US20050015888A1 - Wrinkle resistant composition - Google Patents
Wrinkle resistant composition Download PDFInfo
- Publication number
- US20050015888A1 US20050015888A1 US10/924,539 US92453904A US2005015888A1 US 20050015888 A1 US20050015888 A1 US 20050015888A1 US 92453904 A US92453904 A US 92453904A US 2005015888 A1 US2005015888 A1 US 2005015888A1
- Authority
- US
- United States
- Prior art keywords
- composition
- fabric
- water
- group
- polymers
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M23/00—Treatment of fibres, threads, yarns, fabrics or fibrous goods made from such materials, characterised by the process
- D06M23/06—Processes in which the treating agent is dispersed in a gas, e.g. aerosols
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M13/00—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with non-macromolecular organic compounds; Such treatment combined with mechanical treatment
- D06M13/10—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with non-macromolecular organic compounds; Such treatment combined with mechanical treatment with compounds containing oxygen
- D06M13/224—Esters of carboxylic acids; Esters of carbonic acid
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M13/00—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with non-macromolecular organic compounds; Such treatment combined with mechanical treatment
- D06M13/10—Treating fibres, threads, yarns, fabrics or fibrous goods made from such materials, with non-macromolecular organic compounds; Such treatment combined with mechanical treatment with compounds containing oxygen
- D06M13/224—Esters of carboxylic acids; Esters of carbonic acid
- D06M13/2243—Mono-, di-, or triglycerides
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/01—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with natural macromolecular compounds or derivatives thereof
- D06M15/03—Polysaccharides or derivatives thereof
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/21—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/263—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of unsaturated carboxylic acids; Salts or esters thereof
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/21—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/263—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of unsaturated carboxylic acids; Salts or esters thereof
- D06M15/267—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of unsaturated carboxylic acids; Salts or esters thereof of unsaturated carboxylic esters having amino or quaternary ammonium groups
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/21—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/285—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of unsaturated carboxylic acid amides or imides
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/21—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/356—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of other unsaturated compounds containing nitrogen, sulfur, silicon or phosphorus atoms
- D06M15/3562—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of other unsaturated compounds containing nitrogen, sulfur, silicon or phosphorus atoms containing nitrogen
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/37—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/53—Polyethers
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/37—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/564—Polyureas, polyurethanes or other polymers having ureide or urethane links; Precondensation products forming them
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/37—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/643—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds containing silicon in the main chain
- D06M15/647—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds containing silicon in the main chain containing polyether sequences
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M7/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made of other substances with subsequent freeing of the treated goods from the treating medium, e.g. swelling, e.g. polyolefins
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M2200/00—Functionality of the treatment composition and/or properties imparted to the textile material
- D06M2200/20—Treatment influencing the crease behaviour, the wrinkle resistance, the crease recovery or the ironing ease
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M2200/00—Functionality of the treatment composition and/or properties imparted to the textile material
- D06M2200/40—Reduced friction resistance, lubricant properties; Sizing compositions
Definitions
- the present invention relates to fabric care compositions and to a method for treating fabrics in order to improve various properties of fabrics, in particular in-wear wrinkle resistance.
- Wrinkles in textile fabrics are caused by the bending and creasing of the textile material which places an external portion of a filament in a yarn under tension while the internal portion of that filament in the yarn is placed under compression.
- the hydrogen bonding that occurs between the cellulose molecules contributes to keeping wrinkles in place.
- the wrinkling of fabric, in particular clothing, is therefore subject to the inherent tensional elastic deformation and recovery properties of the fibers which constitute the yam and fabrics.
- U.S. Pat. No. 5,532,023 discloses aqueous wrinkle control compositions containing non-volatile silicone and film-forming polymer.
- Preferred silicones include reactive silicones and amino-functional silicone, known as “amodimethicone”.
- the composition containing such silicones is applied to fabric from a spray dispenser. It is found that in the spray treatment, an appreciable amount of the aqueous composition misses the fabric, and instead falls on flooring surfaces, such as rugs, carpets, concrete floors, tiled floors, linoleum floors, bathtub floors, which leaves a silicone layer that is accumulated on and/or cured on and/or bonded to the flooring surfaces.
- U.S. Pat. No. 5,573,695 discloses an aqueous wrinkle removal composition containing a vegetable oil based cationic quaternary ammonium surfactant, and an anionic fluorosurfactant.
- U.S. Pat. No. 4,661,268 discloses a wrinkle removal spray comprising an aqueous alcoholic composition containing a dialkyl quaternary ammonium salt and a silicone surfactant and/or a fluoro surfactant.
- 5,100,566 discloses a method of reducing wrinkles in fabric by spraying the fabric with an aqueous alcoholic solution of an anionic siliconate alkali metal salt.
- U.S. Pat. No. 4,806,254 discloses fabric wrinkle removal aqueous alcoholic solution containing glycerine and a nonionic surfactant.
- WO98/04772 provides the treatment of fabric against fabric creasing by application of a composition comprising a polycarboxylic acid or derivative thereof; and then curing the composition using a domestic process.
- Starch is also a conventional ingredient of dewrinkling compositions. However, while starch provides a suitable visual benefit onto the treated fabrics, it also gives fabric with an undesired stiff or starchy feeling.
- a lubricant preferably a water-soluble one
- component having a deviation of fabric Wrinkle Recovery Angle (WRA) versus water of at least +15 fulfill such a need.
- WRA fabric Wrinkle Recovery Angle
- This finding is particularly surprising, especially when the component providing such deviation is a polymer.
- the combination of a lubricant, especially a water-soluble one, with polymer is often the cause of phase separation.
- polymer like starch on top of composition comprising a lubricant, preferably a water-soluble one was found to give even worse results on the in-wear performance.
- the addition of a component providing a deviation of fabric WRA of at least +15 overcome such problems.
- the present invention reduces wrinkles in fabrics, including clothing, dry cleanables, linens, bed clothes, and draperies, by ironing.
- the present invention can be used on damp or dry clothing to relax wrinkles and give clothes a ready to wear look with lasting benefits that is demanded by today's fast paced world.
- an additional benefit of the composition of the present invention is an improved garment shape, body and crispness.
- composition of the present invention acts as an excellent ironing aid.
- the present invention makes the task of ironing easier and faster by creating less iron drag.
- the compositions of the present invention help produce a crisp, smooth appearance.
- the present invention is a wrinkle reducing composition
- a wrinkle reducing composition comprising a lubricant, preferably a water-soluble one, and a component having a deviation of fabric Wrinkle Recovery angle (WRA) versus water of at least +15.
- WRA fabric Wrinkle Recovery angle
- an article of manufacture comprising the composition of the invention, such as a sprayer, an aerosol, a foam dispenser, an iron, a refill cartridge thereof which contains the composition.
- a method of treating fabrics for imparting benefits selected from the group consisting of: reducing wrinkles and imparting in-wear resistance to fabrics.
- the composition is sprayed onto a fabric and the fabric is ironed.
- an article of manufacture comprising a container and the composition of the invention in association with usage instructions, in particular, instructions to use in a method where the composition is sprayed onto the fabric and the fabric is ironed.
- One essential component of the invention is a lubricant, preferably a water-soluble one.
- a lubricant preferably a water-soluble one.
- water-soluble is defined as “a component which when dissolved in water at a level of 0.2% by weight, or less, at 25° C., forms a clear, isotropic liquid”.
- Typical water-soluble lubricants include components selected from nonionic silicone containing surfactants, sorbitan esters, ethoxylated sorbitan esters, and mixtures thereof.
- the water-soluble lubricants are preferably present in an amount of from 0.1% to 70% by weight of the composition, more preferably of from 1 to 10% % by weight of the composition for diluted composition and of from 20 to 50% by weight of the composition for concentrated compositions.
- a preferred class of nonionic silicone containing surfactants are the polyalkylene oxide polysiloxanes having a dimethyl polysiloxane hydrophobic moiety and one or more hydrophilic polyalkylene side chains, and having the general formula: R1—(CH3)2SiO—(CH3)2SiO]a—[(CH3)(R1)SiO]b—Si(CH3)2—R1 wherein a+b are from about 1 to about 50, preferably from about 1 to about 30, more preferably from about 1 to about 25, and each R1 is the same or different and is selected from the group consisting of methyl and a poly(ethyleneoxide/propyleneoxide) copolymer group having the general formula: —(CH2)n O(C2 H4 O)c (C3 H6 O)d R2 with at least one R1 being a poly(ethyleneoxy/propyleneoxy) copolymer group, and wherein n is 3 or 4, preferably 3; total c (for all polyalkylene
- Nonlimiting examples of this type of surfactants are the Silwet® surfactants which are available OSI Specialties Inc., a Division of Witco, Danbury, Connecticut.
- Representative Silwet® surfactants which contain only ethyleneoxy (C2H4O) groups are as follows. Name Average MW Average a + b Average total c L-7608 600 1 8 L-7607 1,000 2 17 L-77 600 1 9 L-7605 6,000 20 99 L-7604 4,000 21 53 L-7600 4,000 11 68 L-7657 5,000 20 76 L-7602 3,000 20 29 L-7622 10,000 88 75
- Nonlimiting examples of Silwet® surfactants which contain both ethyleneoxy (C2 H4 O) and propyleneoxy (C3 H6 O) groups are as follows. Name Average MW EO/PO ratio L-720 12,000 50/50 L-7001 20,000 40/60 L-7002 8,000 50/50 L-7210 13,000 20/80 L-7200 19,000 75/25 L-7220 17,000 20/80
- the molecular weight of the polyalkyleneoxy group (R1) is less than or equal to about 10,000.
- the molecular weight of the polyalkyleneoxy group is less than or equal to about 8,000, and most preferably ranges from about 300 to about 5,000.
- the values of c and d can be those numbers which provide molecular weights within these ranges.
- the number of ethyleneoxy units (—C2H4O) in the polyether chain (R1) must be sufficient to render the polyalkylene oxide polysiloxane water-soluble. If propyleneoxy groups are present in the polyalkylenoxy chain, they can be distributed randomly in the chain or exist as blocks.
- Silwet® surfactants which contain both ethyleneoxy and propyleneoxy groups, are also preferred.
- Preferred Silwet® surfactants are the L-7001, L-7087, L-7200, L-7280, L-7600, L-7608, L-7622, L-7657.
- polyalkylene oxide polysiloxanes of the present invention can be prepared according to the procedure set forth in U.S. Pat. No. 3,299,112, incorporated herein by reference.
- polyalkylene oxide polysiloxanes of the surfactant blend of the present invention are readily prepared by an addition reaction between a hydrosiloxane (i.e., a siloxane containing silicon-bonded hydrogen) and an alkenyl ether (e.g., a vinyl, allyl, or methallyl ether) of an alkoxy or hydroxy end-blocked polyalkylene oxide).
- a hydrosiloxane i.e., a siloxane containing silicon-bonded hydrogen
- an alkenyl ether e.g., a vinyl, allyl, or methallyl ether
- reaction conditions employed in addition reactions of this type are well-known in the art and in general involve heating the reactants (e.g., at a temperature of from about 85° C. to 110° C.) in the presence of a platinum catalyst (e.g., chloroplatinic acid) and a solvent (e.g., toluene).
- a platinum catalyst e.g., chloroplatinic acid
- a solvent e.g., toluene
- Still other preferred water-soluble lubricants of the nonionic type are those from the class of sorbitan esters and/or alkylethoxylate sorbitan ester. These ethoxylated sorbitan esters are formed by ethoxylation of sorbitan or its cyclic derivative sorbitan, followed by esterification of one of the available hydroxy groups to introduce one long chain alkyl or alkenyl group, leaving the remaining hydroxy groups free.
- Water-insoluble lubricants are also useful herein. Suitable water-insoluble lubricants include cationic fabric softeners, silicones, and aliphatic and cycloaliphatic hydrocarbons.
- Suitable cationic fabric softening components for use herein include the water-insoluble quaternary-ammonium fabric softeners, the most commonly used having been di-long alkyl chain ammonium chloride or methyl sulfate.
- Preferred cationic softeners among these include the following:
- the quaternary ammonium compounds and amine precursors herein have the formula (I) or (II), below: wherein Q is selected from —O—C(O)—, —C(O)—O—, —O—C(O)—O—, —NR 4 —C(O)—, —C(O)—NR 4 —;
- Non-limiting examples of softener-compatible anions include chloride or methyl sulfate.
- the alkyl, or alkenyl, chain T 1 , T 2 , T 3 , T 4 , T 5 must contain at least 6 carbon atoms, preferably at least 11 carbon atoms, more preferably at least 16 carbon atoms.
- the chain may be straight or branched.
- Tallow is a convenient and inexpensive source of long chain alkyl and alkenyl material.
- the compounds wherein T 1 , T 2 , T 3 , T 4 , T 5 represents the mixture of long chain materials typical for tallow are particularly preferred.
- quaternary ammonium compounds suitable for use in the aqueous fabric softening compositions herein include:
- compounds 1-7 are examples of compounds of Formula (I); compound 8 is a compound of Formula (II).
- N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl ammonium chloride where the tallow chains are at least partially unsaturated.
- the level of unsaturation of the tallow chain can be measured by the Iodine Value (IV) of the corresponding fatty acid, which in the present case should preferably be in the range of from 5 to 100 with two categories of compounds being distinguished, having a IV below or above 25.
- IV Iodine Value
- the anion is merely present as a counterion of the positively charged quaternary ammonium compounds.
- the nature of the counterion is not critical at all to the practice of the present invention.
- the scope of this invention is not considered limited to any particular anion.
- amine precursors thereof is meant the secondary or tertiary amines corresponding to the above quaternary ammonium compounds, said amines being substantially protonated in the present compositions due to the pH values.
- Still other water-insoluble lubricants include polyalkyl or polyaryl siloxanes with the following structure:
- the alkyl or aryl groups substituted on the siloxane chain (R) or at the ends of the siloxane chains (A) can have any structure as long as the resulting silicones remain fluid at room temperature.
- the silicones are hydrophobic, are neither irritating, toxic, nor otherwise harmful when applied to fabrics or when they come in contact with human skin, are compatible with other components of the composition, are chemically stable under normal use and storage conditions, and are capable of being deposited on fabric.
- the R group preferably is a phenyl, a hydroxy, an alkyl or an aryl.
- the two R groups on the silicone atom can represent the same group or different groups. More preferably, the two R groups represent the same group preferably a methyl, an ethyl, a propyl, a phenyl or a hydroxy group, q is prereably an integer from about 7 to about 8,000.
- A represents groups which block the ends of the silicone chains. Suitable A groups include hydrogen, methyl, methoxy, ethoxy, hydroxy, propoxy, and aryloxy.
- the preferred silicones are polydimethyl siloxanes; more preferred silicones are polydimethyl siloxanes having a viscosity of greater than about 10 000 centistokes (cst) at 25° C.
- Suitable methods for preparing these silicone materials are disclosed in U.S. Pat. Nos. 2,826,551 and 3,964,500, incorporated herein by reference. Silicones useful in the present invention are also commercially available. Suitable examples include silicones offered by Dow Corning Corporation.
- hydrocarbons for use herein include, in particular, linear or branched C 8 -C 40 paraffin hydrocarbons or mixtures of different hydrocarbons.
- An important factor in the selection of suitable hydrocarbons is that they should have a liquid to at most wax-like consistency at room temperature.
- a component having a deviation of fabric WRA of at least +15 is another essential component of the invention.
- these components are present in an amount of at least about 0.01%, preferably from about 0.1% to about 20% by weight of the composition, preferably to about 4% by weight of the diluted composition, preferably to about 12% by weight of the concentrated composition.
- the WRA Test method is taken from the AATCC 66-1990. This method is an American National Standard method designed for the determination of the wrinkle recovery of woven fabrics, whereby a test specimen, creased and compressed under controlled conditions of time and load, is suspended in the test instrument for a controlled recovery period, after which the recovery angle is measured. Experimental detail on how to measure this WRA is given in AATCC 66-1990, incorporated herein by reference.
- the WRA method is tested on 100% cotton, woven Oxford pinpoint fabric, free from wrinkles, cut in twelve specimens of 0.59 inch ⁇ 1.57 inch, six with their long dimension parallel to the warp, and six with their long dimensional parallel to the filling. The test is carried out on cloth conditioned for 24 hours at 21° C.
- Tweezers are used to place the test specimen between the leaves of the specimen holder (2 superimposed leaves 0.63 inch wide, but of different lengths and fastened together at one end) with one end directly under the 0.71-inch mark. With the tweezers, the exposed end of the specimen is lifted over and looped back to the 0.71-inch mark on the shorter, thin metal leaf and held with the left thumbnail.
- the holder with the specimen is inserted into a plastic press (2 superimposed leaves of equal length (3.74 inch) and 0.79 inch wide, fastened together at one end ) and a weight of 500 g is applied for 5 minutes so that a crease is formed.
- the plastic press can then be removed and the specimen holder combination can be inserted in the tester with the exposed end of the specimen holder in the mount on the face of the tester.
- the crease should line up with a spot at the center of the tester disk, and the dangling specimen leg should be lined up immediately with the vertical guide line.
- the wrinkle recovery value is read to the nearest degree from the scale. The sum is taken of the average recovery for all warp readings and all filling readings and compared with a cloth treated with water.
- the fabric WRA obtained with the tested component is compared with the fabric WRA obtained with water, thereby giving a deviation ⁇ .
- a component which provide a ⁇ of at least positive(+)15, preferably having a ⁇ within the range of 15-30 is a component suitable for the invention.
- Preferred components which have a deviation of fabric WRA versus water of at least 15 are selected from a) shape retention polymers, b) polymers comprising at least one unit which provide a dye transfer inhibiting benefit, c) polyurethanes, d) Isomaltooligosaccharide, e) polyamine polymers, f) amphoteric polymers, g) aminosilicones, h) curable silicones and mixtures thereof. Most preferred are the polymers which are water-soluble.
- the word “component” is meant to include compounds having a WRA deviation versus water of at least 15, mixtures of such components as well as mixtures of components which per se do not have a WRA deviation versus water of at least 15 but which, in combination do have a WRA deviation versus water of at least 15.
- One such component is disclosed and claimed in co-pending application EP 99870222.9-2413.
- Suitable shape retention polymers can be natural, or synthetic, and can act by forming a film, and/or by providing adhesive properties.
- the present invention can optionally use film-forming and/or adhesive polymer to impart shape retention to fabric, particularly clothing.
- adheresive it is meant that when applied as a solution or a dispersion to a fiber surface and dried, the polymer can attach to the surface.
- the polymer can form a film on the surface, or when residing between two fibers and in contact with the two fibers, it can bind the two fibers together.
- Other polymers such as Isomaltose Oligosaccharide can form a film and/or bond the fibers together when the treated fabric is pressed by a hot iron. Such a film will have adhesive strength, cohesive breaking strength, and cohesive breaking strain.
- Nonlimiting examples for natural polymers are Isomaltose Oligosaccharide and their derivatives, and chitins and their derivatives.
- the synthetic polymers useful in the present invention are comprised of monomers.
- monomers which can be used to form the synthetic polymers of the present invention include: low molecular weight C 1 -C 6 unsaturated organic mono-carboxylic and polycarboxylic acids, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and mixtures thereof; esters of said acids with C 1 -C 12 alcohols, such as methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-1-butanol, 1-methyl-i-butanol, 3-methyl-1-butanol, 1-methyl-1-pentanol, 2-methyl-i-pentanol, 3-methyl-i-pentanol, t-butanol, cyclohexanol, 2-
- Nonlimiting examples of said esters are methyl acrylate, ethyl acrylate, t-butyl acrylate, methyl methacrylate, hydroxyethyl methacrylate, methoxy ethyl methacrylate, and mixtures thereof; amides and imides of said acids, such as N,N-dimethylacrylamide, N-t-butyl acrylamide, maleimides; low molecular weight unsaturated alcohols such as vinyl alcohol (produced by the hydrolysis of vinyl acetate after polymerization), allyl alcohol; esters of said alcohols with low molecular weight carboxylic acids, such as, vinyl acetate, vinyl propionate; ethers of said alcohols such as methyl vinyl ether; aromatic vinyl such as styrene, alpha-methylstyrene, t-butylstyrene, vinyl toluene, polystyrene macromer, and the like; polar vinyl heterocyclics, such as vinyl pyrroli
- said monomers are selected from the group consisting of vinyl alcohol; acrylic acid; methacrylic acid; methyl acrylate; ethyl acrylate; methyl methacrylate; t-butyl acrylate; t-butyl methacrylate; n-butyl acrylate; n-butyl methacrylate; isobutyl methacrylate; 2-ethylhexyl methacrylate; dimethylaminoethyl methacrylate; N,N-dimethyl acrylamide; N,N-dimethyl methacrylamide; N-t-butyl acrylamide; vinylpyrrolidone; vinyl pyridine; adipic acid; diethylenetriamine; salts thereof and alkyl quaternized derivatives thereof, and mixtures thereof.
- said monomers form homopolymers and/or copolymers (i.e., the film-forming and/or adhesive polymer) having a glass transition temperature (Tg) of from about ⁇ 20° C. to about 150° C., preferably from about ⁇ 10° C. to about 150° C., more preferably from about 0° C. to about 100° C., most preferably, the adhesive polymer hereof, when dried to form a film will have a Tg of at least about 25° C., so that they are not unduly sticky, or “tacky” to the touch.
- said polymer is soluble and/or dispersible in water and/or alcohol.
- Said polymer typically has a molecular weight of at least about 500, preferably from about 1,000 to about 2,000,000, more preferably from about 5,000 to about 1,000,000, and even more preferably from about 30,000 to about 300,000 for some polymers.
- adipic acid/dimethylaminohydroxypropyl diethylenetriamine copolymer adipic acid/epoxypropyl diethylenetriamine copolymer
- polyvinyl alcohol polyvinylpyridine n-oxide; methacryloyl ethyl betaine/methacrylates copolymer; ethyl acrylate/methyl methacrylate/methacrylic acid/acrylic acid copolymer
- polyamine resins; and polyquaternary amine resins poly(ethenylformamide); poly(vinylamine) hydrochloride; poly(vinyl alcohol-co-6% vinylamine); poly(vinyl alcohol-co-12% vinylamine); poly(vinyl alcohol-co-6% vinylamine hydrochloride
- said copolymer and/or homopolymers are selected from the group consisting of adipic acid/dimethylaminohydroxypropyl diethylenetriamine copolymer; poly(vinylpyrrolidone/dimethylaminoethyl methacrylate); polyvinyl alcohol; ethyl acrylate/methyl methacrylate/methacrylic acid/acrylic acid copolymer; methacryloyl ethyl betaine/methacrylates copolymer; polyquaternary amine resins; poly(ethenylformamide); poly(vinylamine) hydrochloride; poly(vinyl alcohol-co-6% vinylamine); poly(vinyl alcohol-co-12% vinylamine); poly(vinyl alcohol-co-6% vinylamine hydrochloride); and poly(vinyl alcohol-co-12% vinylamine hydrochloride).
- adipic acid/dimethylaminohydroxypropyl diethylenetriamine copolymer poly(vinylpyrrol
- Preferred polymers useful in the present invention are selected from the group consisting of copolymers of hydrophilic monomers and hydrophobic monomers.
- the polymer can be linear random or block copolymers, and mixtures thereof.
- Such hydrophobicthydrophilic copolymers typically have a hydrophobic monomer/hydrophilic monomer ratio of from about 95:5 to about 20:80, preferably from about 90:10 to about 40:60, more preferably from about 80:20 to about 50:50 by weight of the copolymer.
- the hydrophobic monomer can comprise a single hydrophobic monomer or a mixture of hydrophobic monomers
- the hydrophilic monomer can comprise a single hydrophilic monomer or a mixture of hydrophilic monomers.
- hydrophobic is used herein consistent with its standard meaning of lacking affinity for water, whereas “hydrophilic” is used herein consistent with its standard meaning of having affinity for water.
- hydrophobic means substantially water insoluble; “hydrophilic” means substantially water-soluble.
- substantially water insoluble shall refer to a material that is not soluble in distilled (or equivalent) water, at 25° C., at a concentration of about 0.2% by weight, and preferably not soluble at about 0.1% by weight (calculated on a water plus monomer or polymer weight basis).
- substantially water-soluble shall refer to a material that is soluble in distilled (or equivalent) water, at 25° C., at a concentration of about 0.2% by weight, and are preferably soluble at about 1% by weight.
- soluble refers to the maximum concentration of monomer or polymer, as applicable, that can dissolve in water or other solvents to form a homogeneous solution, as is well understood to those skilled in the art.
- Nonlimiting examples of useful hydrophobic monomers are acrylic acid C 1 -C18 alkyl esters, such as methyl acrylate, ethyl acrylate, t-butyl acrylate; methacrylic C 1 -C18 alkyl esters, such as methyl methacrylate, 2-ethyl hexyl methacrylate, methoxy ethyl methacrylate; vinyl alcohol esters of carboxylic acids, such as, vinyl acetate, vinyl propionate, vinyl neodecanoate; aromatic vinyls, such as styrene, t-butyl styrene, vinyl toluene; vinyl ethers, such as methyl vinyl ether; vinyl chloride; vinylidene chloride; ethylene, propylene and other unsaturated hydrocarbons; and the like; and mixtures thereof.
- acrylic acid C 1 -C18 alkyl esters such as methyl acrylate, ethyl acrylate, t-
- Some preferred hydrophobic monomers are methyl acrylate, methyl methacrylate, t-butyl acrylate, t-butyl methacrylate, n-butyl acrylate, n-butyl methacrylate, and mixtures thereof.
- Nonlimiting examples of useful hydrophilic monomers are unsaturated organic mono-carboxylic and polycarboxylic acids, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid; unsaturated alcohols, such as vinyl alcohol, allyl alcohol; polar vinyl heterocyclics, such as vinyl pyrrolidone, vinyl caprolactam, vinyl pyridine, vinyl imidazole; vinyl amine; vinyl sulfonate; unsaturated amides, such as acrylamides, e.g., N,N-dimethylacrylamide, N-t-butyl acrylamide; hydroxyethyl methacrylate; dimethylaminoethyl methacrylate; salts of acids and amines listed above; and the like; and mixtures thereof.
- unsaturated organic mono-carboxylic and polycarboxylic acids such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters
- Some preferred hydrophilic monomers are acrylic acid, methacrylic acid, N,N-dimethyl acrylamide, N,N-dimethyl methacrylamide, N-t-butyl acrylamide, dimethylaamino ethyl methacrylate, vinyl pyrrolidone, salts thereof and alkyl quaternized derivatives thereof, and mixtures thereof.
- the shape retention copolymers contain hydrophobic monomers and hydrophilic monomers which comprise unsaturated organic mono-carboxylic and polycarboxylic acid monomers, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and salts thereof, and mixtures thereof; and optionally other hydrophilic monomers.
- unsaturated organic mono-carboxylic and polycarboxylic acid monomers such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and salts thereof, and mixtures thereof; and optionally other hydrophilic monomers.
- hydrophilic unsaturated organic mono-carboxylic and polycarboxylic acid monomers are acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and mixtures thereof.
- Nonlimiting examples of the hydrophobic monomers are esters of the unsaturated organic mono-carboxylic and polycarboxylic acids cited hereinabove with C 1 -C 12 alcohols, such as methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-i -butanol, 1-methyl-i -butanol, 3-methyl-1-butanol, 1-methyl-1-pentanol, 2-methyl-1-pentanol, 3-methyl-1-pentanol, t-butanol, cyclohexanol, 2-ethyl-1-butanol, and mixtures thereof, preferably methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, t-butanol, and mixtures thereof.
- Highly preferred adhesive and/or film-forming polymers that are useful in the composition of the present invention actually contain silicone moieties in the polymers themselves.
- These preferred polymers include graft and block copolymers of silicone with moieties containing hydrophilic and/or hydrophobic monomers described hereinbefore.
- the silicone-containing copolymers in the composition of the present invention provide shape retention, body, and/or good, soft fabric feel.
- Suitable silicone copolymers include the following:
- Preferred silicone-containing polymers are the silicone graft copolymers comprising acrylate groups described, along with methods of making them, in U.S. Pat. No. 5,658,557, Bolich et al., issued Aug. 19, 1997, U.S. Pat. No. 4,693,935, Mazurek, issued Sept. 15, 1987, and U.S. Pat. No. 4,728,571, Clemens et al., issued Mar. 1, 1988. Additional silicone-containing polymers are disclosed in U.S. Pat. Nos. 5,480,634, Hayama et al, issued Oct. 2, 1996, 5,166,276, Hayama et al., issued Nov. 24, 1992, 5,061,481, issued Oct.
- These polymers preferably include copolymers having a vinyl polymeric backbone having grafted onto it monovalent siloxane polymeric moieties, and components consisting of non-silicone hydrophilic and hydrophobic monomers.
- the silicone-containing monomers are exemplified by the general formula: X(Y) n Si(R) 3-m Z m wherein X is a polymerizable group, such as a vinyl group, which is part of the backbone of the polymer; Y is a divalent linking group; R is a hydrogen, hydroxyl, lower alkyl (e.g.
- Z is a monovalent polymeric siloxane moiety having an average molecular weight of at least about 500, is essentially unreactive under copolymerization conditions, and is pendant from the vinyl polymeric backbone described above; n is 0 or 1; and m is an integer from 1 to 3.
- the preferred silicone-containing monomer has a weight average molecular weight of from about 1,000 to about 50,000, preferably from about 3,000 to about 40,000, most preferably from about 5,000 to about 20,000.
- Nonlimiting examples of preferred silicone-containing monomers have the following formulas:
- Silicone-containing adhesive and/or film-forming copolymers useful in the present invention comprise from 0% to about 90%, preferably from about 10% to about 80%, more preferably from about 40% to about 75% of hydrophobic monomer, from about 0% to about 90%, preferably from about 5% to about 80% of hydrophilic monomer, and from about 5% to about 50%, preferably from about 10% to about 40%, more preferably from about 15% to about 25% of silicone-containing monomer.
- any particular copolymer will help determine its formulation properties.
- the copolymer can be optimized for inclusion in specific vehicles.
- polymers which are soluble in an aqueous formulation preferably contain from 0% to about 70%, preferably from about 5% to about 70% of hydrophobic monomer, and from about 30% to about 98%, preferably from about 30% to about 80%, of hydrophilic monomer, and from about 1% to about 40% of silicone-containing monomer.
- Polymers which are dispersible preferably contain from 0% to about 70%, more preferably from about 5% to about 70%, of hydrophobic monomer, and from about 20% to about 80%, more preferably from about 20% to about 60%, of hydrophilic monomer, and from about 1% to about 40% of silicone-containing monomer.
- the silicone-containing copolymers preferably have a weight average molecular weight of from about 10,000 to about 1,000,000, preferably from about 30,000 to about 300,000.
- the preferred polymers comprise a vinyl polymeric backbone, preferably having a Tg or a Tm as defined above of about ⁇ 20° C. and, grafted to the backbone, a polydimethylsiloxane macromer having a weight average molecular weight of from about 1,000 to about 50,000, preferably from about 5,000 to about 40,000, most preferably from about 7,000 to about 20,000.
- the polymer is such that when it is formulated into the finished composition, and then dried, the polymer phase separates into a discontinuous phase which includes the polydimethylsiloxane macromer and a continuous phase which includes the backbone.
- Exemplary silicone grafted polymers for use in the present invention include the following, where the composition of the copolymer is given with the approximate weight percentage of each monomer used in the polymerization reaction to prepare the copolymer: N,N-dimethylacrylamide/isobutyl methacrylate/(PDMS macromer ⁇ 20,000 approximate molecular weight) (20/60/20 w/w/w), copolymer of average molecular weight of about 400,000; N,N-dimethylacrylamide/(PDMS macromer ⁇ 20,000 approximate molecular weight) (80/20 w/w), copolymer of average molecular weight of about 300,000; and t-butylacrylate/N,N-dimethylacrylamide/(PDMS macromer ⁇ 10,000 approximate molecular weight) (70/10/20), copolymer of average molecular weight of about 400,000.
- Highly preferred shape retention copolymers of this type contain hydrophobic monomers, silicone-containing monomers and hydrophilic monomers which comprise unsaturated organic mono- and polycarboxylic acid monomers, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and salts thereof, and mixtures thereof. These preferred polymers surprisingly provide control of certain amine type malodors in fabrics, in addition to providing the fabric wrinkle control benefit.
- a nonlimiting example of such copolymer is n-butylmethacrylate /acrylic acid/(polydimethylsiloxane macromer, 20,000 approximate molecular weight) copolymer of average molecular weight of about 100,000, and with an approximate monomer weight ratio of about 70/10/20.
- a highly preferred copolymer is composed of acrylic acid, t-butyl acrylate and silicone-containing monomeric units, preferably with from about 20% to about 90%, preferably from about 30% to about 80%, more preferably from about 50% to about 75% t-butyl acrylate; from about 5% to about 60%, preferably from about 8% to about 45%, more preferably from about 10% to about 30% of acrylic acid; and from about 5% to about 50%, preferably from about 10% to about 40%, more preferably from about 15% to about 30% of polydimethylsiloxane of an average molecular weight of from about 1,000 to about 50,000, preferably from about 5,000 to about 40,000, most preferably from about 7,000 to about 20,000.
- Nonlimiting examples of acrylic acid/tert-butyl acrylate/polydimethyl siloxane macromer copolymers useful in the present invention, with approximate monomer weight ratio, are: t-butylacrylate/acrylic acid/(polydimethylsiloxane macromer, 10,000 approximate molecular weight) (70/10/20 wlwiw), copolymer of average molecular weight of about 300,000; t-butyl acrylate/acrylic acid/(polydimethylsiloxane macromer, 10,000 approximate molecular weight) (63/20/17), copolymer of average molecular weight of from about 120,000 to about 150,000; and n-butylmethacrylate/acrylic acid/ (polydimethylsiloxane macromer ⁇ 20,000 approximate molecular weight) (70/10/20 wlwiw), copolymer of average molecular weight of about 100,000.
- a useful and commercially available copolymer of this type is Diahold® ME from Mitsubishi Chemical Corp., which is a t-butyl acrylate/acrylic acid/ (polydimethylsiloxane macromer, 12,000 approximate molecular weight) (60/20/20), copolymer of average molecular weight of about 128,000.
- silicone block copolymers comprising repeating block units of polysiloxanes.
- silicone-containing block copolymers examples include U.S. Pat. No. 5,523,365, to Geck et al., issued Jun. 4, 1996; U.S. Pat. No. 4,689,289, to Crivello, issued Aug. 25, 1987; U.S. Pat. No. 4,584,356, to Crivello, issued Apr. 22, 1986; Macromolecular Design, Concept & Practice, Ed: M. K. Mishra, Polymer Frontiers International, Inc., Hopewell Jct., N.Y. (1994), and Block Copolymers, A. Noshay and J. E. McGrath, Academic Press, N.Y. (1977), which are all incorporated by reference herein in their entirety.
- Other silicone block copolymers suitable for use herein are those described, along with methods of making them, in the above referenced and incorporated U.S. Pat. No. 5,658,577.
- the silicone-containing block copolymers useful in the present invention can be described by the formulas A-B, A-B-A, and —(A-B) n — wherein n is an integer of 2 or greater.
- A-B represents a diblock structure
- A-B-A represents a triblock structure
- —(A-B) n — represents a multiblock structure.
- the block copolymers can comprise mixtures of diblocks, triblocks, and higher multiblock combinations as well as small amounts of homopolymers.
- the silicone block portion, B can be represented by the following polymeric structure —(SiR 2 O) m —, wherein each R is independently selected from the group consisting of hydrogen, hydroxyl, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 2 -C 6 alkylamino, styryl, phenyl, C 1 -C 6 alkyl or alkoxy-substituted phenyl, preferably methyl; and m is an integer of about 10 or greater, preferably of about 40 or greater, more preferably of about 60 or greater, and most preferably of about 100 or greater.
- the non-silicone block, A comprises monomers selected from the monomers as described hereinabove in reference to the non-silicone hydrophilic and hydrophobic monomers for the silicone grafted copolymers. Vinyl blocks are preferred co-monomers.
- the block copolymers preferably contain one or more non-silicone blocks, and up to about 50%, preferably from about 10% to about 20%, by weight of one or more polydimethyl siloxane blocks.
- sulfur-linked silicone containing copolymers including block copolymers.
- the term “sulfur-linked” means that the copolymer contains a sulfur linkage (i.e., —S—), a disulfide linkage (i.e., —S—S—), or a sulfhydryl group (i.e., —SH).
- A is a vinyl polymeric segment formed from polymerized free radically polymerizable monomers.
- the selection of A is typically based upon the intended uses of the composition, and the properties the copolymer must possess in order to accomplish its intended purpose. If A comprises a block in the case of block copolymers, a polymer having AB and/or ABA architecture will be obtained depending upon whether a mercapto functional group —SH is attached to one or both terminal silicon atoms of the mercapto functional silicone compounds, respectively.
- the weight ratio of vinyl polymer block or segment, to silicone segment of the copolymer can vary.
- the preferred copolymers are those wherein the weight ratio of vinyl polymer segment to silicone segment ranges from about 98:2 to 50:50, in order that the copolymer possesses properties inherent to each of the different polymeric segments while retaining the overall polymer's solubility.
- the preferred polymers comprising at least one unit which provide a dye transfer inhibiting benefit are water-soluble polymers.
- the polymers comprising at least one unit which provide a dye transfer inhibiting benefit useful in the present invention have the formula: [—P(D) m —] n wherein the unit P is a polymer backbone which comprises units which are homopolymeric or copolymeric. D units are defined herein below.
- the term “homopolymeric” is defined as “a polymer backbone which is comprised of units having the same unit composition, i.e., formed from polymerization of the same monomer”.
- copolymeric is defined as “a polymer backbone which is comprised of units having a different unit composition, i.e., formed from the polymerization of two or more monomers”.
- P backbones preferably comprise units having the formula: [—CR 2 —CR 2 ]— or —[(CR 2 ) x —L]— wherein each R unit is independently hydrogen, C 1 -C 12 alkyl, C 6 -C 12 aryl, and D units as described herein below; preferably C 1 -C 4 alkyl.
- Each L unit is independently selected from heteroatom-containing moieties, non-limiting examples of which are selected from the group consisting of: polysiloxane having the formula: wherein the index p is from 1 to about 6; units which have dye transfer inhibition activity: and mixtures thereof; wherein R 1 is hydrogen, C 1l -C 12 alkyl, C 6 -C 12 aryl, and mixtures thereof.
- R 2 is C 1 -C 12 alkyl, C 1 -C 12 alkoxy, C 6 -C 12 aryloxy, and mixtures thereof; preferably methyl and methoxy.
- R 3 is hydrogen C 1 -C 12 alkyl, C 6 -C 12 aryl, and mixtures thereof; preferably hydrogen or C 1 -C 4 alkyl, more preferably hydrogen.
- R 4 is C 1 -C 12 alkyl, C 6 -Cl 2 aryl, and mixtures thereof.
- the backbones of the polymers of the present invention comprise one or more D units which are units which comprise one or more units which provide a dye transfer inhibiting benefit.
- the D unit can be part of the backbone itself as represented in the general formula: [—P(D) m —] n or the D unit may be incorporated into the backbone as a pendant group to a backbone unit having, for example, the formula:
- the number of D units depends upon the formulation. For example, the number of D units will be adjusted to provide water solubility of the polymer as well as efficacy of dye transfer inhibition.
- the molecular weight of the polymers of the present invention are from about 500, preferably from about 1,000, more preferably from about 10,000 most preferably from 200,000 to about 6,000,000, preferably to about 2,000,000, more preferably to about 1,000,000, yet more preferably to about 500,000, most preferably to about 360,000 daltons. Therefore the value of the index n is selected to provide the indicated molecular weight, and providing for a water solubility of at least 100 ppm, preferably at least about 300 ppm, and more preferably at least about 1,000 ppm in water at ambient temperature which is defined herein as 25° C.
- Non-limiting examples of preferred D units are D units which comprise an amide moiety.
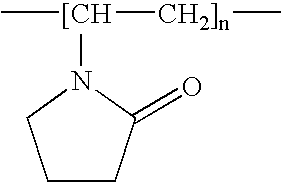
- Examples of polymers wherein an amide unit is introduced into the polymer via a pendant group includes polyvinylpyrrolidone having the formula: polyvinyloxazolidone having the formula: polyvinylmethyloxazolidone having the formula: polyacrylamides and N-substituted polyacrylamides having the formula: wherein each R′ is independently hydrogen, C 1 -C 6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; polymethacrylamides and N-substituted polymethacrylamides having the general formula: wherein each R′ is independently hydrogen, C 1 -C 6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; poly(N-acrylylglycinamide) having the formula: wherein each R′ is independently hydrogen, C 1 -C 6 alkyl, or both R′ units can
- D unit wherein the nitrogen of the dye transfer inhibiting moiety is incorporated into the polymer backbone is a poly(2-ethyl-2-oxazoline) having the formula: wherein the index n indicates the number of monomer residues present.
- the amino-functional polymers of the present invention can comprise any mixture of dye transfer inhibition units which provides the product with suitable properties.
- the preferred polymers which comprise D units which are amide moieties are those which have the nitrogen atoms of the amide unit highly substituted so the nitrogen atoms are in effect shielded to a varying degree by the surrounding non-polar groups. This provides the polymers with an amphiphilic character.
- Non-limiting examples include polyvinyl-pyrrolidones, polyvinyloxazolidones, N,N-disubstituted polyacrylamides, and N,N-disubstituted polymethacrylamides.
- a detailed description of physico-chemical properties of some of these polymers are given in “Water-Soluble Synthetic Polymers: Properties and Behavior”, Philip Molyneux, Vol. 1, CRC Press, (1983) included herein by reference.
- the amide containing polymers may be present partially hydrolyzed and/or crosslinked forms.
- a preferred polymeric compound for the present invention is polyvinylpyrrolidone (PVP). This polymer has an amphiphilic character with a highly polar amide group conferring hydrophilic and polar-attracting properties, and also has non-polar methylene and methine groups, in the backbone and/or the ring, conferring hydrophobic properties. PVP is readily soluble in aqueous and organic solvent systems.
- PVP is available ex ISP, Wayne, New Jersey, and BASF Corp., Parsippany, N.J., as a powder or aqueous solutions in several viscosity grades, designated as, e.g., K-12, K-15, K-25, and K-30. These K-values indicate the viscosity average molecular weight, as shown below: PVP viscosity average molecular weight (in thousands of daltons) K-12 K-15 K-25 K-30 K-60 K-90 2.5 10 24 40 160 360 PVP K-12, K-15, and K-30 are also available ex Polysciences, Inc.
- PVP K-15, K-25, and K-30 and poly(2-ethyl-2-oxazoline) are available ex Aldrich Chemical Co., Inc., Milwaukee, Wis.
- PVP K30 (40,000) through to K90 (360,000) are also commercially available ex BASF under the tradename Luviskol or commercially available ex ISP.
- Still higher molecular PVP like PVP 1.3MM, commercially available ex Aldrich is also suitable for use herein.
- PVP-type of material suitable for use in the present invention are polyvinylpyrrolidone-co-dimethylaminoethylmethacrylate, commercially available ex ISP in a quaternised form under the tradename Gafquat® or commercially available ex Aldrich Chemical Co.
- N-oxide units having the formula: wherein R 1 , R 2 , and R 3 can be any hydrocarbyl unit (for the purposes of the present invention the term “hydrocarbyl” does not include hydrogen atom alone).
- the N-oxide unit may be part of a polymer, such as a polyamine, i.e., polyalkyleneamine backbone, or the N-oxide may be part of a pendant group attached to the polymer backbone.
- An example of a polymer which comprises an the N-oxide unit as a part of the polymer backbone is polyethyleneimine N-oxide.
- Non-limiting examples of groups which can comprise an N-oxide moiety include the N-oxides of certain heterocycles inter alia pyridine, pyrrole, imidazole, pyrazole, pyrazine, pyrimidine, pyridazine, piperidine, pyrrolidine, pyrrolidone, azolidine, morpholine.
- a preferred polymer is poly(4-vinylpyriding N-oxide, PVNO).
- the N-oxide unit may be pendant to the ring, for example, aniline oxide.
- N-oxide comprising polymers of the present invention will preferably have a ratio of N-oxidized amine nitrogen to non-oxidized amine nitrogen of from about 1:0 to about 1:2, preferably to about 1:1, more preferably to about 3:1.
- the amount of N-oxide units can be adjusted by the formulator.
- the formulator may co-polymerize N-oxide comprising monomers with non N-oxide comprising monomers to arrive at the desired ratio of N-oxide to non N-oxide amino units, or the formulator may control the oxidation level of the polymer during preparation.
- the amine oxide unit of the polyamine N-oxides of the present invention have a Pk a less than or equal to 10, preferably less than or equal to 7, more preferably less than or equal to 6.
- the average molecular weight of the N-oxide comprising polymers which provide a dye transfer inhibitor benefit to polymers is from about 500 daltons, preferably from about 10,000 daltons, more preferably from about 20,000 daltons to about 6,000,000 daltons, preferably to about 2,000,000 daltons, more preferably to about 360,000 daltons.
- a further example of polymers which have dye transfer inhibition benefits are polymers which comprise both amide units and N-oxide units as described herein above.
- Non-limiting examples include co-polymers of two monomers wherein the first monomer comprises an amide unit and the second monomer comprises an N-oxide unit.
- oligomers or block polymers comprising these units can be taken together to form the mixed amide/N-oxide polymers.
- the resulting polymers must retain the water solubility requirements described herein above.
- Polymers of the urethane type are also suitable components for use herein.
- a typical disclosure of polyurethane polymer can be found in EP844274A1 as well as in EP839903.
- Isomaltooligosaccharides (including mixtures), the individual components of said mixtures, substituted versions thereof, derivatised versions thereof, and mixtures thereof are suitable components for use herein.
- IMO is used as corn syrup.
- cellulosic fibers/fabrics such as cotton, rayon, ramie, jute, flax, linen, polynosic-fibers, Lyocell (Tencel®), polyester/cotton blends, other cotton blends, and the like, especially cotton, rayon, linen, polyester/cotton blends, and mixtures thereof.
- Suitable fabric improving actives that are useful in the present invention include oligosaccharides with a degree of polymerization (DP) of from about 1 to about 15, preferably from about 2 to about 10, and wherein each monomer is selected from the group consisting of reducing saccharide containing 5 and/or 6 carbon atoms, including isomaltose, isomaltotriose, isomaltotetraose, isomaltooligosaccharide, fructooligosaccharide, levooligosaccharides, galactooligosaccharide, xylooligosaccharide, gentiooligosaccharides, disaccharides, glucose, fructose, galactose, xylose, mannose, arabinose, rhamnose, maltose, sucrose, lactose, maltulose, ribose, lyxose, allose, altrose, gulose, idose, talose, tre
- Oligosaccharides containing b-linkages are also preferred.
- Preferred oligosaccharides are acyclic and have at least one linkage that is not an ⁇ -1,4-glycosidic bond.
- a preferred oligosaccharide is a mixture containing IMO: from 0 to about 20% by weight of glucose, from about 10 to about 65% of isomaltose, from about 1% to about 45% of each of isomaltotriose, isomaltetraose and isomaltopentaose, from 0 to about 3% of each of isomaltohexaose, isomaltoheptaose, isomaltooctaose and isomaltononaose, from about 0.2% to about 15% of each of isomaltohexaose and isomaltoheptaose, and from 0 to about 50% by weight of said mixture being isomaltooligosaccharides of
- Oligosaccharide mixtures are either prepared by enzymatic reactions or separated as natural products from plant materials.
- the enzymatic synthesis of oligosaccharides involves either adding monosaccharides, one at a time, to a di- or higher saccharide to produce branched oligosaccharides, or it can involve the degradation of polysaccharides followed by transfer of saccharides to branching positions.
- Oligosaccharide Mixtures I and II are prepared by enzymatic hydrolysis of starch to maltooligosaccharides, which are then converted to isomaltooligosaccharides by a transglucosidase reaction.
- Oligosaccharide Mixture III for example, is a mixture of oligosaccharides isolated from soybean. Soybean oligosaccharides such as Mixture III, are of pure natural origin.
- Substituted and/or derivatised materials of the oligosaccharides listed hereinabove are also suitable in the present invention.
- Nonlimiting examples of these materials include: carboxyl and hydroxymethyl substitutions (e.g., glucuronic acid instead of glucose); amino oligosaccharides (amine substitution, e.g., glucosamine instead of glucose); cationic quaternized oligosaccharides; C 1 -C 6 alkylated oligosaccharides; acetylated oligosaccharide ethers; oligosaccharides having amino acid residues attached (small fragments of glycoprotein); oligosaccharides containing silicone moieties.
- substituted and/or derivatised oligosaccharides can provide additional benefits, such as: carboxyl and hydroxymethyl substitutions can introduce readily oxidizable materials on and in the fiber, thus reducing the probability of the fiber itself being oxidized by oxidants, such as bleaches; amine substitution can bind and/or condense with oxidatively damaged regions of the fiber to rejuvenate aged fabrics; acetylated sugar ethers can serve as bleach activators in subsequent processes where hydrogen peroxide is present; oligosaccharides having amino acid residues can improve delivery of fabric care benefits for fabrics containing proteinaceous fibers, e.g., wool and silk; and silicone-derivatised oligosaccharides can provide additional fabric softness and lubricity.
- C 6 alkyl oligosaccharide is disclosed (along with other higher, viz., C 6 -C 30 , alkyl polysaccharides) in U.S. Pat. No. 4,565,647.
- Typical disclosure of C 1 -C 6 alkylated oligosaccharides can also be found in U.S. Pat. No. 4,488,981. These patents are incorporated herein by reference.
- One preferred isomaltooligosaccharide is IMO 900 commercially available from Showa Sangyo Co.
- Polyvinylamines polymers are also suitable component giving a deviation of fabric WRA of at least 15.
- Typical polyvinylamines polymers include the quaternized and non-quaternized polyvinylamines having the formula: wherein R is hydrogen, C1-C12 linear or branched alkyl, benzyl, or alkyleneoxy having the formula (R1O)zY, wherein R1 is C1-C6 linear or branched alkylene, Y is hydrogen or an anionic unit, non-limiting examples of which include, —(CH2)fCO2M, —C(O)(CH2)fCO2M, —(CH2)fPO3M, —(CH2)fOPO3M, —(CH2)fSO3M, —CH2(CHSO3M)—(CH2)fSO3M, —CH2(CHSO2M)(CH2)fSO3M, —C(O)CH2CH(SO3M)CO2M, C(O)CH2CH(
- the index x has the value from about 50 to about 1,500; preferably the index x has a value such that the resulting polymeric suds stabilizer has an average molecular weight of from about 2,500, preferably from about 10,000, more preferably from about 20,000 to about 150,000, preferably to about 90,000, more preferably to about 80,000 daltons.
- Most preferred polymers for use in the present invention are water-soluble, including IMO 900 (Isomaltose Oligosaccharide ex. Showa Sangyo Co.), Avalure AC 120 (Polyacrylate ex. BF Goodrich), Luviskol K30, K60 and K85 (Polyvinylpyrrolidone MW 40.000, 400.000 and 1.250.000 ex. BASF), Luvitec VPC 55K65W (copolymer Vinylpyrrolidone & Vinylcaprolactam ex. BASF), Luvitec Quat 73W (copolymer 1-methyl-3-vinyl-imidazolium-methylsulfate & 1-vinyl-2-pyrrolidone ex.
- IMO 900 Isomaltose Oligosaccharide ex. Showa Sangyo Co.
- Avalure AC 120 Polyacrylate ex. BF Goodrich
- Luviskol K30, K60 and K85 Polyvinylpyrrolidone MW 40.000, 400.000 and 1.25
- Luviquat FC 905 copolymer Vinylimidazolium methochloride & Vinylpyrrolidone ex. BASF
- Sedipur 520 modified Polyacrylamide ex. BASF
- Chitanide 222 Chitosan succinamide ex. MIP
- Mirasil ADM-E Amphomer HC (Acrylate/Octylacrylamide copolymer ex. National Starch), and mixtures thereof.
- the water-soluble lubricant and the component, preferably polymer, having a deviation of fabric WRA of at least +15 are present in weight ratios of water-soluble lubricant to component of from 10:1 to 1:1. Indeed, it has been found that within these ratio ranges the resulting composition provides best in wear wrinkle benefit.
- amphoteric polymers i.e., polymers comprising at least one anionic moiety and one cationic moiety, and optionally a non-ionic moiety.
- the anionic moiety comprises a group which is a deprotonated anion of an acid group when the polymer is dissolved/dispersed in water at a pH of about 7 and which can be protonated to form a nonionic acid group when the polymer is dissolved/dispersed in water at an acidic pH.
- Representative examples of such groups include carboxylate, phosphonate, phosphate, phosphite, sulfonate, sulfate groups, and combinations thereof.
- each moiety may be further complexed with a separate, cationic counterion other than hydrogen.
- a separate, cationic counterion other than hydrogen include Na + , Li + , K + , NH4 + or combinations thereof.
- the cationic moiety comprises a protonated cation when the polymer is dissolved/dispersed in water at a pH of about 7 or below and can be deprotonated to a nonionic form when the polymer is dissolved/dispersed in water at a basic pH.
- the cationic moiety comprises a group which is a quaternized group.
- the protonated group include the ammonium functionality, phosphonium functionality, sulfonium functionality, and combinations thereof.
- ammonium refers to a moiety including a nitrogen atom linked to a plurality of moieties (either H, alkyl or aryl groups) by four bonds when dissolved/dispersed in water at a pH of 7.
- sulfonium refers to a moiety including a sulfur atom linked to three other moieties (either H, alkyl or aryl groups) when dispersed in water at a pH of about 7.
- phosphonium refers to a moiety including a phosphorous atom linked to four other moieties (either H, alkyl or aryl groups) when dispersed in water at a pH of about 7.
- ammonium, phosphonium and sulphonium functionality may be presented by the following formulae, respectively:
- R1 represents the polymer backbone and R2 represents hydrogen, alkyl or aryl substituents.
- R2 represents hydrogen, alkyl or aryl substituents.
- all R2 groups represents alkyl or aryl substituents, excluding hydrogen.
- each such second functional group may be further complexed with a separate, anionic counterion.
- counterion include chlorides, sulfates, carbonates, nitrates, formiates, perchlorates, or combinations thereof.
- amphoteric polymers herein comprise a non-ionic moiety.
- a preferred class of amphoteric polymers for use herein are polymers composed of both cationic and anionic vinylmonomers.
- Suitable anionic vinylmonomers for use herein include salts of acrylic acid, methacrylic acid, crotonic acid, maleic acid, fumaric acid, itaconic acid and vinylsulphonic acid.
- Suitable cationic vinylmonomers for use herein include salts of unsaturated amines such as the hydrochloride salt of vinylamine, salts of N,N′-dialkylaminoalkyl (meth) acrylates and N,N′-dialkylaminoalkyl (meth) acrylamides such as the hydrochloride salt of dimethylaminoethylmethacrylate (DMAEMA.HCl) or dimethylaminopropylacrylamide; alkyl quaternized aminoalkyl (meth) acrylates and aminoalky (meth) acrylamides such as trimethylammoniumethyl methacrylatechloride, trimethylammoniumpropyl acrylamidemethylsulfate, alkyl quaternized polar vinyl heterocyclics such
- a non-ionic comonomer can be incorporated, such as amides and imides of organic acids, such as acrylamide, N,N-dialkylacrylarnide, N-t-butylacrylamide, maleimides, vinylformamide, aromatic vinyl monomers such as styrene, vinyltoluene, t-butylstyrene; polar vinyl heterocyclics such as vinyl pyrrolidone, vinyl caprolactam, vinyl pyridine, vinylimidazole; low molecular weight unsaturated hydrocarbons and derivatives such as ethylene, propylene, butadiene, cyclohexadiene, vinylchloride and mixtures thereof.
- organic acids such as acrylamide, N,N-dialkylacrylarnide, N-t-butylacrylamide, maleimides, vinylformamide, aromatic vinyl monomers such as styrene, vinyltoluene, t-butylstyrene; polar vinyl heterocyclics such
- a preferred polymer of this class is based on poly(vinylamine-co-acrylic acid), in molar ratios varying between 1:100 to 100:1, preferably 90:10 to 40:60.
- Polymers of this class preferably have a molecular weight between 20.000 and 5.000.000 preferably between 30.000 and 1.000.000, more preferably between 50.000 and 300.000.
- a second class of polymers which are preferred for use herein are anionically modified polyethyleneimines.
- anionically modified polyethyleneimines include polyethyleneimines grafted with acrylic acid, methacrylic acid, maleic acid, fumaric acid, crotonic acid, itaconic acid, or carboxymethylated.
- anionically modified polyethyleneimines are well known. They can be prepared by reacting ⁇ , ⁇ -unsaturated carboxylic acids ( C ⁇ C—COOH) like acrylic or maleic acid with polyethyleneimine (Michael-type reaction) or by carboxymethylation. The carboxymethylation is carried out by reacting polyethyleneimine either with chloroacetic acid or with formaldehyde and sodium cyanide and subsequent saponification of the resultant aminonitrile. The latter procedure is well-known as the “Strecker Synthesis”.
- Polymers of this class have a degree of substitution of between 5 and 95, preferably 20 and 80, and a molecular weight between 5000 and 2 000 000, preferably 20 000 and 1 000 000.
- the amphoteric polymers can be provided to the clothes in amounts of from 1 ⁇ 10 ⁇ 7 g/g fabric to 0.3 g/g fabric, preferably from 1 ⁇ 10 ⁇ 5 g/g fabric to 0.1 g/g fabric; more preferably from 1 ⁇ 10 ⁇ 3 g/g fabric to 1 ⁇ 10 ⁇ 2 g/g fabric.
- aminosilicones preferably those comprising an amine comprising a sterically hindered functional group.
- any known aminosilicone can be used to treat clothes so as to provide the desired benefit.
- Aminosilicones used in a domestic context have been described in numerous publications, for instance U.S. Pat. No. 5,062,971 and U.S. Pat. No. 5,064,543 as ironing aid; in WO 00/24853, WO/9201773 and EP 300 525 in fabric conditioners, EP 150 867 and EP 150 872 in detergents and there is no need to redescribe such aminosilicones herein.
- aminosilicones can be used to treat clothes, but only in limited amounts such that the yellowing phenomenon does not become too visible, thereby limiting the performance of the composition.
- aminosilicones comprising a sterically hindered functional group.
- aminosilicones have been described in U.S. Pat. No. 5,540,952, EP 659 930, WO 00/5315, U.S. Pat. No. 5,688,889, WO 96/16110, WO 96/16124, WO 96/16127, WO 96/18667 and U.S. Pat. No. 5,792,825, the contents of which are incorporated herein.
- the present invention utilizes amino silicones comprising a sterically hindered functional group, i.e. polyorganosiloxanes having, per mole, at least one unit of general formula: ( R ) a ⁇ ⁇ ( X ) b ⁇ ⁇ Z ⁇ ⁇ Si ⁇ ( O ) 3 - ( a + b ) 2 in which:
- R are identical or different and represent a monovalent hydrocarbon radical chosen from linear or branched alkyl radicals having from 1 to 4 carbon atoms, the phenyl radical, the benzyl radical and the 3,3,3-trifluoropropyl radical;
- X are identical or different and represent a monovalent radical chosen from a hydroxyl group and a linear or branched alkoxy radical having from 1 to 3 carbon atoms;
- U represents —O— or —NR 9 —
- R 9 being a radical chosen from a hydrogen atom, a linear or branched alkyl radical having from 1 to 6 carbon atoms, a divalent radical —R 1 — having the meaning indicated above, one of the valency bonds being connected to the nitrogen of —NR 9 — and the other being connected to a silicon atom and a divalent radical of the formula —R 10 —N(R 1 )—S in which R 1 has the meaning indicated above
- R 10 represents a linear or branched alkylene radical having from 1 to 12 carbon atoms, one of the valency bonds (that of R 10 ) being connected to the nitrogen atom of —NR 9 — and the other (that of R 1 ) being connected to a silicon atom.
- S represents a monovalent group, in which:
- the secondary or tertiary amine function in S is incorporated in a piperidyl group.
- the polyorganosiloxane used can additionally comprise (an) other siloxyl unit(s).
- Such amino silicones comprising a sterically hindered functional group which are suitable for use herein are commercially available from Rhodia under the trade name Rhodorsil ®, in particular Rhodorsil ® H 21645 or Rhodorsil ® H 21650 or Silicex ®, in particular Silicex ® 263.
- the aminosilicones comprising a sterically hindered amino functional group can be provided to the clothes in amounts from 1 ⁇ 10 ⁇ 7 g/g fabric to 0.3 g/g fabric, preferably from 1 ⁇ 10 ⁇ 5 g/g fabric to 0.1 g/g fabric; more preferably from 1 ⁇ 10 ⁇ 3 g/g fabric to 1 ⁇ 10 ⁇ 2 g/g fabric, i.e. in amounts which are greater than the amounts in which other amino silicones can be used.
- a greater benefit can be obtained without observing fabric yellowing.
- curable silicones are also suitable for use herein. “Curable” silicone molecules have the ability to reach one with each other to yield a polymeric elastomer of a much higher molecular weight compared to the original molecule. Thus, “curing” often occurs when two curable silicone molecules or curable silicone polymers react yielding a polymer of a higher molecular weight. This “cure” reaction is define herein as the formation of new silicon-oxygen, silicon-carbon, and/or carbon-carbon linkages. Curable silicones can be cross-linked to some degree before application. That means that the curable silicone has cured to some degree before application but that can still further cure during and after application. Cross-linked curable silicones are preferred.
- curable silicones examples include vinyl-, allyl-, silane-, epoxy-, alkoxy-, and/or silanol-modified polydimethylsiloxanes, and mixtures thereof.
- Some curable silicones may required the cooperative use of a catalyst to induce curing, as in the case of vinyl-,hydrogen-modified silicones which cure via a hydrosilation process catalyzed by platinum compounds or radical catalysts.
- More preferred in this invention are curable silicone able to cure without the addition of a catalysts, such as epoxy-, alkoxy-, and/or silanol-modified polydimethylsiloxanes.
- a catalysts such as epoxy-, alkoxy-, and/or silanol-modified polydimethylsiloxanes.
- silanol-stopped polydimethyl-siloxanes emulsions are preferred.
- Curable silicones can have other organic group modifications as for example, although not restricting, amino or polyalkyleneoxide groups. Curable silicones may content reinforcing fillers. By reinforcing fillers we mean small particles made of inorganic or organic materials added to the curable silicone as additives or intimately linked to silicone molecules via covalent bonds.
- reinforcing fillers we mean small particles made of inorganic or organic materials added to the curable silicone as additives or intimately linked to silicone molecules via covalent bonds.
- silica particles sized from 10 to 100 nanometers present in 10% to 100% by weight based on the weight of the silicone.
- curable silicones are formulated as oil-in-water emulsions.
- Curable silicone emulsions are commercially available; e.g., GE-Bayer SM2112 Silicone Emulsions or Dow Corning Syl-Off® 7922 Catalyst Emulsion.
- curable silicones cure during or/and after application to the fabrics producing a network which will prevent the formation of wrinkles.
- Suitable film-forming polymers for use herein are durable press polymers.
- Durable press polymers are optional components of the invention.
- These polymers can be a cross-linking resin having the property of being cationic.
- cross-linking resin having the property of being cationic it is meant that the resin is at least partially positively charged. It is not however necessary that the reactive part of the molecule carries the positive charge.
- polymeric resins can be based on positively charged monomers which help the deposition on the fibers.
- Cross-linking resins having the property of being cationic suitable for use herein are those commonly known as having wet strength in the paper field. At least two mechanisms have been postulated to account for the mechanism by which wet strength resin act. One is that wet strength resins form covalent bonds between adjacent fibers while another is that the wet strength resin places a layer over the hydrogen bonds formed between adjacent paper fibers and thus prevents water from breaking the hydrogen bonds.
- wet-strength agents suitable for use herein include compounds made of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof.
- amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof.
- the preferred components are the polymeric amine-epichlorohydrin resins selected from the group consisting of a polyamide-epichlorohydrin (PAE) resin, a polyalkylenepolyamine-epichlorohydrin (PAPAE) resin, and an amine polymer-epichlorohydrin (APE) resin, in which the amine groups have been alkylated with epichlorohydrin to produce a polyamine-epichlorohydrin resin that has azetidinium or epoxide functionality.
- PAE polyamide-epichlorohydrin
- PAPAE polyalkylenepolyamine-epichlorohydrin
- APE amine polymer-epichlorohydrin
- the cross-linking resin having cationic properties is a cationic wet strength resin that is produced by reacting a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epichlorohydrin to form a PAE resin.
- a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epic
- cross-linking resin having cationic properties from this class are the wet strength resin Kymene 557H (available from Hercules Incorporated), in which adipic acid is reacted with diethylenetriamine to form a poly(aminoamide) that is alkylated and crosslinked with epichlorohydrin to form a PAE resin.
- Still another preferred cross-linking resin having cationic properties made of epichlorohydrin are Luresin.RTM and Etadurin which both are polyamidoamine-epichlorohydrin resins.
- Amine-aldehyde resins are suitable cross-linking resins for the present invention and are made by condensation of amine or amide monomers with aldehydes such as formaldehyde or glyoxal.
- Preferred amines are those having low molecular weight amines e.g. melamine or polymeric amines e.g. poly-diallylamine, preferably quarteinized.
- Preferred amides are those polymeric amides such as polyacrylamide. All these suitable amine/amide monomers can also be copolymerized with cationic monomers.
- amine-aldehyde cross-linking resin preferred are those from the class of melamine-formaldehyde resin.
- Melamine-formaldehyde resins of this type are known as crosslinking agents of this type in the coating industry and are also described, for example, in German Auslegeschrift Nos. 2,457,387 (U.S. Pat. No. 4,035,213 incorporated herein by reference) and U.S. Pat. No. 1,719,324 and, in particular, in U.S. Pat. No. 3,242,230 incorporated herein by reference.
- Preferred melamine-formaldehyde resin are those commercially available under the tradenames Madurit, and Cassurit from Clariant.
- Still other preferred cross-linking resin having the property of being cationic among the class of amine-aldehyde cross-linking resin are the Poly(acrylamide-glyoxal) resin commercially available under the tradename SOLIDUR1 T KM from Clariant.
- the cross-linking resin having cationic properties have a molecular weight between 200 and 1,000,000, preferably between 500 and 100,000, most preferably between 1000 and 25,000.
- Cross-linking resin having a low molecular weight are most preferred for use in the present invention as they are more water-soluble and have a better fiber penetration.
- low molecular weight it is meant a molecular weight within the range of from 25 to 2000, preferably from 50 to 1000, and more preferably from 50 to 500.
- the level of cross-linking components or derivative thereof is present in an amount of from 0.01% to 60%, preferably from 0.01% to 30% by weight of the total composition
- catalysts includes organic acids such as citric acid, succinic acid, and tartaric acids, as well as conventional Lewis acid such as Al Cl 3 or MgCl 2 , or salts thereof, or mixtures thereof.
- organic acids such as citric acid, succinic acid, and tartaric acids
- Lewis acid such as Al Cl 3 or MgCl 2
- catalyst NKD made of a mixture of salts and organic acid, and commercially available from Hoechst.
- the level of catalyst is from 10% to 50%, preferably from 20 to 40% by weight of the cross-linking components or derivative thereof.
- composition of the invention may also comprise one or more of the following optional ingredients.
- Durable press polymers are optional components of the invention. These polymers can be a cross-linking resin having the property of being cationic. By “cross-linking resin having the property of being cationic”, it is meant that the resin is at least partially positively charged. It is not however necessary that the reactive part of the molecule carries the positive charge. Indeed, polymeric resins can be based on positively charged monomers which help the deposition on the fibers.
- Cross-linking resins having the property of being cationic suitable for use herein are those commonly known as having wet strength in the paper field. At least two mechanisms have been postulated to account for the mechanism by which wet strength resin act. One is that wet strength resins form covalent bonds between adjacent fibers while another is that the wet strength resin places a layer over the hydrogen bonds formed between adjacent paper fibers and thus prevents water from breaking the hydrogen bonds.
- wet-strength agents suitable for use herein include compounds made of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof.
- amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof.
- the preferred components are the polymeric amine-epichlorohydrin resins selected from the group consisting of a polyamide-epichlorohydrin (PAE) resin, a polyalkylenepolyamine-epichlorohydrin (PAPAE) resin, and an amine polymer-epichlorohydrin (APE) resin, in which the amine groups have been alkylated with epichlorohydrin to produce a polyamine-epichlorohydrin resin that has azetidinium or epoxide functionality.
- PAE polyamide-epichlorohydrin
- PAPAE polyalkylenepolyamine-epichlorohydrin
- APE amine polymer-epichlorohydrin
- the cross-linking resin having cationic properties is a cationic wet strength resin that is produced by reacting a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epichlorohydrin to form a PAE resin.
- a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epic
- cross-linking resin having cationic properties from this class are the wet strength resin Kymene 557H (available from Hercules Incorporated), in which adipic acid is reacted with diethylenetriamine to form a poly(aminoamide) that is alkylated and crosslinked with epichlorohydrin to form a PAE resin.
- Still another preferred cross-linking resin having cationic properties made of epichlorohydrin are Luresin.RTM and Etadurin which both are polyamidoamine-epichlorohydrin resins.
- Amine-aldehyde resins are suitable cross-linking resins for the present invention and are made by condensation of amine or amide monomers with aldehydes such as forrnaldehyde or glyoxal.
- Preferred amines are those having low molecular weight amines e.g. melamine or polymeric amines e.g. poly-diallylamine, preferably quarternized.
- Preferred amides are those polymeric amides such as polyacrylamide. All these suitable amine/amide monomers can also be copolymerized with cationic monomers.
- amine-aldehyde cross-linking resin preferred are those from the class of melamine-formaldehyde resin.
- Melamine-formaldehyde resins of this type are known as crosslinking agents of this type in the coating industry and are also described, for example, in German Auslegeschrift Nos. 2,457,387 (U.S. Pat. No. 4,035,213 incorporated herein by reference) and U.S. Pat. No. 1,719,324 and, in particular, in U.S. Pat. No. 3,242,230 incorporated herein by reference.
- Preferred melamine-formaldehyde resin are those commercially available under the tradenames Madurit, and Cassurit from Clariant.
- Still other preferred cross-linking resin having the property of being cationic among the class of amine-aldehyde cross-linking resin are the Poly(acrylamide-glyoxal) resin commercially available under the tradename SOLIDUR1 T KM from Clariant.
- the cross-linking resin having cationic properties have a molecular weight between 200 and 1,000,000, preferably between 500 and 100,000, most preferably between 1000 and 25,000.
- Cross-linking resin having a low molecular weight are most preferred for use in the present invention as they are more water-soluble and have a better fiber penetration.
- low molecular weight it is meant a molecular weight within the range of from 25 to 2000, preferably from 50 to 1000, and more preferably from 50 to 500.
- the level of cross-linking components or derivative thereof is present in an amount of from 0.01% to 60%, preferably from 0.01% to 30% by weight of the total composition
- catalysts includes organic acids such as citric acid, succinic acid, and tartaric acids, as well as conventional Lewis acid such as Al Cl 3 or MgCl 2 , or salts thereof, or mixtures thereof.
- organic acids such as citric acid, succinic acid, and tartaric acids
- Lewis acid such as Al Cl 3 or MgCl 2
- catalyst NKD made of a mixture of salts and organic acid, and commercially available from Hoechst.
- the level of catalyst is from 10% to 50%, preferably from 20 to 40% by weight of the cross-linking components or derivative thereof.
- the liquid carrier employed in the instant compositions is preferably at least primarily water due to its low cost, relative availability, safety, and environmental compatibility.
- the level of water in the liquid carrier is preferably at least about 50%, most preferably at least about 60%, by weight of the carrier.
- Mixtures of water and low molecular weight, e.g., ⁇ about 200, organic solvent, e.g., lower alcohols such as ethanol, propanol, isopropanol or butanol are useful as the carrier liquid.
- Low molecular weight alcohols include monohydric, dihydric (glycol, etc.) trihydric (glycerol, etc.), and higher polyhydric (polyols) alcohols.
- compositions containing both saturated and unsaturated diester quaternary ammonium compounds can be prepared that are stable without the addition of concentration aids.
- the compositions of the present invention may require organic and/or inorganic concentration aids to go to even higher concentrations and/or to meet higher stability standards depending on the other ingredients.
- concentration aids which typically can be viscosity modifiers may be needed, or preferred, for ensuring stability under extreme conditions when particular softener active levels are used.
- the surfactant concentration aids are typically selected from the group consisting of (1) single long chain alkyl cationic surfactants; (2) nonionic surfactants; (3) amine oxides; (4) fatty acids; and (5) mixtures thereof. These aids are described in WO 94/20597, specifically on page 14, line 12 to page 20, line 12, which is herein incorporated by reference.
- the total level is from 0.1% to 20%, preferably from 0.2% to 10%, more preferably from 0.5% to 5%, and even more preferably from 1% to 2% by weight of the composition.
- these materials can either be added as part of the active softener raw material, (I), e.g., the mono-long chain alkyl cationic surfactant and/or the fatty acid which are reactants used to form the biodegradable fabric softener active as discussed hereinbefore, or added as a separate component.
- the total level of dispersibility aid includes any amount that may be present as part of component (I).
- Inorganic viscosity/dispersibility control agents which can also act like or augment the effect of the surfactant concentration aids, include water-soluble, ionizable salts which can also optionally be incorporated into the compositions of the present invention.
- ionizable salts can be used. Examples of suitable salts are the halides of the Group IA and IIA metals of the Periodic Table of the Elements, e.g., calcium chloride, magnesium chloride, sodium chloride, potassium bromide, and lithium chloride.
- the ionizable salts are particularly useful during the process of mixing the ingredients to make the compositions herein, and later to obtain the desired viscosity.
- the amount of ionizable salts used depends on the amount of active ingredients used in the compositions and can be adjusted according to the desires of the formulator. Typical levels of salts used to control the composition viscosity are from about 20 to about 20,000 parts per million (ppm), preferably from about 20 to about 11,000 ppm, by weight of the composition.
- Alkylene polyammonium salts can be incorporated into the composition to give viscosity control in addition to or in place of the water-soluble, ionizable salts above.
- these agents can act as scavengers, forming ion pairs with anionic detergent carried over from the main wash, in the rinse, and on the fabrics, and may improve softness performance. These agents may stabilize the viscosity over a broader range of temperature, especially at low temperatures, compared to the inorganic electrolytes.
- alkylene polyammonium salts include 1-lysine monohydrochloride and 1,5-diammonium 2-methyl pentane dihydrochloride.
- Stabilizers can be present in the compositions of the present invention.
- the term “stabilizer,” as used herein, includes antioxidants and reductive agents. These agents are present at a level of from 0% to about 2%, preferably from about 0.01% to about 0.2%, more preferably from about 0.035% to about 0.1% for antioxidants, and more preferably from about 0.01% to about 0.2% for reductive agents. These assure good odor stability under long term storage conditions for the compositions and compounds stored in molten form.
- the use of antioxidants and reductive agent stabilizers is especially critical for low scent products (low perfume).
- antioxidants examples include a mixture of ascorbic acid, ascorbic palmitate, propyl gallate, available from Eastman Chemical Products, Inc., under the trade names Tenox® PG and Tenox S-1; a mixture of BHT (butylated hydroxytoluene), BHA (butylated hydroxyanisole), propyl gallate, and citric acid, available from Eastman Chemical Products, Inc., under the trade name Tenox-6; butylated hydroxytoluene, available from UOP Process Division under the trade name Sustane® BHT; tertiary butylhydroquinone, Eastman Chemical Products, Inc., as Tenox TBHQ; natural tocopherols, Eastman Chemical Products, Inc., as Tenox GT-1/GT-2; and butylated hydroxyanisole, Eastman Chemical Products, Inc., as BHA; long chain esters (C 8 -C 22 ) of gallic acid, e.g., dodecyl
- reductive agents examples include sodium borohydride, hypophosphorous acid, Irgafos® 168, and mixtures thereof.
- antimicrobial preservative can be added to the composition of the present invention. Contamination by certain microorganisms with subsequent microbial growth can result in an unsightly and/or malodorous solution. Because microbial growth in solutions is highly objectionable when it occurs, it is highly preferable to include an antimicrobial preservative, which is effective for inhibiting and/or regulating microbial growth in order to increase storage stability of the composition.
- a broad spectrum preservative e.g., one that is effective on both bacteria (both gram positive and gram negative) and fungi.
- a limited spectrum preservative e.g., one that is only effective on a single group of microorganisms, e.g., fungi, can be used in combination with a broad spectrum preservative or other limited spectrum preservatives with complimentary and/or supplementary activity.
- a mixture of broad spectrum preservatives can also be used.
- aminocarboxylate chelators such as those described hereinbefore, can be used alone or as potentiators in conjunction with other preservatives.
- chelators which include, e.g., ethylenediaminetetraacetic acid (EDTA), hydroxyethylenediaminetriacetic acid, diethylenetriaminepentaacetic acid, and other aminocarboxylate chelators, and mixtures thereof, and their salts, and mixtures thereof, can increase preservative effectiveness against Gram-negative bacteria, especially Pseudomonas species.
- EDTA ethylenediaminetetraacetic acid
- hydroxyethylenediaminetriacetic acid hydroxyethylenediaminetriacetic acid
- diethylenetriaminepentaacetic acid diethylenetriaminepentaacetic acid
- other aminocarboxylate chelators and mixtures thereof, and their salts, and mixtures thereof, can increase preservative effectiveness against Gram-negative bacteria, especially Pseudomonas species.
- Antimicrobial preservatives useful in the present invention include biocidal compounds, i.e., substances that kill microorganisms, or biostatic compounds, i.e., substances that inhibit and/or regulate the growth of microorganisms.
- biocidal compounds i.e., substances that kill microorganisms
- biostatic compounds i.e., substances that inhibit and/or regulate the growth of microorganisms.
- Well-known preservatives such as short chain alkyl esters of p-hydroxybenzoic acid, commonly known as parabens; N-(4-chlorophenyl)-N′-(3,4-dichlorophenyl) urea, also known as 3,4,4′-trichlorocarbanilide or triclocarban; 2,4,4′-trichloro-2′-hydroxy diphenyl ether, commonly known as triclosan are useful preservative in the present invention.
- Still other preferred preservatives are the water-soluble preservatives, i.e. those that have a solubility in water of at least about 0.3 g per 100 ml of water, i.e., greater than about 0.3% at room temperature, preferably greater than about 0.5% at room temperature.
- the preservative in the present invention is included at an effective amount.
- effective amount means a level sufficient to prevent spoilage, or prevent growth of inadvertently added microorganisms, for a specific period of time.
- the preservative is not being used to kill microorganisms on the surface onto which the composition is deposited in order to eliminate odors produced by microorganisms. Instead, it is preferably being used to prevent spoilage of the solution in order to increase the shelf-life of the composition.
- Preferred levels of preservative are from about 0.0001% to about 0.5%, more preferably from about 0.0002% to about 0.2%, most preferably from about 0.0003% to about 0.1%, by weight of the usage composition.
- the preservative can be any organic preservative material which will not cause damage to fabric appearance, e.g., discoloration, coloration, bleaching.
- Preferred water-soluble preservatives include organic sulfur compounds, halogenated compounds, cyclic organic nitrogen compounds, low molecular weight aldehydes, quaternary ammonium compounds, dehydroacetic acid, phenyl and phenolic compounds, and mixtures thereof.
- Non-limiting examples of preferred water-soluble preservatives for use in the present invention can be found in U.S. Pat. No. 5,714,137, incorporated hereinbefore by reference, as well as co-pending application PCTJUS 98/12154 pages 29 to 36.
- Preferred water-soluble preservatives for use in the present invention are organic sulfur compounds.
- organic sulfur compounds suitable for use in the present invention are:
- a preferred preservative is an antimicrobial, organic preservative containing 3-isothiazolone groups. This class of compounds is disclosed in U.S. Pat. No. 4,265,899, Lewis et al., issued May 5, 1981, and incorporated herein by reference.
- a preferred preservative is a water-soluble mixture of 5-chloro-2-methyl4-isothiazolin-3-one and 2-methyl4-isothiazolin-3-one, more preferably a mixture of about 77% 5-chloro-2-methyl4-isothiazolin-3-one and about 23% 2-methyl4-isothiazolin-3-one, a broad spectrum preservative available as a 1.5% aqueous solution under the trade name Kathon® CG by Rohm and Haas Company.
- Kathon® When Kathon® is used as the preservative in the present invention it is present at a level of from about 0.0001% to about 0.01%, preferably from about 0.0002% to about 0.005%, more preferably from about 0.0003% to about 0.003%, most preferably from about 0.0004% to about 0.002%, by weight of the composition.
- isothiazolins include 1,2-benzisothiazolin-3-one, available under the trade name Proxel® products; and 2-methyl4,5-trimethylene4-isothiazolin-3-one, available under the trade name Promexal®. Both Proxel and Promexal are available from Zeneca. They have stability over a wide pH range (i.e., 4-12). Neither contain active halogen and are not formaldehyde releasing preservatives.
- Proxel and Promexal are effective against typical Gram negative and positive bacteria, fungi and yeasts when used at a level from about 0.001% to about 0.5%, preferably from about 0.005% to about 0.05%, and most preferably from about 0.01% to about 0.02% by weight of the usage composition.
- sodium Pyrithione Another preferred organic sulfur preservative is sodium pyrithione, with water solubility of about 50%.
- sodium pyrithione is typically present at a level of from about 0.0001% to about 0.01%, preferably from about 0.0002% to about 0.005%, more preferably from about 0.0003% to about 0.003%, by weight of the usage composition.
- Mixtures of the preferred organic sulfur compounds can also be used as the preservative in the present invention.
- composition may suitably use an optional solubilized, water-soluble antimicrobial active, useful in providing protection against organisms that become attached to the treated material.
- an optional solubilized, water-soluble antimicrobial active useful in providing protection against organisms that become attached to the treated material.
- the free, uncomplexed antimicrobial, e.g., antibacterial, active provides an optimum antibacterial performance.
- compositions of the present invention containing, antimicrobial materials, e.g., antibacterial halogenated compounds, quaternary compounds, and phenolic compounds.
- antimicrobial materials e.g., antibacterial halogenated compounds, quaternary compounds, and phenolic compounds.
- Some of the more robust antimicrobial halogenated compounds which can function as disinfectants/sanitizers as well as finish product preservatives (vide infra), and are useful in the compositions of the present invention include 1,1′-hexamethylene bis(5-(p-chlorophenyl)biguanide), commonly known as chlorhexidine, and its salts, e.g., with hydrochloric, acetic and gluconic acids.
- the digluconate salt is highly water-soluble, about 70% in water, and the diacetate salt has a solubility of about 1.8% in water.
- chlorhexidine When used as a sanitizer in the present invention it is typically present at a level of from about 0.001% to about 0.4%, preferably from about 0.002% to about 0.3%, and more preferably from about 0.01% to about 0.1%, by weight of the usage composition. In some cases, a level of from about 1% to about 2% may be needed for virucidal activity.
- Other useful biguanide compounds include Cosmoci® CQ®, Vantocil® IB, including poly (hexamethylene biguanide) hydrochloride.
- Other useful cationic antimicrobial agents include the bis-biguanide alkanes.
- Usable water-soluble salts of the above are chlorides, bromides, sulfates, alkyl sulfonates such as methyl sulfonate and ethyl sulfonate, phenylsulfonates such as p-methylphenyl sulfonates, nitrates, acetates, gluconates, and the like.
- the bis biguanide of choice is chlorhexidine and its salts, e.g., digluconate, dihydrochloride, diacetate, and mixtures thereof.
- Quaternary Compounds A wide range of quaternary compounds can also be used as antimicrobial actives, in conjunction with the preferred surfactants, for compositions of the present invention that do not contain cyclodextrin.
- useful quaternary compounds include: (1) benzalkonium chlorides and/or substituted benzalkonium chlorides such as commercially available Barquat® (available from Lonza), Maquat® (available from Mason), Variquat® (available from Witco/Sherex), and Hyamine® (available from Lonza); (2) dialkyl quaternary such as Bardac® products of Lonza, (3) N-(3-chloroallyl) hexaminium chlorides such as Dowicide® and Dowicil® available from Dow; (4) benzethonium chloride such as Hyamine® 1622 from Rohm & Haas; (5) methylbenzethonium chloride represented by Hyamine® 10X supplied by Rohm & Haas, (6) cetylpyr
- Typical concentrations for biocidal effectiveness of these quaternary compounds range from about 0.001% to about 0.8%, preferably from about 0.005% to about 0.3%, more preferably from about 0.01% to 0.2%, by weight of the usage composition.
- the corresponding concentrations for the concentrated compositions are from about 0.003% to about 2%, preferably from about 0.006% to about 1.2%, and more preferably from about 0.1% to about 0.8% by weight of the concentrated compositions.
- the present invention can contain a perfume. Suitable perfumes are disclosed in U.S. Pat. No. 5,500,138, said patent being incorporated herein by reference.
- perfume includes fragrant substance or mixture of substances including natural (i.e., obtained by extraction of flowers, herbs, leaves, roots, barks, wood, blossoms or plants), artificial (i.e., a mixture of different nature oils or oil constituents) and synthetic (i.e., synthetically produced) odoriferous substances.
- natural i.e., obtained by extraction of flowers, herbs, leaves, roots, barks, wood, blossoms or plants
- artificial i.e., a mixture of different nature oils or oil constituents
- synthetic i.e., synthetically produced
- perfumes are complex mixtures of a plurality of organic compounds.
- perfume ingredients useful in the perfumes of the present invention compositions include, but are not limited to, hexyl cinnamic aldehyde; amyl cinnamic aldehyde; amyl salicylate; hexyl salicylate; terpineol; 3,7-dimethyl-cis-2,6-octadien-1-ol; 2,6-dimethyl-2-octanol; 2,6-dimethyl-7-octen-2-ol; 3,7-dimethyl-3-octanol; 3,7-dimethyl-trans-2,6-octadien-1-ol; 3,7-dimethyl-6-octen-1-ol; 3,7-dimethyl-1-octanol; 2-methyl-3-(para-tert-butylphenyl)-propionaldehyde; 4-(4-hydroxy4-methylpentyl)-3-cyclohexene-1-carbox-alde
- fragrance materials include, but are not limited to, orange oil; lemon oil; grapefruit oil; bergamot oil; clove oil; dodecalactone gamma; methyl-2-(2-pentyl-3-oxo-cyclopentyl) acetate; beta-naphthol methylether; methyl-beta-naphthylketone; coumarin; decylaldehyde; benzaldehyde; 4-tert-butylcyclohexyl acetate; alpha,alpha-dimethylphenethyl acetate; methylphenylcarbinyl acetate; Schiff's base of 4-(4-hydroxy4-methylpentyl)-3-cyclohexene-1-carboxaldehyde and methyl anthranilate; cyclic ethyleneglycol diester of tridecandioic acid; 3,7-dimethyl-2,6-octadiene-1-nitrile; i
- perfume components are geraniol; geranyl acetate; linalool; linalyl acetate; tetrahydrolinalool; citronellol; citronellyl acetate; dihydromyrcenol; dihydromyrcenyl acetate; tetrahydromyrcenol; terpinyl acetate; nopol; nopyl acetate; 2-phenylethanol; 2-phenylethyl acetate; benzyl alcohol; benzyl acetate; benzyl salicylate; benzyl benzoate; styrallyl acetate; dimethylbenzylcarbinol; trichloromethylphenylcarbinyl methylphenylcarbinyl acetate; isononyl acetate; vetiveryl acetate; vetiverol; 2-methyl-3-(p-tert-butylphenyl)-propanal; 2-methyl-3-(
- the perfumes useful in the present invention compositions are substantially free of halogenated materials and nitromusks.
- Suitable solvents, diluents or carriers for perfumes ingredients mentioned above are for examples, ethanol, isopropanol, diethylene glycol, monoethyl ether, dipropylene glycol, diethyl phthalate, triethyl citrate, etc.
- the amount of such solvents, diluents or carriers incorporated in the perfumes is preferably kept to the minimum needed to provide a homogeneous perfume solution.
- Perfume can be present at a level of from 0% to 10%, preferably from 0.1% to 5%, and more preferably from 0.2% to 3%, by weight of the finished composition.
- Fabric softener compositions of the present invention provide improved fabric perfume deposition.
- Perfume ingredients may also be suitably added as releasable fragrances, for example, as pro-perfumes or pro-fragrances as described in U.S. Pat. No. 5,652,205 Hartman et al., issued Jul. 29, 1997, WO95/04809, WO96/02625, PCT US97/14610 filed 19 Aug. 1997 and claiming priority of 19 Aug. 1996, EP-A-0,752,465, co-pending application EP 98870227.0, EP 98870226.2, EP 99870026.4, and EP 99870025.6; all incorporated herein by reference.
- Soil Release agents are desirably used in compositions of the instant invention. Any polymeric soil release agent known to those skilled in the art can optionally be employed in the compositions of this invention. Polymeric soil release agents are characterized by having both hydrophilic segments, to hydrophilize the surface of hydrophobic fibers, such as polyester and nylon, and hydrophobic segments, to deposit upon hydrophobic fibers and remain adhered thereto through completion of washing and rinsing cycles and, thus, serve as an anchor for the hydrophilic segments. This can enable stains occurring subsequent to treatment with the soil release agent to be more easily cleaned in later washing procedures.
- soil release agents will generally comprise from about 0.01% to about 10.0%, by weight, of the detergent compositions herein, typically from about 0.1% to about 5%, preferably from about 0.2% to about 3.0%.
- soil release agents include the METOLOSE SM100, METOLOSE SM200 manufactured by Shin-etsu Kagaku Kogyo K. K., SOKALAN type of material, e.g., SOKALAN HP-22, available from BASF (Germany), ZELCON 5126 (from Dupont) and MILEASE T (from ICI).
- compositions according to the present invention are optional requirements of the compositions according to the present invention.
- the pH as measured in the neat compositions at 20° C. is greater than 3, preferably between 3 and 12, more preferably between 4 and 8, most preferably is of 5. This range is preferred for fabric safety.
- the pH of these compositions herein can be regulated by the addition of a Bronsted acid.
- blowing agents selected from the group consisting of ammonium carbonate, ammonium bicarbonate, or mixtures thereof. It is hypothesized that those agents, when present, will generate small amounts of CO 2 when exposed to heat, such as during the ironing process. The CO 2 will be released in the composition which is deposited as a film on the fabric. The film, hence the fabric, will thus acquire more flexibility, resulting in a better ability to resist to the dry formation of wrinkles, when the fabric is stored or worn.
- compositions herein may further comprise void fillers.
- fabric void filler it is meant herein particles having the size and shape suited to fill the structural defects in cotton, and hereby provide lubricating properties.
- Cyclodextrins such as those described in WO 99/55950 can be used as void fillers, as well as polyolefin dispersions, such as those described in U.S. Pat. No. 6,020,302.
- Inorganic particles can also be used to that effect, for instance TiO 2 and Silica.
- the present invention can include optional components conventionally used in textile treatment compositions, for example, humectants like diethylene glycol, and/or salts like lithium salts, colorants, bactericides, optical brighteners, opacifiers, anti-shrinkage agents, germicides, fungicides, anti-oxidants, color protection agent like dye fixing agent as described in EP 931133, enzymes, chelating agents, cyclodextrin as described in WO 98/56888, metallic salts to absorb amine and sulfur-containing compounds and selected from the group consisting of copper salts, zinc salts, and mixtures thereof, water-soluble polyionic polymers, e.g., water-soluble cationic polymer like polyamines, and water-soluble anionic polymers like polyacrylic acids, other antistatic agent, insect and/or moth repelling agents, colorants and dyes, anti-clogging agent, and the like; typical disclosure of which can be found in WO 98/56888.
- compositions are preferably free of any material that would soil or stain fabric, and are also substantially free of starch. Typically, there should be less than about 0.5%, by weight of the composition, preferably less than about 0.3%, more preferably less than about 0.1%, by weight of the composition, of starch and/or modified starch.
- composition of the invention may take a variety of physical form including liquid, liquid-gel, paste-like, foam in either aqueous or non-aqueous form, powder like granular and tablet forms.
- a preferred form of the composition is in a liquid form.
- the composition When in a liquid form, the composition is preferably dispensed by a dispensing means such as a spray dispenser, aerosol dispenser, or refill thereof. Still another preferred dispensing means is by incorporation of the composition of the invention in the ironing tank per se, or via a cartridge preferably adapted for the iron.
- a dispensing means such as a spray dispenser, aerosol dispenser, or refill thereof.
- Still another preferred dispensing means is by incorporation of the composition of the invention in the ironing tank per se, or via a cartridge preferably adapted for the iron.
- the present invention also relates to such compositions incorporated into a spray dispenser to create an article of manufacture that can facilitate treatment of fabric articles and/or surfaces with the compositions according to the invention at a level that is effective.
- the spray dispenser comprises manually activated and non-manual powered (operated) spray means and a container containing the treating composition. Typical disclosure of such spray dispenser can be found in WO 96/04940 page 19 line 21 to page 22 line 27.
- the spray dispenser is selected from spray dispenser comprising battery operated pump, spray dispenser comprising a trigger spray device, spray dispenser comprising a pressurized aerosol spray dispenser.
- the use of the water-soluble lubricant provided a reduction of the WRA compared to water. Accordingly, there is provided a method of increasing the WRA of fabrics, which comprises the steps of contacting the fabrics with a water-soluble lubricant as defined herein before, using a domestic process.
- the use of the water-soluble lubricant or composition of the invention provides surprisingly good benefit on the dewrinkling performance upon wearing. This benefit is particularly achieved while spraying the compound or composition preferably from an iron during an otherwise known fabric ironing process. Accordingly, there is also provided a method of treating fabrics, in particular to provide in wear wrinkle resistance on fabrics, which comprises the steps of contacting the fabrics with a water-soluble lubricant or composition according to the invention, as defined herein before, using a domestic process.
- contacting it is meant any steps that is suitable for providing a contact of the composition with the fabric. This can include by soaking, washing, rinsing, and/or spraying as well as by means of a dryer sheet onto which is adsorbed the composition.
- the contacting occurs after the laundering and optional drying of the fabrics, e.g. by spraying the composition, more preferably by spraying the composition from an iron spray dispenser and/or via the vaporisation holes from an iron sole plate or foam or sprayer which is separate from the iron.
- the composition of the present invention is used as an ironing aid.
- An effective amount of the composition can be sprayed onto the fabric, wherein said fabric should not be sprayed to saturation.
- Still another preferred way of treating the fabrics is when the fabric can be sprayed with an effective amount of the composition, allowed to dry and then ironed, or sprayed and ironed immediately.
- the composition of the invention can also be sprayed onto the fabrics by means of spraying means which are incorporated to an iron, and the composition is incorporated into the iron's water tank or via a cartridge, adapted to fit within the iron.
- spraying means which are incorporated to an iron
- the composition is incorporated into the iron's water tank or via a cartridge, adapted to fit within the iron.
- the spraying means should preferably be capable of providing droplets with a weight average diameter of from about 40 to about 200 ⁇ m, preferably from about 70 to about 150 ⁇ m.
- the loading of moisture on fabrics made of natural and synthetic fibers is from about 5 to about 25%, more preferably from about 5 to about 10% by weight of the dried fabric.
- cylinder test By “wrinkle reducing composition”, it is meant that the composition is tested on 100% cotton, woven Oxford pinpoint fabric according to the procedure given in W. Garner, Textile Laboratory Manual Vol. 6, Ed. 3, Elsevier Inc., 1967, p. 105, so called “cylinder test”.
- Wrinkle reducing compositions are compositions which provide a better crease resistance versus water, i.e, fabrics that have been treated with a composition of the invention show less wrinkles compared to fabrics which have only been treated with water.
- the composition can be sprayed onto fabrics by an in-home de-wrinkling chamber containing the fabric to be dewrinkled, thereby providing ease of operation.
- Conventional personal as well as industrial de-wrinkling apparatuses are suitable for use herein. Traditionally, these apparatuses act by a steaming process which effects a relaxation of the fibers. Examples of home dewrinkling chambers include shower stalls.
- the spraying of the composition or compounds onto the fabrics can then occur within the chamber of the apparatus or before placing the fabrics into the chamber.
- the spraying means should preferably be capable of providing droplets with a weight average diameter of from about 8 to about 100 ⁇ m, preferably from about 10 to about 50 ⁇ m.
- the loading of moisture on fabrics made of natural and synthetic fibers is from about 5 to about 25%, more preferably from about 5 to about 10% by weight of the dried fabric.
- Other conventional steps that can be carried out in the dewrinkling apparatus can be applied such as heating which will provide the curing step and drying.
- the temperature profile inside the chamber ranges from about 40° C. to about 80° C., more preferably from about 50° C. to about 70° C.
- the preferred length of the drying cycle is from about 15 to about 60 minutes, more preferably from about 20 to about 45 minutes.
- the steaming step in the dewrinkling apparatus can also be eliminated if the composition is maintained at a temperature range from about 22° C. (about 72° F.) to about 76° C. (170° F.) before spraying.
- the present invention encompasses the method of spraying a mist of an effective amount of solution of the invention composition onto fabric and/or fabric articles.
- said fabric and/or fabric articles include, but are not limited to, clothes, curtains, drapes, upholstered furniture, carpeting, bed linens, bath linens, tablecloths, sleeping bags, tents, car interiors, etc.
- compositions herein are especially useful, when used to treat garments for extending the time before another wash cycle is needed, and/or even reducing the time involved in ironing.
- Such garments include uniforms and other garments which are normally treated in an industrial process, which can be dewrinkled and the time between treatments extended.
- an article of manufacture comprising a container and the composition of the invention in association with a set of instructions to use the composition in an amount effective to provide a solution to problems involving and/or provision of a benefit related to those selected from reducing wrinkles; imparting in-wear resistance to fabrics. It is important that the consumer be aware of these additional benefits, since otherwise the consumer would not know that the composition would solve these problems and/or provide these benefits.
- the phrase “in association with” means the set of instructions are either directly printed on the container itself or presented in a separate manner including, but not limited to, a brochure, print advertisement, electronic advertisement, and/or verbal communication, so as to communicate the set of instructions to a consumer of the article of manufacture.
- the set of instructions preferably comprises the instruction to apply an effective amount of the composition, preferably by spraying, to provide the indicated benefit, e.g. wrinkles reduction; imparting in-wear resistance to fabrics.
- the set of instructions preferably comprises instructions to spray the composition on the fabrics and iron the fabrics.
- Polymer 1 Isomalto oligosaccharide available from Showa Sangyo Co. under the trade name IMO 900
- Polymer 2 Polyvinylpyrrolidone available from BASF under the trade name Luviskol K30
- Polymer 3 Co-polymer of vinylpyrrolidone and vinylcaprolactame available from BASF under the trade name Luvitec VPC
- Polymer 4 Co-polymer of vinylpyrrolidone and vinylimidazolinium methachloride available from BASF under the trade name Luviquat FC 905
- Lubricant 1 Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7200 from OSI Chem./Witco
- Lubricant 2 Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7657 from OSI Chem.[Witco
- Lubricant 3 Polyethoxylated (20 moles) sorbitan monolaurate commercially available under the tradename of Radiasurf 7137 from FINA
- Lubricant 4 Polyethoxylated (20 moles) sorbitan tristearate commercially available under the tradename of Tween 65
- Wetting agent 1 Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7600 from OSI Chem./Witco
- Wetting agent 1 Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet L 77 from OSI Chem./Witco
- Emulsifier 1 CAE 10 (coconut alcohol condensed with an average of 10 moles of ethylene oxide) A B C D E F Polymer #1 5% — — — — 1% Polymer #2 — 1% — 2% — — Polymer #3 — — 2% — — — Polymer #4 — — — — — 0.5% — Lubricant #1 14% 4% — — 1.5% — Lubricant #2 — — 6% 2% 1.5% — Lubricant #3 8% 2% — — — 5% Lubricant #4 — — — 3% — — Wetting agent 1 3% 0.5 — — 0.2% — Wetting agent 2 — — 0.5% — — — Dipropyleneglycol — 0.3 — — — — — Emulsifier 1 0.6% 0.2 — — — — — Cyclodextrin — 0.5 1% — — — — Preserv
Abstract
There is provided a composition comprising a lubricant, preferably a water-soluble one, and components having a deviation of fabric wrinkle recovery angle versus water of at least +15, whereby the combination imparts in-wear wrinkle resistance to the fabric treated therewith.
Description
- This application is a continuation of U.S. patent application Ser. No. 10/111,491, filed Apr. 25, 2002, the disclosure of which is incorporated by reference herein.
- The present invention relates to fabric care compositions and to a method for treating fabrics in order to improve various properties of fabrics, in particular in-wear wrinkle resistance.
- Wrinkles in textile fabrics are caused by the bending and creasing of the textile material which places an external portion of a filament in a yarn under tension while the internal portion of that filament in the yarn is placed under compression. Particularly with cotton fabrics, the hydrogen bonding that occurs between the cellulose molecules contributes to keeping wrinkles in place. The wrinkling of fabric, in particular clothing, is therefore subject to the inherent tensional elastic deformation and recovery properties of the fibers which constitute the yam and fabrics.
- In the modern world, with the increase of hustle and bustle and travel, there is a demand for a quick fix which will help to diminish the labor involved in home laundering and/or the cost and time involved in dry cleaning or commercial laundering. Further, it is well-known that alternating cycles of using and laundering fabrics and textiles, such as articles of worn clothing and apparel, will inevitably adversely affect the appearance and integrity of the fabric and textile items so used and laundered. Fabrics and textiles simply wear out over time and with use. Laundering of fabrics and textiles is necessary to remove soils and stains which accumulate therein and thereon during ordinary use. However, the laundering operation itself, over many cycles, can accentuate and contribute to the deterioration of the integrity and the appearance of such fabrics and textiles. Accordingly, this has brought additional pressure to bear on textile technologists to produce a product that will sufficiently reduce wrinkles in fabrics, especially clothing, whilst still producing a good appearance through a simple, convenient application of a product.
- The prior art contains numerous examples of compositions for reducing wrinkles. U.S. Pat. No. 5,532,023, discloses aqueous wrinkle control compositions containing non-volatile silicone and film-forming polymer. Preferred silicones include reactive silicones and amino-functional silicone, known as “amodimethicone”. The composition containing such silicones is applied to fabric from a spray dispenser. It is found that in the spray treatment, an appreciable amount of the aqueous composition misses the fabric, and instead falls on flooring surfaces, such as rugs, carpets, concrete floors, tiled floors, linoleum floors, bathtub floors, which leaves a silicone layer that is accumulated on and/or cured on and/or bonded to the flooring surfaces. Such silicones that are accumulated on such surfaces, and especially those that are bonded to such surfaces are difficult to remove. Flooring surfaces thus become slippery and can present a safety hazard to the household members. U.S. Pat. No. 5,573,695 discloses an aqueous wrinkle removal composition containing a vegetable oil based cationic quaternary ammonium surfactant, and an anionic fluorosurfactant. Similarly, U.S. Pat. No. 4,661,268 discloses a wrinkle removal spray comprising an aqueous alcoholic composition containing a dialkyl quaternary ammonium salt and a silicone surfactant and/or a fluoro surfactant. U.S. Pat. No. 5,100,566 discloses a method of reducing wrinkles in fabric by spraying the fabric with an aqueous alcoholic solution of an anionic siliconate alkali metal salt. U.S. Pat. No. 4,806,254 discloses fabric wrinkle removal aqueous alcoholic solution containing glycerine and a nonionic surfactant. WO98/04772 provides the treatment of fabric against fabric creasing by application of a composition comprising a polycarboxylic acid or derivative thereof; and then curing the composition using a domestic process. Starch is also a conventional ingredient of dewrinkling compositions. However, while starch provides a suitable visual benefit onto the treated fabrics, it also gives fabric with an undesired stiff or starchy feeling. These patents are incorporated herein by reference.
- Accordingly, the domestic treatment of fabric is a problem known in the art to the formulator of laundry compositions. Therefore, there is a need for a wrinkle reducing composition which reduces the above mentioned negatives.
- Further, most of the focus in the dewrinkling area has been on providing compositions with instant dewrinkling. However, with the current trends of reducing the labor involved in ironing, it has now been found that there is a need for a composition that would additionally provide in-wear wrinkle resistance, i.e. a composition that would provide long-lasting benefit upon ironing, and wearing.
- Moreover, there is also a need for an efficient and economical composition.
- It has now surprisingly been found that the combination of a lubricant, preferably a water-soluble one, and component having a deviation of fabric Wrinkle Recovery Angle (WRA) versus water of at least +15 fulfill such a need. This finding is particularly surprising, especially when the component providing such deviation is a polymer. Indeed, it is known that the combination of a lubricant, especially a water-soluble one, with polymer is often the cause of phase separation. Further, often the addition of polymer like starch on top of composition comprising a lubricant, preferably a water-soluble one, was found to give even worse results on the in-wear performance. Surprisingly, it has been found that the addition of a component providing a deviation of fabric WRA of at least +15 overcome such problems.
- Accordingly, the present invention reduces wrinkles in fabrics, including clothing, dry cleanables, linens, bed clothes, and draperies, by ironing. The present invention can be used on damp or dry clothing to relax wrinkles and give clothes a ready to wear look with lasting benefits that is demanded by today's fast paced world.
- In a preferred aspect, an additional benefit of the composition of the present invention is an improved garment shape, body and crispness.
- The composition of the present invention acts as an excellent ironing aid. The present invention makes the task of ironing easier and faster by creating less iron drag. The compositions of the present invention help produce a crisp, smooth appearance.
- The present invention is a wrinkle reducing composition comprising a lubricant, preferably a water-soluble one, and a component having a deviation of fabric Wrinkle Recovery angle (WRA) versus water of at least +15.
- In another aspect of the invention, there is provided an article of manufacture comprising the composition of the invention, such as a sprayer, an aerosol, a foam dispenser, an iron, a refill cartridge thereof which contains the composition.
- Still in a further aspect of the invention, there is provided a method of treating fabrics for imparting benefits selected from the group consisting of: reducing wrinkles and imparting in-wear resistance to fabrics. In a preferred method, the composition is sprayed onto a fabric and the fabric is ironed.
- In a further aspect of the invention, there is provided an article of manufacture comprising a container and the composition of the invention in association with usage instructions, in particular, instructions to use in a method where the composition is sprayed onto the fabric and the fabric is ironed.
- 1)-lubricant:
- One essential component of the invention is a lubricant, preferably a water-soluble one. By means of this component, the composition provides an ease of ironing whilst still avoiding the staining of fabric and/or presenting safety hazard to the household members.
- For the purposes of the present invention the term “water-soluble” is defined as “a component which when dissolved in water at a level of 0.2% by weight, or less, at 25° C., forms a clear, isotropic liquid”.
- Typical water-soluble lubricants include components selected from nonionic silicone containing surfactants, sorbitan esters, ethoxylated sorbitan esters, and mixtures thereof. The water-soluble lubricants are preferably present in an amount of from 0.1% to 70% by weight of the composition, more preferably of from 1 to 10% % by weight of the composition for diluted composition and of from 20 to 50% by weight of the composition for concentrated compositions.
- A preferred class of nonionic silicone containing surfactants are the polyalkylene oxide polysiloxanes having a dimethyl polysiloxane hydrophobic moiety and one or more hydrophilic polyalkylene side chains, and having the general formula:
R1—(CH3)2SiO—(CH3)2SiO]a—[(CH3)(R1)SiO]b—Si(CH3)2—R1
wherein a+b are from about 1 to about 50, preferably from about 1 to about 30, more preferably from about 1 to about 25, and each R1 is the same or different and is selected from the group consisting of methyl and a poly(ethyleneoxide/propyleneoxide) copolymer group having the general formula:
—(CH2)n O(C2 H4 O)c (C3 H6 O)d R2
with at least one R1 being a poly(ethyleneoxy/propyleneoxy) copolymer group, and wherein n is 3 or 4, preferably 3; total c (for all polyalkyleneoxy side groups) has a value of from 1 to about 100, preferably from about 6 to about 100; total d is from 0 to about 14, preferably from 0 to about 3; and more preferably d is 0; total c+d has a value of from about 5 to about 150, preferably from about 7 to about 100 and each R2 is the same or different and is selected from the group consisting of hydrogen, an alkyl having 1 to 4 carbon atoms, and an acetyl group, preferably hydrogen and methyl group. Each polyalkylene oxide polysiloxane has at least one R1 group being a poly(ethyleneoxide/propyleneoxide) copolymer group. - Nonlimiting examples of this type of surfactants are the Silwet® surfactants which are available OSI Specialties Inc., a Division of Witco, Danbury, Connecticut. Representative Silwet® surfactants which contain only ethyleneoxy (C2H4O) groups are as follows.
Name Average MW Average a + b Average total c L-7608 600 1 8 L-7607 1,000 2 17 L-77 600 1 9 L-7605 6,000 20 99 L-7604 4,000 21 53 L-7600 4,000 11 68 L-7657 5,000 20 76 L-7602 3,000 20 29 L-7622 10,000 88 75 - Nonlimiting examples of Silwet® surfactants which contain both ethyleneoxy (C2 H4 O) and propyleneoxy (C3 H6 O) groups are as follows.
Name Average MW EO/PO ratio L-720 12,000 50/50 L-7001 20,000 40/60 L-7002 8,000 50/50 L-7210 13,000 20/80 L-7200 19,000 75/25 L-7220 17,000 20/80 - The molecular weight of the polyalkyleneoxy group (R1) is less than or equal to about 10,000. Preferably, the molecular weight of the polyalkyleneoxy group is less than or equal to about 8,000, and most preferably ranges from about 300 to about 5,000. Thus, the values of c and d can be those numbers which provide molecular weights within these ranges. However, the number of ethyleneoxy units (—C2H4O) in the polyether chain (R1) must be sufficient to render the polyalkylene oxide polysiloxane water-soluble. If propyleneoxy groups are present in the polyalkylenoxy chain, they can be distributed randomly in the chain or exist as blocks. Mixtures of Silwet® surfactants which contain both ethyleneoxy and propyleneoxy groups, are also preferred. Preferred Silwet® surfactants are the L-7001, L-7087, L-7200, L-7280, L-7600, L-7608, L-7622, L-7657.
- The preparation of polyalkylene oxide polysiloxanes is well-known in the art. Polyalkylene oxide polysiloxanes of the present invention can be prepared according to the procedure set forth in U.S. Pat. No. 3,299,112, incorporated herein by reference. Typically, polyalkylene oxide polysiloxanes of the surfactant blend of the present invention are readily prepared by an addition reaction between a hydrosiloxane (i.e., a siloxane containing silicon-bonded hydrogen) and an alkenyl ether (e.g., a vinyl, allyl, or methallyl ether) of an alkoxy or hydroxy end-blocked polyalkylene oxide). The reaction conditions employed in addition reactions of this type are well-known in the art and in general involve heating the reactants (e.g., at a temperature of from about 85° C. to 110° C.) in the presence of a platinum catalyst (e.g., chloroplatinic acid) and a solvent (e.g., toluene).
- Still other preferred water-soluble lubricants of the nonionic type are those from the class of sorbitan esters and/or alkylethoxylate sorbitan ester. These ethoxylated sorbitan esters are formed by ethoxylation of sorbitan or its cyclic derivative sorbitan, followed by esterification of one of the available hydroxy groups to introduce one long chain alkyl or alkenyl group, leaving the remaining hydroxy groups free. Compounds of this type are included in the range commercially available under the Registered Trade Mark TWEEN from Aldrich and from ICI United States Inc, but are also available from other suppliers e.g Radiasurf 7137 (Polyethoxylated (20 moles) sorbitan monolaurate), Radiasurf 7147 (Polysorbate 60), Radiasurf 7157 (Polysorbate 80) commercially available from FINA and Tween 65 (Polyethoxylated (20 moles) sorbitan tristearate), Tween 20 (Polyethoxylated (20 moles) sorbitan monolaurate, Tween 21 (Polyethoxylated (4 moles) sorbitan monolaurate), Tween 40 (Polyethoxylated (20 moles) sorbitan palmitate), commercially available from Aldrich.
- Water-insoluble lubricants are also useful herein. Suitable water-insoluble lubricants include cationic fabric softeners, silicones, and aliphatic and cycloaliphatic hydrocarbons.
- Suitable cationic fabric softening components for use herein include the water-insoluble quaternary-ammonium fabric softeners, the most commonly used having been di-long alkyl chain ammonium chloride or methyl sulfate.
- Preferred cationic softeners among these include the following:
-
- 1) ditallow dimethylammonium chloride (DTDMAC);
- 2) dihydrogenated tallow dimethylammonium chloride;
- 3) dihydrogenated tallow dimethylammonium methylsulfate;
- 4) distearyl dimethylammonium chloride;
- 5) dioleyl dimethylammonium chloride;
- 6) dipalmityl hydroxyethyl methylammonium chloride;
- 7) stearyl benzyl dimethylammonium chloride;
- 8) tallow trimethylammonium chloride;
- 9) hydrogenated tallow trimethylammonium chloride;
- 10) C12-14 alkyl hydroxyethyl dimethylammonium chloride;
- 11) C12-18 alkyl dihydroxyethyl methylammonium chloride;
- 12) di(stearoyloxyethyl) dimethylammonium chloride (DSOEDMAC);
- 13) di(tallowoyloxyethyl) dimethylammonium chloride;
- 14) ditallow imidazolinium methylsulfate;
- 15) 1-(2-tallowylamidoethyl)-2-tallowyl imidazolinium methylsulfate.
- However, in recent years, the need has arisen for more environmentally-friendly materials, and rapidly biodegradable quaternary ammonium compounds have been presented as alternatives to the traditionally used di-long alkyl chain ammonium chlorides and methyl sulfates. Such quaternary ammonium compounds contain long chain alk(en)yl groups interrupted by functional groups such as carboxy groups. Said materials and fabric softening compositions containing them are disclosed in numerous publications such as EP-A-0,040,562, and EP-A-0,239,910.
-
-
- R1 is (CH2)n-Q-T2 or T3;
- R2 is (CH2)m-Q-T4 or T5 or R3;
- R3 is C1-C4 alkyl or C1-C4 hydroxyalkyl or H;
- R4 is H or C1-C4 alkyl or C1-C4 hydroxyalkyl;
- T1, T2, T3, T4, T5 are independently C6-C22 alkyl or alkenyl;
- n and m are integers from 1 to 4; and
- X− is a softener-compatible anion.
- Non-limiting examples of softener-compatible anions include chloride or methyl sulfate.
- The alkyl, or alkenyl, chain T1, T2, T3, T4, T5 must contain at least 6 carbon atoms, preferably at least 11 carbon atoms, more preferably at least 16 carbon atoms. The chain may be straight or branched.
- Tallow is a convenient and inexpensive source of long chain alkyl and alkenyl material. The compounds wherein T1, T2, T3, T4, T5 represents the mixture of long chain materials typical for tallow are particularly preferred.
- Specific examples of quaternary ammonium compounds suitable for use in the aqueous fabric softening compositions herein include:
-
- 1) N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl ammonium chloride;
- 2) N,N-di(tallowoyl-oxy-ethyl)-N-methyl, N-(2-hydroxyethyl) ammonium methyl sulfate;
- 3) N,N-di(2-tallowoyl-oxy-2-oxo-ethyl)-N,N-dimethyl ammonium chloride;
- 4) N,N-di(2-tallowoyl-oxy-ethylcarbonyl-oxy-ethyl)-N,N-dimethyl ammonium chloride;
- 5) N-(2-tallowoyl-oxy-2-ethyl)-N-(2-tallowyl-oxy-2-oxo-ethyl)-N,N-dimethyl ammonium chloride;
- 6) N,N,N-tri(tallowoyl-oxy-ethyl)-N-methyl ammonium chloride;
- 7) N-(2-tallowoyl-oxy-2-oxo-ethyl)-N-(tallowyl-N,N-dimethyl-ammonium chloride; and
- 8) 1,2-ditallowoyl-oxy-3-trimethylammoniopropane chloride; and mixtures of any of the above materials.
- Of these, compounds 1-7 are examples of compounds of Formula (I); compound 8 is a compound of Formula (II).
- Particularly preferred is N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl ammonium chloride, where the tallow chains are at least partially unsaturated.
- The level of unsaturation of the tallow chain can be measured by the Iodine Value (IV) of the corresponding fatty acid, which in the present case should preferably be in the range of from 5 to 100 with two categories of compounds being distinguished, having a IV below or above 25.
- Indeed, for compounds of Formula (I) made from tallow fatty acids having a IV of from 5 to 25, preferably 15 to 20, it has been found that a cis/trans isomer weight ratio greater than 30/70, preferably greater than 50/50 and more preferably greater than 70/30 provides optimal concentrability.
- For compounds of Formula (I) made from tallow fatty acids having a IV of above 25, the ratio of cis to trans isomers has been found to be less critical unless very high concentrations are needed. Other examples of suitable quaternary ammoniums of Formula (I) and (II) are obtained by, e.g.:
-
- replacing “tallow” in the above compounds with, for example, coco, palm, lauryl, oleyl, ricinoleyl, stearyl, palmityl, or the like, said fatty acyl chains being either fully saturated, or preferably at least partly unsaturated;
- replacing “methyl” in the above compounds with ethyl, ethoxy, propyl, propoxy, isopropyl, butyl, isobutyl or t-butyl;
- replacing “chloride” in the above compounds with bromide, methylsulfate, formate, sulfate, nitrate, and the like.
- In fact, the anion is merely present as a counterion of the positively charged quaternary ammonium compounds. The nature of the counterion is not critical at all to the practice of the present invention. The scope of this invention is not considered limited to any particular anion. By “amine precursors thereof” is meant the secondary or tertiary amines corresponding to the above quaternary ammonium compounds, said amines being substantially protonated in the present compositions due to the pH values.
-
- the alkyl or aryl groups substituted on the siloxane chain (R) or at the ends of the siloxane chains (A) can have any structure as long as the resulting silicones remain fluid at room temperature. Preferably, the silicones are hydrophobic, are neither irritating, toxic, nor otherwise harmful when applied to fabrics or when they come in contact with human skin, are compatible with other components of the composition, are chemically stable under normal use and storage conditions, and are capable of being deposited on fabric.
- The R group preferably is a phenyl, a hydroxy, an alkyl or an aryl. The two R groups on the silicone atom can represent the same group or different groups. More preferably, the two R groups represent the same group preferably a methyl, an ethyl, a propyl, a phenyl or a hydroxy group, q is prereably an integer from about 7 to about 8,000.
- “A” represents groups which block the ends of the silicone chains. Suitable A groups include hydrogen, methyl, methoxy, ethoxy, hydroxy, propoxy, and aryloxy. The preferred silicones are polydimethyl siloxanes; more preferred silicones are polydimethyl siloxanes having a viscosity of greater than about 10 000 centistokes (cst) at 25° C.
- Suitable methods for preparing these silicone materials are disclosed in U.S. Pat. Nos. 2,826,551 and 3,964,500, incorporated herein by reference. Silicones useful in the present invention are also commercially available. Suitable examples include silicones offered by Dow Corning Corporation.
- Still other water-insoluble lubricants for use herein are hydrocarbons. Suitable hydrocarbons for use herein include, in particular, linear or branched C8-C40 paraffin hydrocarbons or mixtures of different hydrocarbons. An important factor in the selection of suitable hydrocarbons is that they should have a liquid to at most wax-like consistency at room temperature.
- 2)-component having a deviation of fabric WRA versus water of at least +15:
- A component having a deviation of fabric WRA of at least +15 is another essential component of the invention. Typically, these components are present in an amount of at least about 0.01%, preferably from about 0.1% to about 20% by weight of the composition, preferably to about 4% by weight of the diluted composition, preferably to about 12% by weight of the concentrated composition.
- The WRA Test method is taken from the AATCC 66-1990. This method is an American National Standard method designed for the determination of the wrinkle recovery of woven fabrics, whereby a test specimen, creased and compressed under controlled conditions of time and load, is suspended in the test instrument for a controlled recovery period, after which the recovery angle is measured. Experimental detail on how to measure this WRA is given in AATCC 66-1990, incorporated herein by reference. The WRA method is tested on 100% cotton, woven Oxford pinpoint fabric, free from wrinkles, cut in twelve specimens of 0.59 inch×1.57 inch, six with their long dimension parallel to the warp, and six with their long dimensional parallel to the filling. The test is carried out on cloth conditioned for 24 hours at 21° C. (70° F.) and 65% RH. Three specimens from each set are creased on one side and three on the other. Tweezers are used to place the test specimen between the leaves of the specimen holder (2 superimposed leaves 0.63 inch wide, but of different lengths and fastened together at one end) with one end directly under the 0.71-inch mark. With the tweezers, the exposed end of the specimen is lifted over and looped back to the 0.71-inch mark on the shorter, thin metal leaf and held with the left thumbnail. The holder with the specimen is inserted into a plastic press (2 superimposed leaves of equal length (3.74 inch) and 0.79 inch wide, fastened together at one end ) and a weight of 500 g is applied for 5 minutes so that a crease is formed. The plastic press can then be removed and the specimen holder combination can be inserted in the tester with the exposed end of the specimen holder in the mount on the face of the tester. The crease should line up with a spot at the center of the tester disk, and the dangling specimen leg should be lined up immediately with the vertical guide line. In order to eliminate gravitation effects, keep the dangling specimen leg aligned with the vertical guide line during the 5-min recovery period. Adjust every 15 seconds for the first minute, and once a minute thereafter. Five minutes after the removal of the creasing load, the wrinkle recovery value is read to the nearest degree from the scale. The sum is taken of the average recovery for all warp readings and all filling readings and compared with a cloth treated with water.
- Components defined by their WRA are well-known in the art. For example, in JAPS, Vol.15, pp.341-349 (1971) as well as in Textile Research Journal, pp. 199-201, February 1970, are given various examples of components defined by a WRA, all of which are included within the scope of the present invention.
- The fabric WRA obtained with the tested component is compared with the fabric WRA obtained with water, thereby giving a deviation Δ. A component which provide a Δ of at least positive(+)15, preferably having a Δ within the range of 15-30 is a component suitable for the invention.
- The following represents the WRA deviation versus water of different polymers suitable for use in the present invention and according to the above procedure. In each case, numbers are arithmetic averages of 9 replicates and the results are statistically significantly different at 95% confidence level:
Polymer ΔWRA IMO 900 19 Avalure AC 120 21 Luviquat FC 905 15
IMO 900: Isomaltose Oligosaccharide cx. Showa Sangyo Co.
Avalure AC 120: Polyacrylate ex. BF Goodrich
Luviquat FC 905: copolymer Vinylimidazolium methochloride & Vinylpyrrolidone ex. BASF
-
- IMO 900: Isomaltose Oligosaccharide ex. Showa Sangyo Co.
- Avalure AC 120 :Polyacrylate ex. BF Goodrich
- Luviquat FC 905 :copolymer Vinylimidazolium methochloride & Vinylpyrrolidone ex. BASF
- Preferred components which have a deviation of fabric WRA versus water of at least 15 are selected from a) shape retention polymers, b) polymers comprising at least one unit which provide a dye transfer inhibiting benefit, c) polyurethanes, d) Isomaltooligosaccharide, e) polyamine polymers, f) amphoteric polymers, g) aminosilicones, h) curable silicones and mixtures thereof. Most preferred are the polymers which are water-soluble. Furthermore, as used herein, the word “component” is meant to include compounds having a WRA deviation versus water of at least 15, mixtures of such components as well as mixtures of components which per se do not have a WRA deviation versus water of at least 15 but which, in combination do have a WRA deviation versus water of at least 15. One such component is disclosed and claimed in co-pending application EP 99870222.9-2413.
- a)-Shape Retention Polymer
- Suitable shape retention polymers can be natural, or synthetic, and can act by forming a film, and/or by providing adhesive properties. E.g., the present invention can optionally use film-forming and/or adhesive polymer to impart shape retention to fabric, particularly clothing. By “adhesive” it is meant that when applied as a solution or a dispersion to a fiber surface and dried, the polymer can attach to the surface. The polymer can form a film on the surface, or when residing between two fibers and in contact with the two fibers, it can bind the two fibers together. Other polymers such as Isomaltose Oligosaccharide can form a film and/or bond the fibers together when the treated fabric is pressed by a hot iron. Such a film will have adhesive strength, cohesive breaking strength, and cohesive breaking strain.
- Nonlimiting examples for natural polymers are Isomaltose Oligosaccharide and their derivatives, and chitins and their derivatives.
- The synthetic polymers useful in the present invention are comprised of monomers. Some nonlimiting examples of monomers which can be used to form the synthetic polymers of the present invention include: low molecular weight C1-C6 unsaturated organic mono-carboxylic and polycarboxylic acids, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and mixtures thereof; esters of said acids with C1-C12 alcohols, such as methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-1-butanol, 1-methyl-i-butanol, 3-methyl-1-butanol, 1-methyl-1-pentanol, 2-methyl-i-pentanol, 3-methyl-i-pentanol, t-butanol, cyclohexanol, 2-ethyl-1-butanol, neodecanol, 3-heptanol, benzyl alcohol, 2-octanol, 6-methyl-1-heptanol, 2-ethyl-1-hexanol, 3,5-dimethyl-1-hexanol, 3,5,5-trimethyl-1-hexanol, 1-decanol, 1-dodecanol, and the like, and mixtures thereof. Nonlimiting examples of said esters are methyl acrylate, ethyl acrylate, t-butyl acrylate, methyl methacrylate, hydroxyethyl methacrylate, methoxy ethyl methacrylate, and mixtures thereof; amides and imides of said acids, such as N,N-dimethylacrylamide, N-t-butyl acrylamide, maleimides; low molecular weight unsaturated alcohols such as vinyl alcohol (produced by the hydrolysis of vinyl acetate after polymerization), allyl alcohol; esters of said alcohols with low molecular weight carboxylic acids, such as, vinyl acetate, vinyl propionate; ethers of said alcohols such as methyl vinyl ether; aromatic vinyl such as styrene, alpha-methylstyrene, t-butylstyrene, vinyl toluene, polystyrene macromer, and the like; polar vinyl heterocyclics, such as vinyl pyrrolidone, vinyl caprolactam, vinyl pyridine, vinyl imidazole, and mixtures thereof; other unsaturated amines and amides, such as vinyl amine, diethylene triamine, dimethylaminoethyl methacrylate, ethenyl formamide; vinyl sulfonate; salts of acids and arnines listed above; low molecular weight unsaturated hydrocarbons and derivatives such as ethylene, propylene, butadiene, cyclohexadiene, vinyl chloride; vinylidene chloride; and mixtures thereof and alkyl quaternized derivatives thereof, and mixtures thereof. Preferably, said monomers are selected from the group consisting of vinyl alcohol; acrylic acid; methacrylic acid; methyl acrylate; ethyl acrylate; methyl methacrylate; t-butyl acrylate; t-butyl methacrylate; n-butyl acrylate; n-butyl methacrylate; isobutyl methacrylate; 2-ethylhexyl methacrylate; dimethylaminoethyl methacrylate; N,N-dimethyl acrylamide; N,N-dimethyl methacrylamide; N-t-butyl acrylamide; vinylpyrrolidone; vinyl pyridine; adipic acid; diethylenetriamine; salts thereof and alkyl quaternized derivatives thereof, and mixtures thereof.
- Preferably, said monomers form homopolymers and/or copolymers (i.e., the film-forming and/or adhesive polymer) having a glass transition temperature (Tg) of from about −20° C. to about 150° C., preferably from about −10° C. to about 150° C., more preferably from about 0° C. to about 100° C., most preferably, the adhesive polymer hereof, when dried to form a film will have a Tg of at least about 25° C., so that they are not unduly sticky, or “tacky” to the touch. Preferably said polymer is soluble and/or dispersible in water and/or alcohol. Said polymer typically has a molecular weight of at least about 500, preferably from about 1,000 to about 2,000,000, more preferably from about 5,000 to about 1,000,000, and even more preferably from about 30,000 to about 300,000 for some polymers.
- Some non-limiting examples of homopolymers and copolymers which can be used as film-forming and/or adhesive polymers of the present invention are: adipic acid/dimethylaminohydroxypropyl diethylenetriamine copolymer; adipic acid/epoxypropyl diethylenetriamine copolymer; poly(vinylpyrrolidone/ dimethylaminoethyl methacrylate); polyvinyl alcohol; polyvinylpyridine n-oxide; methacryloyl ethyl betaine/methacrylates copolymer; ethyl acrylate/methyl methacrylate/methacrylic acid/acrylic acid copolymer; polyamine resins; and polyquaternary amine resins; poly(ethenylformamide); poly(vinylamine) hydrochloride; poly(vinyl alcohol-co-6% vinylamine); poly(vinyl alcohol-co-12% vinylamine); poly(vinyl alcohol-co-6% vinylamine hydrochloride); and poly(vinyl alcohol-co-12% vinylamine hydrochloride). Preferably, said copolymer and/or homopolymers are selected from the group consisting of adipic acid/dimethylaminohydroxypropyl diethylenetriamine copolymer; poly(vinylpyrrolidone/dimethylaminoethyl methacrylate); polyvinyl alcohol; ethyl acrylate/methyl methacrylate/methacrylic acid/acrylic acid copolymer; methacryloyl ethyl betaine/methacrylates copolymer; polyquaternary amine resins; poly(ethenylformamide); poly(vinylamine) hydrochloride; poly(vinyl alcohol-co-6% vinylamine); poly(vinyl alcohol-co-12% vinylamine); poly(vinyl alcohol-co-6% vinylamine hydrochloride); and poly(vinyl alcohol-co-12% vinylamine hydrochloride).
- Preferred polymers useful in the present invention are selected from the group consisting of copolymers of hydrophilic monomers and hydrophobic monomers. The polymer can be linear random or block copolymers, and mixtures thereof. Such hydrophobicthydrophilic copolymers typically have a hydrophobic monomer/hydrophilic monomer ratio of from about 95:5 to about 20:80, preferably from about 90:10 to about 40:60, more preferably from about 80:20 to about 50:50 by weight of the copolymer. The hydrophobic monomer can comprise a single hydrophobic monomer or a mixture of hydrophobic monomers, and the hydrophilic monomer can comprise a single hydrophilic monomer or a mixture of hydrophilic monomers. The term “hydrophobic” is used herein consistent with its standard meaning of lacking affinity for water, whereas “hydrophilic” is used herein consistent with its standard meaning of having affinity for water. As used herein in relation to monomer units and polymeric materials, including the copolymers, “hydrophobic” means substantially water insoluble; “hydrophilic” means substantially water-soluble. In this regard, “substantially water insoluble” shall refer to a material that is not soluble in distilled (or equivalent) water, at 25° C., at a concentration of about 0.2% by weight, and preferably not soluble at about 0.1% by weight (calculated on a water plus monomer or polymer weight basis). “Substantially water-soluble” shall refer to a material that is soluble in distilled (or equivalent) water, at 25° C., at a concentration of about 0.2% by weight, and are preferably soluble at about 1% by weight. The terms “soluble”, “solubility” and the like, for purposes hereof, corresponds to the maximum concentration of monomer or polymer, as applicable, that can dissolve in water or other solvents to form a homogeneous solution, as is well understood to those skilled in the art.
- Nonlimiting examples of useful hydrophobic monomers are acrylic acid C1-C18 alkyl esters, such as methyl acrylate, ethyl acrylate, t-butyl acrylate; methacrylic C1-C18 alkyl esters, such as methyl methacrylate, 2-ethyl hexyl methacrylate, methoxy ethyl methacrylate; vinyl alcohol esters of carboxylic acids, such as, vinyl acetate, vinyl propionate, vinyl neodecanoate; aromatic vinyls, such as styrene, t-butyl styrene, vinyl toluene; vinyl ethers, such as methyl vinyl ether; vinyl chloride; vinylidene chloride; ethylene, propylene and other unsaturated hydrocarbons; and the like; and mixtures thereof. Some preferred hydrophobic monomers are methyl acrylate, methyl methacrylate, t-butyl acrylate, t-butyl methacrylate, n-butyl acrylate, n-butyl methacrylate, and mixtures thereof.
- Nonlimiting examples of useful hydrophilic monomers are unsaturated organic mono-carboxylic and polycarboxylic acids, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid; unsaturated alcohols, such as vinyl alcohol, allyl alcohol; polar vinyl heterocyclics, such as vinyl pyrrolidone, vinyl caprolactam, vinyl pyridine, vinyl imidazole; vinyl amine; vinyl sulfonate; unsaturated amides, such as acrylamides, e.g., N,N-dimethylacrylamide, N-t-butyl acrylamide; hydroxyethyl methacrylate; dimethylaminoethyl methacrylate; salts of acids and amines listed above; and the like; and mixtures thereof. Some preferred hydrophilic monomers are acrylic acid, methacrylic acid, N,N-dimethyl acrylamide, N,N-dimethyl methacrylamide, N-t-butyl acrylamide, dimethylaamino ethyl methacrylate, vinyl pyrrolidone, salts thereof and alkyl quaternized derivatives thereof, and mixtures thereof.
- Preferably, the shape retention copolymers contain hydrophobic monomers and hydrophilic monomers which comprise unsaturated organic mono-carboxylic and polycarboxylic acid monomers, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and salts thereof, and mixtures thereof; and optionally other hydrophilic monomers. These preferred polymers of the current invention surprisingly provide control of certain amine type malodors in fabrics, in addition to providing the fabric wrinkle control benefit. Examples of the hydrophilic unsaturated organic mono-carboxylic and polycarboxylic acid monomers are acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and mixtures thereof. Nonlimiting examples of the hydrophobic monomers are esters of the unsaturated organic mono-carboxylic and polycarboxylic acids cited hereinabove with C1-C12 alcohols, such as methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-i -butanol, 1-methyl-i -butanol, 3-methyl-1-butanol, 1-methyl-1-pentanol, 2-methyl-1-pentanol, 3-methyl-1-pentanol, t-butanol, cyclohexanol, 2-ethyl-1-butanol, and mixtures thereof, preferably methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-methyl-1-propanol, t-butanol, and mixtures thereof. Compositions containing these polymers also can additionally comprise perfume, antibacterial active, odor control agent, static control agent, and mixtures thereof.
- It is not intended to exclude the use of higher or lower levels of the polymers, as long as an effective amount is used to provide adhesive and film-forming properties to the composition and the composition can be formulated and effectively applied for its intended purpose.
- Highly preferred adhesive and/or film-forming polymers that are useful in the composition of the present invention actually contain silicone moieties in the polymers themselves. These preferred polymers include graft and block copolymers of silicone with moieties containing hydrophilic and/or hydrophobic monomers described hereinbefore. The silicone-containing copolymers in the composition of the present invention provide shape retention, body, and/or good, soft fabric feel.
- Both silicone-containing graft and block copolymers useful in the present invention have the following properties:
-
- (1) the silicone portion is covalently attached to the non-silicone portion;
- (2) the molecular weight of the silicone portion is from about 1,000 to about 50,000; and
- (3) the non-silicone portion must render the entire copolymer soluble or dispersible in the wrinkle control composition vehicle and permit the copolymer to deposit on/adhere to the treated fabrics.
- Suitable silicone copolymers include the following:
- Preferred silicone-containing polymers are the silicone graft copolymers comprising acrylate groups described, along with methods of making them, in U.S. Pat. No. 5,658,557, Bolich et al., issued Aug. 19, 1997, U.S. Pat. No. 4,693,935, Mazurek, issued Sept. 15, 1987, and U.S. Pat. No. 4,728,571, Clemens et al., issued Mar. 1, 1988. Additional silicone-containing polymers are disclosed in U.S. Pat. Nos. 5,480,634, Hayama et al, issued Oct. 2, 1996, 5,166,276, Hayama et al., issued Nov. 24, 1992, 5,061,481, issued Oct. 29, 1991, Suzuki et al., 5,106,609, Bolich et al., issued Apr. 21, 1992, 5,100,658, Bolich et al., issued Mar. 31, 1992, 5,100,657, Ansher-Jackson, et al., issued Mar. 31, 1992, 5,104,646, Bolich et al., issued Apr. 14, 1992, all of which are incorporated herein by reference.
- These polymers preferably include copolymers having a vinyl polymeric backbone having grafted onto it monovalent siloxane polymeric moieties, and components consisting of non-silicone hydrophilic and hydrophobic monomers.
- The silicone-containing monomers are exemplified by the general formula:
X(Y)n Si(R)3-m Zm
wherein X is a polymerizable group, such as a vinyl group, which is part of the backbone of the polymer; Y is a divalent linking group; R is a hydrogen, hydroxyl, lower alkyl (e.g. C1-C4), aryl, alkaryl, alkoxy, or alkylamino; Z is a monovalent polymeric siloxane moiety having an average molecular weight of at least about 500, is essentially unreactive under copolymerization conditions, and is pendant from the vinyl polymeric backbone described above; n is 0 or 1; and m is an integer from 1 to 3. - The preferred silicone-containing monomer has a weight average molecular weight of from about 1,000 to about 50,000, preferably from about 3,000 to about 40,000, most preferably from about 5,000 to about 20,000.
-
- In these structures m is an integer from 1 to 3, preferably 1; p is 0 or 1; q is an integer from 2 to 6; n is an integer from 0 to 4, preferably 0 or 1, more preferably 0; R1 is hydrogen, lower alkyl, alkoxy, hydroxyl, aryl, alkylamino, preferably R1 is alkyl; R″ is alkyl or hydrogen; X is CH(R3)══C(R4)—
-
- R3 is hydrogen or —COOH, preferably hydrogen; R4 is hydrogen, methyl or —CH2COOH, preferably methyl; Z is
- R5—[Si(R6)(R7)]r
wherein R5, R6, and R7, independently are lower alkyl, alkoxy, alkylamino, hydrogen or hydroxyl, preferably alkyl; and r is an integer of from about 5 to about 700, preferably from about 60 to about 400, more preferably from about 100 to about 300. Most preferably, R5, R6, and R7 are methyl, p=0, and q=3.
- Silicone-containing adhesive and/or film-forming copolymers useful in the present invention comprise from 0% to about 90%, preferably from about 10% to about 80%, more preferably from about 40% to about 75% of hydrophobic monomer, from about 0% to about 90%, preferably from about 5% to about 80% of hydrophilic monomer, and from about 5% to about 50%, preferably from about 10% to about 40%, more preferably from about 15% to about 25% of silicone-containing monomer.
- The composition of any particular copolymer will help determine its formulation properties. In fact, by appropriate selection and combination of particular hydrophobic, hydrophilic and silicone-containing components, the copolymer can be optimized for inclusion in specific vehicles. For example, polymers which are soluble in an aqueous formulation preferably contain from 0% to about 70%, preferably from about 5% to about 70% of hydrophobic monomer, and from about 30% to about 98%, preferably from about 30% to about 80%, of hydrophilic monomer, and from about 1% to about 40% of silicone-containing monomer. Polymers which are dispersible preferably contain from 0% to about 70%, more preferably from about 5% to about 70%, of hydrophobic monomer, and from about 20% to about 80%, more preferably from about 20% to about 60%, of hydrophilic monomer, and from about 1% to about 40% of silicone-containing monomer.
- The silicone-containing copolymers preferably have a weight average molecular weight of from about 10,000 to about 1,000,000, preferably from about 30,000 to about 300,000.
- The preferred polymers comprise a vinyl polymeric backbone, preferably having a Tg or a Tm as defined above of about −20° C. and, grafted to the backbone, a polydimethylsiloxane macromer having a weight average molecular weight of from about 1,000 to about 50,000, preferably from about 5,000 to about 40,000, most preferably from about 7,000 to about 20,000. The polymer is such that when it is formulated into the finished composition, and then dried, the polymer phase separates into a discontinuous phase which includes the polydimethylsiloxane macromer and a continuous phase which includes the backbone. Exemplary silicone grafted polymers for use in the present invention include the following, where the composition of the copolymer is given with the approximate weight percentage of each monomer used in the polymerization reaction to prepare the copolymer: N,N-dimethylacrylamide/isobutyl methacrylate/(PDMS macromer −20,000 approximate molecular weight) (20/60/20 w/w/w), copolymer of average molecular weight of about 400,000; N,N-dimethylacrylamide/(PDMS macromer −20,000 approximate molecular weight) (80/20 w/w), copolymer of average molecular weight of about 300,000; and t-butylacrylate/N,N-dimethylacrylamide/(PDMS macromer −10,000 approximate molecular weight) (70/10/20), copolymer of average molecular weight of about 400,000.
- Highly preferred shape retention copolymers of this type contain hydrophobic monomers, silicone-containing monomers and hydrophilic monomers which comprise unsaturated organic mono- and polycarboxylic acid monomers, such as acrylic acid, methacrylic acid, crotonic acid, maleic acid and its half esters, itaconic acid, and salts thereof, and mixtures thereof. These preferred polymers surprisingly provide control of certain amine type malodors in fabrics, in addition to providing the fabric wrinkle control benefit. A nonlimiting example of such copolymer is n-butylmethacrylate /acrylic acid/(polydimethylsiloxane macromer, 20,000 approximate molecular weight) copolymer of average molecular weight of about 100,000, and with an approximate monomer weight ratio of about 70/10/20. A highly preferred copolymer is composed of acrylic acid, t-butyl acrylate and silicone-containing monomeric units, preferably with from about 20% to about 90%, preferably from about 30% to about 80%, more preferably from about 50% to about 75% t-butyl acrylate; from about 5% to about 60%, preferably from about 8% to about 45%, more preferably from about 10% to about 30% of acrylic acid; and from about 5% to about 50%, preferably from about 10% to about 40%, more preferably from about 15% to about 30% of polydimethylsiloxane of an average molecular weight of from about 1,000 to about 50,000, preferably from about 5,000 to about 40,000, most preferably from about 7,000 to about 20,000. Nonlimiting examples of acrylic acid/tert-butyl acrylate/polydimethyl siloxane macromer copolymers useful in the present invention, with approximate monomer weight ratio, are: t-butylacrylate/acrylic acid/(polydimethylsiloxane macromer, 10,000 approximate molecular weight) (70/10/20 wlwiw), copolymer of average molecular weight of about 300,000; t-butyl acrylate/acrylic acid/(polydimethylsiloxane macromer, 10,000 approximate molecular weight) (63/20/17), copolymer of average molecular weight of from about 120,000 to about 150,000; and n-butylmethacrylate/acrylic acid/ (polydimethylsiloxane macromer −20,000 approximate molecular weight) (70/10/20 wlwiw), copolymer of average molecular weight of about 100,000. A useful and commercially available copolymer of this type is Diahold® ME from Mitsubishi Chemical Corp., which is a t-butyl acrylate/acrylic acid/ (polydimethylsiloxane macromer, 12,000 approximate molecular weight) (60/20/20), copolymer of average molecular weight of about 128,000.
- Silicone Block Copolymers
- Also useful herein are silicone block copolymers comprising repeating block units of polysiloxanes.
- Examples of silicone-containing block copolymers are found in U.S. Pat. No. 5,523,365, to Geck et al., issued Jun. 4, 1996; U.S. Pat. No. 4,689,289, to Crivello, issued Aug. 25, 1987; U.S. Pat. No. 4,584,356, to Crivello, issued Apr. 22, 1986; Macromolecular Design, Concept & Practice, Ed: M. K. Mishra, Polymer Frontiers International, Inc., Hopewell Jct., N.Y. (1994), and Block Copolymers, A. Noshay and J. E. McGrath, Academic Press, N.Y. (1977), which are all incorporated by reference herein in their entirety. Other silicone block copolymers suitable for use herein are those described, along with methods of making them, in the above referenced and incorporated U.S. Pat. No. 5,658,577.
- The silicone-containing block copolymers useful in the present invention can be described by the formulas A-B, A-B-A, and —(A-B)n— wherein n is an integer of 2 or greater. A-B represents a diblock structure, A-B-A represents a triblock structure, and —(A-B)n— represents a multiblock structure. The block copolymers can comprise mixtures of diblocks, triblocks, and higher multiblock combinations as well as small amounts of homopolymers.
- The silicone block portion, B, can be represented by the following polymeric structure
—(SiR2O)m—,
wherein each R is independently selected from the group consisting of hydrogen, hydroxyl, C1-C6 alkyl, C1-C6 alkoxy, C2-C6 alkylamino, styryl, phenyl, C1-C6 alkyl or alkoxy-substituted phenyl, preferably methyl; and m is an integer of about 10 or greater, preferably of about 40 or greater, more preferably of about 60 or greater, and most preferably of about 100 or greater. - The non-silicone block, A, comprises monomers selected from the monomers as described hereinabove in reference to the non-silicone hydrophilic and hydrophobic monomers for the silicone grafted copolymers. Vinyl blocks are preferred co-monomers. The block copolymers preferably contain one or more non-silicone blocks, and up to about 50%, preferably from about 10% to about 20%, by weight of one or more polydimethyl siloxane blocks.
- Also useful herein are sulfur-linked silicone containing copolymers, including block copolymers. As used herein in reference to silicone containing copolymers, the term “sulfur-linked” means that the copolymer contains a sulfur linkage (i.e., —S—), a disulfide linkage (i.e., —S—S—), or a sulfhydryl group (i.e., —SH).
-
-
- each G5 and G6 is independently selected from the group consisting of alkyl, aryl, alkaryl, alkoxy, alkylamino, fluoroalkyl, hydrogen, and —ZSA, wherein A represents a vinyl polymeric segment consisting essentially of polymerized free radically polymerizable monomer, and Z is a divalent linking group (Useful divalent linking groups Z include but are not limited to the following: C1 to C10 alkylene, alkarylene, arylene, and alkoxyalkylene. Preferably, Z is selected from the group consisting of methylene and propylene for reasons of commercial availability.);
- each G2 comprises A;
- each G4 comprises A;
- each R1 is a monovalent moiety selected from the group consisting of alkyl, aryl, alkaryl, alkoxy, alkylamino, fluoroalkyl, hydrogen, and hydroxyl (Preferably, R1 represents monovalent moieties which can independently be the same or different selected from the group consisting of C1-4 alkyl and hydroxyl for reasons of commercial availability. Most preferably, R1 is methyl.);
- each R2 is a divalent linking group (Suitable divalent linking groups include but are not limited to the following: C1 to C10 alkylene, arylene, alkarylene, and alkoxyalkylene. Preferably, R2 is selected from the group consisting of C13 alkylene and C7-C10 alkarylene due to ease of synthesis of the compound. Most preferably, R2 is selected from the group consisting of —CH2—, 1,3-propylene, and
- each R3 represents monovalent moieties which can independently be the same or different and are selected from the group consisting of alkyl, aryl, alkaryl, alkoxy, alkylamino, fluoroalkyl, hydrogen, and hydroxyl (Preferably, R3 represents monovalent moieties which can independently be the same or different selected from the group consisting of C1-4 alkyl and hydroxyl for reasons of commercial availability. Most preferably, R3 is methyl.);
- each R4 is a divalent linking group(Suitable divalent linking groups include but are not limited to the following: C1 to C10 alkylene, arylene, alkarylene, and alkoxyalkylene. Preferably, R4 is selected from the group consisting of C1-3 alkylene and C7-C10 alkarylene for ease of synthesis. Most preferably, R4 is selected from the group consisting of —CH2—, 1,3-propylene, and
- x is an integer of 0-3;
- y is an integer of 5 or greater(preferably y is an integer ranging from about 14 to about 700, preferably from about 20 to about 200); and
- q is an integer of 0-3;
wherein at least one of the following is true: - q is an integer of at least 1;
- x is an integer of at least 1;
- G5 comprises at least one—ZSA moiety; or
- G6 comprises at least one—ZSA moiety.
- As noted above, A is a vinyl polymeric segment formed from polymerized free radically polymerizable monomers. The selection of A is typically based upon the intended uses of the composition, and the properties the copolymer must possess in order to accomplish its intended purpose. If A comprises a block in the case of block copolymers, a polymer having AB and/or ABA architecture will be obtained depending upon whether a mercapto functional group —SH is attached to one or both terminal silicon atoms of the mercapto functional silicone compounds, respectively. The weight ratio of vinyl polymer block or segment, to silicone segment of the copolymer can vary. The preferred copolymers are those wherein the weight ratio of vinyl polymer segment to silicone segment ranges from about 98:2 to 50:50, in order that the copolymer possesses properties inherent to each of the different polymeric segments while retaining the overall polymer's solubility.
- Sulfur linked silicone copolymers are described in more detail in U.S. Pat. No. 5,468,477, to Kumar et al., issued Nov. 21, 1995, and PCT Application No. WO 95/03776, assigned to 3M, published Feb. 9, 1995, which are incorporated by reference herein in their entirety.
- b)- Polymers Comprising at Least One Unit which Provide a Dye Transfer Inhibiting Benefit
- The preferred polymers comprising at least one unit which provide a dye transfer inhibiting benefit are water-soluble polymers.
- The polymers comprising at least one unit which provide a dye transfer inhibiting benefit useful in the present invention have the formula:
[—P(D)m—]n
wherein the unit P is a polymer backbone which comprises units which are homopolymeric or copolymeric. D units are defined herein below. For the purposes of the present invention the term “homopolymeric” is defined as “a polymer backbone which is comprised of units having the same unit composition, i.e., formed from polymerization of the same monomer”. For the purposes of the present invention the term “copolymeric” is defined as “a polymer backbone which is comprised of units having a different unit composition, i.e., formed from the polymerization of two or more monomers”. - P backbones preferably comprise units having the formula:
[—CR2—CR2]— or —[(CR2)x—L]—
wherein each R unit is independently hydrogen, C1-C12 alkyl, C6-C12 aryl, and D units as described herein below; preferably C1-C4 alkyl. - Each L unit is independently selected from heteroatom-containing moieties, non-limiting examples of which are selected from the group consisting of:
polysiloxane having the formula:
wherein the index p is from 1 to about 6; units which have dye transfer inhibition activity:
and mixtures thereof; wherein R1 is hydrogen, C1l -C 12 alkyl, C6-C12 aryl, and mixtures thereof. R2 is C1-C12 alkyl, C1-C12 alkoxy, C6-C12 aryloxy, and mixtures thereof; preferably methyl and methoxy. R3 is hydrogen C1-C12 alkyl, C6-C12 aryl, and mixtures thereof; preferably hydrogen or C1-C4 alkyl, more preferably hydrogen. R4 is C1-C12 alkyl, C6-Cl2 aryl, and mixtures thereof. - The backbones of the polymers of the present invention comprise one or more D units which are units which comprise one or more units which provide a dye transfer inhibiting benefit. The D unit can be part of the backbone itself as represented in the general formula:
[—P(D)m—]n
or the D unit may be incorporated into the backbone as a pendant group to a backbone unit having, for example, the formula:
However, the number of D units depends upon the formulation. For example, the number of D units will be adjusted to provide water solubility of the polymer as well as efficacy of dye transfer inhibition. The molecular weight of the polymers of the present invention are from about 500, preferably from about 1,000, more preferably from about 10,000 most preferably from 200,000 to about 6,000,000, preferably to about 2,000,000, more preferably to about 1,000,000, yet more preferably to about 500,000, most preferably to about 360,000 daltons. Therefore the value of the index n is selected to provide the indicated molecular weight, and providing for a water solubility of at least 100 ppm, preferably at least about 300 ppm, and more preferably at least about 1,000 ppm in water at ambient temperature which is defined herein as 25° C. - Non-limiting examples of preferred D units are D units which comprise an amide moiety. Examples of polymers wherein an amide unit is introduced into the polymer via a pendant group includes polyvinylpyrrolidone having the formula:
polyvinyloxazolidone having the formula:
polyvinylmethyloxazolidone having the formula:
polyacrylamides and N-substituted polyacrylamides having the formula:
wherein each R′ is independently hydrogen, C1-C6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; polymethacrylamides and N-substituted polymethacrylamides having the general formula:
wherein each R′ is independently hydrogen, C1-C6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; poly(N-acrylylglycinamide) having the formula:
wherein each R′ is independently hydrogen, C1-C6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; poly(N-methacrylylglycinamide) having the formula:
wherein each R′ is independently hydrogen, C1-C6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms; polyvinylurethanes having the formula:
wherein each R′ is independently hydrogen, C1-C6 alkyl, or both R′ units can be taken together to form a ring comprising 4-6 carbon atoms. -
- The amino-functional polymers of the present invention can comprise any mixture of dye transfer inhibition units which provides the product with suitable properties.
- The preferred polymers which comprise D units which are amide moieties are those which have the nitrogen atoms of the amide unit highly substituted so the nitrogen atoms are in effect shielded to a varying degree by the surrounding non-polar groups. This provides the polymers with an amphiphilic character. Non-limiting examples include polyvinyl-pyrrolidones, polyvinyloxazolidones, N,N-disubstituted polyacrylamides, and N,N-disubstituted polymethacrylamides. A detailed description of physico-chemical properties of some of these polymers are given in “Water-Soluble Synthetic Polymers: Properties and Behavior”, Philip Molyneux, Vol. 1, CRC Press, (1983) included herein by reference.
- The amide containing polymers may be present partially hydrolyzed and/or crosslinked forms. A preferred polymeric compound for the present invention is polyvinylpyrrolidone (PVP). This polymer has an amphiphilic character with a highly polar amide group conferring hydrophilic and polar-attracting properties, and also has non-polar methylene and methine groups, in the backbone and/or the ring, conferring hydrophobic properties. PVP is readily soluble in aqueous and organic solvent systems. PVP is available ex ISP, Wayne, New Jersey, and BASF Corp., Parsippany, N.J., as a powder or aqueous solutions in several viscosity grades, designated as, e.g., K-12, K-15, K-25, and K-30. These K-values indicate the viscosity average molecular weight, as shown below:
PVP viscosity average molecular weight (in thousands of daltons) K-12 K-15 K-25 K-30 K-60 K-90 2.5 10 24 40 160 360
PVP K-12, K-15, and K-30 are also available ex Polysciences, Inc. Warrington, Pa., PVP K-15, K-25, and K-30 and poly(2-ethyl-2-oxazoline) are available ex Aldrich Chemical Co., Inc., Milwaukee, Wis. PVP K30 (40,000) through to K90 (360,000) are also commercially available ex BASF under the tradename Luviskol or commercially available ex ISP. Still higher molecular PVP like PVP 1.3MM, commercially available ex Aldrich is also suitable for use herein. Yet further PVP-type of material suitable for use in the present invention are polyvinylpyrrolidone-co-dimethylaminoethylmethacrylate, commercially available ex ISP in a quaternised form under the tradename Gafquat® or commercially available ex Aldrich Chemical Co. having a molecular weight of approximately 1.0MM; copolymer of 3-methyl-1-vinyl-1H-imidazolium chloride and 1-vinyl-2-pyrrolidone (30:70) ex BASF under the tradename Luviquat FC370, polyvinylpyrrolidone-co-vinyl acetate, available ex BASF under the tradename Luviskol®, available in vinylpyrrolidone:vinylacetate ratios of from 3:7 to 7:3; polyvinylpyrrolidine-co-vinylimidazoliumquat, commercially available ex BASF under the tradename Luviquat®. - Another D unit which provides dye transfer inhibition enhancement to the polymers described herein, are N-oxide units having the formula:
wherein R1, R2, and R3 can be any hydrocarbyl unit (for the purposes of the present invention the term “hydrocarbyl” does not include hydrogen atom alone). The N-oxide unit may be part of a polymer, such as a polyamine, i.e., polyalkyleneamine backbone, or the N-oxide may be part of a pendant group attached to the polymer backbone. An example of a polymer which comprises an the N-oxide unit as a part of the polymer backbone is polyethyleneimine N-oxide. Non-limiting examples of groups which can comprise an N-oxide moiety include the N-oxides of certain heterocycles inter alia pyridine, pyrrole, imidazole, pyrazole, pyrazine, pyrimidine, pyridazine, piperidine, pyrrolidine, pyrrolidone, azolidine, morpholine. A preferred polymer is poly(4-vinylpyriding N-oxide, PVNO). In addition, the N-oxide unit may be pendant to the ring, for example, aniline oxide. - N-oxide comprising polymers of the present invention will preferably have a ratio of N-oxidized amine nitrogen to non-oxidized amine nitrogen of from about 1:0 to about 1:2, preferably to about 1:1, more preferably to about 3:1. The amount of N-oxide units can be adjusted by the formulator. For example, the formulator may co-polymerize N-oxide comprising monomers with non N-oxide comprising monomers to arrive at the desired ratio of N-oxide to non N-oxide amino units, or the formulator may control the oxidation level of the polymer during preparation. The amine oxide unit of the polyamine N-oxides of the present invention have a Pka less than or equal to 10, preferably less than or equal to 7, more preferably less than or equal to 6. The average molecular weight of the N-oxide comprising polymers which provide a dye transfer inhibitor benefit to polymers is from about 500 daltons, preferably from about 10,000 daltons, more preferably from about 20,000 daltons to about 6,000,000 daltons, preferably to about 2,000,000 daltons, more preferably to about 360,000 daltons.
- A further example of polymers which have dye transfer inhibition benefits are polymers which comprise both amide units and N-oxide units as described herein above. Non-limiting examples include co-polymers of two monomers wherein the first monomer comprises an amide unit and the second monomer comprises an N-oxide unit. In addition, oligomers or block polymers comprising these units can be taken together to form the mixed amide/N-oxide polymers. However, the resulting polymers must retain the water solubility requirements described herein above.
- c)-Urethanes Polymers
- Polymers of the urethane type are also suitable components for use herein. A typical disclosure of polyurethane polymer can be found in EP844274A1 as well as in EP839903.
- d)-Isomaltooligosaccharide
- Isomaltooligosaccharides (IMO) (including mixtures), the individual components of said mixtures, substituted versions thereof, derivatised versions thereof, and mixtures thereof are suitable components for use herein. Currently IMO is used as corn syrup. These components are particularly suitable where cellulosic fibers/fabrics are used, such as cotton, rayon, ramie, jute, flax, linen, polynosic-fibers, Lyocell (Tencel®), polyester/cotton blends, other cotton blends, and the like, especially cotton, rayon, linen, polyester/cotton blends, and mixtures thereof.
- Suitable fabric improving actives that are useful in the present invention include oligosaccharides with a degree of polymerization (DP) of from about 1 to about 15, preferably from about 2 to about 10, and wherein each monomer is selected from the group consisting of reducing saccharide containing 5 and/or 6 carbon atoms, including isomaltose, isomaltotriose, isomaltotetraose, isomaltooligosaccharide, fructooligosaccharide, levooligosaccharides, galactooligosaccharide, xylooligosaccharide, gentiooligosaccharides, disaccharides, glucose, fructose, galactose, xylose, mannose, arabinose, rhamnose, maltose, sucrose, lactose, maltulose, ribose, lyxose, allose, altrose, gulose, idose, talose, trehalose, nigerose, kojibiose, lactulose, oligosaccharides, maltooligosaccharides, trisaccharides, tetrasaccharides, pentasaccharides, hexasaccharides, oligosaccharides from partial hydrolysates of natural polysaccharide sources, and the like, and mixtures thereof, preferably mixtures of isomaltooligosaccharides, especially mixtures including isomaltooligosaccharides, comprising from about 3 to about 7 units of glucose, respectively, and which are linked by 1,2-α, 1,3-α, 1,4-α- and 1,6-α-linkages, and mixtures of these linkages. Oligosaccharides containing b-linkages are also preferred. Preferred oligosaccharides are acyclic and have at least one linkage that is not an α-1,4-glycosidic bond. A preferred oligosaccharide is a mixture containing IMO: from 0 to about 20% by weight of glucose, from about 10 to about 65% of isomaltose, from about 1% to about 45% of each of isomaltotriose, isomaltetraose and isomaltopentaose, from 0 to about 3% of each of isomaltohexaose, isomaltoheptaose, isomaltooctaose and isomaltononaose, from about 0.2% to about 15% of each of isomaltohexaose and isomaltoheptaose, and from 0 to about 50% by weight of said mixture being isomaltooligosaccharides of 2 to 7 glucose units and from 0 to about 10% by weight of said mixture being isomaltooligosaccharides of about 7 to about 10 glucose units. Other nonlimiting examples of preferred acyclic oligosaccharides, with approximate content by weight percent, are:
Isomaltooligosaccharide Mixture I Trisaccharides (maltotriose, panose, isomaltotriose) 40-65% Disaccharides (maltose, isomaltose) 5-15% Monosaccharide (glucose) 0-20% Higher branched sugars (4 < DP < 10) 10-30% Isomaltooligosaccharide Mixture II Trisaccharides (maltotriose, panose, isomaltotriose) 10-25% Disaccharides (maltose, isomaltose) 10-55% Monosaccharide (glucose) 10-20% Higher branched sugars (4 < DP < 10) 5-10% Isomaltooligosaccharide Mixture III Tetrasaccharides (stachyose) 10-40% Trisaccharides (raffinose) 0-10% Disaccharides (sucrose, trehalose) 10-50% Monosaccharide (glucose, fructose) 0-10% Other higher branched sugars (4 < DP < 10) 0-5% - Oligosaccharide mixtures are either prepared by enzymatic reactions or separated as natural products from plant materials. The enzymatic synthesis of oligosaccharides involves either adding monosaccharides, one at a time, to a di- or higher saccharide to produce branched oligosaccharides, or it can involve the degradation of polysaccharides followed by transfer of saccharides to branching positions. For instance, Oligosaccharide Mixtures I and II are prepared by enzymatic hydrolysis of starch to maltooligosaccharides, which are then converted to isomaltooligosaccharides by a transglucosidase reaction. Oligosaccharide Mixture III, for example, is a mixture of oligosaccharides isolated from soybean. Soybean oligosaccharides such as Mixture III, are of pure natural origin.
- Substituted and/or derivatised materials of the oligosaccharides listed hereinabove are also suitable in the present invention. Nonlimiting examples of these materials include: carboxyl and hydroxymethyl substitutions (e.g., glucuronic acid instead of glucose); amino oligosaccharides (amine substitution, e.g., glucosamine instead of glucose); cationic quaternized oligosaccharides; C1-C6 alkylated oligosaccharides; acetylated oligosaccharide ethers; oligosaccharides having amino acid residues attached (small fragments of glycoprotein); oligosaccharides containing silicone moieties. These substituted and/or derivatised oligosaccharides can provide additional benefits, such as: carboxyl and hydroxymethyl substitutions can introduce readily oxidizable materials on and in the fiber, thus reducing the probability of the fiber itself being oxidized by oxidants, such as bleaches; amine substitution can bind and/or condense with oxidatively damaged regions of the fiber to rejuvenate aged fabrics; acetylated sugar ethers can serve as bleach activators in subsequent processes where hydrogen peroxide is present; oligosaccharides having amino acid residues can improve delivery of fabric care benefits for fabrics containing proteinaceous fibers, e.g., wool and silk; and silicone-derivatised oligosaccharides can provide additional fabric softness and lubricity. C6 alkyl oligosaccharide is disclosed (along with other higher, viz., C6-C30, alkyl polysaccharides) in U.S. Pat. No. 4,565,647. Typical disclosure of C1-C6 alkylated oligosaccharides can also be found in U.S. Pat. No. 4,488,981. These patents are incorporated herein by reference.
- One preferred isomaltooligosaccharide is IMO 900 commercially available from Showa Sangyo Co.
- e)-Polyvinylamines Polymers
- Polyvinylamines polymers are also suitable component giving a deviation of fabric WRA of at least 15. Typical polyvinylamines polymers include the quaternized and non-quaternized polyvinylamines having the formula:
wherein R is hydrogen, C1-C12 linear or branched alkyl, benzyl, or alkyleneoxy having the formula (R1O)zY, wherein R1 is C1-C6 linear or branched alkylene, Y is hydrogen or an anionic unit, non-limiting examples of which include, —(CH2)fCO2M, —C(O)(CH2)fCO2M, —(CH2)fPO3M, —(CH2)fOPO3M, —(CH2)fSO3M, —CH2(CHSO3M)—(CH2)fSO3M, —CH2(CHSO2M)(CH2)fSO3M, —C(O)CH2CH(SO3M)CO2M, C(O)CH2CH(CO2M)NHCH(CO2M)CH2CO2M, —C(O)CH2CH(CO2M)NHCH2CO2M, —CH2CH(OZ)CH2O(R1O)tZ, —(CH2)fCH[O(R2O)tZ]CH2O(R2O)tZ, and mixtures thereof, wherein Z is hydrogen or an anionic unit non-limiting examples of which include —(CH2)fCO2M, —C(O)(CH2)fCO2M, —(CH2)fPO3M, —(CH2)fOPO3M, —(CH2)fSO3M, —CH2(CHSO3M)—(CH2)fSO3M, —CH2(CHSO2M)(CH2)fSO3M, —C(O)CH2CH(SO3M)CO2M, —C(O)CH2CH(CO2M)NHCH(CO2M)CH2CO2M, and mixtures thereof, M is a cation which provides charge neutrality; and the index f is from 0 to 6, t is 0 or 1, z is from 1 to 50. - The index x has the value from about 50 to about 1,500; preferably the index x has a value such that the resulting polymeric suds stabilizer has an average molecular weight of from about 2,500, preferably from about 10,000, more preferably from about 20,000 to about 150,000, preferably to about 90,000, more preferably to about 80,000 daltons.
- Most preferred polymers for use in the present invention are water-soluble, including IMO 900 (Isomaltose Oligosaccharide ex. Showa Sangyo Co.), Avalure AC 120 (Polyacrylate ex. BF Goodrich), Luviskol K30, K60 and K85 (Polyvinylpyrrolidone MW 40.000, 400.000 and 1.250.000 ex. BASF), Luvitec VPC 55K65W (copolymer Vinylpyrrolidone & Vinylcaprolactam ex. BASF), Luvitec Quat 73W (copolymer 1-methyl-3-vinyl-imidazolium-methylsulfate & 1-vinyl-2-pyrrolidone ex. BASF), Luviquat FC 905 (copolymer Vinylimidazolium methochloride & Vinylpyrrolidone ex. BASF), Sedipur 520 (modified Polyacrylamide ex. BASF), Chitanide 222 (Chitosan succinamide ex. MIP), Mirasil ADM-E (Aminodimethicone ex. Rhone-Poulenc), Percol 370 (diallyl amine polymer ex. CIBA), Amphomer HC (Acrylate/Octylacrylamide copolymer ex. National Starch), and mixtures thereof.
- More preferably, the water-soluble lubricant and the component, preferably polymer, having a deviation of fabric WRA of at least +15 are present in weight ratios of water-soluble lubricant to component of from 10:1 to 1:1. Indeed, it has been found that within these ratio ranges the resulting composition provides best in wear wrinkle benefit.
- f)-Amphoteric Polymers
- Suitable for use herein are amphoteric polymers, i.e., polymers comprising at least one anionic moiety and one cationic moiety, and optionally a non-ionic moiety. The anionic moiety comprises a group which is a deprotonated anion of an acid group when the polymer is dissolved/dispersed in water at a pH of about 7 and which can be protonated to form a nonionic acid group when the polymer is dissolved/dispersed in water at an acidic pH. Representative examples of such groups include carboxylate, phosphonate, phosphate, phosphite, sulfonate, sulfate groups, and combinations thereof.
- Optionally, each moiety may be further complexed with a separate, cationic counterion other than hydrogen. When used, representative examples of such counterions, include Na+, Li+, K+, NH4+ or combinations thereof.
- The cationic moiety comprises a protonated cation when the polymer is dissolved/dispersed in water at a pH of about 7 or below and can be deprotonated to a nonionic form when the polymer is dissolved/dispersed in water at a basic pH. Alternatively, the cationic moiety comprises a group which is a quaternized group.
- Representative examples of the protonated group include the ammonium functionality, phosphonium functionality, sulfonium functionality, and combinations thereof. The term ammonium refers to a moiety including a nitrogen atom linked to a plurality of moieties (either H, alkyl or aryl groups) by four bonds when dissolved/dispersed in water at a pH of 7. The term sulfonium refers to a moiety including a sulfur atom linked to three other moieties (either H, alkyl or aryl groups) when dispersed in water at a pH of about 7. The term phosphonium refers to a moiety including a phosphorous atom linked to four other moieties (either H, alkyl or aryl groups) when dispersed in water at a pH of about 7.
-
- In these formulae, R1 represents the polymer backbone and R2 represents hydrogen, alkyl or aryl substituents. In case the cationic moiety exists as a quaternized group, all R2 groups represents alkyl or aryl substituents, excluding hydrogen.
- As an option, each such second functional group may be further complexed with a separate, anionic counterion. When used, representative examples of such counterion, include chlorides, sulfates, carbonates, nitrates, formiates, perchlorates, or combinations thereof.
- Optionally, amphoteric polymers herein comprise a non-ionic moiety. A preferred class of amphoteric polymers for use herein are polymers composed of both cationic and anionic vinylmonomers.
- Suitable anionic vinylmonomers for use herein include salts of acrylic acid, methacrylic acid, crotonic acid, maleic acid, fumaric acid, itaconic acid and vinylsulphonic acid. Suitable cationic vinylmonomers for use herein include salts of unsaturated amines such as the hydrochloride salt of vinylamine, salts of N,N′-dialkylaminoalkyl (meth) acrylates and N,N′-dialkylaminoalkyl (meth) acrylamides such as the hydrochloride salt of dimethylaminoethylmethacrylate (DMAEMA.HCl) or dimethylaminopropylacrylamide; alkyl quaternized aminoalkyl (meth) acrylates and aminoalky (meth) acrylamides such as trimethylammoniumethyl methacrylatechloride, trimethylammoniumpropyl acrylamidemethylsulfate, alkyl quaternized polar vinyl heterocyclics such as based on pyridinium or imidazolium such as alkylvinylpyridinium, alkylvinylimidazolium and mixtures thereof.
- Optionally, a non-ionic comonomer can be incorporated, such as amides and imides of organic acids, such as acrylamide, N,N-dialkylacrylarnide, N-t-butylacrylamide, maleimides, vinylformamide, aromatic vinyl monomers such as styrene, vinyltoluene, t-butylstyrene; polar vinyl heterocyclics such as vinyl pyrrolidone, vinyl caprolactam, vinyl pyridine, vinylimidazole; low molecular weight unsaturated hydrocarbons and derivatives such as ethylene, propylene, butadiene, cyclohexadiene, vinylchloride and mixtures thereof.
- A preferred polymer of this class is based on poly(vinylamine-co-acrylic acid), in molar ratios varying between 1:100 to 100:1, preferably 90:10 to 40:60. Polymers of this class preferably have a molecular weight between 20.000 and 5.000.000 preferably between 30.000 and 1.000.000, more preferably between 50.000 and 300.000.
- A second class of polymers which are preferred for use herein are anionically modified polyethyleneimines. Examples of anionically modified polyethyleneimines include polyethyleneimines grafted with acrylic acid, methacrylic acid, maleic acid, fumaric acid, crotonic acid, itaconic acid, or carboxymethylated.
- The processes for the preparation of anionically modified polyethyleneimines are well known. They can be prepared by reacting α,β-unsaturated carboxylic acids ( C═C—COOH) like acrylic or maleic acid with polyethyleneimine (Michael-type reaction) or by carboxymethylation. The carboxymethylation is carried out by reacting polyethyleneimine either with chloroacetic acid or with formaldehyde and sodium cyanide and subsequent saponification of the resultant aminonitrile. The latter procedure is well-known as the “Strecker Synthesis”.
- Polymers of this class have a degree of substitution of between 5 and 95, preferably 20 and 80, and a molecular weight between 5000 and 2 000 000, preferably 20 000 and 1 000 000.
- In the present invention, the amphoteric polymers can be provided to the clothes in amounts of from 1×10−7 g/g fabric to 0.3 g/g fabric, preferably from 1×10−5 g/g fabric to 0.1 g/g fabric; more preferably from 1×10−3 g/g fabric to 1×10−2 g/g fabric.
- g)-Aminosilicones
- Suitable for use herein are aminosilicones, preferably those comprising an amine comprising a sterically hindered functional group.
- In the present invention, any known aminosilicone can be used to treat clothes so as to provide the desired benefit. Aminosilicones used in a domestic context have been described in numerous publications, for instance U.S. Pat. No. 5,062,971 and U.S. Pat. No. 5,064,543 as ironing aid; in WO 00/24853, WO/9201773 and EP 300 525 in fabric conditioners, EP 150 867 and EP 150 872 in detergents and there is no need to redescribe such aminosilicones herein.
- However, a particular problem that arises with most aminosilicones is that they eventually yellow fabrics. The phenomenon for such yellowing is not well understood, but it does create a practical limitation to the use of aminosilicones to treat clothes: amino silicones can be used to treat clothes, but only in limited amounts such that the yellowing phenomenon does not become too visible, thereby limiting the performance of the composition.
- It has now been found that there exists a particular class of amino silicones which is suitable for use in a domestic context and which does not yellow fabrics. Such silicones have been discussed in, e.g. U.S. Pat. No. 5,688,889 as well as U.S. Pat. No. 5,540,952, but only for use in an industrial context, and for a different benefit. In particular, in example 3 of those documents, a process is described in which fabrics are immersed in a solution of the amino silicone in white spirit, and the fabrics are subsequently dried at 40° C. for 15 minutes in a ventilated oven and then heated at 160° C. for 30 min. This pad-dry-cure process is a standard process in textile industry, but it cannot be performed in a domestic context. This particular class of amino silicones is referred throughout this description as aminosilicones comprising a sterically hindered functional group. Such aminosilicones have been described in U.S. Pat. No. 5,540,952, EP 659 930, WO 00/5315, U.S. Pat. No. 5,688,889, WO 96/16110, WO 96/16124, WO 96/16127, WO 96/18667 and U.S. Pat. No. 5,792,825, the contents of which are incorporated herein.
- The present invention utilizes amino silicones comprising a sterically hindered functional group, i.e. polyorganosiloxanes having, per mole, at least one unit of general formula:
in which: - The symbols R are identical or different and represent a monovalent hydrocarbon radical chosen from linear or branched alkyl radicals having from 1 to 4 carbon atoms, the phenyl radical, the benzyl radical and the 3,3,3-trifluoropropyl radical;
- The symbols X are identical or different and represent a monovalent radical chosen from a hydroxyl group and a linear or branched alkoxy radical having from 1 to 3 carbon atoms;
- The symbol Z represents a monovalent group of the formula R1—U—S in which:
-
- R′ is a divalent hydrocarbon radical chosen from:
- linear or branched alkylene radicals having from 2 to 18 carbon atoms;
- alkylenecarbonyl radicals in which the linear or branched alkylene part contains 2 to 20 carbon atoms;
- alkylenecyclohexylene radicals in which the linear or branched alkylene part contains from 2 to 12 carbon atoms and the cyclohexylene part contains an —OH group and optionally 1 or 2 alkyl radicals having from 1 to 4 carbon atoms;
- radicals of the formula R2—O—R3— in which the radicals R2 and R3, which are identical or different, represent alkylene radicals having 1 to 12 carbon atoms;
- radicals of the formula R2—O—R3— in which the radicals R2 and R3 have the meanings indicated above and one of them or both are substituted by one or two —OH group(s);
- radicals of the formula R2—COO—R3— and R2—OCO—R3 in which the radicals R2 and R3 have the meanings above;
- radicals of the formula R4—O—R5—O—CO—R6— in which the radicals R4 , R5 and R6, which are identical or different, represent alkylene radicals having 2 to 12 carbon atoms and the radical R5 is optionally substituted by a hydroxyl group;
- radicals of the formula
in which the radical R7 represents alkylene radicals having 1 to 4 carbon atoms, and the radical R8 represents linear or branched alkylene radicals having 1 to 4 carbon atoms, the phenyl radical and the phenylalkyl radical where the linear or branched alkyl part contains 1 to 3 carbon atoms; and where x is a number chosen between 0, 1 and 2.
- R′ is a divalent hydrocarbon radical chosen from:
- U represents —O— or —NR9—, R9 being a radical chosen from a hydrogen atom, a linear or branched alkyl radical having from 1 to 6 carbon atoms, a divalent radical —R1— having the meaning indicated above, one of the valency bonds being connected to the nitrogen of —NR9— and the other being connected to a silicon atom and a divalent radical of the formula —R10—N(R1)—S in which R1 has the meaning indicated above, and R10 represents a linear or branched alkylene radical having from 1 to 12 carbon atoms, one of the valency bonds (that of R10) being connected to the nitrogen atom of —NR9— and the other (that of R1) being connected to a silicon atom.
- S represents a monovalent group, in which:
-
- the free valency is a carbon atom, carrying a secondary or tertiary amine function, comprised in a cyclic hydrocarbon chain or in a heterocyclic chain comprising from 6 to 30 carbon atoms, in which the two atoms of the cyclic chain in the positions α and α′ relative to the nitrogen atom, do not comprise any hydrogen atom;
- the free valency is a carbon atom, carrying a secondary or tertiary amine function, comprised in a linear hydrocarbon chain comprising 6 to 40 carbon atoms, in which the two atoms of the cyclic chain in the positions α and α′ relative to the nitrogen atom, do not comprise any hydrogen atom.
- Preferably, the secondary or tertiary amine function in S is incorporated in a piperidyl group.
-
- a is a number chosen from 0, 1 and 2;
- b is a number chosen from 0, 1 and 2;
- the sum a +b is not greater than 2.
- The polyorganosiloxane used can additionally comprise (an) other siloxyl unit(s).
- Such amino silicones comprising a sterically hindered functional group which are suitable for use herein are commercially available from Rhodia under the trade name Rhodorsil ®, in particular Rhodorsil ® H 21645 or Rhodorsil ® H 21650 or Silicex ®, in particular Silicex ® 263.
- In the present invention, thanks to their ability not to yellow fabrics, the aminosilicones comprising a sterically hindered amino functional group can be provided to the clothes in amounts from 1×10−7 g/g fabric to 0.3 g/g fabric, preferably from 1×10−5 g/g fabric to 0.1 g/g fabric; more preferably from 1×10−3 g/g fabric to 1×10−2 g/g fabric, i.e. in amounts which are greater than the amounts in which other amino silicones can be used. Thus, a greater benefit can be obtained without observing fabric yellowing.
- h)-Curable Silicones
- Also suitable for use herein are curable silicones. “Curable” silicone molecules have the ability to reach one with each other to yield a polymeric elastomer of a much higher molecular weight compared to the original molecule. Thus, “curing” often occurs when two curable silicone molecules or curable silicone polymers react yielding a polymer of a higher molecular weight. This “cure” reaction is define herein as the formation of new silicon-oxygen, silicon-carbon, and/or carbon-carbon linkages. Curable silicones can be cross-linked to some degree before application. That means that the curable silicone has cured to some degree before application but that can still further cure during and after application. Cross-linked curable silicones are preferred.
- Examples of curable silicones are vinyl-, allyl-, silane-, epoxy-, alkoxy-, and/or silanol-modified polydimethylsiloxanes, and mixtures thereof. Some curable silicones may required the cooperative use of a catalyst to induce curing, as in the case of vinyl-,hydrogen-modified silicones which cure via a hydrosilation process catalyzed by platinum compounds or radical catalysts. More preferred in this invention are curable silicone able to cure without the addition of a catalysts, such as epoxy-, alkoxy-, and/or silanol-modified polydimethylsiloxanes. Most preferred are silanol-stopped polydimethyl-siloxanes emulsions.
- Curable silicones can have other organic group modifications as for example, although not restricting, amino or polyalkyleneoxide groups. Curable silicones may content reinforcing fillers. By reinforcing fillers we mean small particles made of inorganic or organic materials added to the curable silicone as additives or intimately linked to silicone molecules via covalent bonds. One example, although not restricting, are silica particles sized from 10 to 100 nanometers present in 10% to 100% by weight based on the weight of the silicone.
- It is preferred that curable silicones are formulated as oil-in-water emulsions. Curable silicone emulsions are commercially available; e.g., GE-Bayer SM2112 Silicone Emulsions or Dow Corning Syl-Off® 7922 Catalyst Emulsion.
- It is believed that curable silicones cure during or/and after application to the fabrics producing a network which will prevent the formation of wrinkles.
- Other suitable film-forming polymers for use herein are durable press polymers. Durable press polymers are optional components of the invention. These polymers can be a cross-linking resin having the property of being cationic. By “cross-linking resin having the property of being cationic”, it is meant that the resin is at least partially positively charged. It is not however necessary that the reactive part of the molecule carries the positive charge. Indeed, polymeric resins can be based on positively charged monomers which help the deposition on the fibers.
- Cross-linking resins having the property of being cationic suitable for use herein are those commonly known as having wet strength in the paper field. At least two mechanisms have been postulated to account for the mechanism by which wet strength resin act. One is that wet strength resins form covalent bonds between adjacent fibers while another is that the wet strength resin places a layer over the hydrogen bonds formed between adjacent paper fibers and thus prevents water from breaking the hydrogen bonds.
- Conventional wet-strength agents suitable for use herein include compounds made of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof. For use within the meaning of the present invention, there can also be used materials of the above-mentioned classes of substances which admittedly do not themselves possess any outstanding wet-strength properties but, nevertheless, have the same durable press effect as do the wet-strength agents as described therein.
- Among the class of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, the preferred components are the polymeric amine-epichlorohydrin resins selected from the group consisting of a polyamide-epichlorohydrin (PAE) resin, a polyalkylenepolyamine-epichlorohydrin (PAPAE) resin, and an amine polymer-epichlorohydrin (APE) resin, in which the amine groups have been alkylated with epichlorohydrin to produce a polyamine-epichlorohydrin resin that has azetidinium or epoxide functionality. Preferably, for use herein, the cross-linking resin having cationic properties is a cationic wet strength resin that is produced by reacting a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epichlorohydrin to form a PAE resin.
- These polyamide/polyamine/epichlorohydrin wet-strength resins are fully described by Carr, Doane, Hamerstrand and Hofreiter, in an article appearing in the Journal of Applied Polymer Science Vol. 17, pp. 721-735 (1973). Such resins are available as KYMENE from Hercules, Inc. A commercial synthesis of such resins from adipic acid, diethylene triamine and epichlorohydrin is described in the Carr et al publication, ibid., and is U.S. Pat. No. 2,926,154 (Feb. 23, 1960) to G. I. Keim or U.S. Pat. No. 4,240,995. Reference can be made to these publications for further details regarding the preparation of polyamide/polyamine/epichlorohydrin resins.
- Most preferred cross-linking resin having cationic properties from this class are the wet strength resin Kymene 557H (available from Hercules Incorporated), in which adipic acid is reacted with diethylenetriamine to form a poly(aminoamide) that is alkylated and crosslinked with epichlorohydrin to form a PAE resin. Still another preferred cross-linking resin having cationic properties made of epichlorohydrin are Luresin.RTM and Etadurin which both are polyamidoamine-epichlorohydrin resins.
- Amine-aldehyde resins are suitable cross-linking resins for the present invention and are made by condensation of amine or amide monomers with aldehydes such as formaldehyde or glyoxal. Preferred amines are those having low molecular weight amines e.g. melamine or polymeric amines e.g. poly-diallylamine, preferably quarteinized. Preferred amides are those polymeric amides such as polyacrylamide. All these suitable amine/amide monomers can also be copolymerized with cationic monomers.
- Among the class of amine-aldehyde cross-linking resin, preferred are those from the class of melamine-formaldehyde resin. Melamine-formaldehyde resins of this type are known as crosslinking agents of this type in the coating industry and are also described, for example, in German Auslegeschrift Nos. 2,457,387 (U.S. Pat. No. 4,035,213 incorporated herein by reference) and U.S. Pat. No. 1,719,324 and, in particular, in U.S. Pat. No. 3,242,230 incorporated herein by reference.
- Preferred melamine-formaldehyde resin are those commercially available under the tradenames Madurit, and Cassurit from Clariant.
- Still other preferred cross-linking resin having the property of being cationic among the class of amine-aldehyde cross-linking resin are the Poly(acrylamide-glyoxal) resin commercially available under the tradename SOLIDUR1 T KM from Clariant.
- According to the present invention, there can also be used a mixture of wet-strength agents of the above-mentioned types or equivalent compounds.
- Preferably for the purpose of the invention, the cross-linking resin having cationic properties have a molecular weight between 200 and 1,000,000, preferably between 500 and 100,000, most preferably between 1000 and 25,000. Cross-linking resin having a low molecular weight are most preferred for use in the present invention as they are more water-soluble and have a better fiber penetration. By low molecular weight it is meant a molecular weight within the range of from 25 to 2000, preferably from 50 to 1000, and more preferably from 50 to 500.
- It is desirable if the level of cross-linking components or derivative thereof is present in an amount of from 0.01% to 60%, preferably from 0.01% to 30% by weight of the total composition
- It is advantageous for aldehyde containing cross-linking resins if a catalyst is used with compositions of the invention. Preferred catalysts includes organic acids such as citric acid, succinic acid, and tartaric acids, as well as conventional Lewis acid such as Al Cl3 or MgCl2, or salts thereof, or mixtures thereof. A typical example of catalyst is the catalyst NKD made of a mixture of salts and organic acid, and commercially available from Hoechst.
- It is preferred if the level of catalyst is from 10% to 50%, preferably from 20 to 40% by weight of the cross-linking components or derivative thereof.
- For other cross-linking resins like the Kymene, the use of a catalyst is not necessary.
- 3)-Optionals:
- The composition of the invention may also comprise one or more of the following optional ingredients.
- a) Durable Press Polymer
- Durable press polymers are optional components of the invention. These polymers can be a cross-linking resin having the property of being cationic. By “cross-linking resin having the property of being cationic”, it is meant that the resin is at least partially positively charged. It is not however necessary that the reactive part of the molecule carries the positive charge. Indeed, polymeric resins can be based on positively charged monomers which help the deposition on the fibers.
- Cross-linking resins having the property of being cationic suitable for use herein are those commonly known as having wet strength in the paper field. At least two mechanisms have been postulated to account for the mechanism by which wet strength resin act. One is that wet strength resins form covalent bonds between adjacent fibers while another is that the wet strength resin places a layer over the hydrogen bonds formed between adjacent paper fibers and thus prevents water from breaking the hydrogen bonds.
- Conventional wet-strength agents suitable for use herein include compounds made of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, amine-aldehyde resins such as melamine-formaldehyde resin, amide-aldehyde resins, and mixtures thereof. For use within the meaning of the present invention, there can also be used materials of the above-mentioned classes of substances which admittedly do not themselves possess any outstanding wet-strength properties but, nevertheless, have the same durable press effect as do the wet-strength agents as described therein.
- Among the class of epichlorohydrin adducts of polyamine resins, polyethyleneimine resins, cationic starch, polydiallyldimethylammonium chloride, and mixtures thereof, the preferred components are the polymeric amine-epichlorohydrin resins selected from the group consisting of a polyamide-epichlorohydrin (PAE) resin, a polyalkylenepolyamine-epichlorohydrin (PAPAE) resin, and an amine polymer-epichlorohydrin (APE) resin, in which the amine groups have been alkylated with epichlorohydrin to produce a polyamine-epichlorohydrin resin that has azetidinium or epoxide functionality. Preferably, for use herein, the cross-linking resin having cationic properties is a cationic wet strength resin that is produced by reacting a saturated aliphatic dicarboxylic acid containing three to ten carbon atoms with a polyalkylenepolyamine, containing from two to four ethylene groups, two primary amine groups, and one to three secondary amine groups (such as diethylenetriamine, triethylenetetramine and tetraethylenepentamine), to form a poly(aminoamide) having secondary amine groups that are alkylated with epichlorohydrin to form a PAE resin.
- These polyamide/polyamine/epichlorohydrin wet-strength resins are fully described by Carr, Doane, Hamerstrand and Hofreiter, in an article appearing in the Journal of Applied Polymer Science Vol. 17, pp. 721-735 (1973). Such resins are available as KYMENE from Hercules, Inc. A commercial synthesis of such resins from adipic acid, diethylene triamine and epichlorohydrin is described in the Carr et al publication, ibid., and is U.S. Pat. No. 2,926,154 (Feb. 23, 1960) to G. I. Keim or U.S. Pat. No. 4,240,995. Reference can be made to these publications for further details regarding the preparation of polyamide/polyamine/epichlorohydrin resins.
- Most preferred cross-linking resin having cationic properties from this class are the wet strength resin Kymene 557H (available from Hercules Incorporated), in which adipic acid is reacted with diethylenetriamine to form a poly(aminoamide) that is alkylated and crosslinked with epichlorohydrin to form a PAE resin. Still another preferred cross-linking resin having cationic properties made of epichlorohydrin are Luresin.RTM and Etadurin which both are polyamidoamine-epichlorohydrin resins.
- Amine-aldehyde resins are suitable cross-linking resins for the present invention and are made by condensation of amine or amide monomers with aldehydes such as forrnaldehyde or glyoxal. Preferred amines are those having low molecular weight amines e.g. melamine or polymeric amines e.g. poly-diallylamine, preferably quarternized. Preferred amides are those polymeric amides such as polyacrylamide. All these suitable amine/amide monomers can also be copolymerized with cationic monomers.
- Among the class of amine-aldehyde cross-linking resin, preferred are those from the class of melamine-formaldehyde resin. Melamine-formaldehyde resins of this type are known as crosslinking agents of this type in the coating industry and are also described, for example, in German Auslegeschrift Nos. 2,457,387 (U.S. Pat. No. 4,035,213 incorporated herein by reference) and U.S. Pat. No. 1,719,324 and, in particular, in U.S. Pat. No. 3,242,230 incorporated herein by reference.
- Preferred melamine-formaldehyde resin are those commercially available under the tradenames Madurit, and Cassurit from Clariant.
- Still other preferred cross-linking resin having the property of being cationic among the class of amine-aldehyde cross-linking resin are the Poly(acrylamide-glyoxal) resin commercially available under the tradename SOLIDUR1 T KM from Clariant.
- According to the present invention, there can also be used a mixture of wet-strength agents of the above-mentioned types or equivalent compounds.
- Preferably for the purpose of the invention, the cross-linking resin having cationic properties have a molecular weight between 200 and 1,000,000, preferably between 500 and 100,000, most preferably between 1000 and 25,000. Cross-linking resin having a low molecular weight are most preferred for use in the present invention as they are more water-soluble and have a better fiber penetration. By low molecular weight it is meant a molecular weight within the range of from 25 to 2000, preferably from 50 to 1000, and more preferably from 50 to 500.
- It is desirable if the level of cross-linking components or derivative thereof is present in an amount of from 0.01% to 60%, preferably from 0.01% to 30% by weight of the total composition
- It is advantageous for aldehyde containing cross-linking resins if a catalyst is used with compositions of the invention. Preferred catalysts includes organic acids such as citric acid, succinic acid, and tartaric acids, as well as conventional Lewis acid such as Al Cl3 or MgCl2, or salts thereof, or mixtures thereof. A typical example of catalyst is the catalyst NKD made of a mixture of salts and organic acid, and commercially available from Hoechst.
- It is preferred if the level of catalyst is from 10% to 50%, preferably from 20 to 40% by weight of the cross-linking components or derivative thereof.
- For other cross-linking resins like the Kymene, the use of a catalyst is not necessary.
- b) Liquid Carrier
- Another optional, but preferred, ingredient is a liquid carrier. The liquid carrier employed in the instant compositions is preferably at least primarily water due to its low cost, relative availability, safety, and environmental compatibility. The level of water in the liquid carrier is preferably at least about 50%, most preferably at least about 60%, by weight of the carrier. Mixtures of water and low molecular weight, e.g., <about 200, organic solvent, e.g., lower alcohols such as ethanol, propanol, isopropanol or butanol are useful as the carrier liquid. Low molecular weight alcohols include monohydric, dihydric (glycol, etc.) trihydric (glycerol, etc.), and higher polyhydric (polyols) alcohols.
- c) Dispersibility Aids
- Relatively concentrated compositions containing both saturated and unsaturated diester quaternary ammonium compounds can be prepared that are stable without the addition of concentration aids. However, the compositions of the present invention may require organic and/or inorganic concentration aids to go to even higher concentrations and/or to meet higher stability standards depending on the other ingredients. These concentration aids which typically can be viscosity modifiers may be needed, or preferred, for ensuring stability under extreme conditions when particular softener active levels are used. The surfactant concentration aids are typically selected from the group consisting of (1) single long chain alkyl cationic surfactants; (2) nonionic surfactants; (3) amine oxides; (4) fatty acids; and (5) mixtures thereof. These aids are described in WO 94/20597, specifically on page 14, line 12 to page 20, line 12, which is herein incorporated by reference.
- When said dispersibility aids are present, the total level is from 0.1% to 20%, preferably from 0.2% to 10%, more preferably from 0.5% to 5%, and even more preferably from 1% to 2% by weight of the composition. These materials can either be added as part of the active softener raw material, (I), e.g., the mono-long chain alkyl cationic surfactant and/or the fatty acid which are reactants used to form the biodegradable fabric softener active as discussed hereinbefore, or added as a separate component. The total level of dispersibility aid includes any amount that may be present as part of component (I).
- Inorganic viscosity/dispersibility control agents which can also act like or augment the effect of the surfactant concentration aids, include water-soluble, ionizable salts which can also optionally be incorporated into the compositions of the present invention. A wide variety of ionizable salts can be used. Examples of suitable salts are the halides of the Group IA and IIA metals of the Periodic Table of the Elements, e.g., calcium chloride, magnesium chloride, sodium chloride, potassium bromide, and lithium chloride. The ionizable salts are particularly useful during the process of mixing the ingredients to make the compositions herein, and later to obtain the desired viscosity. The amount of ionizable salts used depends on the amount of active ingredients used in the compositions and can be adjusted according to the desires of the formulator. Typical levels of salts used to control the composition viscosity are from about 20 to about 20,000 parts per million (ppm), preferably from about 20 to about 11,000 ppm, by weight of the composition. Alkylene polyammonium salts can be incorporated into the composition to give viscosity control in addition to or in place of the water-soluble, ionizable salts above. In addition, these agents can act as scavengers, forming ion pairs with anionic detergent carried over from the main wash, in the rinse, and on the fabrics, and may improve softness performance. These agents may stabilize the viscosity over a broader range of temperature, especially at low temperatures, compared to the inorganic electrolytes.
- Specific examples of alkylene polyammonium salts include 1-lysine monohydrochloride and 1,5-diammonium 2-methyl pentane dihydrochloride.
- d) Stabilizers
- Stabilizers can be present in the compositions of the present invention. The term “stabilizer,” as used herein, includes antioxidants and reductive agents. These agents are present at a level of from 0% to about 2%, preferably from about 0.01% to about 0.2%, more preferably from about 0.035% to about 0.1% for antioxidants, and more preferably from about 0.01% to about 0.2% for reductive agents. These assure good odor stability under long term storage conditions for the compositions and compounds stored in molten form. The use of antioxidants and reductive agent stabilizers is especially critical for low scent products (low perfume).
- Examples of antioxidants that can be added to the compositions of this invention include a mixture of ascorbic acid, ascorbic palmitate, propyl gallate, available from Eastman Chemical Products, Inc., under the trade names Tenox® PG and Tenox S-1; a mixture of BHT (butylated hydroxytoluene), BHA (butylated hydroxyanisole), propyl gallate, and citric acid, available from Eastman Chemical Products, Inc., under the trade name Tenox-6; butylated hydroxytoluene, available from UOP Process Division under the trade name Sustane® BHT; tertiary butylhydroquinone, Eastman Chemical Products, Inc., as Tenox TBHQ; natural tocopherols, Eastman Chemical Products, Inc., as Tenox GT-1/GT-2; and butylated hydroxyanisole, Eastman Chemical Products, Inc., as BHA; long chain esters (C8-C22) of gallic acid, e.g., dodecyl gallate; Irganox® 1010; Irganox® 1035; Irganox® B 1171; Irganox® 1425; Irganox( 3114; Irganox® 3125; and mixtures thereof; preferably Irganox® 3125, Irganox® 1425, Irganox@ 3114, and mixtures thereof; more preferably Irganox® 3125 alone. The chemical names and CAS numbers for some of the above stabilizers are listed in Table II below.
TABLE II Chemical Name used in Code of Federal Antioxidant CAS No. Regulations Irganox ® 6683-19-8 Tetrakis(methylene(3,5-di-tert-butyl-4 1010 hydroxyhydrocinnamate))methane Irganox ® 41484-35-9 Thiodiethylene bis(3,5-di-tert-butyl-4- 1035 hydroxyhydrocinnamate Irganox ® 23128-74-7 N,N′-Hexamethylene bis(3,5-di-tert-butyl-4- 1098 hydroxyhydrocinnamamide Irganox ® 31570-04-4 B 1171 23128-74-7 1:1 Blend of Irganox ® 1098 and Irgafos ® 168 Irganox ® 65140-91-2 Calcium bis(monoethyl(3,5-di-tert-butyl-4- 1425 hydroxybenzyl)phosphonate) Irganox ® 65140-91-2 Calcium bis(monoethyl(3,5-di-tert-butyl-4- 3114 hydroxybenzyl)phosphonate) Irganox ® 34137-09-2 3,5-Di-tert-butyl-4-hydroxy-hydrocinnamic 3125 acid triester with 1,3,5-tris(2-hydroxyethyl)- S-triazine-2,4,6-(1H, 3H, 5H)-trione Irgafos ® 31570-04-4 Tris(2,4-di-tert-butyl-phenyl)phosphite 168 - Examples of reductive agents include sodium borohydride, hypophosphorous acid, Irgafos® 168, and mixtures thereof.
- e) Preservative
- Optionally, but preferably, antimicrobial preservative can be added to the composition of the present invention. Contamination by certain microorganisms with subsequent microbial growth can result in an unsightly and/or malodorous solution. Because microbial growth in solutions is highly objectionable when it occurs, it is highly preferable to include an antimicrobial preservative, which is effective for inhibiting and/or regulating microbial growth in order to increase storage stability of the composition.
- It is preferable to use a broad spectrum preservative, e.g., one that is effective on both bacteria (both gram positive and gram negative) and fungi. A limited spectrum preservative, e.g., one that is only effective on a single group of microorganisms, e.g., fungi, can be used in combination with a broad spectrum preservative or other limited spectrum preservatives with complimentary and/or supplementary activity. A mixture of broad spectrum preservatives can also be used. In some cases where a specific group of microbial contaminants is problematic (such as Gram negatives), aminocarboxylate chelators, such as those described hereinbefore, can be used alone or as potentiators in conjunction with other preservatives. These chelators which include, e.g., ethylenediaminetetraacetic acid (EDTA), hydroxyethylenediaminetriacetic acid, diethylenetriaminepentaacetic acid, and other aminocarboxylate chelators, and mixtures thereof, and their salts, and mixtures thereof, can increase preservative effectiveness against Gram-negative bacteria, especially Pseudomonas species.
- Antimicrobial preservatives useful in the present invention include biocidal compounds, i.e., substances that kill microorganisms, or biostatic compounds, i.e., substances that inhibit and/or regulate the growth of microorganisms. Well-known preservatives such as short chain alkyl esters of p-hydroxybenzoic acid, commonly known as parabens; N-(4-chlorophenyl)-N′-(3,4-dichlorophenyl) urea, also known as 3,4,4′-trichlorocarbanilide or triclocarban; 2,4,4′-trichloro-2′-hydroxy diphenyl ether, commonly known as triclosan are useful preservative in the present invention.
- Still other preferred preservatives are the water-soluble preservatives, i.e. those that have a solubility in water of at least about 0.3 g per 100 ml of water, i.e., greater than about 0.3% at room temperature, preferably greater than about 0.5% at room temperature.
- The preservative in the present invention is included at an effective amount. The term “effective amount” as herein defined means a level sufficient to prevent spoilage, or prevent growth of inadvertently added microorganisms, for a specific period of time. In other words, the preservative is not being used to kill microorganisms on the surface onto which the composition is deposited in order to eliminate odors produced by microorganisms. Instead, it is preferably being used to prevent spoilage of the solution in order to increase the shelf-life of the composition. Preferred levels of preservative are from about 0.0001% to about 0.5%, more preferably from about 0.0002% to about 0.2%, most preferably from about 0.0003% to about 0.1%, by weight of the usage composition.
- The preservative can be any organic preservative material which will not cause damage to fabric appearance, e.g., discoloration, coloration, bleaching. Preferred water-soluble preservatives include organic sulfur compounds, halogenated compounds, cyclic organic nitrogen compounds, low molecular weight aldehydes, quaternary ammonium compounds, dehydroacetic acid, phenyl and phenolic compounds, and mixtures thereof. Non-limiting examples of preferred water-soluble preservatives for use in the present invention can be found in U.S. Pat. No. 5,714,137, incorporated hereinbefore by reference, as well as co-pending application PCTJUS 98/12154 pages 29 to 36.
- Preferred water-soluble preservatives for use in the present invention are organic sulfur compounds. Some non-limiting examples of organic sulfur compounds suitable for use in the present invention are:
- 3-Isothiazolone Compounds: A preferred preservative is an antimicrobial, organic preservative containing 3-isothiazolone groups. This class of compounds is disclosed in U.S. Pat. No. 4,265,899, Lewis et al., issued May 5, 1981, and incorporated herein by reference. A preferred preservative is a water-soluble mixture of 5-chloro-2-methyl4-isothiazolin-3-one and 2-methyl4-isothiazolin-3-one, more preferably a mixture of about 77% 5-chloro-2-methyl4-isothiazolin-3-one and about 23% 2-methyl4-isothiazolin-3-one, a broad spectrum preservative available as a 1.5% aqueous solution under the trade name Kathon® CG by Rohm and Haas Company.
- When Kathon® is used as the preservative in the present invention it is present at a level of from about 0.0001% to about 0.01%, preferably from about 0.0002% to about 0.005%, more preferably from about 0.0003% to about 0.003%, most preferably from about 0.0004% to about 0.002%, by weight of the composition.
- Other isothiazolins include 1,2-benzisothiazolin-3-one, available under the trade name Proxel® products; and 2-methyl4,5-trimethylene4-isothiazolin-3-one, available under the trade name Promexal®. Both Proxel and Promexal are available from Zeneca. They have stability over a wide pH range (i.e., 4-12). Neither contain active halogen and are not formaldehyde releasing preservatives. Both Proxel and Promexal are effective against typical Gram negative and positive bacteria, fungi and yeasts when used at a level from about 0.001% to about 0.5%, preferably from about 0.005% to about 0.05%, and most preferably from about 0.01% to about 0.02% by weight of the usage composition.
- Sodium Pyrithione : Another preferred organic sulfur preservative is sodium pyrithione, with water solubility of about 50%. When sodium pyrithione is used as the preservative in the present invention it is typically present at a level of from about 0.0001% to about 0.01%, preferably from about 0.0002% to about 0.005%, more preferably from about 0.0003% to about 0.003%, by weight of the usage composition.
- Mixtures of the preferred organic sulfur compounds can also be used as the preservative in the present invention.
- f) Antimicrobial Active
- The composition may suitably use an optional solubilized, water-soluble antimicrobial active, useful in providing protection against organisms that become attached to the treated material. The free, uncomplexed antimicrobial, e.g., antibacterial, active provides an optimum antibacterial performance.
- Sanitization of fabrics can be achieved by the compositions of the present invention containing, antimicrobial materials, e.g., antibacterial halogenated compounds, quaternary compounds, and phenolic compounds.
- Biguanides. Some of the more robust antimicrobial halogenated compounds which can function as disinfectants/sanitizers as well as finish product preservatives (vide infra), and are useful in the compositions of the present invention include 1,1′-hexamethylene bis(5-(p-chlorophenyl)biguanide), commonly known as chlorhexidine, and its salts, e.g., with hydrochloric, acetic and gluconic acids. The digluconate salt is highly water-soluble, about 70% in water, and the diacetate salt has a solubility of about 1.8% in water. When chlorhexidine is used as a sanitizer in the present invention it is typically present at a level of from about 0.001% to about 0.4%, preferably from about 0.002% to about 0.3%, and more preferably from about 0.01% to about 0.1%, by weight of the usage composition. In some cases, a level of from about 1% to about 2% may be needed for virucidal activity.
- Other useful biguanide compounds include Cosmoci® CQ®, Vantocil® IB, including poly (hexamethylene biguanide) hydrochloride. Other useful cationic antimicrobial agents include the bis-biguanide alkanes. Usable water-soluble salts of the above are chlorides, bromides, sulfates, alkyl sulfonates such as methyl sulfonate and ethyl sulfonate, phenylsulfonates such as p-methylphenyl sulfonates, nitrates, acetates, gluconates, and the like.
- As stated hereinbefore, the bis biguanide of choice is chlorhexidine and its salts, e.g., digluconate, dihydrochloride, diacetate, and mixtures thereof.
- Quaternary Compounds. A wide range of quaternary compounds can also be used as antimicrobial actives, in conjunction with the preferred surfactants, for compositions of the present invention that do not contain cyclodextrin. Non-limiting examples of useful quaternary compounds include: (1) benzalkonium chlorides and/or substituted benzalkonium chlorides such as commercially available Barquat® (available from Lonza), Maquat® (available from Mason), Variquat® (available from Witco/Sherex), and Hyamine® (available from Lonza); (2) dialkyl quaternary such as Bardac® products of Lonza, (3) N-(3-chloroallyl) hexaminium chlorides such as Dowicide® and Dowicil® available from Dow; (4) benzethonium chloride such as Hyamine® 1622 from Rohm & Haas; (5) methylbenzethonium chloride represented by Hyamine® 10X supplied by Rohm & Haas, (6) cetylpyridinium chloride such as Cepacol chloride available from of Merrell Labs. Typical concentrations for biocidal effectiveness of these quaternary compounds range from about 0.001% to about 0.8%, preferably from about 0.005% to about 0.3%, more preferably from about 0.01% to 0.2%, by weight of the usage composition. The corresponding concentrations for the concentrated compositions are from about 0.003% to about 2%, preferably from about 0.006% to about 1.2%, and more preferably from about 0.1% to about 0.8% by weight of the concentrated compositions.
- Other preservatives which are conventional in the art, such as described in U.S. Pat. No. 5,593, 670 incorporated herein by reference, may also be used herein.
- g) Perfume
- The present invention can contain a perfume. Suitable perfumes are disclosed in U.S. Pat. No. 5,500,138, said patent being incorporated herein by reference.
- As used herein, perfume includes fragrant substance or mixture of substances including natural (i.e., obtained by extraction of flowers, herbs, leaves, roots, barks, wood, blossoms or plants), artificial (i.e., a mixture of different nature oils or oil constituents) and synthetic (i.e., synthetically produced) odoriferous substances. Such materials are often accompanied by auxiliary materials, such as fixatives, extenders, stabilizers and solvents. These auxiliaries are also included within the meaning of “perfume”, as used herein. Typically, perfumes are complex mixtures of a plurality of organic compounds.
- Examples of perfume ingredients useful in the perfumes of the present invention compositions include, but are not limited to, hexyl cinnamic aldehyde; amyl cinnamic aldehyde; amyl salicylate; hexyl salicylate; terpineol; 3,7-dimethyl-cis-2,6-octadien-1-ol; 2,6-dimethyl-2-octanol; 2,6-dimethyl-7-octen-2-ol; 3,7-dimethyl-3-octanol; 3,7-dimethyl-trans-2,6-octadien-1-ol; 3,7-dimethyl-6-octen-1-ol; 3,7-dimethyl-1-octanol; 2-methyl-3-(para-tert-butylphenyl)-propionaldehyde; 4-(4-hydroxy4-methylpentyl)-3-cyclohexene-1-carbox-aldehyde; tricyclodecenyl propionate; tricyclodecenyl acetate; anisaldehyde; 2-methyl-2-(para-iso-propylphenyl)-propionaldehyde; ethyl-3-methyl-3-phenyl glycidate; 4-(para-hydroxyphenyl)-butan-2-one; 1-(2,6,6-trimethyl-2-cyclohexen-1-yl)-2-buten-1-one; para-methoxyacetophenone; para-methoxy-alpha-phenylpropene; methyl-2-n-hexyl-3-oxo-cyclopentane carboxylate; undecalac-tone gamma.
- Additional examples of fragrance materials include, but are not limited to, orange oil; lemon oil; grapefruit oil; bergamot oil; clove oil; dodecalactone gamma; methyl-2-(2-pentyl-3-oxo-cyclopentyl) acetate; beta-naphthol methylether; methyl-beta-naphthylketone; coumarin; decylaldehyde; benzaldehyde; 4-tert-butylcyclohexyl acetate; alpha,alpha-dimethylphenethyl acetate; methylphenylcarbinyl acetate; Schiff's base of 4-(4-hydroxy4-methylpentyl)-3-cyclohexene-1-carboxaldehyde and methyl anthranilate; cyclic ethyleneglycol diester of tridecandioic acid; 3,7-dimethyl-2,6-octadiene-1-nitrile; ionone gamma methyl; ionone alpha; ionone beta; petitgrain; methyl cedrylone; 7-acetyl-1,2,3,4,5,6,7,8-octahydro-1,1,6,7-tetramethyl-naphthalene; ionone methyl; methyl-1,6,10-trimethyl-2,5,9-cyclododecatrien-1-yl ketone; 7-acetyl-1,1,3,4,4,6-hexamethyl tetralin; 4-acetyl-6-tert-butyl-1,1-dimethyl indane; benzophenone; 6-acetyl-1,1,2,3,3,5-hexamethyl indane; 5-acetyl-3-isopropyl-1,1,2,6-tetramethyl indane; 1-dodecanal; 7-hydroxy-3,7-dimethyl octanal; 10-undecen-1-al; iso-hexenyl cyclohexyl carboxaldehyde; formyl tricyclodecan; cyclopentadecanolide; 16-hydroxy-9-hexadecenoic acid lactone; 1,3,4,6,7,8-hexahydro4,6,6,7,8,8-hexamethylcyclopenta-gamma-2-benzopyrane; ambroxane; dodecahydro-3a,6,6,9a-tetramethylnaphtho-[2,1b]furan; cedrol; 5-(2,2,3-trimethylcyclopent-3-enyl)-3-methylpentan-2-ol; 2-ethyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-buten-1-ol; caryophyllene alcohol; cedryl acetate; para-tert-butylcyclohexyl acetate; patchouli; olibanum resinoid; labdanum; vetivert; copaiba balsam; fir balsam; and condensation products of: hydroxycitronellal and methyl anthranilate; hydroxycitronellal and indol; phenyl acetaldehyde and indol; 4-(4-hydroxy-4-methyl pentyl)-3-cyclohexene-1-carboxaldehyde and methyl anthranilate.
- More examples of perfume components are geraniol; geranyl acetate; linalool; linalyl acetate; tetrahydrolinalool; citronellol; citronellyl acetate; dihydromyrcenol; dihydromyrcenyl acetate; tetrahydromyrcenol; terpinyl acetate; nopol; nopyl acetate; 2-phenylethanol; 2-phenylethyl acetate; benzyl alcohol; benzyl acetate; benzyl salicylate; benzyl benzoate; styrallyl acetate; dimethylbenzylcarbinol; trichloromethylphenylcarbinyl methylphenylcarbinyl acetate; isononyl acetate; vetiveryl acetate; vetiverol; 2-methyl-3-(p-tert-butylphenyl)-propanal; 2-methyl-3-(p-isopropylphenyl)-propanal; 3-(p-tert-butylphenyl)-propanal; 4-(4-methyl-3-pentenyl)-3-cyclohexenecarbaldehyde; 4-acetoxy-3-pentyltetrahydropyran; methyl dihydrojasmonate; 2-n-heptylcyclopentanone; 3-methyl-2-pentyl-cyclopentanone; n-decanal; n-dodecanal; 9-decenol-1; phenoxyethyl isobutyrate; phenylacetaldehyde dimethylacetal; phenylacetaldehyde diethylacetal; geranonitrile; citronellonitrile; cedryl acetal; 3-isocamphylcyclohexanol; cedryl methylether; isolongifolanone; aubepine nitrile; aubepine; heliotropine; eugenol; vanillin; diphenyl oxide; hydroxycitronellal ionones; methyl ionones; isomethyl ionomes; irones; cis-3-hexenol and esters thereof; indane musk fragrances; tetralin musk fragrances; isochroman musk fragrances; macrocyclic ketones; macrolactone musk fragrances; ethylene brassylate.
- The perfumes useful in the present invention compositions are substantially free of halogenated materials and nitromusks.
- Suitable solvents, diluents or carriers for perfumes ingredients mentioned above are for examples, ethanol, isopropanol, diethylene glycol, monoethyl ether, dipropylene glycol, diethyl phthalate, triethyl citrate, etc. The amount of such solvents, diluents or carriers incorporated in the perfumes is preferably kept to the minimum needed to provide a homogeneous perfume solution.
- Perfume can be present at a level of from 0% to 10%, preferably from 0.1% to 5%, and more preferably from 0.2% to 3%, by weight of the finished composition. Fabric softener compositions of the present invention provide improved fabric perfume deposition.
- Perfume ingredients may also be suitably added as releasable fragrances, for example, as pro-perfumes or pro-fragrances as described in U.S. Pat. No. 5,652,205 Hartman et al., issued Jul. 29, 1997, WO95/04809, WO96/02625, PCT US97/14610 filed 19 Aug. 1997 and claiming priority of 19 Aug. 1996, EP-A-0,752,465, co-pending application EP 98870227.0, EP 98870226.2, EP 99870026.4, and EP 99870025.6; all incorporated herein by reference.
- h) Soil Release Agent
- Soil Release agents are desirably used in compositions of the instant invention. Any polymeric soil release agent known to those skilled in the art can optionally be employed in the compositions of this invention. Polymeric soil release agents are characterized by having both hydrophilic segments, to hydrophilize the surface of hydrophobic fibers, such as polyester and nylon, and hydrophobic segments, to deposit upon hydrophobic fibers and remain adhered thereto through completion of washing and rinsing cycles and, thus, serve as an anchor for the hydrophilic segments. This can enable stains occurring subsequent to treatment with the soil release agent to be more easily cleaned in later washing procedures.
- If utilized, soil release agents will generally comprise from about 0.01% to about 10.0%, by weight, of the detergent compositions herein, typically from about 0.1% to about 5%, preferably from about 0.2% to about 3.0%.
- The following, all included herein by reference, describe soil release polymers suitable for use in the present invention. U.S. Pat. No. 3,959,230 Hays, issued May 25, 1976; U.S. Pat. No. 3,893,929 Basadur, issued Jul. 8, 1975; U.S. Pat. No. 4,000,093, Nicol, et al., issued Dec. 28, 1976; U.S. Pat. No. 4,702,857 Gosselink, issued Oct. 27, 1987; U.S. Pat. No. 4,968,451, Scheibel et al., issued November 6; U.S. Pat. No. 4,702,857, Gosselink, issued Oct. 27, 1987; U.S. Pat. No. 4,711,730, Gosselink et al., issued Dec. 8, 1987; U.S. Pat. No. 4,721,580, Gosselink, issued Jan. 26, 1988; U.S. Pat. No. 4,877,896, Maldonado et al., issued Oct. 31, 1989; U.S. Pat. No. 4,956,447, Gosselink et al., issued Sep. 11, 1990; U.S. Pat. No. 5,415,807 Gosselink et al., issued May 16, 1995; European Patent Application 0 219 048, published Apr. 22, 1987 by Kud, et al.
- Further suitable soil release agents are described in U.S. Pat. No. 4,201,824, Violland et al.; U.S. Pat. No. 4,240,918 Lagasse et al.; U.S. Pat. No. 4,525,524 Tung et al.; U.S. Pat. No. 4,579,681, Ruppert et al.; U.S. Pat. No. 4,240,918; U.S. Pat. No. 4,787,989; U.S. Pat. No. 4,525,524; EP 279,134 A, 1988, to Rhone-Poulenc Chemie; EP 457,205 A to BASF (1991); and DE 2,335,044 to Unilever N. V., 1974 all incorporated herein by reference.
- Commercially available soil release agents include the METOLOSE SM100, METOLOSE SM200 manufactured by Shin-etsu Kagaku Kogyo K. K., SOKALAN type of material, e.g., SOKALAN HP-22, available from BASF (Germany), ZELCON 5126 (from Dupont) and MILEASE T (from ICI).
- i)-pH
- An optional requirement of the compositions according to the present invention is that the pH as measured in the neat compositions at 20° C., is greater than 3, preferably between 3 and 12, more preferably between 4 and 8, most preferably is of 5. This range is preferred for fabric safety. The pH of these compositions herein can be regulated by the addition of a Bronsted acid.
- j)-Blowing Agents
- Also suitable for use herein are blowing agents selected from the group consisting of ammonium carbonate, ammonium bicarbonate, or mixtures thereof. It is hypothesized that those agents, when present, will generate small amounts of CO2 when exposed to heat, such as during the ironing process. The CO2 will be released in the composition which is deposited as a film on the fabric. The film, hence the fabric, will thus acquire more flexibility, resulting in a better ability to resist to the dry formation of wrinkles, when the fabric is stored or worn.
- k) Void Fillers
- The compositions herein may further comprise void fillers. By fabric void filler, it is meant herein particles having the size and shape suited to fill the structural defects in cotton, and hereby provide lubricating properties. Cyclodextrins such as those described in WO 99/55950 can be used as void fillers, as well as polyolefin dispersions, such as those described in U.S. Pat. No. 6,020,302. Inorganic particles can also be used to that effect, for instance TiO2 and Silica.
- 1) Other Optional Ingredients
- The present invention can include optional components conventionally used in textile treatment compositions, for example, humectants like diethylene glycol, and/or salts like lithium salts, colorants, bactericides, optical brighteners, opacifiers, anti-shrinkage agents, germicides, fungicides, anti-oxidants, color protection agent like dye fixing agent as described in EP 931133, enzymes, chelating agents, cyclodextrin as described in WO 98/56888, metallic salts to absorb amine and sulfur-containing compounds and selected from the group consisting of copper salts, zinc salts, and mixtures thereof, water-soluble polyionic polymers, e.g., water-soluble cationic polymer like polyamines, and water-soluble anionic polymers like polyacrylic acids, other antistatic agent, insect and/or moth repelling agents, colorants and dyes, anti-clogging agent, and the like; typical disclosure of which can be found in WO 98/56888. Still other suitable optional ingredients are ingredients which provide shield protection against stain like hydroxypropylcellulose as well as other cellulosic polymer like carboxymethylcellulose. The compositions are preferably free of any material that would soil or stain fabric, and are also substantially free of starch. Typically, there should be less than about 0.5%, by weight of the composition, preferably less than about 0.3%, more preferably less than about 0.1%, by weight of the composition, of starch and/or modified starch.
- 4)-Form of the Composition:
- The composition of the invention may take a variety of physical form including liquid, liquid-gel, paste-like, foam in either aqueous or non-aqueous form, powder like granular and tablet forms. A preferred form of the composition is in a liquid form.
- When in a liquid form, the composition is preferably dispensed by a dispensing means such as a spray dispenser, aerosol dispenser, or refill thereof. Still another preferred dispensing means is by incorporation of the composition of the invention in the ironing tank per se, or via a cartridge preferably adapted for the iron.
- 5)-Spray Dispenser:
- The present invention also relates to such compositions incorporated into a spray dispenser to create an article of manufacture that can facilitate treatment of fabric articles and/or surfaces with the compositions according to the invention at a level that is effective. The spray dispenser comprises manually activated and non-manual powered (operated) spray means and a container containing the treating composition. Typical disclosure of such spray dispenser can be found in WO 96/04940 page 19 line 21 to page 22 line 27. Preferably, the spray dispenser is selected from spray dispenser comprising battery operated pump, spray dispenser comprising a trigger spray device, spray dispenser comprising a pressurized aerosol spray dispenser.
- 6)-Method of Use:
- It has been found that the use of the water-soluble lubricant provided a reduction of the WRA compared to water. Accordingly, there is provided a method of increasing the WRA of fabrics, which comprises the steps of contacting the fabrics with a water-soluble lubricant as defined herein before, using a domestic process.
- It has also been found that the use of the water-soluble lubricant or composition of the invention provides surprisingly good benefit on the dewrinkling performance upon wearing. This benefit is particularly achieved while spraying the compound or composition preferably from an iron during an otherwise known fabric ironing process. Accordingly, there is also provided a method of treating fabrics, in particular to provide in wear wrinkle resistance on fabrics, which comprises the steps of contacting the fabrics with a water-soluble lubricant or composition according to the invention, as defined herein before, using a domestic process.
- By “contacting”, it is meant any steps that is suitable for providing a contact of the composition with the fabric. This can include by soaking, washing, rinsing, and/or spraying as well as by means of a dryer sheet onto which is adsorbed the composition. Preferably, the contacting occurs after the laundering and optional drying of the fabrics, e.g. by spraying the composition, more preferably by spraying the composition from an iron spray dispenser and/or via the vaporisation holes from an iron sole plate or foam or sprayer which is separate from the iron. Accordingly, in this instance, the composition of the present invention is used as an ironing aid. An effective amount of the composition can be sprayed onto the fabric, wherein said fabric should not be sprayed to saturation. Still another preferred way of treating the fabrics is when the fabric can be sprayed with an effective amount of the composition, allowed to dry and then ironed, or sprayed and ironed immediately.
- Accordingly, in a further aspect of the invention, the composition of the invention can also be sprayed onto the fabrics by means of spraying means which are incorporated to an iron, and the composition is incorporated into the iron's water tank or via a cartridge, adapted to fit within the iron. Such irons are disclosed for instance in WO 00/08247 and WO 99/27176. As for the method of spraying via the iron, the spraying means should preferably be capable of providing droplets with a weight average diameter of from about 40 to about 200 μm, preferably from about 70 to about 150 μm. Preferably, the loading of moisture on fabrics made of natural and synthetic fibers is from about 5 to about 25%, more preferably from about 5 to about 10% by weight of the dried fabric.
- By “wrinkle reducing composition”, it is meant that the composition is tested on 100% cotton, woven Oxford pinpoint fabric according to the procedure given in W. Garner, Textile Laboratory Manual Vol. 6, Ed. 3, Elsevier Inc., 1967, p. 105, so called “cylinder test”. The cylinder test consists in taking a 12×14 inch of treated cloth, rolling it round a plastic tube, placing the roll in a 360 ml measuring cylinder (r=0.67 inch, I=15.7 inch), withdrawing the tube, and pushing the fabric down to occupy a volume of about 90 ml by means of a plastic tube which is an easy sliding fit for the cylinder. This test is carried out on cloth conditioned for 24 hours at 21° C. (70° F.) and 65% RH. The cloth is left 1 minute in the cylinder, opened immediately, inspected visually, and then compared with a cloth only treated with water. The results obtained are compared against fabrics which have only been treated with water. Wrinkle reducing compositions are compositions which provide a better crease resistance versus water, i.e, fabrics that have been treated with a composition of the invention show less wrinkles compared to fabrics which have only been treated with water.
- In a still further aspect of the invention, the composition can be sprayed onto fabrics by an in-home de-wrinkling chamber containing the fabric to be dewrinkled, thereby providing ease of operation. Conventional personal as well as industrial de-wrinkling apparatuses are suitable for use herein. Traditionally, these apparatuses act by a steaming process which effects a relaxation of the fibers. Examples of home dewrinkling chambers include shower stalls. The spraying of the composition or compounds onto the fabrics can then occur within the chamber of the apparatus or before placing the fabrics into the chamber. As for the manual method of spraying, the spraying means should preferably be capable of providing droplets with a weight average diameter of from about 8 to about 100 μm, preferably from about 10 to about 50 μm. Preferably, the loading of moisture on fabrics made of natural and synthetic fibers is from about 5 to about 25%, more preferably from about 5 to about 10% by weight of the dried fabric. Other conventional steps that can be carried out in the dewrinkling apparatus can be applied such as heating which will provide the curing step and drying. Preferably, for optimum dewrinkling benefit, the temperature profile inside the chamber ranges from about 40° C. to about 80° C., more preferably from about 50° C. to about 70° C. The preferred length of the drying cycle is from about 15 to about 60 minutes, more preferably from about 20 to about 45 minutes.
- The steaming step in the dewrinkling apparatus can also be eliminated if the composition is maintained at a temperature range from about 22° C. (about 72° F.) to about 76° C. (170° F.) before spraying.
- The present invention encompasses the method of spraying a mist of an effective amount of solution of the invention composition onto fabric and/or fabric articles. Preferably, said fabric and/or fabric articles include, but are not limited to, clothes, curtains, drapes, upholstered furniture, carpeting, bed linens, bath linens, tablecloths, sleeping bags, tents, car interiors, etc.
- The compositions herein are especially useful, when used to treat garments for extending the time before another wash cycle is needed, and/or even reducing the time involved in ironing. Such garments include uniforms and other garments which are normally treated in an industrial process, which can be dewrinkled and the time between treatments extended.
- 7)-Article of Manufacture:
- Also provided herein is an article of manufacture comprising a container and the composition of the invention in association with a set of instructions to use the composition in an amount effective to provide a solution to problems involving and/or provision of a benefit related to those selected from reducing wrinkles; imparting in-wear resistance to fabrics. It is important that the consumer be aware of these additional benefits, since otherwise the consumer would not know that the composition would solve these problems and/or provide these benefits.
- As used herein, the phrase “in association with” means the set of instructions are either directly printed on the container itself or presented in a separate manner including, but not limited to, a brochure, print advertisement, electronic advertisement, and/or verbal communication, so as to communicate the set of instructions to a consumer of the article of manufacture. The set of instructions preferably comprises the instruction to apply an effective amount of the composition, preferably by spraying, to provide the indicated benefit, e.g. wrinkles reduction; imparting in-wear resistance to fabrics. The set of instructions preferably comprises instructions to spray the composition on the fabrics and iron the fabrics.
- The invention is illustrated in the following non limiting examples, in which all percentages are on a weight basis unless otherwise stated.
- In the examples, the abbreviated component identifications have the following meanings:
- Polymer 1: Isomalto oligosaccharide available from Showa Sangyo Co. under the trade name IMO 900
- Polymer 2: Polyvinylpyrrolidone available from BASF under the trade name Luviskol K30
- Polymer 3: Co-polymer of vinylpyrrolidone and vinylcaprolactame available from BASF under the trade name Luvitec VPC
- Polymer 4: Co-polymer of vinylpyrrolidone and vinylimidazolinium methachloride available from BASF under the trade name Luviquat FC 905
- Lubricant 1: Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7200 from OSI Chem./Witco
- Lubricant 2: Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7657 from OSI Chem.[Witco
- Lubricant 3: Polyethoxylated (20 moles) sorbitan monolaurate commercially available under the tradename of Radiasurf 7137 from FINA
- Lubricant 4: Polyethoxylated (20 moles) sorbitan tristearate commercially available under the tradename of Tween 65
- Wetting agent 1: Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet 7600 from OSI Chem./Witco
- Wetting agent 1: Polyalkylene oxide polysiloxane commercially available under the tradename of Silwet L 77 from OSI Chem./Witco
- Emulsifier 1: CAE 10 (coconut alcohol condensed with an average of 10 moles of ethylene oxide)
A B C D E F Polymer #1 5% — — — — 1% Polymer #2 — 1% — 2% — — Polymer #3 — — 2% — — — Polymer #4 — — — — 0.5% — Lubricant #1 14% 4% — — 1.5% — Lubricant #2 — — 6% 2% 1.5% — Lubricant #3 8% 2% — — — 5% Lubricant #4 — — — 3% — — Wetting agent 1 3% 0.5 — — 0.2% — Wetting agent 2 — — 0.5% — — — Dipropyleneglycol — 0.3 — — — — Emulsifier 1 0.6% 0.2 — — — — Cyclodextrin — 0.5 1% — — — Preservative 3 ppm 3 ppm — 3 ppm — — Perfume 0.5% 0.1 0.1% — — 0.2% Water Balance Balance Balance Balance Bal- Bal- ance ance
Claims (16)
1. A domestic fabric wrinkle reducing composition comprising a water-soluble lubricant and a component having a deviation of fabric Wrinkle Recovery Angle (WRA) versus water of at least +15, wherein said composition is contained in a device selected from the group consisting of a spray dispenser, an aerosol dispenser, a foam dispenser, an iron, and a refill cartridge thereof.
2. A composition according to claim 1 , wherein said water-soluble lubricant is selected from the group consisting of nonionic silicone containing surfactants and ethoxylated sorbitan esters.
3. A composition according to claim 1 , wherein said water-soluble lubricant is present in an amount of from 0.1% to 70% by weight of said composition.
4. A composition according claim 1 , wherein said component having a deviation of fabric WRA versus water of at least +15 is a material selected from the group consisting of shape retention polymers, polymers comprising at least one unit which provide a dye transfer inhibiting benefit, urethane polymers, isomalto oligosaccharide, polyvinylamine polymers, amphoteric polymers, aminosilicones, curable silicones, and mixtures thereof.
5. A composition according to claim 1 , wherein said component having a deviation of fabric WRA versus water of at least +15 is present in an amount of at least about 0.01% by weight of the composition.
6. A composition according to claim 1 , wherein said water-soluble lubricant and said component having a deviation of fabric WRA versus water of at least +15 are present in a weight ratio of from 10:1 to 1:1.
7. A composition according to claim 1 , wherein said composition is a liquid composition.
8. A domestic article of manufacture comprising a fabric wrinkle reducing composition comprising a water-soluble lubricant and a component having a deviation of fabric Wrinkle Recovery Angle (WRA) versus water of at least +15 wherein said article is selected from the group consisting of an aerosol dispenser, a spray dispenser, an iron, a foam dispenser, and a refill cartridge thereof.
9. The article of manufacture of claim 8 , wherein said article is a spray dispenser selected from the group consisting of spray dispenser comprising battery operated pump, spray dispenser comprising a trigger spray device, spray dispenser comprising a pressurized aerosol spray dispenser, and spray dispenser comprising a non-manually operated spray dispenser.
10. A method for treating fabrics domestically which comprises the step of contacting fabrics with a fabric wrinkle reducing composition comprising a water-soluble lubricant, said composition being dispensed from a device selected from the group consisting of a spray dispenser, an aerosol dispenser, a foam dispenser, an iron, and a refill cartridge thereof.
11. A method according to claim 10 , wherein said method provides a reduction of the time and/or effort involved to iron fabrics.
12. A method according to claim 10 , wherein said method increases the fabric WRA.
13. A method according to claim 10 , wherein said method provides in-wear resistance to treated fabrics.
14. A method according to claim 10 , wherein said method is performed in an in-home dewrinkling apparatus.
15. A method according to claim 10 , wherein said composition is sprayed onto said fabric and said fabric is ironed.
16. A domestic article of manufacture comprising a container and a fabric reducing composition, said fabric reducing composition comprising a water-soluble lubricant, wherein said container is selected from the group consisting of a spray dispenser, an aerosol dispenser, a foam dispenser, an iron, and a refill cartridge thereof, in association with instructions to use an effective amount of said composition on fabric to provide at least one benefit selected from the group consisting of: reducing wrinkles; reducing the time and/or effort involved to iron fabrics, imparting in-wear resistance to fabrics.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/924,539 US20050015888A1 (en) | 1999-10-27 | 2004-08-24 | Wrinkle resistant composition |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP99870223A EP1096060A1 (en) | 1999-10-27 | 1999-10-27 | Wrinkle resistant composition |
| EP99870223.7 | 1999-10-27 | ||
| US11149102A | 2002-04-25 | 2002-04-25 | |
| US10/924,539 US20050015888A1 (en) | 1999-10-27 | 2004-08-24 | Wrinkle resistant composition |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2000/029769 Continuation WO2001031112A2 (en) | 1999-10-27 | 2000-10-27 | Wrinkle resistant composition |
| US11149102A Continuation | 1999-10-27 | 2002-04-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20050015888A1 true US20050015888A1 (en) | 2005-01-27 |
Family
ID=34081837
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/924,539 Abandoned US20050015888A1 (en) | 1999-10-27 | 2004-08-24 | Wrinkle resistant composition |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US20050015888A1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050028293A1 (en) * | 2002-09-09 | 2005-02-10 | Cedric Geffroy | Rinsing formulation for textiles |
| US20050035493A1 (en) * | 2003-07-02 | 2005-02-17 | Ansell Healthcare Products Inc. | Textured surface coating for gloves and method of making |
| US20060150300A1 (en) * | 2005-01-12 | 2006-07-13 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip and method for in-line processing thereof |
| US20070093408A1 (en) * | 2004-02-17 | 2007-04-26 | Moore John W | Compositions useful as fabric softeners |
| US20070122441A1 (en) * | 2005-11-18 | 2007-05-31 | Hironobu Murata | Biocidal surfaces, articles with biocidal surface agents and methods of synthesizing and evaluating biocidal surface agents |
| US20070151684A1 (en) * | 2005-12-29 | 2007-07-05 | Grigoriev Vladimir A | Creping adhesives comprising blends of polyaminoamide epihalolhydrin resins and polyamides |
| US20090084402A1 (en) * | 2007-09-17 | 2009-04-02 | Christopher Andrew Morrison | Process for treating hard surface |
| US20120046383A1 (en) * | 2010-08-19 | 2012-02-23 | Terumo Kabushiki Kaisha | Silicone rubber composition |
| US20130288941A1 (en) * | 2012-04-25 | 2013-10-31 | Basf Se | Formulations, their use as or for producing dishwashing compositions and their preparation |
| US20140127276A1 (en) * | 2010-02-15 | 2014-05-08 | Philadelphia University | Methods for Reducing Airborne Bacteria and Mycetes and Apparatus for the Same |
| US9695292B2 (en) | 2013-11-26 | 2017-07-04 | Ansell Limited | Effervescent texturing |
| EP3275983A1 (en) * | 2016-07-25 | 2018-01-31 | Henkel AG & Co. KGaA | Polymers of vinylpyrrolidone and/or vinylacetate as textile care agents |
| US10292440B2 (en) | 2015-03-10 | 2019-05-21 | Ansell Limited | Supported glove having an abrasion resistant nitrile coating |
Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3841832A (en) * | 1971-12-06 | 1974-10-15 | Cotton Inc | Process for treating cellulosic material with formaldehyde in liquid phase and sulfur dioxide |
| US3892681A (en) * | 1973-02-16 | 1975-07-01 | Procter & Gamble | Detergent compositions containing water insoluble starch |
| US4007005A (en) * | 1975-06-19 | 1977-02-08 | Redken Laboratories, Inc. | Hair setting compositions which display high resistance to high humidity |
| US4051046A (en) * | 1973-02-16 | 1977-09-27 | The Procter & Gamble Company | Detergent compositions containing insoluble particulate materials having fabric conditioning properties |
| US4116854A (en) * | 1977-02-14 | 1978-09-26 | The Procter & Gamble Company | Detergent compositions containing starch |
| US4162983A (en) * | 1978-03-13 | 1979-07-31 | The Procter & Gamble Company | Fabric care composition containing starch and surfactant |
| US4169064A (en) * | 1977-12-23 | 1979-09-25 | The Procter & Gamble Company | Detergent compositions containing starch |
| US4376802A (en) * | 1980-01-24 | 1983-03-15 | Allied Corporation | Finish composition for polyester yarn |
| US4661268A (en) * | 1985-12-24 | 1987-04-28 | Very Incredible Products, Inc. | Wrinkle removing solution and process for using same |
| US4806254A (en) * | 1987-05-26 | 1989-02-21 | Colgate-Palmolive Co. | Composition and method for removal of wrinkles in fabrics |
| US5100566A (en) * | 1991-02-04 | 1992-03-31 | Dow Corning Corporation | Fabric wrinkle reduction composition and method |
| US5342875A (en) * | 1990-04-30 | 1994-08-30 | The Procter & Gamble Company | Polycationic latex wet strength agent |
| US5417867A (en) * | 1991-04-24 | 1995-05-23 | Dow Corning Toray Silicone Co., Ltd. | Fiber treatment agent |
| US5532023A (en) * | 1994-11-10 | 1996-07-02 | The Procter & Gamble Company | Wrinkle reducing composition |
| US5573695A (en) * | 1995-12-19 | 1996-11-12 | Targosz; Eugene F. | Compositions for removal of wrinkles in fabrics |
| US5665804A (en) * | 1996-02-05 | 1997-09-09 | Dow Corning Corporation | Silicone latex solvent thickening |
| US5798107A (en) * | 1994-11-10 | 1998-08-25 | The Procter & Gamble Company | Wrinkle reducing composition |
| US5939478A (en) * | 1997-07-21 | 1999-08-17 | Dow Corning Corporation | Silicone polyether stabilized silicone latex solvent thickening |
| US5965517A (en) * | 1996-07-25 | 1999-10-12 | Lever Brothers Company, Division Of Conopco,Inc. | Fabric treatment composition |
| US6201093B1 (en) * | 1998-01-21 | 2001-03-13 | Huels Aktiengesellschaft | Amino-functional polyorganosiloxanes, their production and use |
| US6369024B1 (en) * | 1997-09-15 | 2002-04-09 | The Procter & Gamble Company | Laundry detergent compositions with linear amine based polymers to provide appearance and integrity benefits to fabrics laundered therewith |
| US6376456B1 (en) * | 1998-10-27 | 2002-04-23 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Wrinkle reduction laundry product compositions |
| US6384011B1 (en) * | 1997-09-15 | 2002-05-07 | The Procter & Gamble Company | Laundry detergent compositions with cellulosic based polymers to provide appearance and integrity benefits to fabrics laundered therewith |
| US6403548B1 (en) * | 1998-10-27 | 2002-06-11 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Wrinkle reduction laundry product compositions |
-
2004
- 2004-08-24 US US10/924,539 patent/US20050015888A1/en not_active Abandoned
Patent Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3841832A (en) * | 1971-12-06 | 1974-10-15 | Cotton Inc | Process for treating cellulosic material with formaldehyde in liquid phase and sulfur dioxide |
| US3892681A (en) * | 1973-02-16 | 1975-07-01 | Procter & Gamble | Detergent compositions containing water insoluble starch |
| US4051046A (en) * | 1973-02-16 | 1977-09-27 | The Procter & Gamble Company | Detergent compositions containing insoluble particulate materials having fabric conditioning properties |
| US4007005A (en) * | 1975-06-19 | 1977-02-08 | Redken Laboratories, Inc. | Hair setting compositions which display high resistance to high humidity |
| US4116854A (en) * | 1977-02-14 | 1978-09-26 | The Procter & Gamble Company | Detergent compositions containing starch |
| US4169064A (en) * | 1977-12-23 | 1979-09-25 | The Procter & Gamble Company | Detergent compositions containing starch |
| US4162983A (en) * | 1978-03-13 | 1979-07-31 | The Procter & Gamble Company | Fabric care composition containing starch and surfactant |
| US4376802A (en) * | 1980-01-24 | 1983-03-15 | Allied Corporation | Finish composition for polyester yarn |
| US4661268A (en) * | 1985-12-24 | 1987-04-28 | Very Incredible Products, Inc. | Wrinkle removing solution and process for using same |
| US4806254A (en) * | 1987-05-26 | 1989-02-21 | Colgate-Palmolive Co. | Composition and method for removal of wrinkles in fabrics |
| US5342875A (en) * | 1990-04-30 | 1994-08-30 | The Procter & Gamble Company | Polycationic latex wet strength agent |
| US5100566A (en) * | 1991-02-04 | 1992-03-31 | Dow Corning Corporation | Fabric wrinkle reduction composition and method |
| US5417867A (en) * | 1991-04-24 | 1995-05-23 | Dow Corning Toray Silicone Co., Ltd. | Fiber treatment agent |
| US5532023A (en) * | 1994-11-10 | 1996-07-02 | The Procter & Gamble Company | Wrinkle reducing composition |
| US5798107A (en) * | 1994-11-10 | 1998-08-25 | The Procter & Gamble Company | Wrinkle reducing composition |
| US5573695A (en) * | 1995-12-19 | 1996-11-12 | Targosz; Eugene F. | Compositions for removal of wrinkles in fabrics |
| US5665804A (en) * | 1996-02-05 | 1997-09-09 | Dow Corning Corporation | Silicone latex solvent thickening |
| US5965517A (en) * | 1996-07-25 | 1999-10-12 | Lever Brothers Company, Division Of Conopco,Inc. | Fabric treatment composition |
| US5939478A (en) * | 1997-07-21 | 1999-08-17 | Dow Corning Corporation | Silicone polyether stabilized silicone latex solvent thickening |
| US6369024B1 (en) * | 1997-09-15 | 2002-04-09 | The Procter & Gamble Company | Laundry detergent compositions with linear amine based polymers to provide appearance and integrity benefits to fabrics laundered therewith |
| US6384011B1 (en) * | 1997-09-15 | 2002-05-07 | The Procter & Gamble Company | Laundry detergent compositions with cellulosic based polymers to provide appearance and integrity benefits to fabrics laundered therewith |
| US6201093B1 (en) * | 1998-01-21 | 2001-03-13 | Huels Aktiengesellschaft | Amino-functional polyorganosiloxanes, their production and use |
| US6376456B1 (en) * | 1998-10-27 | 2002-04-23 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Wrinkle reduction laundry product compositions |
| US6403548B1 (en) * | 1998-10-27 | 2002-06-11 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Wrinkle reduction laundry product compositions |
Cited By (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050028293A1 (en) * | 2002-09-09 | 2005-02-10 | Cedric Geffroy | Rinsing formulation for textiles |
| US7771644B2 (en) | 2003-07-02 | 2010-08-10 | Ansell Healthcare Products Llc | Textured surface coating for gloves and method of making |
| US20070192929A1 (en) * | 2003-07-02 | 2007-08-23 | Ansell Healthcare Products Llc | Textured surface coating for gloves and method of making |
| US20160360808A1 (en) * | 2003-07-02 | 2016-12-15 | Ansell Limited | Textured surface articles and method of making |
| US20070118967A1 (en) * | 2003-07-02 | 2007-05-31 | Ansell Healthcare Products Llc | Textured surface coating for gloves and method of making |
| US20050035493A1 (en) * | 2003-07-02 | 2005-02-17 | Ansell Healthcare Products Inc. | Textured surface coating for gloves and method of making |
| US20170000201A1 (en) * | 2003-07-02 | 2017-01-05 | Ansell Limited | Textured surface articles and method of making |
| US7662765B2 (en) | 2004-02-17 | 2010-02-16 | Optimer, Inc. | Compositions useful as fabric softener |
| US7402555B2 (en) * | 2004-02-17 | 2008-07-22 | Optimer, Inc. | Compositions useful as fabric softeners |
| US20080312126A1 (en) * | 2004-02-17 | 2008-12-18 | Optimer, Inc. | Compositions useful as fabric softener |
| US20070093408A1 (en) * | 2004-02-17 | 2007-04-26 | Moore John W | Compositions useful as fabric softeners |
| US20060150300A1 (en) * | 2005-01-12 | 2006-07-13 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip and method for in-line processing thereof |
| US7814570B2 (en) | 2005-01-12 | 2010-10-19 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip method for in-line processing thereof |
| US7378043B2 (en) * | 2005-01-12 | 2008-05-27 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip and method for in-line processing thereof |
| US20080244809A1 (en) * | 2005-01-12 | 2008-10-09 | Noorman Bin Abu Hassan | Latex Gloves and Articles with Geometrically Defined Surface Texture Providing Enhanced Grip Method for In-Line Processing Thereof |
| US8522363B2 (en) | 2005-01-12 | 2013-09-03 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip and method for in-line processing thereof |
| US20110088140A1 (en) * | 2005-01-12 | 2011-04-21 | Ansell Healthcare Products Llc | Latex gloves and articles with geometrically defined surface texture providing enhanced grip and method for in-line processing thereof |
| WO2007061954A3 (en) * | 2005-11-18 | 2007-11-15 | Univ Pittsburgh | Biocidal surfaces, articles with biocidal surface agents and methods of synthesizing and evaluating biocidal surface agents |
| US20070122441A1 (en) * | 2005-11-18 | 2007-05-31 | Hironobu Murata | Biocidal surfaces, articles with biocidal surface agents and methods of synthesizing and evaluating biocidal surface agents |
| WO2007061954A2 (en) * | 2005-11-18 | 2007-05-31 | University Of Pittsburgh | Biocidal surfaces, articles with biocidal surface agents and methods of synthesizing and evaluating biocidal surface agents |
| US20070151684A1 (en) * | 2005-12-29 | 2007-07-05 | Grigoriev Vladimir A | Creping adhesives comprising blends of polyaminoamide epihalolhydrin resins and polyamides |
| US8066847B2 (en) * | 2005-12-29 | 2011-11-29 | Nalco Corporation | Creping adhesives comprising blends of polyaminoamide epihalolhydrin resins and polyamides |
| US8008240B2 (en) * | 2007-09-17 | 2011-08-30 | The Procter & Gamble Company | Process for treating a hard surface using an EO/PO trisiloxane |
| US8334251B2 (en) | 2007-09-17 | 2012-12-18 | The Procter & Gamble Company | Process of treating a hard surface with a polyalkoxylated trisiloxane |
| US20090084402A1 (en) * | 2007-09-17 | 2009-04-02 | Christopher Andrew Morrison | Process for treating hard surface |
| US20140127276A1 (en) * | 2010-02-15 | 2014-05-08 | Philadelphia University | Methods for Reducing Airborne Bacteria and Mycetes and Apparatus for the Same |
| US20120046383A1 (en) * | 2010-08-19 | 2012-02-23 | Terumo Kabushiki Kaisha | Silicone rubber composition |
| US8846593B2 (en) * | 2012-04-25 | 2014-09-30 | Basf Se | Dishwashing composition comprising a covalently modified alkyleneimine polymer |
| US20130288941A1 (en) * | 2012-04-25 | 2013-10-31 | Basf Se | Formulations, their use as or for producing dishwashing compositions and their preparation |
| US9695292B2 (en) | 2013-11-26 | 2017-07-04 | Ansell Limited | Effervescent texturing |
| US10292440B2 (en) | 2015-03-10 | 2019-05-21 | Ansell Limited | Supported glove having an abrasion resistant nitrile coating |
| EP3275983A1 (en) * | 2016-07-25 | 2018-01-31 | Henkel AG & Co. KGaA | Polymers of vinylpyrrolidone and/or vinylacetate as textile care agents |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2580665C (en) | Fabric care compositions comprising polyol based fabric care materials and deposition agents | |
| US7893014B2 (en) | Fabric treatment for stain release | |
| US20050015888A1 (en) | Wrinkle resistant composition | |
| US20060276370A1 (en) | Fabric care compositions | |
| US20070256252A1 (en) | Fabric Treatment for Stain Release | |
| US6908962B1 (en) | Stable silicone oil emulsion composition, article of manufacture, and method of fabric wrinkle control | |
| EP1238137A2 (en) | Wrinkle resistant composition | |
| EP1096056A1 (en) | Wrinkle resistant composition | |
| EP0978556B1 (en) | Wrinkle resistant composition | |
| CA2330604C (en) | Wrinkle and malodour reducing composition | |
| AU3762399A (en) | Fabric wrinkle control composition and method | |
| US6573233B1 (en) | Wrinkle and malodour reducing composition | |
| US10577743B2 (en) | Laundry additive for providing antimicrobial effects to fabrics and interior surfaces of washing machine | |
| US20190233786A1 (en) | Dryer sheets comprising branched polyester polymers | |
| EP1204793B1 (en) | Stable silicone oil emulsion composition, article of manufacture, and method of fabric wrinkle control | |
| US20210115357A1 (en) | Fabric Treatment Method for Stain Release | |
| US6514932B1 (en) | Wrinkle resistant composition | |
| US6723253B2 (en) | Domestic treatment of fabrics with film-forming materials and blowing agents | |
| US10822577B2 (en) | Fabric treatment method for stain release | |
| US10900168B2 (en) | Fabric treatment for stain repellency | |
| MXPA01001329A (en) | Wrinkle resistant composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |