grain boundaries significantly increase resistance.
- Next, the resistance of the resistors was measured with a multimeter. Using the value of resistivity from above, the line width of each resistor was determined. The line widths were also measured using an optical microscope to an accuracy of about ±1 μm. The results of for two different resistor slides are shown in Table 4.
TABLE 4 Resistance measurements and calculated, measured, and designed line widths. Computed Re- R Line Measured Design sistor # L (um) (Ohms) Width (um) (um) (um) Slide 11 3000 1020 8.34 8 3 2 2500 845 8.39 8 3 3 500 185 7.66 8 3 4 1000 347 8.17 8 3 5 1000 272 10.42 10 4 6 2000 672 8.44 9 3 7 1500 504 8.44 8 3 8 1000 260 10.90 10 5 Slide 31 3000 850 10.01 10 6 2 2500 728 9.74 10 6 3 500 247 5.74 6 3 4 1000 479 5.92 6 3 5 1000 316 8.97 9 6 6 2000 620 9.15 9 6 7 1500 450 9.45 10 6 8 1000 270 10.50 10 6 - From this it was determined that the measured and calculated line widths were within the range of error for the measurements, confirming the resistivity calculation. The resulting plot of normalized resistance versus temperature is shown in FIG. 22. The resistance was normalized using the resistance at room temperature. This curve was used later to predict the temperature of a resistor, knowing the resistance at room temperature and measuring the resistance during operation.
- Using the cross-sectional area of the resistors, the maximum current before electromigration was calculated. It was known that maximum current density before electromigration is 9×10 6 A/cm2. Using this the maximum current for each resistor was calcualted. The results of this are shown in Table 5.
TABLE 5 Computed electromigration limits for resistors. Computed Line Maximum Resistor # Width (um) Current (mA) Slide 11 8.3 71.3 2 8.4 71.7 3 7.7 65.5 4 8.2 69.9 5 10.4 89.1 6 8.4 72.1 7 8.4 72.1 8 10.9 93.2 Slide 31 10.0 85.6 2 9.7 83.2 3 5.7 49.1 4 5.9 50.6 5 9.0 76.7 6 9.1 78.2 7 9.5 80.8 8 10.5 89.8 - These results were used as guidelines during testing of microbubble devices to avoid burning out the resistors.
- The main objective for the resistors was that they be able to reach high enough temperatures to boil water. The resistors were tested on a probe station using an HP4145b to vary the voltage and measure the resulting current through the resistor. A PDMS gasket was place on top of the slide and filled with water. The gasket contained the water and kept it from touching the electrical contacts and probes. FIG. 23 is a schematic of this configuration.
- Upon ramping the voltage across resistors from zero to about 20-30 V, there was violent bubbling originating not from the hot part of the resistor, but from the edges of the wide connector lines. It was evident that the bubbles were gas bubbles and not water vapor bubbles because the bubbles did not condense when the heater was turned off. Further experimentation revealed that electrolysis of the water was occurring and the water was being broken down into hydrogen and oxygen. After flushing the slides, gaskets, and glassware for several minutes with deionized water, and testing again, the problem of electrolysis was eliminated.
- When the problem of electrolysis was eliminated, the resistors were once again tested in water. When the resistor reached a sufficient temperature, boiling occurred along the length of the heater. After the power was turned off, small air bubbles remained on the resistor due to the dissolved gas coming out of solution, as described previously. In subsequent tests, the air bubbles served as nucleation sites for boiling, the inception of boiling occurred at a much lower temperature. When boiling begins and bubbles form on the resistor, the heat dissipation into the water increases drastically. This is a favorable phenomenon for the operation of the device because the onset of boiling is represented as a sharp increase in current on the I-V curve. This is because when the heat dissipation increases, the temperature decreases, resulting in a lower resistance and thus a higher current through the resistor. An I-V curve for the onset of boiling on a line resistor is shown in FIG. 24.
- In this I-V curve it is shown that for the first run when no bubbles were present on the line, there is a sharp jump in current at the onset of boiling. For the second run, residual bubbles were left on the heater and served as nucleation sites for boiling resulting in a smooth I-V curve with boiling beginning at a lower temperature. The two curves are very close after the boiling begins for
run 1. - In later tests, when no dissolved gas came out of solution, the jump in the I-V curve occurred during each heating cycle for the resistors, since there were no residual air bubbles left when the power was turned off.
- Using the calibration given in FIG. 22 for the temperature-resistance relationship of the resistor, the temperature of the resistor for each current was plotted to find the boiling temperature. The current vs. temperature plot corresponding to the I-V curve shown above is in FIG. 25. On this plot water is shown to boil at approximately 308° C., at which point the temperature drops rapidly due to the increased convective heat transfer associated with boiling.
- The boiling points for the 5 resistors tested ranged from 250° C. to 308° C. The lowest calculated value for the superheat limit of water was found to be 273° C., so these measured boiling points suggest that the bubble nucleation occurs either in the homogeneous regime, or by a weak heterogeneous mechanism.
- After a considerable amount of testing of the resistors characterized above, a drift in the boiling temperature became apparent. In order to determine the reason for this, the resistors were recalibrated as described in the previous section. The temperature versus normalized resistance curve is shown in FIG. 26. The dramatic change in temperature-resistance characterization led to the testing of a second generation of resistors. It is thought that these change characteristics are caused over time by the heating of the resistors. The operation of the resistors effectively caused them to anneal themselves. Annealing changed the geography of the platinum grain boundaries and thus changed the resistivity of the resistors.
- In order to avoid this effect in future testing, new resistor slides were annealed at 600° C. for 1 hour as the last step in their process. This temperature is higher than operating temperatures are likely to reach, but not so high that major agglomeration will result. Once the anneal was complete, the new resistors were characterized as described above for the first generation resistors.
- First, the resistivity of the platinum at room temperature was found to be 2.056×10 −7 Ωm, less than the unannealed resistors that were 2.41×10−7 Ωm. Next the resistances were measured using a multimeter, and the line widths were computed as before, as shown in Table 6.
TABLE 6 Measured resistances and computed line widths of second generation resistors. Computed Design Resistor # L (um) R (Ohms) Line Width (um) (um) Slide 31 3000 553 13.12 6 2 2500 481 12.57 6 3 500 146 8.28 3 4 1000 281 8.61 3 5 1000 205 11.80 6 6 2000 409 11.83 6 7 1500 310 11.70 6 8 1000 186 13.00 6 - The temperature-resistance characteristic or the resistors was then measured on a hotplate as described above, and is shown in FIG. 27.
- At this point, the bubble formation characteristics of the resistors were tested as described previously with boiled, deionized water. Voltages were ramped up by 0.5V steps with delay times of 1 ms using the HP4145b, as before. None of these tests resulted in residual gas bubbles since the delay time was short, and the maximum voltage used was just above the bubble nucleation voltage, determined by testing. All resulting vapor bubbles condensed back into the liquid phase within on minute of stop of current flow.
- A resulting I-V curve is shown in FIG. 28, and the corresponding temperature curve is shown in FIG. 29. From the curve we can see that the onset of boiling occurred at about 200° C. a much lower temperature than for the first generation resistors, and well below the superheat limit of water. For the 8 second generation resistors tested, boiling points ranged from 128° C.-200° C. with the majority of the temperatures above 180° C. This suggests that the boiling is in the heterogeneous nucleation regime as discussed earlier. The cavity radii corresponding to these boiling inception temperatures are calculated from Equation (1-59).
- The result of this calculation are shown in Table7.
TABLE 7 Bubble nucleation cavity radii corresponding to measured boiling temperatures. Resistor # Boiling Temperature (C) Cavity Radius (um) 1 200.7 0.33 2 198.3 0.34 3 170.4 0.47 4 183.2 0.40 5 128 1.19 6 188 0.38 7 189 0.37 8 169 0.48 - From this we can see that bubbles were nucleated in radii ranging from 0.3-1.2 μm. As discussed previously, these cavities were most likely formed during the 600° C. anneal, during which the grooves at the grain boundaries widened creating cavities.
- The second generation resistors were also tested for the repeatability of their boiling temperatures. I-V curves were measured as in the previous section, and then remeasured for the same conditions several times. Between measurements, time was given for the vapor bubbles to dissipate so that the characteristic jump in the I-V curve at boiling could be observed with each measurement. The boiling point was found to be very repeatable, and an example of the results is shown in FIG. 30. This result demonstrated the potential of a control system based on a jump in the I-V curve at the onset of boiling, since the boiling point remained fixed.
- Another interesting result from this testing is that for a particular resistor, the bubbles tended to nucleate in the same locations on the resistor each time. This strengthens the hypothesis that the bubbles are nucleating in the heterogeneous regime, in cavities created by thermal grooving caused by the annealing.
- The cell chip was attached to the glass resistor slide as described earlier, and then tested in two ways. First tests were done with stagnant fluid on the device. Then the device was put into the flow chamber for testing. The results of these tests are described below.
- For these tests, several drops of bulk solution were placed on top of the cell chip, and contained by the PDMS gasket. A drop of the polystyrene bead solution was then added to the bulk fluid and allowed to settle. The bulk solution was a 0.05% solution of Triton x-100 surfactant in deionized water. The bead solution was about 1% beads diluted in the same bulk solution. Some of the beads settled into wells, as shown in FIG. 42. When voltage across the resistor was ramped up by the HP4145b, an I-V curve with a jump similar to that in FIG. 24 was produced, demonstrating that boiling had occurred. Consequently, the bubble formation under the well caused a volume expansion which rapidly ejected the beads from the well. First the beads are in the well, and then they are rapidly expelled. This sequence was also captured on videotape, and the process was repeated multiple times with the same success.
- Preliminary dynamic testing was performed in the flow chamber. Beads were ejected in a similar way to the static test, and carried away in the flow. The preliminary tests suggested that the beads are held in the wells against a reasonable flow rate, and are ejected into the flow when a microbubble forms.
- While the invention has been particularly shown and described above with reference to several preferred embodiments and variations thereon, it is to be understood that additional variations could be made in the invention by those skilled in the art while still remaining within the spirit and scope of the invention, and that the invention is intended to include any such variations, being limited only by the scope of the appended claims.
Claims (48)
1. A method for analyzing a cell population, comprising:
providing a cell sorting apparatus having an array of sites, each site including a capture mechanism comprising a well that is capable of capturing a single cell, and a release mechanism comprising an actuator coupled to the well for selectively releasing the single cell from its site;
introducing a fluid medium containing a plurality of cells onto the apparatus;
capturing at least one cell in a well;
identifying a cellular response in the at least one captured cell; and
selecting at least one captured cell based on the cellular response.
2. The method of claim 1 , wherein the step of capturing further comprises inducing a fluid medium with a cell population to flow across the substrate.
3. The method of claim 1 , wherein the cell is captured in the well by negative pressure.
4. The method of claim 1 , wherein the cell is captured in the well by well geometry.
5. The method of claim 1 , wherein the cell is captured in the well by gravitational forces.
6. The method of claim 1 , wherein the cell is captured in the well by electric field forces.
7. The method of claim 1 , wherein the cell is captured in the well by dielectrophoretic forces.
8. The method of claim 1 , wherein the step of identifying a cellular response further includes applying an optical probe to the captured cell and detecting an optical signal from the captured cell.
9. The method of claim 1 , wherein a plurality of cells are captured, and the method further comprises the step of applying a fluorescent agent to the plurality of cells.
10. The method of claim 9 , further including the step of detecting fluorescence exhibited by captured cells within the sorting apparatus.
11. The method of claim 10 , further including the step of measuring intensity of fluorescence at individual sites in the sorting apparatus over time.
12. The method of claim 1 , wherein the step of identifying a cellular response further comprises coupling a photometric array to the cell sorting apparatus to detect fluorescence at sites in the sorting apparatus.
13. The method of claim 12 , further including the step of comparing the fluorescence intensity at particular sites.
14. The method of claim 13 , wherein the step of selecting a cell further comprises selecting a cell based on its fluorescence intensity, and releasing the cell from its well.
15. The method of claim 13 , wherein a receptor-ligand binding event at a site in the sorting apparatus is identified by fluorescence intensity.
16. The method of claim 13 , wherein a protein to protein interaction at a site in the sorting apparatus is identified by fluorescence intensity.
17. The method of claim 13 , wherein cellular signal transduction at a site in the sorting apparatus is identified by fluorescence intensity.
18. The method of claim 13 , wherein intracellular calcium transport at a site in the sorting apparatus is identified by fluorescence intensity.
19. The method of claim 1 , wherein the step of selecting at least one captured cell further comprises releasing the captured cell from its well by activating the release mechanism of the well.
20. The method of claim 19 , wherein the step of releasing comprises forming a microbubble to displace the captured cell from its well.
21. The method of claim 19 , wherein the step of releasing comprises lowering an energy field surrounding the captured cell.
22. A method for sorting individual cells in a cell population, comprising:
providing a cell sorting apparatus having an array of sites, each site including a capture mechanism comprising a well that is capable of capturing a single cell, and a release mechanism comprising an actuator coupled to the well for selectively releasing the single cell from its site;
introducing a fluid medium containing a plurality of cells onto the apparatus;
capturing at least one cell in a well;
selecting a captured cell; and
releasing the selected, captured cell from the apparatus.
23. The method of claim 22 , wherein the step of capturing comprises inducing a fluid medium with a cell population to flow across the substrate.
24. The method of claim 22 , wherein the cell is captured in the well by negative pressure.
25. The method of claim 22 , wherein the cell is captured in the well by well geometry.
26. The method of claim 22 , wherein the cell is captured in the well by gravitational forces.
27. The method of claim 22 , wherein the cell is captured in the well by electric field forces.
28. The method of claim 22 , wherein the cell is captured in the well by dielectrophoretic forces.
29. The method of claim 22 , wherein a plurality of cells are captured within wells.
30. The method of claim 22 , wherein the step of releasing the captured cell further comprises activating the actuator so as to release the captured cell from its well.
31. The method of claim 30 , wherein the step of activating further comprises forming a microbubble to displace the captured cell from its well.
32. The method of claim 22 , wherein the step of releasing the captured cell further comprises lowering an energy field surrounding the captured cell.
33. The method of claim 22 , wherein the step of releasing the captured cell further comprises releasing the selected, captured cell into a fluid flow around the well.
34. The method of claim 22 , further including the step of collecting at least one released cell.
35. The method of claim 22 , further including the step of coupling a photometric array to the apparatus to detect fluorescence at sites in the sorting apparatus.
36. The method of claim 35 , further including the step of applying a fluorescent agent to the captured cell.
37. The method of claim 36 , further including the step of detecting fluorescence at sites within the sorting array.
38. The method of claim 37 , wherein a temporal response of captured cells to stimuli is monitored.
39. The method of claim 22 , wherein the step of selecting a captured cell from the sorting apparatus further comprises selecting the cell selected based on its response to at least one stimulus.
40. The method of claim 39 , further including the step of observing a temporal response to stimulus.
41. The method of claim 39 , wherein the captured cell is selected based on detection of a receptor-ligand binding event at a site in the sorting apparatus.
42. The method of claim 39 , wherein the step of selecting the captured cell is based on detection of a protein to protein interaction at a site in the sorting apparatus.
43. The method of claim 39 , wherein the captured cell is selected based detection of cellular signal induction at a site in the sorting apparatus.
44. The method of claim 39 , wherein the captured cell is selected based on detection of intracellular calcium transport at a site in the sorting apparatus.
45. The method of claim 39 , wherein the captured cell is selected based on detection of a drug response at a site in the sorting apparatus.
46. The method of claim 39 , wherein the captured cell is selected based on detection of a phenotypical response at a site in the sorting apparatus.
47. The method of claim 46 , wherein the phenotypical response of the selected cell is different from that of other cells captured in the sorting apparatus.
48. The method of claim 39 , wherein the captured cell is selected based on a reporter-gene based assay.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/778,831 US20040166555A1 (en) | 1999-11-10 | 2004-02-13 | Cell sorting apparatus and methods for manipulating cells using the same |
| US11/146,581 US20060128006A1 (en) | 1999-11-10 | 2005-06-07 | Hydrodynamic capture and release mechanisms for particle manipulation |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16464399P | 1999-11-10 | 1999-11-10 | |
| US09/710,032 US6692952B1 (en) | 1999-11-10 | 2000-11-10 | Cell analysis and sorting apparatus for manipulation of cells |
| US10/778,831 US20040166555A1 (en) | 1999-11-10 | 2004-02-13 | Cell sorting apparatus and methods for manipulating cells using the same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/710,032 Continuation US6692952B1 (en) | 1999-11-10 | 2000-11-10 | Cell analysis and sorting apparatus for manipulation of cells |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/146,581 Continuation-In-Part US20060128006A1 (en) | 1999-11-10 | 2005-06-07 | Hydrodynamic capture and release mechanisms for particle manipulation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040166555A1 true US20040166555A1 (en) | 2004-08-26 |
Family
ID=31190633
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/710,032 Expired - Fee Related US6692952B1 (en) | 1999-11-10 | 2000-11-10 | Cell analysis and sorting apparatus for manipulation of cells |
| US10/778,831 Abandoned US20040166555A1 (en) | 1999-11-10 | 2004-02-13 | Cell sorting apparatus and methods for manipulating cells using the same |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/710,032 Expired - Fee Related US6692952B1 (en) | 1999-11-10 | 2000-11-10 | Cell analysis and sorting apparatus for manipulation of cells |
Country Status (1)
| Country | Link |
|---|---|
| US (2) | US6692952B1 (en) |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060073470A1 (en) * | 2002-05-30 | 2006-04-06 | Naohiro Noda | Method of counting microorganisms or cells |
| US20060128006A1 (en) * | 1999-11-10 | 2006-06-15 | Gerhardt Antimony L | Hydrodynamic capture and release mechanisms for particle manipulation |
| US20090258383A1 (en) * | 2006-08-31 | 2009-10-15 | Joseph Kovac | Opto-fluidic architecture for particle manipulation and sorting |
| US20100120077A1 (en) * | 2002-04-01 | 2010-05-13 | Fluidigm Corporation | Microfluidic particle-analysis systems |
| US8021614B2 (en) | 2005-04-05 | 2011-09-20 | The General Hospital Corporation | Devices and methods for enrichment and alteration of cells and other particles |
| US8137912B2 (en) | 2006-06-14 | 2012-03-20 | The General Hospital Corporation | Methods for the diagnosis of fetal abnormalities |
| WO2012048113A2 (en) | 2010-10-07 | 2012-04-12 | The General Hospital Corporation | Biomarkers of cancer |
| US20120094324A1 (en) * | 2010-04-19 | 2012-04-19 | Durack Gary P | System and method for high throughput cell analysis and sorting |
| US8168389B2 (en) | 2006-06-14 | 2012-05-01 | The General Hospital Corporation | Fetal cell analysis using sample splitting |
| US8195415B2 (en) | 2008-09-20 | 2012-06-05 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8304230B2 (en) | 2002-09-27 | 2012-11-06 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| WO2012151501A3 (en) * | 2011-05-05 | 2013-04-25 | Anpac Bio-Medical Science Co., Ltd. | Apparatus for detecting tumor cells |
| WO2013063035A1 (en) | 2011-10-24 | 2013-05-02 | The General Hospital Corporation | Biomarkers of cancer |
| EP2594631A1 (en) | 2005-04-05 | 2013-05-22 | Cellpoint Diagnostics | Devices and method for detecting circulating tumor cells and other particles |
| WO2014052685A3 (en) * | 2012-09-26 | 2014-05-30 | Quantumcyte, Inc. | Devices and methods for single cell analysis |
| US8921102B2 (en) | 2005-07-29 | 2014-12-30 | Gpb Scientific, Llc | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| WO2015200756A1 (en) * | 2014-06-26 | 2015-12-30 | President And Fellows Of Harvard College | Pore-based bubble chamber |
| US10258741B2 (en) | 2016-12-28 | 2019-04-16 | Cequr Sa | Microfluidic flow restrictor and system |
| US10324011B2 (en) | 2013-03-15 | 2019-06-18 | The Trustees Of Princeton University | Methods and devices for high throughput purification |
| US10591391B2 (en) | 2006-06-14 | 2020-03-17 | Verinata Health, Inc. | Diagnosis of fetal abnormalities using polymorphisms including short tandem repeats |
| US10704090B2 (en) | 2006-06-14 | 2020-07-07 | Verinata Health, Inc. | Fetal aneuploidy detection by sequencing |
| US10976232B2 (en) | 2015-08-24 | 2021-04-13 | Gpb Scientific, Inc. | Methods and devices for multi-step cell purification and concentration |
| US11085923B2 (en) | 2011-03-24 | 2021-08-10 | Anpac Bio-Medical Science Co., Ltd | Micro-devices for disease detection |
| US11142746B2 (en) | 2013-03-15 | 2021-10-12 | University Of Maryland, Baltimore | High efficiency microfluidic purification of stem cells to improve transplants |
| US11493428B2 (en) | 2013-03-15 | 2022-11-08 | Gpb Scientific, Inc. | On-chip microfluidic processing of particles |
Families Citing this family (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6692952B1 (en) * | 1999-11-10 | 2004-02-17 | Massachusetts Institute Of Technology | Cell analysis and sorting apparatus for manipulation of cells |
| US6838056B2 (en) * | 2002-07-08 | 2005-01-04 | Innovative Micro Technology | Method and apparatus for sorting biological cells with a MEMS device |
| US7229838B2 (en) * | 2002-07-08 | 2007-06-12 | Innovative Micro Technology | MEMS actuator and method of manufacture for MEMS particle sorting device |
| US7220594B2 (en) * | 2002-07-08 | 2007-05-22 | Innovative Micro Technology | Method and apparatus for sorting particles with a MEMS device |
| US7534601B2 (en) * | 2002-08-27 | 2009-05-19 | Vanderbilt University | Capillary perfused bioreactors with multiple chambers |
| JP2004305076A (en) * | 2003-04-04 | 2004-11-04 | Canon Inc | Modification device for object |
| AU2004250131A1 (en) * | 2003-06-13 | 2004-12-29 | The General Hospital Corporation | Microfluidic systems for size based removal of red blood cells and platelets from blood |
| WO2005014792A2 (en) * | 2003-08-08 | 2005-02-17 | The General Hospital Corporation D/B/A Massachusetts General Hospital | Preservation of biomaterials with transported preservation agents |
| WO2005078425A1 (en) * | 2004-02-04 | 2005-08-25 | The Johns Hopkins University | Methods and systems for producing arrays of particles |
| US20050266433A1 (en) * | 2004-03-03 | 2005-12-01 | Ravi Kapur | Magnetic device for isolation of cells and biomolecules in a microfluidic environment |
| CA2600899C (en) * | 2004-07-16 | 2014-04-01 | Simon Fraser University | Microfluidic device and method of using same |
| US20060134772A1 (en) * | 2004-11-18 | 2006-06-22 | The Regents Of The University Of California | System for locating cells and for cellular analysis |
| JP2006197880A (en) * | 2005-01-21 | 2006-08-03 | Fujitsu Ltd | Apparatus for catching cell and method for catching cell |
| US20070026417A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026413A1 (en) * | 2005-07-29 | 2007-02-01 | Mehmet Toner | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026415A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026414A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| GB0508983D0 (en) | 2005-05-03 | 2005-06-08 | Oxford Gene Tech Ip Ltd | Cell analyser |
| CA2616404A1 (en) * | 2005-07-26 | 2007-02-01 | Oregon Health & Science University | Nanoparticle probes for capture, sorting and placement of targets |
| US20090181421A1 (en) * | 2005-07-29 | 2009-07-16 | Ravi Kapur | Diagnosis of fetal abnormalities using nucleated red blood cells |
| US20070026416A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070059680A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for cell enrichment |
| US20070059683A1 (en) * | 2005-09-15 | 2007-03-15 | Tom Barber | Veterinary diagnostic system |
| US20070059781A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for size based separation and analysis |
| US20070059719A1 (en) * | 2005-09-15 | 2007-03-15 | Michael Grisham | Business methods for prenatal Diagnosis |
| US20070059774A1 (en) * | 2005-09-15 | 2007-03-15 | Michael Grisham | Kits for Prenatal Testing |
| US20070059718A1 (en) * | 2005-09-15 | 2007-03-15 | Mehmet Toner | Systems and methods for enrichment of analytes |
| EP2024512A4 (en) | 2006-06-14 | 2009-12-09 | Artemis Health Inc | Methods for the diagnosis of fetal abnormalities |
| DK2029778T3 (en) | 2006-06-14 | 2018-08-20 | Verinata Health Inc | DIAGNOSIS OF Fetal ABNORMITIES |
| EP4108780A1 (en) | 2006-06-14 | 2022-12-28 | Verinata Health, Inc. | Rare cell analysis using sample splitting and dna tags |
| WO2010036912A2 (en) * | 2008-09-26 | 2010-04-01 | The General Hospital Corporation | Capturing particles |
| US20100081926A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Histological facilitation systems and methods |
| US20100081916A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware. | Histological facilitation systems and methods |
| US20100081924A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Histological facilitation systems and methods |
| US20100081927A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Histological facilitation systems and methods |
| US20100081928A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Histological Facilitation systems and methods |
| US20100081915A1 (en) * | 2008-09-29 | 2010-04-01 | Searete Llc, Alimited Liability Corporation Of The State Of Delaware | Histological facilitation systems and methods |
| US8367018B2 (en) * | 2009-12-07 | 2013-02-05 | Southern Taiwan University Of Technology | Chip with tri-layer electrode and micro-cavity arrays for control of bioparticle and manufacturing method thereof |
| US8187979B2 (en) * | 2009-12-23 | 2012-05-29 | Varian Semiconductor Equipment Associates, Inc. | Workpiece patterning with plasma sheath modulation |
| US20130143197A1 (en) * | 2010-08-15 | 2013-06-06 | Gpb Scientific, Llc | Microfluidic Cell Separation in the Assay of Blood |
| US10466160B2 (en) | 2011-08-01 | 2019-11-05 | Celsee Diagnostics, Inc. | System and method for retrieving and analyzing particles |
| EP2739587B1 (en) | 2011-08-01 | 2020-05-27 | Denovo Sciences | Cell capture system |
| US9174216B2 (en) | 2013-03-13 | 2015-11-03 | DeNovo Science, Inc. | System for capturing and analyzing cells |
| US9404864B2 (en) | 2013-03-13 | 2016-08-02 | Denovo Sciences, Inc. | System for imaging captured cells |
| JP6169111B2 (en) | 2012-02-29 | 2017-07-26 | フリューダイム・コーポレイション | Methods, systems, and devices for capturing and processing multiple single cells using microfluidics |
| US9752181B2 (en) | 2013-01-26 | 2017-09-05 | Denovo Sciences, Inc. | System and method for capturing and analyzing cells |
| US9707562B2 (en) | 2013-03-13 | 2017-07-18 | Denovo Sciences, Inc. | System for capturing and analyzing cells |
| US9856535B2 (en) | 2013-05-31 | 2018-01-02 | Denovo Sciences, Inc. | System for isolating cells |
| US10391490B2 (en) | 2013-05-31 | 2019-08-27 | Celsee Diagnostics, Inc. | System and method for isolating and analyzing cells |
| GB201617722D0 (en) | 2016-10-19 | 2016-11-30 | Univ London Queen Mary | Method for determining prognosis of cancer |
| GB201617723D0 (en) | 2016-10-19 | 2016-11-30 | Univ London Queen Mary | Method for predicting prostate cancer metastasis |
| AU2018323449B2 (en) | 2017-08-29 | 2020-09-03 | Bio-Rad Laboratories, Inc. | System and method for isolating and analyzing cells |
| US10633693B1 (en) | 2019-04-16 | 2020-04-28 | Celsee Diagnostics, Inc. | System and method for leakage control in a particle capture system |
| US11273439B2 (en) | 2019-05-07 | 2022-03-15 | Bio-Rad Laboratories, Inc. | System and method for target material retrieval from microwells |
| JP7413408B2 (en) | 2019-05-07 | 2024-01-15 | バイオ-ラッド ラボラトリーズ インコーポレイテッド | Systems and methods for automated single cell processing |
| AU2020290981B2 (en) | 2019-06-14 | 2024-03-07 | Bio-Rad Laboratories, Inc. | System and method for automated single cell processing and analyses |
| JP7215366B2 (en) * | 2019-07-17 | 2023-01-31 | 株式会社島津製作所 | Asymmetric flow flow field fractionator |
| US11504719B2 (en) | 2020-03-12 | 2022-11-22 | Bio-Rad Laboratories, Inc. | System and method for receiving and delivering a fluid for sample processing |
Citations (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4009435A (en) * | 1973-10-19 | 1977-02-22 | Coulter Electronics, Inc. | Apparatus for preservation and identification of particles analyzed by flow-through apparatus |
| US4190535A (en) * | 1978-02-27 | 1980-02-26 | Corning Glass Works | Means for separating lymphocytes and monocytes from anticoagulated blood |
| US4434156A (en) * | 1981-10-26 | 1984-02-28 | The Salk Institute For Biological Studies | Monoclonal antibodies specific for the human transferrin receptor glycoprotein |
| US4729949A (en) * | 1982-05-10 | 1988-03-08 | Bar-Ilan University | System and methods for cell selection |
| US4800159A (en) * | 1986-02-07 | 1989-01-24 | Cetus Corporation | Process for amplifying, detecting, and/or cloning nucleic acid sequences |
| US4814098A (en) * | 1986-09-06 | 1989-03-21 | Bellex Corporation | Magnetic material-physiologically active substance conjugate |
| US4894343A (en) * | 1986-11-19 | 1990-01-16 | Hitachi, Ltd. | Chamber plate for use in cell fusion and a process for production thereof |
| US4895805A (en) * | 1987-08-31 | 1990-01-23 | Hitachi, Ltd. | Cell manipulating apparatus |
| US4906439A (en) * | 1986-03-25 | 1990-03-06 | Pb Diagnostic Systems, Inc. | Biological diagnostic device and method of use |
| US4984574A (en) * | 1988-11-23 | 1991-01-15 | Seth Goldberg | Noninvasive fetal oxygen monitor using NMR |
| US4999283A (en) * | 1986-01-10 | 1991-03-12 | University Of Kentucky Research Foundation | Method for x and y spermatozoa separation |
| US5183744A (en) * | 1988-10-26 | 1993-02-02 | Hitachi, Ltd. | Cell handling method for cell fusion processor |
| US5275933A (en) * | 1992-09-25 | 1994-01-04 | The Board Of Trustees Of The Leland Stanford Junior University | Triple gradient process for recovering nucleated fetal cells from maternal blood |
| US5296375A (en) * | 1992-05-01 | 1994-03-22 | Trustees Of The University Of Pennsylvania | Mesoscale sperm handling devices |
| US5486335A (en) * | 1992-05-01 | 1996-01-23 | Trustees Of The University Of Pennsylvania | Analysis based on flow restriction |
| US5489506A (en) * | 1992-10-26 | 1996-02-06 | Biolife Systems, Inc. | Dielectrophoretic cell stream sorter |
| US5498392A (en) * | 1992-05-01 | 1996-03-12 | Trustees Of The University Of Pennsylvania | Mesoscale polynucleotide amplification device and method |
| US5707801A (en) * | 1988-08-31 | 1998-01-13 | Aprogenex, Inc. | Manual in situ hybridization assay |
| US5707799A (en) * | 1994-09-30 | 1998-01-13 | Abbott Laboratories | Devices and methods utilizing arrays of structures for analyte capture |
| US5709943A (en) * | 1995-05-04 | 1998-01-20 | Minnesota Mining And Manufacturing Company | Biological adsorption supports |
| US5714325A (en) * | 1993-09-24 | 1998-02-03 | New England Medical Center Hospitals | Prenatal diagnosis by isolation of fetal granulocytes from maternal blood |
| US5715946A (en) * | 1995-06-07 | 1998-02-10 | Reichenbach; Steven H. | Method and apparatus for sorting particles suspended in a fluid |
| US5726026A (en) * | 1992-05-01 | 1998-03-10 | Trustees Of The University Of Pennsylvania | Mesoscale sample preparation device and systems for determination and processing of analytes |
| US5731156A (en) * | 1996-10-21 | 1998-03-24 | Applied Imaging, Inc. | Use of anti-embryonic hemoglobin antibodies to identify fetal cells |
| US5856174A (en) * | 1995-06-29 | 1999-01-05 | Affymetrix, Inc. | Integrated nucleic acid diagnostic device |
| US5858188A (en) * | 1990-02-28 | 1999-01-12 | Aclara Biosciences, Inc. | Acrylic microchannels and their use in electrophoretic applications |
| US5858195A (en) * | 1994-08-01 | 1999-01-12 | Lockheed Martin Energy Research Corporation | Apparatus and method for performing microfluidic manipulations for chemical analysis and synthesis |
| US5858187A (en) * | 1996-09-26 | 1999-01-12 | Lockheed Martin Energy Systems, Inc. | Apparatus and method for performing electrodynamic focusing on a microchip |
| US5858649A (en) * | 1992-07-17 | 1999-01-12 | Aprogenex, Inc. | Amplification of mRNA for distinguishing fetal cells in maternal blood |
| US5863502A (en) * | 1996-01-24 | 1999-01-26 | Sarnoff Corporation | Parallel reaction cassette and associated devices |
| US5866345A (en) * | 1992-05-01 | 1999-02-02 | The Trustees Of The University Of Pennsylvania | Apparatus for the detection of an analyte utilizing mesoscale flow systems |
| US5869004A (en) * | 1997-06-09 | 1999-02-09 | Caliper Technologies Corp. | Methods and apparatus for in situ concentration and/or dilution of materials in microfluidic systems |
| US5879624A (en) * | 1997-01-15 | 1999-03-09 | Boehringer Laboratories, Inc. | Method and apparatus for collecting and processing blood |
| US5882465A (en) * | 1997-06-18 | 1999-03-16 | Caliper Technologies Corp. | Method of manufacturing microfluidic devices |
| US6013188A (en) * | 1996-06-07 | 2000-01-11 | Immunivest Corporation | Methods for biological substance analysis employing internal magnetic gradients separation and an externally-applied transport force |
| US6036857A (en) * | 1998-02-20 | 2000-03-14 | Florida State University Research Foundation, Inc. | Apparatus for continuous magnetic separation of components from a mixture |
| US6043027A (en) * | 1997-10-28 | 2000-03-28 | Glaxo Wellcome Inc. | Multi-well single-membrane permeation device and methods |
| US6169816B1 (en) * | 1997-05-14 | 2001-01-02 | Applied Imaging, Inc. | Identification of objects of interest using multiple illumination schemes and finding overlap of features in corresponding multiple images |
| US6174683B1 (en) * | 1999-04-26 | 2001-01-16 | Biocept, Inc. | Method of making biochips and the biochips resulting therefrom |
| US6176962B1 (en) * | 1990-02-28 | 2001-01-23 | Aclara Biosciences, Inc. | Methods for fabricating enclosed microchannel structures |
| US6184043B1 (en) * | 1992-09-14 | 2001-02-06 | FODSTAD øYSTEIN | Method for detection of specific target cells in specialized or mixed cell population and solutions containing mixed cell populations |
| US6186660B1 (en) * | 1997-10-09 | 2001-02-13 | Caliper Technologies Corp. | Microfluidic systems incorporating varied channel dimensions |
| US6197523B1 (en) * | 1997-11-24 | 2001-03-06 | Robert A. Levine | Method for the detection, identification, enumeration and confirmation of circulating cancer and/or hematologic progenitor cells in whole blood |
| US6200765B1 (en) * | 1998-05-04 | 2001-03-13 | Pacific Northwest Cancer Foundation | Non-invasive methods to detect prostate cancer |
| US20020006621A1 (en) * | 1989-11-13 | 2002-01-17 | Children's Medical Center Corporation | Non-invasive method for isolation and detection of fetal DNA |
| US20020005354A1 (en) * | 1997-09-23 | 2002-01-17 | California Institute Of Technology | Microfabricated cell sorter |
| US20020009738A1 (en) * | 2000-04-03 | 2002-01-24 | Houghton Raymond L. | Methods, compositions and kits for the detection and monitoring of breast cancer |
| US20020012931A1 (en) * | 2000-03-27 | 2002-01-31 | Waldman Scott A. | High specificity marker detection |
| US6344326B1 (en) * | 1996-07-30 | 2002-02-05 | Aclara Bio Sciences, Inc. | Microfluidic method for nucleic acid purification and processing |
| US20020019001A1 (en) * | 1999-10-15 | 2002-02-14 | Ventana Medical Systems, Inc. | Method of detecting single gene copies in-situ |
| US6355491B1 (en) * | 1999-03-15 | 2002-03-12 | Aviva Biosciences | Individually addressable micro-electromagnetic unit array chips |
| US6361958B1 (en) * | 1999-11-12 | 2002-03-26 | Motorola, Inc. | Biochannel assay for hybridization with biomaterial |
| US20030017514A1 (en) * | 2001-06-02 | 2003-01-23 | Katharina Pachmann | Method for quantitative detection of vital epithelial tumor cells in a body fluid |
| US6521188B1 (en) * | 2000-11-22 | 2003-02-18 | Industrial Technology Research Institute | Microfluidic actuator |
| US20030036054A1 (en) * | 2000-04-17 | 2003-02-20 | Purdue Research Foundation | Biosensor and related method |
| US20030036100A1 (en) * | 2001-04-10 | 2003-02-20 | Imperial College Innovations Ltd. | Simultaneous determination of phenotype and genotype |
| US6524456B1 (en) * | 1999-08-12 | 2003-02-25 | Ut-Battelle, Llc | Microfluidic devices for the controlled manipulation of small volumes |
| US6529835B1 (en) * | 1998-06-25 | 2003-03-04 | Caliper Technologies Corp. | High throughput methods, systems and apparatus for performing cell based screening assays |
| US20030049563A1 (en) * | 2001-08-03 | 2003-03-13 | Nec Corporation | Fractionating apparatus having colonies of pillars arranged in migration passage at interval and process for fabricating pillars |
| US6537505B1 (en) * | 1998-02-20 | 2003-03-25 | Bio Dot, Inc. | Reagent dispensing valve |
| US6674525B2 (en) * | 2001-04-03 | 2004-01-06 | Micronics, Inc. | Split focusing cytometer |
| US6673541B1 (en) * | 1998-09-18 | 2004-01-06 | Micromet Ag | DNA amplification of a single cell |
| US20040009471A1 (en) * | 2002-04-25 | 2004-01-15 | Bo Cao | Methods and kits for detecting a target cell |
| US20040018116A1 (en) * | 2002-07-26 | 2004-01-29 | Desmond Sean M. | Microfluidic size-exclusion devices, systems, and methods |
| US20040018611A1 (en) * | 2002-07-23 | 2004-01-29 | Ward Michael Dennis | Microfluidic devices for high gradient magnetic separation |
| US6685841B2 (en) * | 2001-02-14 | 2004-02-03 | Gabriel P. Lopez | Nanostructured devices for separation and analysis |
| US20040023222A1 (en) * | 2002-07-31 | 2004-02-05 | Russell Thomas R. | Methods and reagents for improved selection of biological materials |
| US6689615B1 (en) * | 2000-10-04 | 2004-02-10 | James Murto | Methods and devices for processing blood samples |
| US6692952B1 (en) * | 1999-11-10 | 2004-02-17 | Massachusetts Institute Of Technology | Cell analysis and sorting apparatus for manipulation of cells |
| US20040043506A1 (en) * | 2002-08-30 | 2004-03-04 | Horst Haussecker | Cascaded hydrodynamic focusing in microfluidic channels |
| US20050003351A1 (en) * | 2003-04-03 | 2005-01-06 | Monaliza Medical Ltd. | Non-invasive prenatal genetic diagnosis using transcervical cells |
| US20050014208A1 (en) * | 2001-09-06 | 2005-01-20 | Alf-Andreas Krehan | Method and kit for diagnosing or controlling the treatment of breast cancer |
| US6849423B2 (en) * | 2000-11-29 | 2005-02-01 | Picoliter Inc | Focused acoustics for detection and sorting of fluid volumes |
| US6858439B1 (en) * | 1999-03-15 | 2005-02-22 | Aviva Biosciences | Compositions and methods for separation of moieties on chips |
| US20050042685A1 (en) * | 2001-09-06 | 2005-02-24 | Winfried Albert | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| US20050049793A1 (en) * | 2001-04-30 | 2005-03-03 | Patrizia Paterlini-Brechot | Prenatal diagnosis method on isolated foetal cell of maternal blood |
| US20050069886A1 (en) * | 2001-11-07 | 2005-03-31 | Zairen Sun | Prostate cancer genes |
| US20060008807A1 (en) * | 2002-08-23 | 2006-01-12 | O'hara Shawn M | Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample |
| US20060008824A1 (en) * | 2004-05-20 | 2006-01-12 | Leland Stanford Junior University | Methods and compositions for clonal amplification of nucleic acid |
| US20060019235A1 (en) * | 2001-07-02 | 2006-01-26 | The Board Of Trustees Of The Leland Stanford Junior University | Molecular and functional profiling using a cellular microarray |
| US6991917B2 (en) * | 2000-11-29 | 2006-01-31 | Picoliter Inc. | Spatially directed ejection of cells from a carrier fluid |
| US20060051265A1 (en) * | 2004-09-08 | 2006-03-09 | Health Research, Inc. | Apparatus and method for sorting microstructures in a fluid medium |
| US20060060767A1 (en) * | 2001-04-27 | 2006-03-23 | Wang Mark M | Methods and apparatus for use of optical forces for identification, characterization and/or sorting of particles |
| US20070026418A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026417A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026415A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026419A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026414A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026416A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026413A1 (en) * | 2005-07-29 | 2007-02-01 | Mehmet Toner | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026381A1 (en) * | 2005-04-05 | 2007-02-01 | Huang Lotien R | Devices and methods for enrichment and alteration of cells and other particles |
| US20070026469A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070059680A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for cell enrichment |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5310674A (en) | 1982-05-10 | 1994-05-10 | Bar-Ilan University | Apertured cell carrier |
| WO1998022819A1 (en) * | 1996-11-16 | 1998-05-28 | Nmi Naturwissenschaftliches Und Medizinisches Institut An Der Universität Tübingen In Reutlingen Stiftung Bürgerlichen Rechts | Array of microelements, method of contacting cells in a liquid environment and method for the production of an array of microelements |
| DE19712309A1 (en) | 1996-11-16 | 1998-05-20 | Nmi Univ Tuebingen | Microelement arrangement, method for contacting cells in a liquid environment and method for producing a microelement arrangement |
| DE59801410D1 (en) | 1997-12-17 | 2001-10-11 | Ecole Polytech | POSITIONING AND ELECTROPHYSIOLOGICAL CHARACTERIZATION OF INDIVIDUAL CELLS AND RECONSTRUCTED MEMBRANE SYSTEMS ON MICROSTRUCTURED CARRIERS |
-
2000
- 2000-11-10 US US09/710,032 patent/US6692952B1/en not_active Expired - Fee Related
-
2004
- 2004-02-13 US US10/778,831 patent/US20040166555A1/en not_active Abandoned
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4009435A (en) * | 1973-10-19 | 1977-02-22 | Coulter Electronics, Inc. | Apparatus for preservation and identification of particles analyzed by flow-through apparatus |
| US4190535A (en) * | 1978-02-27 | 1980-02-26 | Corning Glass Works | Means for separating lymphocytes and monocytes from anticoagulated blood |
| US4434156A (en) * | 1981-10-26 | 1984-02-28 | The Salk Institute For Biological Studies | Monoclonal antibodies specific for the human transferrin receptor glycoprotein |
| US4729949A (en) * | 1982-05-10 | 1988-03-08 | Bar-Ilan University | System and methods for cell selection |
| US4999283A (en) * | 1986-01-10 | 1991-03-12 | University Of Kentucky Research Foundation | Method for x and y spermatozoa separation |
| US4800159A (en) * | 1986-02-07 | 1989-01-24 | Cetus Corporation | Process for amplifying, detecting, and/or cloning nucleic acid sequences |
| US4906439A (en) * | 1986-03-25 | 1990-03-06 | Pb Diagnostic Systems, Inc. | Biological diagnostic device and method of use |
| US4814098A (en) * | 1986-09-06 | 1989-03-21 | Bellex Corporation | Magnetic material-physiologically active substance conjugate |
| US4894343A (en) * | 1986-11-19 | 1990-01-16 | Hitachi, Ltd. | Chamber plate for use in cell fusion and a process for production thereof |
| US4895805A (en) * | 1987-08-31 | 1990-01-23 | Hitachi, Ltd. | Cell manipulating apparatus |
| US5707801A (en) * | 1988-08-31 | 1998-01-13 | Aprogenex, Inc. | Manual in situ hybridization assay |
| US5183744A (en) * | 1988-10-26 | 1993-02-02 | Hitachi, Ltd. | Cell handling method for cell fusion processor |
| US4984574A (en) * | 1988-11-23 | 1991-01-15 | Seth Goldberg | Noninvasive fetal oxygen monitor using NMR |
| US20020006621A1 (en) * | 1989-11-13 | 2002-01-17 | Children's Medical Center Corporation | Non-invasive method for isolation and detection of fetal DNA |
| US20040018509A1 (en) * | 1989-11-13 | 2004-01-29 | Bianchi Diana W. | Non-invasive method for isolation and detection of fetal DNA |
| US20060051775A1 (en) * | 1989-11-13 | 2006-03-09 | The Children's Hospital | Non-invasive method for isolation and detection of fetal DNA |
| US6176962B1 (en) * | 1990-02-28 | 2001-01-23 | Aclara Biosciences, Inc. | Methods for fabricating enclosed microchannel structures |
| US5858188A (en) * | 1990-02-28 | 1999-01-12 | Aclara Biosciences, Inc. | Acrylic microchannels and their use in electrophoretic applications |
| US5726026A (en) * | 1992-05-01 | 1998-03-10 | Trustees Of The University Of Pennsylvania | Mesoscale sample preparation device and systems for determination and processing of analytes |
| US5866345A (en) * | 1992-05-01 | 1999-02-02 | The Trustees Of The University Of Pennsylvania | Apparatus for the detection of an analyte utilizing mesoscale flow systems |
| US6184029B1 (en) * | 1992-05-01 | 2001-02-06 | Trustees Of The University Of Pennsylvania | Mesoscale sample preparation device and systems for determination and processing of analytes |
| US5296375A (en) * | 1992-05-01 | 1994-03-22 | Trustees Of The University Of Pennsylvania | Mesoscale sperm handling devices |
| US5486335A (en) * | 1992-05-01 | 1996-01-23 | Trustees Of The University Of Pennsylvania | Analysis based on flow restriction |
| US5498392A (en) * | 1992-05-01 | 1996-03-12 | Trustees Of The University Of Pennsylvania | Mesoscale polynucleotide amplification device and method |
| US5858649A (en) * | 1992-07-17 | 1999-01-12 | Aprogenex, Inc. | Amplification of mRNA for distinguishing fetal cells in maternal blood |
| US5861253A (en) * | 1992-07-17 | 1999-01-19 | Aprogenex, Inc. | Intracellular antigens for identifying fetal cells in maternal blood |
| US6184043B1 (en) * | 1992-09-14 | 2001-02-06 | FODSTAD øYSTEIN | Method for detection of specific target cells in specialized or mixed cell population and solutions containing mixed cell populations |
| US5275933A (en) * | 1992-09-25 | 1994-01-04 | The Board Of Trustees Of The Leland Stanford Junior University | Triple gradient process for recovering nucleated fetal cells from maternal blood |
| US5489506A (en) * | 1992-10-26 | 1996-02-06 | Biolife Systems, Inc. | Dielectrophoretic cell stream sorter |
| US5714325A (en) * | 1993-09-24 | 1998-02-03 | New England Medical Center Hospitals | Prenatal diagnosis by isolation of fetal granulocytes from maternal blood |
| US6033546A (en) * | 1994-08-01 | 2000-03-07 | Lockheed Martin Energy Research Corporation | Apparatus and method for performing microfluidic manipulations for chemical analysis and synthesis |
| US5858195A (en) * | 1994-08-01 | 1999-01-12 | Lockheed Martin Energy Research Corporation | Apparatus and method for performing microfluidic manipulations for chemical analysis and synthesis |
| US5707799A (en) * | 1994-09-30 | 1998-01-13 | Abbott Laboratories | Devices and methods utilizing arrays of structures for analyte capture |
| US5709943A (en) * | 1995-05-04 | 1998-01-20 | Minnesota Mining And Manufacturing Company | Biological adsorption supports |
| US5715946A (en) * | 1995-06-07 | 1998-02-10 | Reichenbach; Steven H. | Method and apparatus for sorting particles suspended in a fluid |
| US5856174A (en) * | 1995-06-29 | 1999-01-05 | Affymetrix, Inc. | Integrated nucleic acid diagnostic device |
| US5863502A (en) * | 1996-01-24 | 1999-01-26 | Sarnoff Corporation | Parallel reaction cassette and associated devices |
| US6013188A (en) * | 1996-06-07 | 2000-01-11 | Immunivest Corporation | Methods for biological substance analysis employing internal magnetic gradients separation and an externally-applied transport force |
| US6344326B1 (en) * | 1996-07-30 | 2002-02-05 | Aclara Bio Sciences, Inc. | Microfluidic method for nucleic acid purification and processing |
| US5858187A (en) * | 1996-09-26 | 1999-01-12 | Lockheed Martin Energy Systems, Inc. | Apparatus and method for performing electrodynamic focusing on a microchip |
| US5731156A (en) * | 1996-10-21 | 1998-03-24 | Applied Imaging, Inc. | Use of anti-embryonic hemoglobin antibodies to identify fetal cells |
| US5879624A (en) * | 1997-01-15 | 1999-03-09 | Boehringer Laboratories, Inc. | Method and apparatus for collecting and processing blood |
| US6169816B1 (en) * | 1997-05-14 | 2001-01-02 | Applied Imaging, Inc. | Identification of objects of interest using multiple illumination schemes and finding overlap of features in corresponding multiple images |
| US5869004A (en) * | 1997-06-09 | 1999-02-09 | Caliper Technologies Corp. | Methods and apparatus for in situ concentration and/or dilution of materials in microfluidic systems |
| US5882465A (en) * | 1997-06-18 | 1999-03-16 | Caliper Technologies Corp. | Method of manufacturing microfluidic devices |
| US20020005354A1 (en) * | 1997-09-23 | 2002-01-17 | California Institute Of Technology | Microfabricated cell sorter |
| US6186660B1 (en) * | 1997-10-09 | 2001-02-13 | Caliper Technologies Corp. | Microfluidic systems incorporating varied channel dimensions |
| US6517234B1 (en) * | 1997-10-09 | 2003-02-11 | Caliper Technologies Corp. | Microfluidic systems incorporating varied channel dimensions |
| US6043027A (en) * | 1997-10-28 | 2000-03-28 | Glaxo Wellcome Inc. | Multi-well single-membrane permeation device and methods |
| US6197523B1 (en) * | 1997-11-24 | 2001-03-06 | Robert A. Levine | Method for the detection, identification, enumeration and confirmation of circulating cancer and/or hematologic progenitor cells in whole blood |
| US6036857A (en) * | 1998-02-20 | 2000-03-14 | Florida State University Research Foundation, Inc. | Apparatus for continuous magnetic separation of components from a mixture |
| US6537505B1 (en) * | 1998-02-20 | 2003-03-25 | Bio Dot, Inc. | Reagent dispensing valve |
| US6200765B1 (en) * | 1998-05-04 | 2001-03-13 | Pacific Northwest Cancer Foundation | Non-invasive methods to detect prostate cancer |
| US6529835B1 (en) * | 1998-06-25 | 2003-03-04 | Caliper Technologies Corp. | High throughput methods, systems and apparatus for performing cell based screening assays |
| US6673541B1 (en) * | 1998-09-18 | 2004-01-06 | Micromet Ag | DNA amplification of a single cell |
| US6355491B1 (en) * | 1999-03-15 | 2002-03-12 | Aviva Biosciences | Individually addressable micro-electromagnetic unit array chips |
| US6858439B1 (en) * | 1999-03-15 | 2005-02-22 | Aviva Biosciences | Compositions and methods for separation of moieties on chips |
| US6174683B1 (en) * | 1999-04-26 | 2001-01-16 | Biocept, Inc. | Method of making biochips and the biochips resulting therefrom |
| US6524456B1 (en) * | 1999-08-12 | 2003-02-25 | Ut-Battelle, Llc | Microfluidic devices for the controlled manipulation of small volumes |
| US20020019001A1 (en) * | 1999-10-15 | 2002-02-14 | Ventana Medical Systems, Inc. | Method of detecting single gene copies in-situ |
| US6692952B1 (en) * | 1999-11-10 | 2004-02-17 | Massachusetts Institute Of Technology | Cell analysis and sorting apparatus for manipulation of cells |
| US6361958B1 (en) * | 1999-11-12 | 2002-03-26 | Motorola, Inc. | Biochannel assay for hybridization with biomaterial |
| US20020012931A1 (en) * | 2000-03-27 | 2002-01-31 | Waldman Scott A. | High specificity marker detection |
| US20020009738A1 (en) * | 2000-04-03 | 2002-01-24 | Houghton Raymond L. | Methods, compositions and kits for the detection and monitoring of breast cancer |
| US20030036054A1 (en) * | 2000-04-17 | 2003-02-20 | Purdue Research Foundation | Biosensor and related method |
| US6689615B1 (en) * | 2000-10-04 | 2004-02-10 | James Murto | Methods and devices for processing blood samples |
| US6521188B1 (en) * | 2000-11-22 | 2003-02-18 | Industrial Technology Research Institute | Microfluidic actuator |
| US6991917B2 (en) * | 2000-11-29 | 2006-01-31 | Picoliter Inc. | Spatially directed ejection of cells from a carrier fluid |
| US6849423B2 (en) * | 2000-11-29 | 2005-02-01 | Picoliter Inc | Focused acoustics for detection and sorting of fluid volumes |
| US6685841B2 (en) * | 2001-02-14 | 2004-02-03 | Gabriel P. Lopez | Nanostructured devices for separation and analysis |
| US6674525B2 (en) * | 2001-04-03 | 2004-01-06 | Micronics, Inc. | Split focusing cytometer |
| US20030036100A1 (en) * | 2001-04-10 | 2003-02-20 | Imperial College Innovations Ltd. | Simultaneous determination of phenotype and genotype |
| US20060060767A1 (en) * | 2001-04-27 | 2006-03-23 | Wang Mark M | Methods and apparatus for use of optical forces for identification, characterization and/or sorting of particles |
| US20050049793A1 (en) * | 2001-04-30 | 2005-03-03 | Patrizia Paterlini-Brechot | Prenatal diagnosis method on isolated foetal cell of maternal blood |
| US20030017514A1 (en) * | 2001-06-02 | 2003-01-23 | Katharina Pachmann | Method for quantitative detection of vital epithelial tumor cells in a body fluid |
| US20060019235A1 (en) * | 2001-07-02 | 2006-01-26 | The Board Of Trustees Of The Leland Stanford Junior University | Molecular and functional profiling using a cellular microarray |
| US20030049563A1 (en) * | 2001-08-03 | 2003-03-13 | Nec Corporation | Fractionating apparatus having colonies of pillars arranged in migration passage at interval and process for fabricating pillars |
| US20050014208A1 (en) * | 2001-09-06 | 2005-01-20 | Alf-Andreas Krehan | Method and kit for diagnosing or controlling the treatment of breast cancer |
| US20050042685A1 (en) * | 2001-09-06 | 2005-02-24 | Winfried Albert | Method and diagnosis kit for selecting and or qualitative and/or quantitative detection of cells |
| US20050069886A1 (en) * | 2001-11-07 | 2005-03-31 | Zairen Sun | Prostate cancer genes |
| US20040009471A1 (en) * | 2002-04-25 | 2004-01-15 | Bo Cao | Methods and kits for detecting a target cell |
| US20040018611A1 (en) * | 2002-07-23 | 2004-01-29 | Ward Michael Dennis | Microfluidic devices for high gradient magnetic separation |
| US20040018116A1 (en) * | 2002-07-26 | 2004-01-29 | Desmond Sean M. | Microfluidic size-exclusion devices, systems, and methods |
| US20040023222A1 (en) * | 2002-07-31 | 2004-02-05 | Russell Thomas R. | Methods and reagents for improved selection of biological materials |
| US20060008807A1 (en) * | 2002-08-23 | 2006-01-12 | O'hara Shawn M | Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample |
| US20040043506A1 (en) * | 2002-08-30 | 2004-03-04 | Horst Haussecker | Cascaded hydrodynamic focusing in microfluidic channels |
| US20050003351A1 (en) * | 2003-04-03 | 2005-01-06 | Monaliza Medical Ltd. | Non-invasive prenatal genetic diagnosis using transcervical cells |
| US20060008824A1 (en) * | 2004-05-20 | 2006-01-12 | Leland Stanford Junior University | Methods and compositions for clonal amplification of nucleic acid |
| US20060051265A1 (en) * | 2004-09-08 | 2006-03-09 | Health Research, Inc. | Apparatus and method for sorting microstructures in a fluid medium |
| US20070026381A1 (en) * | 2005-04-05 | 2007-02-01 | Huang Lotien R | Devices and methods for enrichment and alteration of cells and other particles |
| US20070026419A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026415A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026417A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026414A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026416A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026413A1 (en) * | 2005-07-29 | 2007-02-01 | Mehmet Toner | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026418A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070026469A1 (en) * | 2005-07-29 | 2007-02-01 | Martin Fuchs | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US20070059680A1 (en) * | 2005-09-15 | 2007-03-15 | Ravi Kapur | System for cell enrichment |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060128006A1 (en) * | 1999-11-10 | 2006-06-15 | Gerhardt Antimony L | Hydrodynamic capture and release mechanisms for particle manipulation |
| US9926521B2 (en) | 2000-06-27 | 2018-03-27 | Fluidigm Corporation | Microfluidic particle-analysis systems |
| US20100120077A1 (en) * | 2002-04-01 | 2010-05-13 | Fluidigm Corporation | Microfluidic particle-analysis systems |
| US8658418B2 (en) * | 2002-04-01 | 2014-02-25 | Fluidigm Corporation | Microfluidic particle-analysis systems |
| US20060073470A1 (en) * | 2002-05-30 | 2006-04-06 | Naohiro Noda | Method of counting microorganisms or cells |
| US8304230B2 (en) | 2002-09-27 | 2012-11-06 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US11052392B2 (en) | 2002-09-27 | 2021-07-06 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US10081014B2 (en) | 2002-09-27 | 2018-09-25 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US8986966B2 (en) | 2002-09-27 | 2015-03-24 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US8895298B2 (en) | 2002-09-27 | 2014-11-25 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US8372579B2 (en) | 2002-09-27 | 2013-02-12 | The General Hospital Corporation | Microfluidic device for cell separation and uses thereof |
| US9174222B2 (en) | 2005-04-05 | 2015-11-03 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| EP2594631A1 (en) | 2005-04-05 | 2013-05-22 | Cellpoint Diagnostics | Devices and method for detecting circulating tumor cells and other particles |
| US8021614B2 (en) | 2005-04-05 | 2011-09-20 | The General Hospital Corporation | Devices and methods for enrichment and alteration of cells and other particles |
| US9956562B2 (en) | 2005-04-05 | 2018-05-01 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US8585971B2 (en) | 2005-04-05 | 2013-11-19 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| US10786817B2 (en) | 2005-04-05 | 2020-09-29 | The General Hospital Corporation | Devices and method for enrichment and alteration of cells and other particles |
| WO2006133208A3 (en) * | 2005-06-07 | 2007-11-22 | Massachusetts Inst Technology | Hydrodynamic capture and release mechanisms for particle manipulation |
| WO2006133208A2 (en) * | 2005-06-07 | 2006-12-14 | Massachusetts Institute Of Technology | Hydrodynamic capture and release mechanisms for particle manipulation |
| US8921102B2 (en) | 2005-07-29 | 2014-12-30 | Gpb Scientific, Llc | Devices and methods for enrichment and alteration of circulating tumor cells and other particles |
| US9273355B2 (en) | 2006-06-14 | 2016-03-01 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US10704090B2 (en) | 2006-06-14 | 2020-07-07 | Verinata Health, Inc. | Fetal aneuploidy detection by sequencing |
| US10591391B2 (en) | 2006-06-14 | 2020-03-17 | Verinata Health, Inc. | Diagnosis of fetal abnormalities using polymorphisms including short tandem repeats |
| US10155984B2 (en) | 2006-06-14 | 2018-12-18 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US8168389B2 (en) | 2006-06-14 | 2012-05-01 | The General Hospital Corporation | Fetal cell analysis using sample splitting |
| US8372584B2 (en) | 2006-06-14 | 2013-02-12 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US11674176B2 (en) | 2006-06-14 | 2023-06-13 | Verinata Health, Inc | Fetal aneuploidy detection by sequencing |
| US8137912B2 (en) | 2006-06-14 | 2012-03-20 | The General Hospital Corporation | Methods for the diagnosis of fetal abnormalities |
| US9017942B2 (en) | 2006-06-14 | 2015-04-28 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US11781187B2 (en) | 2006-06-14 | 2023-10-10 | The General Hospital Corporation | Rare cell analysis using sample splitting and DNA tags |
| US9347100B2 (en) | 2006-06-14 | 2016-05-24 | Gpb Scientific, Llc | Rare cell analysis using sample splitting and DNA tags |
| US8691151B2 (en) * | 2006-08-31 | 2014-04-08 | Massachusetts Institute Of Technology | Opto-fluidic architecture for particle manipulation and sorting |
| US20090258383A1 (en) * | 2006-08-31 | 2009-10-15 | Joseph Kovac | Opto-fluidic architecture for particle manipulation and sorting |
| US8682594B2 (en) | 2008-09-20 | 2014-03-25 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US9353414B2 (en) | 2008-09-20 | 2016-05-31 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US9404157B2 (en) | 2008-09-20 | 2016-08-02 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8195415B2 (en) | 2008-09-20 | 2012-06-05 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8296076B2 (en) | 2008-09-20 | 2012-10-23 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuoploidy by sequencing |
| US10669585B2 (en) | 2008-09-20 | 2020-06-02 | The Board Of Trustees Of The Leland Stanford Junior University | Noninvasive diagnosis of fetal aneuploidy by sequencing |
| US8569069B2 (en) * | 2010-04-19 | 2013-10-29 | Sony Corporation | System and method for high throughput cell analysis and sorting |
| US20120094324A1 (en) * | 2010-04-19 | 2012-04-19 | Durack Gary P | System and method for high throughput cell analysis and sorting |
| WO2012048113A2 (en) | 2010-10-07 | 2012-04-12 | The General Hospital Corporation | Biomarkers of cancer |
| US11085923B2 (en) | 2011-03-24 | 2021-08-10 | Anpac Bio-Medical Science Co., Ltd | Micro-devices for disease detection |
| US9408565B2 (en) | 2011-05-05 | 2016-08-09 | Shanghai Xinshenpai Technology Co., Ltd. | Apparatus for detecting tumor cells |
| WO2012151501A3 (en) * | 2011-05-05 | 2013-04-25 | Anpac Bio-Medical Science Co., Ltd. | Apparatus for detecting tumor cells |
| US10895573B2 (en) | 2011-05-05 | 2021-01-19 | Anpac Bio-Medical Science Co., Ltd. | Apparatus for detecting tumor cells |
| WO2013063035A1 (en) | 2011-10-24 | 2013-05-02 | The General Hospital Corporation | Biomarkers of cancer |
| WO2014052685A3 (en) * | 2012-09-26 | 2014-05-30 | Quantumcyte, Inc. | Devices and methods for single cell analysis |
| US10852220B2 (en) | 2013-03-15 | 2020-12-01 | The Trustees Of Princeton University | Methods and devices for high throughput purification |
| US11142746B2 (en) | 2013-03-15 | 2021-10-12 | University Of Maryland, Baltimore | High efficiency microfluidic purification of stem cells to improve transplants |
| US11486802B2 (en) | 2013-03-15 | 2022-11-01 | University Of Maryland, Baltimore | Methods and devices for high throughput purification |
| US11493428B2 (en) | 2013-03-15 | 2022-11-08 | Gpb Scientific, Inc. | On-chip microfluidic processing of particles |
| US10324011B2 (en) | 2013-03-15 | 2019-06-18 | The Trustees Of Princeton University | Methods and devices for high throughput purification |
| WO2015200756A1 (en) * | 2014-06-26 | 2015-12-30 | President And Fellows Of Harvard College | Pore-based bubble chamber |
| US10976232B2 (en) | 2015-08-24 | 2021-04-13 | Gpb Scientific, Inc. | Methods and devices for multi-step cell purification and concentration |
| US10258741B2 (en) | 2016-12-28 | 2019-04-16 | Cequr Sa | Microfluidic flow restrictor and system |
Also Published As
| Publication number | Publication date |
|---|---|
| US6692952B1 (en) | 2004-02-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6692952B1 (en) | Cell analysis and sorting apparatus for manipulation of cells | |
| EP1236032A2 (en) | Cell analysis and sorting apparatus for manipulation of cells | |
| US20060128006A1 (en) | Hydrodynamic capture and release mechanisms for particle manipulation | |
| Chen et al. | A multichannel neural probe for selective chemical delivery at the cellular level | |
| US7425253B2 (en) | Microscale sorting cytometer | |
| US8895292B2 (en) | Microfluidic chip devices and their use | |
| US8585916B2 (en) | Micropores and methods of making and using thereof | |
| KR20050106408A (en) | Microfluidic device with thin-film electronic devices | |
| US20040219732A1 (en) | Thermal micro-valves for micro-integrated devices | |
| US20090066315A1 (en) | Dynamic modulation for multiplexation of microfluidic and nanofluidic based biosensors | |
| JP2009539105A (en) | Transparent microfluidic device | |
| CN109112063A (en) | A kind of detection of nucleic acids micro-fluidic chip and preparation method thereof | |
| JP2005513455A (en) | Dielectric gate for injecting and controlling fluids | |
| Bavière et al. | An experimental study of water flow in smooth and rough rectangular micro-channels | |
| EP2764347A2 (en) | Devices for detecting a particle in a sample and methods for use thereof | |
| Mahabadi et al. | Fabrication of PMMA micro-and nanofluidic channels by proton beam writing: electrokinetic and morphological characterization | |
| Xu et al. | A MEMS multi-sensor chip for gas flow sensing | |
| WO2012011810A1 (en) | Lab-on-a-chip device, for instance for use of the analysis of semen | |
| Monserrat Lopez et al. | Direct electrification of silicon microfluidics for electric field applications | |
| Davaji et al. | In-vivo single cell protein interaction investigation using microfluidic platform | |
| Huchet et al. | Multi-scale analysis of hydrodynamics inside a network of crossing minichannels using electrodiffusion method and PIV measurements | |
| Okamoto et al. | Thermal flow sensor with a bidirectional thermal reference | |
| Jachowicz et al. | 1C4. 18P | |
| Höjer | Characterization of Agarose Microdroplet Properties for Acoustic Trapping of Encapsulated Bacteria and On-chip DNA Extraction | |
| Basu et al. | Trapping and manipulation of particles and droplets using micro-toroidal convection currents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |