US20100107476A1 - Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives - Google Patents
Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives Download PDFInfo
- Publication number
- US20100107476A1 US20100107476A1 US12/262,420 US26242008A US2010107476A1 US 20100107476 A1 US20100107476 A1 US 20100107476A1 US 26242008 A US26242008 A US 26242008A US 2010107476 A1 US2010107476 A1 US 2010107476A1
- Authority
- US
- United States
- Prior art keywords
- fuel
- fuels
- hexahydro
- group
- triazine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- UYDVVRHVWYKIIW-UHFFFAOYSA-N C1=CC=C(CN2CN(CC3=CC=CC=C3)CN(CC3=CC=CC=C3)C2)C=C1.C1=CN(CCCN2CN(CCCN3C=CN=C3)CN(CCCN3C=CN=C3)C2)C=N1.C1CCN(CCCN2CN(CCCN3CCCCC3)CN(CCCN3CCCCC3)C2)CC1 Chemical compound C1=CC=C(CN2CN(CC3=CC=CC=C3)CN(CC3=CC=CC=C3)C2)C=C1.C1=CN(CCCN2CN(CCCN3C=CN=C3)CN(CCCN3C=CN=C3)C2)C=N1.C1CCN(CCCN2CN(CCCN3CCCCC3)CN(CCCN3CCCCC3)C2)CC1 UYDVVRHVWYKIIW-UHFFFAOYSA-N 0.000 description 5
- RTDJADAEDVQOMN-UHFFFAOYSA-N C1CCN(CCCN2CN(CCCN3CCCC3)CN(CCCN3CCCC3)C2)C1.CCCCCCN1CN(CCCCCC)CN(CCCCCC)C1.CCCCCCN1CN(CCCCCC)CN(CCN2CCNCC2)C1.CCCCN1CN(CCCC)CN(CCCC)C1 Chemical compound C1CCN(CCCN2CN(CCCN3CCCC3)CN(CCCN3CCCC3)C2)C1.CCCCCCN1CN(CCCCCC)CN(CCCCCC)C1.CCCCCCN1CN(CCCCCC)CN(CCN2CCNCC2)C1.CCCCN1CN(CCCC)CN(CCCC)C1 RTDJADAEDVQOMN-UHFFFAOYSA-N 0.000 description 5
- LOLUKZDTFFEREM-UHFFFAOYSA-N CC(C)N(CCN1CN(CCN(C(C)C)C(C)C)CN(CCN(C(C)C)C(C)C)C1)C(C)C.CCCCCCCCCCCCN1CN(CCCCCCCCCCCC)CN(CCCCCCCCCCCC)C1.CCCCCCCCN1CN(CCCCCCCC)CN(CCCCCCCC)C1 Chemical compound CC(C)N(CCN1CN(CCN(C(C)C)C(C)C)CN(CCN(C(C)C)C(C)C)C1)C(C)C.CCCCCCCCCCCCN1CN(CCCCCCCCCCCC)CN(CCCCCCCCCCCC)C1.CCCCCCCCN1CN(CCCCCCCC)CN(CCCCCCCC)C1 LOLUKZDTFFEREM-UHFFFAOYSA-N 0.000 description 5
- GAPDXYSKMGAHST-UHFFFAOYSA-N C1CCN(CCN2CN(CCN3CCOCC3)CN(CCN3CCOCC3)C2)CC1 Chemical compound C1CCN(CCN2CN(CCN3CCOCC3)CN(CCN3CCOCC3)C2)CC1 GAPDXYSKMGAHST-UHFFFAOYSA-N 0.000 description 4
- 0 CCCCCC*1CN(CCNCC*)C*(CCCCCC)C1 Chemical compound CCCCCC*1CN(CCNCC*)C*(CCCCCC)C1 0.000 description 4
- KWUXDHLHOGKCRG-UHFFFAOYSA-N CCCCCCCCN1CN(CCCCCCCC)CN(CC2=CC=CC=C2)C1.CCCCCCCCN1CN(CCCCCCCC)CN(CCN2CCOCC2)C1.CCCCCCN1CN(CCCCCC)CN(CCNCCO)C1 Chemical compound CCCCCCCCN1CN(CCCCCCCC)CN(CC2=CC=CC=C2)C1.CCCCCCCCN1CN(CCCCCCCC)CN(CCN2CCOCC2)C1.CCCCCCN1CN(CCCCCC)CN(CCNCCO)C1 KWUXDHLHOGKCRG-UHFFFAOYSA-N 0.000 description 4
- KFQQXPASYLXSKA-UHFFFAOYSA-N CCCCCCCCN1CN(CCN2CCOCC2)CN(CCCCCCCC)C1 Chemical compound CCCCCCCCN1CN(CCN2CCOCC2)CN(CCCCCCCC)C1 KFQQXPASYLXSKA-UHFFFAOYSA-N 0.000 description 2
- QDJPQWYQXYSMGX-UHFFFAOYSA-N C1CN(CCN2CN(CCN3CCNCC3)CN(CCN3CCNCC3)C2)CCN1.CCCCCCCCN1CN(CCCCCCCC)CN(CC(CC)CCCC)C1.CCCCCCCCN1CN(CCCN2C=CN=C2)CN(CCCN2C=CN=C2)C1 Chemical compound C1CN(CCN2CN(CCN3CCNCC3)CN(CCN3CCNCC3)C2)CCN1.CCCCCCCCN1CN(CCCCCCCC)CN(CC(CC)CCCC)C1.CCCCCCCCN1CN(CCCN2C=CN=C2)CN(CCCN2C=CN=C2)C1 QDJPQWYQXYSMGX-UHFFFAOYSA-N 0.000 description 1
- XIDQLVVZFFJREM-UHFFFAOYSA-N C1CN(CCN2CN(CCN3CCOCC3)CN(CCN3CCOCC3)C2)CCO1 Chemical compound C1CN(CCN2CN(CCN3CCOCC3)CN(CCN3CCOCC3)C2)CCO1 XIDQLVVZFFJREM-UHFFFAOYSA-N 0.000 description 1
- QYQFBXJJRHCTNI-UHFFFAOYSA-N CC(C)OCCCN1CN(CCCOC(C)C)CN(CCCOC(C)C)C1.CCCOCCCN1CN(CCCOCCC)CN(CCCOCCC)C1.OCCNCCN1CN(CCNCCO)CN(CCNCCO)C1 Chemical compound CC(C)OCCCN1CN(CCCOC(C)C)CN(CCCOC(C)C)C1.CCCOCCCN1CN(CCCOCCC)CN(CCCOCCC)C1.OCCNCCN1CN(CCNCCO)CN(CCNCCO)C1 QYQFBXJJRHCTNI-UHFFFAOYSA-N 0.000 description 1
- ZCBOVGMEYIEWLI-UHFFFAOYSA-N CCCCC(CC)CN1CN(CC(CC)CCCC)CN(CC(CC)CCCC)C1.COC(CN1CN(CC(OC)OC)CN(CC(OC)OC)C1)OC Chemical compound CCCCC(CC)CN1CN(CC(CC)CCCC)CN(CC(CC)CCCC)C1.COC(CN1CN(CC(OC)OC)CN(CC(OC)OC)C1)OC ZCBOVGMEYIEWLI-UHFFFAOYSA-N 0.000 description 1
- FRCXBHBOJMLHKY-UHFFFAOYSA-N CCCCCCN1CN(CCNCCO)CN(CCCCCC)C1 Chemical compound CCCCCCN1CN(CCNCCO)CN(CCCCCC)C1 FRCXBHBOJMLHKY-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/06—Use of additives to fuels or fires for particular purposes for facilitating soot removal
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/143—Organic compounds mixtures of organic macromolecular compounds with organic non-macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/232—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/232—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring
- C10L1/233—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring containing nitrogen and oxygen in the ring, e.g. oxazoles
- C10L1/2335—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring containing nitrogen and oxygen in the ring, e.g. oxazoles morpholino, and derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L3/00—Gaseous fuels; Natural gas; Synthetic natural gas obtained by processes not covered by subclass C10G, C10K; Liquefied petroleum gas
- C10L3/003—Additives for gaseous fuels
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/198—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
- C10L1/2383—Polyamines or polyimines, or derivatives thereof (poly)amines and imines; derivatives thereof (substituted by a macromolecular group containing 30C)
Definitions
- the disclosure is directed to certain fuel additives, fuel additive concentrates, and fuels that include a hexahydrotriazine.
- the disclosure is directed a fuel additive that is effective to reduce deposits in fuel injectors useful in diesel and/or gasoline direct injection engines.
- Dispersants are suitable for keeping soot and sludge suspended in a fluid, however dispersants are not particularly effective for cleaning surfaces once deposits have formed on the surfaces. Hence, fuel compositions that include dispersants often still produce undesirable deposits on diesel engine injectors. Accordingly, improved compositions that can prevent deposit build up, maintaining “as new” cleanliness for the vehicle life are desired. Ideally, the same composition that can clean up dirty fuel injectors restoring performance to the previous “as new” condition would be equally desirable and valuable in the attempt to reduce air borne exhaust emissions.
- exemplary embodiments provide a fuel, a fuel additive concentrate, a fuel soluble deposit control additive, and a method for improving the performance of fuel injectors for a direct injection engine.
- the additive comprises at least one hexahydrotriazine.
- a fuel suitable for use in a fuel injection engine may comprise a major amount of a fuel, and a minor amount of an additive concentrate comprising a hexahydro triazine.
- an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring. In some embodiments, the alkyl group is linear. In some embodiments, the alkyl group is branched at a position after the beta position to the at least one nitrogen.
- the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
- the hexahydro triazine has a molecular weight of from about 100 to about 700. In some embodiments, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
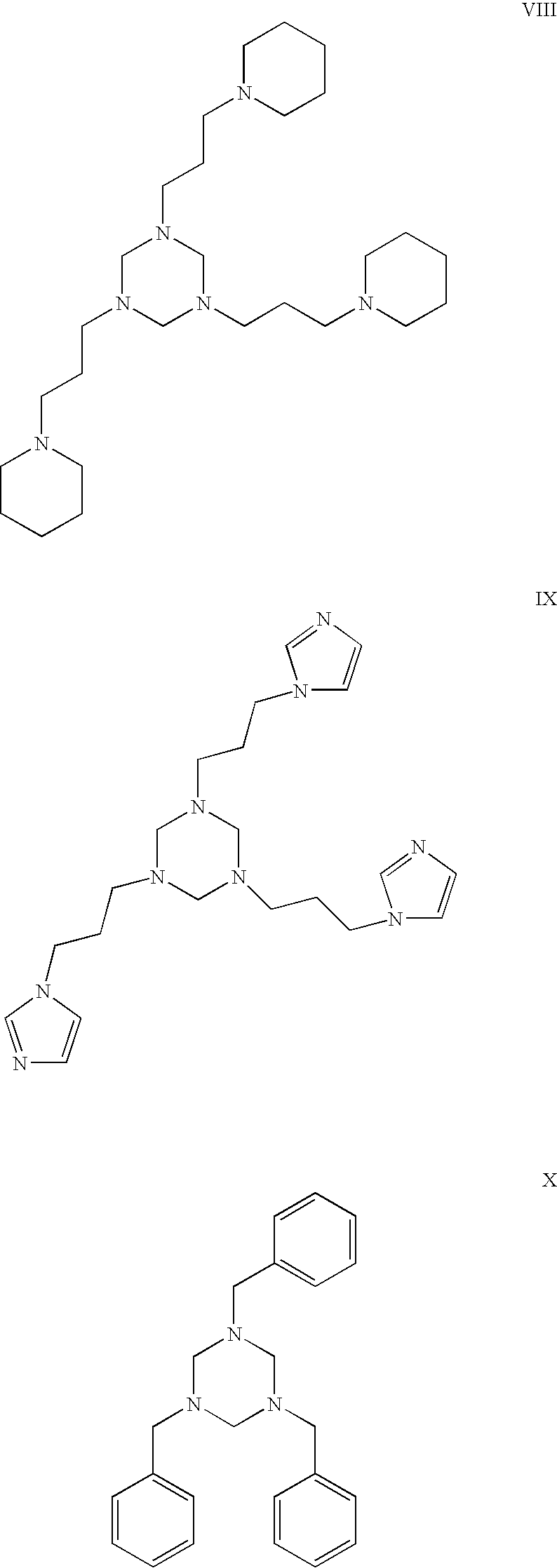
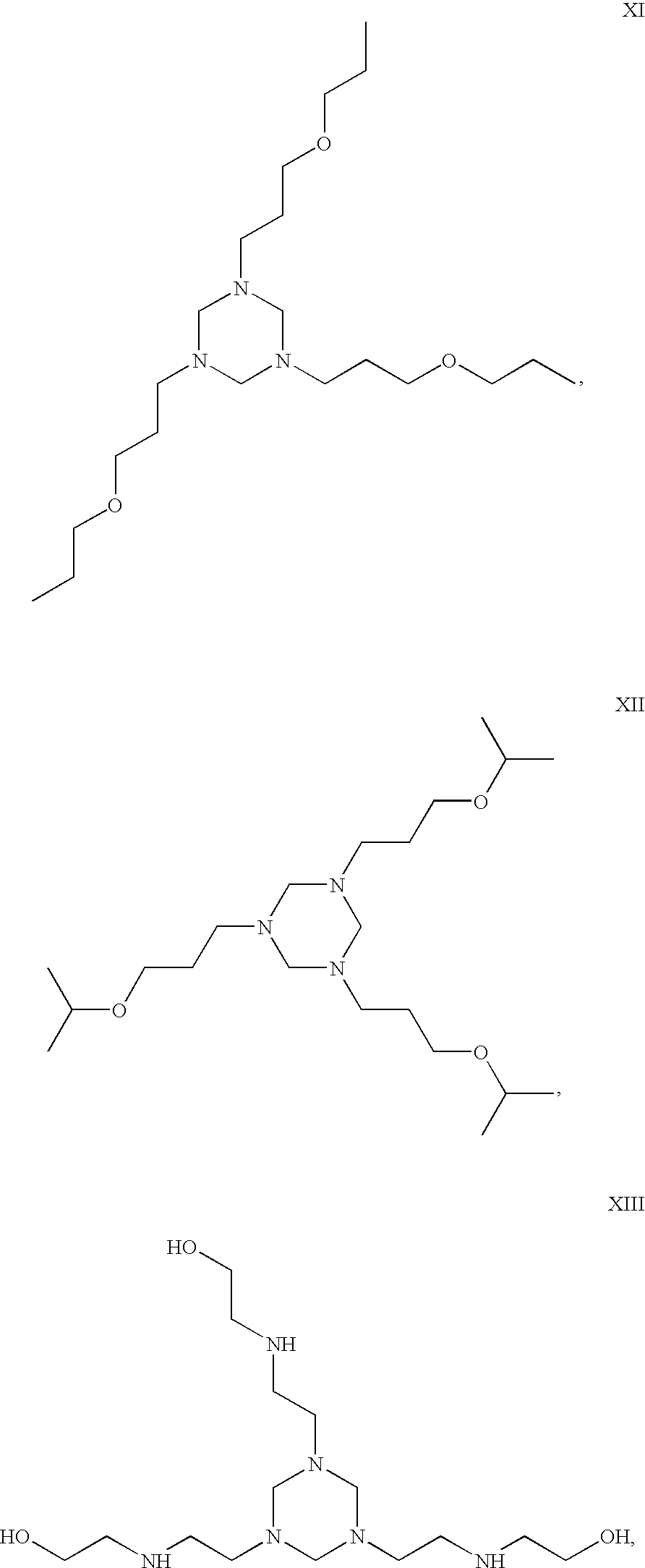
- the hexahydro triazine is selected from the group consisting of at least one of the following structures:
- the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
- the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm. In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
- the additive concentrate further comprises a dispersant/detergent.
- the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, a polyisobutylene amine dispersant, or a Mannich base dispersant.
- the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no.
- middle distillate fuels diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no.
- low sulfur fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- synthetic fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- a method for removing and/or preventing the occurrence of deposits in a fuel injector of a fuel injection engine may comprise adding to and operating in an injection engine having fuel injectors a fuel composition comprising (a) a major amount of fuel and (b) a minor amount of an additive concentrate comprising a hexahydro triazine.
- an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring.
- the alkyl group is linear.
- the alkyl group is branched at a position after the beta position to the at least one nitrogen.
- the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
- the hexahydro triazine has a molecular weight of from about 100 to about 700. In some embodiments, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
- the hexahydro triazine is selected from the group consisting of at least one of the structures I to XX, disclosed herein.
- the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
- the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm. In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
- the additive concentrate further comprises a dispersant/detergent.
- the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, polyisobutylene amine dispersant, or a Mannich base dispersant.
- the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no.
- middle distillate fuels diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no.
- low sulfur fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- synthetic fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- the injection engine comprises a direct injection engine.
- a fuel additive concentrate for removing and/or preventing the occurrence of deposits in a fuel injector of an injection engine may comprise a hexahydro triazine.
- the hexahydro-triazine is a member of the group consisting of octyl hexahydro triazine and dodecylamine hexahydro triazine.
- the hexahydro-triazine is selected from the group consisting of at least one of the structures I to XX, disclosed herein.
- injection engine is intended to describe any injection engine, including but not limited to injection engines suitable for use with diesel- and/or gasoline-based fuels.
- the injection may be by throttle body injection, central fuel injection, single-point injection, continuous injection, central port injection, multi-point injection, or direct injection.
- fuel injectors are intended to describe the part of an injection engine comprising a nozzle and a valve.
- fuel injector deposit is intended to describe a hydrocarbon deposit formation, such as coke.
- the term “unhindered” is used in the ordinary sense and is understood by one of skill in the art.
- “unhindered” may be used to describe a lack of steric hindrance.
- at least one nitrogen in the hexahydro triazine ring has an alkyl chain with no branching until at least after the beta position to the ringed nitrogen.
- the alkyl chain is linear.
- fuel soluble means that the substance under discussion should be sufficiently soluble at 20° C. in the base fuel selected for use to reach at least the minimum concentration required to enable the substance to serve its intended function. However, the substance need not dissolve in the fuel in all proportions.
- hydrocarbyl group or “hydrocarbyl” is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of a molecule and having a predominantly hydrocarbon character. Examples of hydrocarbyl groups include:
- hydrocarbon substituents that is, aliphatic (e.g., alkyl or alkenyl), alicyclic (e.g., cycloalkyl, cycloalkenyl) substituents, and aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents, as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form an alicyclic radical);
- aliphatic e.g., alkyl or alkenyl
- alicyclic e.g., cycloalkyl, cycloalkenyl
- aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form an alicyclic radical);
- substituted hydrocarbon substituents that is, substituents containing non-hydrocarbon groups which, in the context of the description herein, do not alter the predominantly hydrocarbon substituent (e.g., halo (especially chloro and fluoro), hydroxy, alkoxy, mercapto, alkylmercapto, nitro, nitroso, and sulfoxy);
- hetero-substituents that is, substituents which, while having a predominantly hydrocarbon character, in the context of this description, contain other than carbon in a ring or chain otherwise composed of carbon atoms.
- Hetero-atoms include sulfur, oxygen, nitrogen, and encompass substituents such as pyridyl, furyl, thienyl, and imidazolyl.
- substituents such as pyridyl, furyl, thienyl, and imidazolyl.
- no more than two, or as a further example, no more than one, non-hydrocarbon substituent will be present for every ten carbon atoms in the hydrocarbyl group; in some embodiments, there will be no non-hydrocarbon substituent in the hydrocarbyl group.
- the term “major amount” is understood to mean an amount greater than or equal to 50 wt. %, for example from about 80 to about 98 wt. % relative to the total weight of the composition. Moreover, as used herein, the term “minor amount” is understood to mean an amount less than 50 wt. % relative to the total weight of the composition.
- substantially free of is intended to mean a trace amount or an amount that does not affect performance or a method as described herein.
- the additive may not only reduce the amount of deposits forming on fuel injectors, but the additive may also be effective to clean up dirty fuel injectors.
- the deposit reduction and cleaning effect of the additive is demonstrated in the present Examples. Further the fuel additive is not water soluble and is chemically stable to aqueous media.
- dispersant/detergent is used to describe a dispersant or a detergent, for example, but not limited to, those particular dispersants/detergents described below. Additional embodiments and advantages of the disclosure will be set forth in part in the detailed description which follows, and/or can be learned by practice of the disclosure. It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive of the disclosure, as claimed.
- the fuel compositions of the present application may be suitable for use in a fuel injection engine.
- the fuel composition may comprise a major amount of a fuel and a minor amount of an additive concentrate comprising a hexahydro triazine, wherein the hexahydro triazine is present in an amount sufficient to remove and/or reduce the occurrence of deposits in a fuel injector of an injection engine.
- Suitable hexahydro triazines may be oil soluble and thermally stable.
- the hexahydro triazine ring may be the reaction product of or the product of combining an alkylamine and formaldehyde or a mixture of amines and formaldehyde.
- Suitable amines may comprise any primary amines.
- the hexahydro triazine may, but is not limited to, be alkylated or alkoxylated. At least one nitrogen on the hexahydro triazine ring may be unhindered.
- the hexahydro triazine ring may be saturated.
- Suitable hexahydro triazines include but are not limited to octyl hexahydro triazine and dodecyl hexahydro triazine.
- a triazine suitable for use in present embodiments is free of or substantially free of triazines made from diamines.
- Embodiments may include, but are not limited to the following structures:
- Suitable hexahydro triazines may be unhindered.
- hexahydro triazines may lack steric hindrance.
- an alkyl group attached to a ringed nitrogen may be branched at a position after the beta position to the ringed nitrogen. In the Comparative Structures I and 11, below, there is branching at the beta position to at least one ringed nitrogen.
- the hexahydro triazine has a molecular weight of from about 100 to about 700. In another embodiment, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- the hexahydro triazine additive components may be added to a fuel composition in an amount sufficient to provide control, including removal or prevention of, deposits.
- the hexahydro triazine additive components may be added to a fuel in proportions effective to reduce the volume of injector deposits in a direct injection gasoline engine operated on said fuel containing said hexahydro triazine additive components to below the volume of injector deposits in said engine operated in the same manner on the same fuel except that it is devoid of said hexahydro triazine additive components.
- one or more hexahydro triazine additive component(s) may be present in a fuel in an amount from about 1 ppm to about 100 ppm. In other embodiments, one or more hexahydro triazine additive component(s) may be present in a fuel in an amount from about 5 ppm to about 20 ppm.
- the fuels may contain conventional quantities of cetane improvers, corrosion inhibitors, cold flow improvers (CFPP additive), pour point depressants, solvents, demulsifiers, emulsifiers, lubricity additives, friction modifiers, amine stabilizers, combustion improvers, dispersant/detergents, antioxidants, heat stabilizers, conductivity improvers, metal deactivators, markers, dyes, organic nitrate ignition accelerators, cyclomatic manganese tricarbonyl compounds, carrier fluids, biocides, antistatic additives, drag reducing agents, dehazers, anti-icing additives, antiknock additives, anti-valve-seat recession additives, surfactants, and the like.
- CFPP additive cold flow improvers
- pour point depressants solvents
- demulsifiers emulsifiers
- lubricity additives lubricity additives
- friction modifiers amine stabilizers
- combustion improvers dispersant/detergents
- compositions described herein may contain about 10 weight percent or less, or in other aspects, about 5 weight percent or less, based on the total weight of the additive concentrate, of one or more of the above additives.
- the fuels may contain suitable amounts of conventional fuel blending components such as methanol, ethanol, dialkyl ethers, and the like.
- the fuel additive concentrate may comprise a dispersant/detergent.
- the dispersant/detergent may comprise an ashless dispersant, a metal-containing dispersant, or a Mannich dispersant.
- a suitable dispersant/detergent may include at least one oil-soluble ashless dispersant having a basic nitrogen and/or at least one hydroxyl group in the molecule.
- Other suitable dispersants/detergents include alkenyl succinimides, alkenyl succinic acid esters, alkenyl succinic ester-amides, and Mannich bases.
- suitable dispersants/detergents may comprise, but are not limited to ethylene diamine or dibutyl amine Mannich base dispersants.
- a suitable dispersant may have a molecular weight of from about 750 to about 3000.
- Suitable amine detergents include those well known in the art for use in fuels for MPI engines to control intake valve deposits.
- Suitable amine detergents include nitrogen-containing derivatives of hydrocarbyl succinic acylating agents, Mannich condensation products, hydrocarbyl amines and polyetheramines. When used, the amine detergents are typically present in an amount sufficient to control intake valve deposits and are typically present in an amount of from about 5 to about 100 pounds by weight of additive per thousand barrels by volume of fuel.
- the nitrogen-containing derivatives of hydrocarbyl succinic acylating agents suitable for use in the present embodiments may include hydrocarbyl succinimides, succinamides, succinimide-amides and succinimide-esters.
- the nitrogen-containing derivatives of hydrocarbyl succinic acylating agents are typically prepared by reacting a hydrocarbyl-substituted succinic acylating agent with a polyamine.
- the hydrocarbyl-substituted succinic acylating agents include the hydrocarbyl-substituted succinic acids, the hydrocarbyl-substituted succinic anhydrides, the hydrocarbyl-substituted succinic acid halides (especially the acid fluorides and acid chlorides), the esters of the hydrocarbyl-substituted succinic acids and lower alcohols (e.g., those containing up to 7 carbon atoms), that is, hydrocarbyl-substituted compounds which can function as carboxylic acylating agents, and mixtures of hydrocarbyl-substituted succinic acids and hydrocarbyl-substituted succinic anhydrides.
- Amines which may be reacted with the alkenyl succinic anhydride to form the hydrocarbyl-succinimide include any that have at least one primary amine group that can react to form an imide group.
- a few representative examples are: methylamine, 2-ethylhexylamine, n-dodecylamine, stearylamine, N,N-dimethyl-propanediamine, N-(3aminopropyl)morpholine, N-dodecyl propanediamine, N-aminopropyl piperazine ethanolamine, N-ethanol ethylene diamine and the like.
- Suitable amines include the alkylene polyamines such as propylene diamine, dipropylene triamine, di-(1,2-butylene)triamine, tetra-(1,2-propylene)pentaamine.
- ethylene polyamines which have the formula H 2 N(CH 2 CH 2 NH) n H wherein n is an integer from one to ten.
- ethylene polyamines include ethylene diamine, diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, pentaethylene hexaamine, and the like, including mixtures thereof in which case n is the average value of the mixture.
- ethylene polyamines have a primary amine group at each end so can form mono-alkenylsuccinimides and bis-alkenylsuccinimides.
- suitable hydrocarbyl succinimides may include the products of reaction of a polyethylenepolyamine, e.g.
- a hydrocarbon substituted carboxylic acid or anhydride made by reaction of a polyolefin, for example polyisobutene, having a molecular weight of 500 to 2,000, especially 700 to 1500, with an unsaturated polycarboxylic acid or anhydride, e.g. maleic anhydride.
- Suitable Mannich base detergents include the reaction products of a high molecular weight alkyl-substituted hydroxyaromatic compound, aldehydes, and amines.
- the alkyl-substituted hydroxyaromatic compound, aldehydes, and amines used in making the Mannich reaction products may be any suitable such compounds.
- the high molecular weight alkyl substituents on the benzene ring of the hydroxyaromatic compound may be derived from polyolefin having a number average molecular weight (M n ) of from about 500 to about 3000, or from about 700 to about 2100, as determined by gel permeation chromatography (GPC).
- M n number average molecular weight
- the polyolefin may have a polydispersity (weight average molecular weight/number average molecular weight) in the range of about 1 to about 4 (for example from about 1 to about 2) as determined by GPC.
- the alkylation of the hydroxyaromatic compound is typically performed in the presence of an alkylating catalyst at a temperature in the range of about 0 to about 200° C., for example from 0 to 100° C.
- Acidic catalysts are generally used to promote Friedel-Crafts alkylation.
- Typical catalysts used in commercial production include sulphuric acid, BF 3 , aluminum phenoxide, methanesulphonic acid, cationic exchange resin, acidic clays, and modified zeolites.
- Polyolefins suitable for forming the high molecular weight alkyl-substituted hydroxyaromatic compounds include polypropylene, polybutenes, polyisobutylene, copolymers of butylene and/or butylene and propylene, copolymers of butylene and/or isobutylene and/or propylene, and one or more mono-olefinic comonomers copolymerizable therewith (e.g., ethylene, 1-pentene, 1-hexene, 1-octene, 1-decene, etc.) where the copolymer molecule contains at least 50% by weight, of butylene and/or isobutylene and/or propylene units.
- mono-olefinic comonomers e.g., ethylene, 1-pentene, 1-hexene, 1-octene, 1-decene, etc.
- the comonomers polymerized with propylene or such butenes may be aliphatic and can also contain non-aliphatic groups, e.g., styrene, o-methylstyrene, p-methylstyrene, divinyl benzene, and the like.
- non-aliphatic groups e.g., styrene, o-methylstyrene, p-methylstyrene, divinyl benzene, and the like.
- the resulting polymers and copolymers used in forming the high molecular weight alkyl-substituted hydroxyaromatic compounds are substantially aliphatic hydrocarbon polymers.
- polybutylene is used in a generic sense to include polymers made from “pure” or “substantially pure” 1-butene or isobutene, and polymers made from mixtures of two or all three of 1-butene, 2-butene, and isobutene. Commercial grades of such polymers may also contain insignificant amounts of other olefins. So-called high reactivity polyisobutenes having relatively high proportions of polymer molecules having a terminal vinylidene group are also suitable for use in forming the long chain alkylated phenol reactant.
- Suitable high-reactivity polyisobutenes include those polyisobutenes that comprise at least about 20% of the more reactive methylvinylidene isomer, for example at least 50%, and as a further example at least 70%.
- Suitable polyisobutenes include those prepared using BF 3 catalysts. The preparation of such polyisobutenes in which the methylvinylidene isomer comprises a high percentage of the total composition is described in U.S. Pat. Nos. 4,152,499 and 4,605,808.
- the Mannich detergent may be made from a high molecular weight alkylphenol or alkylcresol.
- other phenolic compounds may be used including high molecular weight alkyl-substituted derivatives of resorcinol, hydroquinone, catechol, hydroxydiphenyl, benzylphenol, phenethylphenol, naphthol, tolylnaphthol, among others.
- the polyalkylphenol and polyalkylcresol reactants e.g., polypropylphenol, polybutylphenol, polypropylcresol and polybutylcresol, wherein the alkyl group has a number average molecular weight of about 500 to about 2100, and as another example, the alkyl group is a polybutyl group derived from polyisobutylene having a number average molecular weight in the range of about 700 to about 1300.
- a suitable configuration of the high molecular weight alkyl-substituted hydroxyaromatic compound is that of a para-substituted mono-alkylphenol or a para-substituted mono-alkyl ortho-cresol.
- any hydroxyaromatic compound readily reactive in the Mannich condensation reaction may be employed.
- Mannich products made from hydroxyaromatic compounds having only one ring alkyl substituent, or two or more ring alkyl substituents are suitable.
- the long chain alkyl substituents may contain some residual unsaturation, but in general, are substantially saturated alkyl groups.
- Representative amine reactants include, but are not limited to, alkylene polyamines having at least one suitably reactive primary or secondary amino group in the molecule. Other substituents such as hydroxyl, cyano, amido, etc., can be present in the polyamine.
- the alkylene polyamine is a polyethylene polyamine.
- Suitable alkylene polyamine reactants include ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, and mixtures of such amines having nitrogen contents corresponding to alkylene polyamines of the formula H 2 N-(A-NH—) n H, where A is divalent ethylene or propylene and n is an integer of from I to 10, or, as another example, from 1 to 4.
- the alkylene polyamines may be obtained by the reaction of ammonia and dihalo alkanes, such as dichloro alkanes.
- the amine may also be an aliphatic diamine having one primary or secondary amino group and at least one tertiary amino group in the molecule.
- suitable polyamines include N,N,N′′,N′′-tetraalkyldialkylenetriamines (two terminal tertiary amino groups and one central secondary amino group), N,N,N′,N′′-tetraalkyltrialkylenetetramines (one terminal tertiary amino group, two internal tertiary amino groups and one terminal primary amino group), N,N,N′,N′′,N′′′-pentaalkyltrialkylenetetramines (one terminal tertiary amino group, two internal tertiary amino groups and one terminal secondary amino group), N,N-dihydroxyalkyl-alpha, omega-alkylenediamines (one terminal tertiary amino group and one terminal primary amino group), N,N,N′-trihydroxyalkyl-alpha, omega-alkylenediamine
- alkyl groups may be methyl and/or ethyl groups.
- Suitable polyamine reactants are N,N-dialkyl-alpha, omega-alkylenediamine, such as those having from 3 to about 6 carbon atoms in the alkylene group and from 1 to about 12 carbon atoms in each of the alkyl groups, which may be the same but which can be different.
- Suitable examples include N,N-dimethyl-1,3-propanediamine and N-methyl piperazine.
- polyamines having one reactive primary or secondary amino group that can participate in the Mannich condensation reaction, and at least one sterically hindered amino group that cannot participate directly in the Mannich condensation reaction to any appreciable extent include N-(tert-butyl)-1,3-propanediamine, N-neopentyl-1,3-propanediamine, N-(tert-butyl)-1-methyl-1,2-ethanediamine, N-(tert-butyl)-1-methyl-1,3-propanediamine, and 3,5-di(tert-butyl)aminoethylpiperazine.
- aldehydes for use in the preparation of the Mannich base products include the aliphatic aldehydes such as formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, valeraldehyde, caproaldehyde, heptaldehyde, stearaldehyde.
- Aromatic aldehydes which may be used include benzaldehyde and salicylaldehyde.
- Illustrative heterocyclic aldehydes for use herein are furfural and thiophene aldehyde, etc.
- formaldehyde-producing reagents such as paraformaldehyde, or aqueous formaldehyde solutions such as formalin.
- Suitable Mannich base detergents may include those detergents taught in U.S. Pat. Nos. 4,231,759; 5,514,190; 5,634,951; 5,697,988; 5,725,612; and 5,876,468, the disclosures of which are incorporated herein by reference.
- the additive concentrates may comprise a fuel soluble carrier.
- Such carriers may be of various types, such as liquids or solids, e.g., waxes.
- liquid carriers include, but are not limited to, mineral oil and oxygenates, such as liquid polyalkoxylated ethers (also known as polyalkylene glycols or polyalkylene ethers), liquid polyalkoxylated phenols, liquid polyalkoxylated esters, liquid polyalkoxylated amines, and mixtures thereof.
- oxygenate carriers may be found in U.S. Pat. No. 5,752,989, issued May 19, 1998 to Henly et. al., the description of which carriers is herein incorporated by reference in its entirety.
- oxygenate carriers include alkyl-substituted aryl polyalkoxylates described in U.S. Patent Publication No. 2003/0131527, published Jul. 17, 2003 to Colucci et. al., the description of which is herein incorporated by reference in its entirety.
- Suitable carriers can be of various types, such as for example liquid poly-alpha-olefin oligomers, mineral oils, liquid poly(oxyalkylene) compounds, liquid alcohols or polyols, polyalkenes, liquid esters, and similar liquid carriers. Mixtures of two or more such carriers can be employed.
- Suitable liquid carriers may include 1) a mineral oil or a blend of mineral oils that have a viscosity index of less than about 120, 2) one or more poly-alpha-olefin oligomers, 3) one or more poly(oxyalkylene) compounds having an average molecular weight in the range of about 500 to about 3000, 4) polyalkenes, 5) polyalkyl-substituted hydroxyaromatic compounds, or 6) mixtures thereof.
- the mineral oil carrier fluids that can be used include paraffinic, naphthenic, and asphaltic oils, and can be derived from various petroleum crude oils and processed in any suitable manner.
- the mineral oils may be solvent extracted or hydrotreated oils. Reclaimed mineral oils can also be used.
- the mineral oil used may have a viscosity at 40° C. of less than about 1600 SUS, and as another example between about 300 and 1500 SUS at 40° C.
- Paraffinic mineral oils may have viscosities at 40° C. in the range of about 475 SUS to about 700 SUS.
- the mineral oil may have a viscosity index of less than about 100, as a further example, less than about 70, and, as an even further example, in the range of from about 30 to about 60.
- the ratio (wt/wt) of detergent to carrier fluid(s) is typically in the range of from 1:0.1 to 1:3.
- compositions of the present application may not contain a carrier.
- some compositions of the present application may not contain mineral oil or oxygenates, such as those oxygenates described above.
- organic nitrate ignition accelerators that include aliphatic or cycloaliphatic nitrates in which the aliphatic or cycloaliphatic group is saturated, and that contain up to about 12 carbons may be used.
- organic nitrate ignition accelerators examples include methyl nitrate, ethyl nitrate, propyl nitrate, isopropyl nitrate, allyl nitrate, butyl nitrate, isobutyl nitrate, sec-butyl nitrate, tert-butyl nitrate, amyl nitrate, isoamyl nitrate, 2-amyl nitrate, 3-amyl nitrate, hexyl nitrate, heptyl nitrate, 2-heptyl nitrate, octyl nitrate, isooctyl nitrate, 2-ethylhexyl nitrate, nonyl nitrate, decyl nitrate, undecyl nitrate, dodecyl nitrate, cyclopentyl nitrate, cyclohexyl
- metal deactivators useful in the compositions of the present application are disclosed in U.S. Pat. No. 4,482,357, issued Nov. 13, 1984, the disclosure of which is herein incorporated by reference in its entirety.
- metal deactivators include, for example, salicylideneaminophenol, disalicylidene ethylenediamine, disalicylidene propylenediamine, and N,N′-disalicylidene-1,2-diaminopropane.
- Suitable optional cyclomatic manganese tricarbonyl compounds which may be employed in the compositions of the present application include, for example, cyclopentadienyl manganese tricarbonyl, methylcyclopentadienyl manganese tricarbonyl, indenyl manganese tricarbonyl, and ethylcyclopentadienyl manganese tricarbonyl.
- suitable cyclomatic manganese tricarbonyl compounds are disclosed in U.S. Pat. No. 5,575,823, issued Nov. 19, 1996, and U.S. Pat. No. 3,015,668, issued Jan. 2, 1962, both of which disclosures are herein incorporated by reference in their entirety.
- the base fuels suitable for use in formulating the fuel compositions of embodiments described herein may include any base fuels suitable for use in the operation of direct injection engine such as leaded or unleaded motor gasolines, and so-called reformulated gasolines which typically contain both hydrocarbons of the gasoline boiling range and fuel-soluble oxygenated blending agents (“oxygenates”), such as alcohols, ethers, and other suitable oxygen-containing organic compounds.
- the fuel may be a mixture of hydrocarbons boiling in the gasoline boiling range.
- the fuel may consist of straight chain or branch chain paraffins, cycloparaffins, olefins, aromatic hydrocarbons, or any mixture of these.
- the fuel may be a gasoline derived from straight run naphtha, polymer gasoline, natural gasoline or from catalytically reformed stocks boiling in the range from about 80 to about 450° F.
- the octane level of the gasoline is not critical and any conventional gasoline may be employed.
- the diesel fuel may be applicable to the operation of both stationary diesel engines (e.g., engines used in electrical power generation installations, in pumping stations, etc.) and ambulatory diesel engines (e.g., engines used as prime movers in automobiles, trucks, road-grading equipment, military vehicles, etc.).
- Suitable fuels may include any known hydrocarbon fuel or mixture thereof. Suitable fuels include, but are not limited to, any and all middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no.
- GTL gas-to-liquid
- low sulfur fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- synthetic fuels such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- Biorenewable fuels as used herein is understood to mean any fuel which is derived from resources other than petroleum.
- the biorenewable fuel can comprise monohydroxy alcohols, such as those comprising from 1 to about 5 carbon atoms.
- suitable monohydroxy alcohols include methanol, ethanol, bioethanol, biobutanol, propanol, n-butanol, isobutanol, t-butyl alcohol, amyl alcohol, and isoamyl alcohol.
- Suitable oxygenates include methanol, ethanol, isopropanol, t-butanol, mixed C1 to C5 alcohols, methyl tertiary butyl ether, tertiary amyl methyl ether, ethyl tertiary butyl ether, and mixed ethers.
- Oxygenates when used, will normally be present in the base fuel in an amount below about 30% by volume, and in an amount that provides an oxygen content in the overall fuel in the range of about 0.5 to about 5 percent by volume.
- the additives may be employed in amounts sufficient to remove, reduce, inhibit, and/or prevent deposit formation in an injection engine.
- the fuels may contain minor amounts of the above described hexahydro triazine compound that removes, reduces, inhibits, or prevents the formation of engine deposits, for example injector deposits.
- the fuels of this application may contain, on an active ingredient basis, an amount of the hexahydro triazine compound in the range of about 1 ppm to about 100 ppm in a fuel.
- a fuel may contain from about 5 ppm to about 20 ppm of the hexahydro triazine.
- the additives of the present application may be blended into the base fuel individually or in various sub-combinations.
- the additive components of the present application may be blended into the fuel concurrently using an additive concentrate, as this takes advantage of the mutual compatibility and convenience afforded by the combination of ingredients when in the form of an additive concentrate. Also, use of a concentrate may reduce blending time and lessen the possibility of blending errors.
- the hexahydrotriazine may be combined with one or more additives, such as a dispersant, to form a top treat.
- the top treat may be added to an additive concentrate, which is then added to a fuel. Alternatively, the top treat may be added directly to a fuel that either does or does not contain a separate additive concentrate.
- the top treat or after-market additive may be added to a fuel in the vehicle or to a fuel storage facility.
- aspects of the present application are directed to methods for removing, reducing, inhibiting, and/or preventing the occurrence of and/or amount of injector deposits in an injection engine.
- the improvements may also be observed in direct fuel injectors.
- the methods comprise injecting a hydrocarbon-based compression ignition fuel comprising the hexahydro triazine compound additive of the present application, through the injectors of the engine.
- the method may also comprise mixing into the fuel at least one of the optional additional ingredients described above.
- the additive concentrate may be provided as a top treat, or after-market additive package, that may be added to a fuel in the vehicle, at a fuel storage facility, to a fuel storage receptacle, or the like.
- Inventive and comparative samples were prepared and tested to demonstrate the effectiveness of the inventive additive concentrations in reducing deposits in injection engines. Tests were conducted in a Mitsubishi 4G93 GDI engine. The vehicle engine specifications are as follows:
- Valve train DOHC 4 valves (2 intake and 2 exhaust valves) per cylinder
- test procedure “The Evaluation of Deposits in a Direct Injection Gasoline Engine,” developed by CEC, OACIS, and CRC. Each cycle lasted about 100 minutes, and each test had 30 cycles. A complete test takes about 2 days. Injectors are cleaned and re-used after each test. All test conditions are defined by this procedure except the test cycle. A Quad-4 cycle was used in the disclosed tests.
- the treat rate of the additive package and added component(s) is given in PPM (weight based) added to the base fuel.
- the treat rate (PPM, wt) of the chemical structure or comparative structure is used in addition to the treat rate of the additive package.
- the chemical structure numerical reference is the same numerical reference disclosed herein.
- the plugging average (%) is a measurement of injector flow loss compared to that of a new, unused (and, consequently, unplugged) injector. It is provided as an average of 4 injectors plugged due to deposit during the test.
- Test 1 The data in the following table illustrates the advantage in performance of the inventive examples (Tests 3 and 5) versus unadditized fuel (Test 1), versus fuel containing a dispersant package (Test 2), and versus a comparative triazine (Test 4).
- a base fuel without any additive caused injector plugging at an average of 7.5%.
- a fluid containing only an additive package at 292 ppm (wt) resulted in injector plugging of 9%, as shown in Test 2.
- injector plugging dropped to 0.75% and 0.24%, as shown in Tests 3 and 5, respectively.
- a comparative chemical structure added to the fuel at the same treat rate led to injector plugging of 1.93%, which was less effective than the presently disclosed chemical structures.
- the comparative example demonstrates less than desirable performance when relatively hindered triazines, with branching only one carbon away from the ring-nitrogen, are used. That feature appears to negatively impact performance, as the nitrogen of the ring is not exposed and therefore less available to keep clean or clean injectors. Further, it seems that the hindrance of the triazine ring overrides the polarity effect.
- a dispersant includes one, two, or more different dispersants.
- the term “include” and its grammatical variants are intended to be non-limiting, such that recitation of items in a list is not to the exclusion of other like items that can be substituted or added to the listed items.
Abstract
A fuel, fuel additive concentrate, and method for removing and/or preventing the occurrence of deposits in a fuel injector of an injection engine are provided. The additive concentrate contains at least one hexahydro triazine.
Description
- The disclosure is directed to certain fuel additives, fuel additive concentrates, and fuels that include a hexahydrotriazine. In particular the disclosure is directed a fuel additive that is effective to reduce deposits in fuel injectors useful in diesel and/or gasoline direct injection engines.
- It has long been desired to maximize fuel economy, power, and drivability of vehicles while enhancing acceleration, reducing emissions, and preventing hesitation. While it is known to enhance engine performance by employing dispersants to keep valves and fuel injectors clean, such dispersants may be only minimally effective.
- Dispersants are suitable for keeping soot and sludge suspended in a fluid, however dispersants are not particularly effective for cleaning surfaces once deposits have formed on the surfaces. Hence, fuel compositions that include dispersants often still produce undesirable deposits on diesel engine injectors. Accordingly, improved compositions that can prevent deposit build up, maintaining “as new” cleanliness for the vehicle life are desired. Ideally, the same composition that can clean up dirty fuel injectors restoring performance to the previous “as new” condition would be equally desirable and valuable in the attempt to reduce air borne exhaust emissions.
- In accordance with the disclosure, exemplary embodiments provide a fuel, a fuel additive concentrate, a fuel soluble deposit control additive, and a method for improving the performance of fuel injectors for a direct injection engine. The additive comprises at least one hexahydrotriazine.
- In an embodiment, a fuel suitable for use in a fuel injection engine may comprise a major amount of a fuel, and a minor amount of an additive concentrate comprising a hexahydro triazine.
- In some embodiments, an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring. In some embodiments, the alkyl group is linear. In some embodiments, the alkyl group is branched at a position after the beta position to the at least one nitrogen.
- In some embodiments, the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
- In some embodiments, the hexahydro triazine has a molecular weight of from about 100 to about 700. In some embodiments, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- In some embodiments, the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
- In some embodiments, the hexahydro triazine is selected from the group consisting of at least one of the following structures:
- In some embodiments, the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
- In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm. In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
- In some embodiments, the additive concentrate further comprises a dispersant/detergent.
- In some embodiments, the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, a polyisobutylene amine dispersant, or a Mannich base dispersant.
- In some embodiments, the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no. 6), low sulfur fuels, synthetic fuels, such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- In another embodiment, a method for removing and/or preventing the occurrence of deposits in a fuel injector of a fuel injection engine may comprise adding to and operating in an injection engine having fuel injectors a fuel composition comprising (a) a major amount of fuel and (b) a minor amount of an additive concentrate comprising a hexahydro triazine.
- In some embodiments, an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring.
- In some embodiments, the alkyl group is linear.
- In some embodiments, the alkyl group is branched at a position after the beta position to the at least one nitrogen.
- In some embodiments, the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
- In some embodiments, the hexahydro triazine has a molecular weight of from about 100 to about 700. In some embodiments, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- In some embodiments, the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
- In some embodiments, the hexahydro triazine is selected from the group consisting of at least one of the structures I to XX, disclosed herein.
- In some embodiments, the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
- In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm. In some embodiments, the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
- In some embodiments, the additive concentrate further comprises a dispersant/detergent.
- In some embodiments, the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, polyisobutylene amine dispersant, or a Mannich base dispersant.
- In some embodiments, the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no. 6), low sulfur fuels, synthetic fuels, such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
- In some embodiments, the injection engine comprises a direct injection engine.
- In yet another embodiment, a fuel additive concentrate for removing and/or preventing the occurrence of deposits in a fuel injector of an injection engine may comprise a hexahydro triazine.
- In some embodiments, the hexahydro-triazine is a member of the group consisting of octyl hexahydro triazine and dodecylamine hexahydro triazine.
- In some embodiments, the hexahydro-triazine is selected from the group consisting of at least one of the structures I to XX, disclosed herein.
- As used herein, injection engine is intended to describe any injection engine, including but not limited to injection engines suitable for use with diesel- and/or gasoline-based fuels. The injection may be by throttle body injection, central fuel injection, single-point injection, continuous injection, central port injection, multi-point injection, or direct injection.
- As used herein, fuel injectors are intended to describe the part of an injection engine comprising a nozzle and a valve.
- As used herein, the term fuel injector deposit is intended to describe a hydrocarbon deposit formation, such as coke.
- As used herein, the term “unhindered” is used in the ordinary sense and is understood by one of skill in the art. For example, “unhindered” may be used to describe a lack of steric hindrance. In some embodiments described herein, at least one nitrogen in the hexahydro triazine ring has an alkyl chain with no branching until at least after the beta position to the ringed nitrogen. In some embodiments, the alkyl chain is linear.
- As used herein, the term “fuel soluble” means that the substance under discussion should be sufficiently soluble at 20° C. in the base fuel selected for use to reach at least the minimum concentration required to enable the substance to serve its intended function. However, the substance need not dissolve in the fuel in all proportions.
- As used herein, the term “hydrocarbyl group” or “hydrocarbyl” is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of a molecule and having a predominantly hydrocarbon character. Examples of hydrocarbyl groups include:
- (1) hydrocarbon substituents, that is, aliphatic (e.g., alkyl or alkenyl), alicyclic (e.g., cycloalkyl, cycloalkenyl) substituents, and aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents, as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form an alicyclic radical);
- (2) substituted hydrocarbon substituents, that is, substituents containing non-hydrocarbon groups which, in the context of the description herein, do not alter the predominantly hydrocarbon substituent (e.g., halo (especially chloro and fluoro), hydroxy, alkoxy, mercapto, alkylmercapto, nitro, nitroso, and sulfoxy);
- (3) hetero-substituents, that is, substituents which, while having a predominantly hydrocarbon character, in the context of this description, contain other than carbon in a ring or chain otherwise composed of carbon atoms. Hetero-atoms include sulfur, oxygen, nitrogen, and encompass substituents such as pyridyl, furyl, thienyl, and imidazolyl. In general, no more than two, or as a further example, no more than one, non-hydrocarbon substituent will be present for every ten carbon atoms in the hydrocarbyl group; in some embodiments, there will be no non-hydrocarbon substituent in the hydrocarbyl group.
- As used herein, the term “major amount” is understood to mean an amount greater than or equal to 50 wt. %, for example from about 80 to about 98 wt. % relative to the total weight of the composition. Moreover, as used herein, the term “minor amount” is understood to mean an amount less than 50 wt. % relative to the total weight of the composition.
- As used herein, the term substantially free of is intended to mean a trace amount or an amount that does not affect performance or a method as described herein.
- An advantage of the fuel additive described herein is that the additive may not only reduce the amount of deposits forming on fuel injectors, but the additive may also be effective to clean up dirty fuel injectors. The deposit reduction and cleaning effect of the additive is demonstrated in the present Examples. Further the fuel additive is not water soluble and is chemically stable to aqueous media.
- As used herein, dispersant/detergent is used to describe a dispersant or a detergent, for example, but not limited to, those particular dispersants/detergents described below. Additional embodiments and advantages of the disclosure will be set forth in part in the detailed description which follows, and/or can be learned by practice of the disclosure. It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive of the disclosure, as claimed.
- The fuel compositions of the present application may be suitable for use in a fuel injection engine. The fuel composition may comprise a major amount of a fuel and a minor amount of an additive concentrate comprising a hexahydro triazine, wherein the hexahydro triazine is present in an amount sufficient to remove and/or reduce the occurrence of deposits in a fuel injector of an injection engine. Suitable hexahydro triazines may be oil soluble and thermally stable.
- The hexahydro triazine ring may be the reaction product of or the product of combining an alkylamine and formaldehyde or a mixture of amines and formaldehyde. Suitable amines may comprise any primary amines. The hexahydro triazine may, but is not limited to, be alkylated or alkoxylated. At least one nitrogen on the hexahydro triazine ring may be unhindered. The hexahydro triazine ring may be saturated. Suitable hexahydro triazines include but are not limited to octyl hexahydro triazine and dodecyl hexahydro triazine. In some embodiments, a triazine suitable for use in present embodiments is free of or substantially free of triazines made from diamines. Embodiments may include, but are not limited to the following structures:
- Suitable hexahydro triazines may be unhindered. For example, hexahydro triazines may lack steric hindrance. For example, an alkyl group attached to a ringed nitrogen may be branched at a position after the beta position to the ringed nitrogen. In the Comparative Structures I and 11, below, there is branching at the beta position to at least one ringed nitrogen.
- In some embodiments the hexahydro triazine has a molecular weight of from about 100 to about 700. In another embodiment, the hexahydro triazine has a molecular weight of from about 400 to about 600.
- The hexahydro triazine additive components may be added to a fuel composition in an amount sufficient to provide control, including removal or prevention of, deposits. For example, the hexahydro triazine additive components may be added to a fuel in proportions effective to reduce the volume of injector deposits in a direct injection gasoline engine operated on said fuel containing said hexahydro triazine additive components to below the volume of injector deposits in said engine operated in the same manner on the same fuel except that it is devoid of said hexahydro triazine additive components. Economically, it is desirable to use the least amount of additive effective for the desired purpose.
- In some embodiments, one or more hexahydro triazine additive component(s) may be present in a fuel in an amount from about 1 ppm to about 100 ppm. In other embodiments, one or more hexahydro triazine additive component(s) may be present in a fuel in an amount from about 5 ppm to about 20 ppm.
- One or more additional optional compounds may be present in the fuel compositions of the disclosed embodiments. For example, the fuels may contain conventional quantities of cetane improvers, corrosion inhibitors, cold flow improvers (CFPP additive), pour point depressants, solvents, demulsifiers, emulsifiers, lubricity additives, friction modifiers, amine stabilizers, combustion improvers, dispersant/detergents, antioxidants, heat stabilizers, conductivity improvers, metal deactivators, markers, dyes, organic nitrate ignition accelerators, cyclomatic manganese tricarbonyl compounds, carrier fluids, biocides, antistatic additives, drag reducing agents, dehazers, anti-icing additives, antiknock additives, anti-valve-seat recession additives, surfactants, and the like. In some aspects, the compositions described herein may contain about 10 weight percent or less, or in other aspects, about 5 weight percent or less, based on the total weight of the additive concentrate, of one or more of the above additives. Similarly, the fuels may contain suitable amounts of conventional fuel blending components such as methanol, ethanol, dialkyl ethers, and the like.
- In some embodiments, the fuel additive concentrate may comprise a dispersant/detergent. The dispersant/detergent may comprise an ashless dispersant, a metal-containing dispersant, or a Mannich dispersant. A suitable dispersant/detergent may include at least one oil-soluble ashless dispersant having a basic nitrogen and/or at least one hydroxyl group in the molecule. Other suitable dispersants/detergents include alkenyl succinimides, alkenyl succinic acid esters, alkenyl succinic ester-amides, and Mannich bases. For example, suitable dispersants/detergents may comprise, but are not limited to ethylene diamine or dibutyl amine Mannich base dispersants. An example of a dibutyl amine Mannich base dispersant is HiTEC® 6416 Performance Additive Package (available from Afton Chemical Corporation). In some embodiments, a suitable dispersant may have a molecular weight of from about 750 to about 3000.
- Suitable amine detergents include those well known in the art for use in fuels for MPI engines to control intake valve deposits. Suitable amine detergents include nitrogen-containing derivatives of hydrocarbyl succinic acylating agents, Mannich condensation products, hydrocarbyl amines and polyetheramines. When used, the amine detergents are typically present in an amount sufficient to control intake valve deposits and are typically present in an amount of from about 5 to about 100 pounds by weight of additive per thousand barrels by volume of fuel.
- The nitrogen-containing derivatives of hydrocarbyl succinic acylating agents suitable for use in the present embodiments may include hydrocarbyl succinimides, succinamides, succinimide-amides and succinimide-esters. The nitrogen-containing derivatives of hydrocarbyl succinic acylating agents are typically prepared by reacting a hydrocarbyl-substituted succinic acylating agent with a polyamine.
- The hydrocarbyl-substituted succinic acylating agents include the hydrocarbyl-substituted succinic acids, the hydrocarbyl-substituted succinic anhydrides, the hydrocarbyl-substituted succinic acid halides (especially the acid fluorides and acid chlorides), the esters of the hydrocarbyl-substituted succinic acids and lower alcohols (e.g., those containing up to 7 carbon atoms), that is, hydrocarbyl-substituted compounds which can function as carboxylic acylating agents, and mixtures of hydrocarbyl-substituted succinic acids and hydrocarbyl-substituted succinic anhydrides.
- Amines which may be reacted with the alkenyl succinic anhydride to form the hydrocarbyl-succinimide include any that have at least one primary amine group that can react to form an imide group. A few representative examples are: methylamine, 2-ethylhexylamine, n-dodecylamine, stearylamine, N,N-dimethyl-propanediamine, N-(3aminopropyl)morpholine, N-dodecyl propanediamine, N-aminopropyl piperazine ethanolamine, N-ethanol ethylene diamine and the like. Suitable amines include the alkylene polyamines such as propylene diamine, dipropylene triamine, di-(1,2-butylene)triamine, tetra-(1,2-propylene)pentaamine.
- Further suitable amines are the ethylene polyamines which have the formula H2N(CH2CH2NH)nH wherein n is an integer from one to ten. These ethylene polyamines include ethylene diamine, diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, pentaethylene hexaamine, and the like, including mixtures thereof in which case n is the average value of the mixture. These ethylene polyamines have a primary amine group at each end so can form mono-alkenylsuccinimides and bis-alkenylsuccinimides. Thus suitable hydrocarbyl succinimides may include the products of reaction of a polyethylenepolyamine, e.g. triethylene tetramine or tetraethylene pentamine, with a hydrocarbon substituted carboxylic acid or anhydride made by reaction of a polyolefin, for example polyisobutene, having a molecular weight of 500 to 2,000, especially 700 to 1500, with an unsaturated polycarboxylic acid or anhydride, e.g. maleic anhydride.
- Suitable Mannich base detergents include the reaction products of a high molecular weight alkyl-substituted hydroxyaromatic compound, aldehydes, and amines. The alkyl-substituted hydroxyaromatic compound, aldehydes, and amines used in making the Mannich reaction products may be any suitable such compounds.
- The high molecular weight alkyl substituents on the benzene ring of the hydroxyaromatic compound may be derived from polyolefin having a number average molecular weight (Mn) of from about 500 to about 3000, or from about 700 to about 2100, as determined by gel permeation chromatography (GPC). The polyolefin may have a polydispersity (weight average molecular weight/number average molecular weight) in the range of about 1 to about 4 (for example from about 1 to about 2) as determined by GPC.
- The alkylation of the hydroxyaromatic compound is typically performed in the presence of an alkylating catalyst at a temperature in the range of about 0 to about 200° C., for example from 0 to 100° C. Acidic catalysts are generally used to promote Friedel-Crafts alkylation. Typical catalysts used in commercial production include sulphuric acid, BF3, aluminum phenoxide, methanesulphonic acid, cationic exchange resin, acidic clays, and modified zeolites.
- Polyolefins suitable for forming the high molecular weight alkyl-substituted hydroxyaromatic compounds include polypropylene, polybutenes, polyisobutylene, copolymers of butylene and/or butylene and propylene, copolymers of butylene and/or isobutylene and/or propylene, and one or more mono-olefinic comonomers copolymerizable therewith (e.g., ethylene, 1-pentene, 1-hexene, 1-octene, 1-decene, etc.) where the copolymer molecule contains at least 50% by weight, of butylene and/or isobutylene and/or propylene units. The comonomers polymerized with propylene or such butenes may be aliphatic and can also contain non-aliphatic groups, e.g., styrene, o-methylstyrene, p-methylstyrene, divinyl benzene, and the like. Thus in any case the resulting polymers and copolymers used in forming the high molecular weight alkyl-substituted hydroxyaromatic compounds are substantially aliphatic hydrocarbon polymers.
- Unless otherwise specified herein, the term “polybutylene” is used in a generic sense to include polymers made from “pure” or “substantially pure” 1-butene or isobutene, and polymers made from mixtures of two or all three of 1-butene, 2-butene, and isobutene. Commercial grades of such polymers may also contain insignificant amounts of other olefins. So-called high reactivity polyisobutenes having relatively high proportions of polymer molecules having a terminal vinylidene group are also suitable for use in forming the long chain alkylated phenol reactant. Suitable high-reactivity polyisobutenes include those polyisobutenes that comprise at least about 20% of the more reactive methylvinylidene isomer, for example at least 50%, and as a further example at least 70%. Suitable polyisobutenes include those prepared using BF3 catalysts. The preparation of such polyisobutenes in which the methylvinylidene isomer comprises a high percentage of the total composition is described in U.S. Pat. Nos. 4,152,499 and 4,605,808.
- The Mannich detergent may be made from a high molecular weight alkylphenol or alkylcresol. However, other phenolic compounds may be used including high molecular weight alkyl-substituted derivatives of resorcinol, hydroquinone, catechol, hydroxydiphenyl, benzylphenol, phenethylphenol, naphthol, tolylnaphthol, among others. In the preparation of the Mannich detergents are the polyalkylphenol and polyalkylcresol reactants, e.g., polypropylphenol, polybutylphenol, polypropylcresol and polybutylcresol, wherein the alkyl group has a number average molecular weight of about 500 to about 2100, and as another example, the alkyl group is a polybutyl group derived from polyisobutylene having a number average molecular weight in the range of about 700 to about 1300.
- A suitable configuration of the high molecular weight alkyl-substituted hydroxyaromatic compound is that of a para-substituted mono-alkylphenol or a para-substituted mono-alkyl ortho-cresol. However, any hydroxyaromatic compound readily reactive in the Mannich condensation reaction may be employed. Thus, Mannich products made from hydroxyaromatic compounds having only one ring alkyl substituent, or two or more ring alkyl substituents are suitable. The long chain alkyl substituents may contain some residual unsaturation, but in general, are substantially saturated alkyl groups.
- Representative amine reactants include, but are not limited to, alkylene polyamines having at least one suitably reactive primary or secondary amino group in the molecule. Other substituents such as hydroxyl, cyano, amido, etc., can be present in the polyamine. In an embodiment, the alkylene polyamine is a polyethylene polyamine. Suitable alkylene polyamine reactants include ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, and mixtures of such amines having nitrogen contents corresponding to alkylene polyamines of the formula H2N-(A-NH—)nH, where A is divalent ethylene or propylene and n is an integer of from I to 10, or, as another example, from 1 to 4. The alkylene polyamines may be obtained by the reaction of ammonia and dihalo alkanes, such as dichloro alkanes.
- The amine may also be an aliphatic diamine having one primary or secondary amino group and at least one tertiary amino group in the molecule. Examples of suitable polyamines include N,N,N″,N″-tetraalkyldialkylenetriamines (two terminal tertiary amino groups and one central secondary amino group), N,N,N′,N″-tetraalkyltrialkylenetetramines (one terminal tertiary amino group, two internal tertiary amino groups and one terminal primary amino group), N,N,N′,N″,N′″-pentaalkyltrialkylenetetramines (one terminal tertiary amino group, two internal tertiary amino groups and one terminal secondary amino group), N,N-dihydroxyalkyl-alpha, omega-alkylenediamines (one terminal tertiary amino group and one terminal primary amino group), N,N,N′-trihydroxyalkyl-alpha, omega-alkylenediamines (one terminal tertiary amino group and one terminal secondary amino group), tris(dialkylaminoalkyl)aminoalkylmethanes (three terminal tertiary amino groups and one terminal primary amino group), and similar compounds, wherein the alkyl groups are the same or different and typically contain no more than about 12 carbon atoms each, and which may contain from 1 to 4 carbon atoms each. These alkyl groups may be methyl and/or ethyl groups. Suitable polyamine reactants are N,N-dialkyl-alpha, omega-alkylenediamine, such as those having from 3 to about 6 carbon atoms in the alkylene group and from 1 to about 12 carbon atoms in each of the alkyl groups, which may be the same but which can be different. Suitable examples include N,N-dimethyl-1,3-propanediamine and N-methyl piperazine.
- Examples of polyamines having one reactive primary or secondary amino group that can participate in the Mannich condensation reaction, and at least one sterically hindered amino group that cannot participate directly in the Mannich condensation reaction to any appreciable extent include N-(tert-butyl)-1,3-propanediamine, N-neopentyl-1,3-propanediamine, N-(tert-butyl)-1-methyl-1,2-ethanediamine, N-(tert-butyl)-1-methyl-1,3-propanediamine, and 3,5-di(tert-butyl)aminoethylpiperazine.
- Representative aldehydes for use in the preparation of the Mannich base products include the aliphatic aldehydes such as formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, valeraldehyde, caproaldehyde, heptaldehyde, stearaldehyde. Aromatic aldehydes which may be used include benzaldehyde and salicylaldehyde. Illustrative heterocyclic aldehydes for use herein are furfural and thiophene aldehyde, etc. Also useful are formaldehyde-producing reagents such as paraformaldehyde, or aqueous formaldehyde solutions such as formalin.
- Suitable Mannich base detergents may include those detergents taught in U.S. Pat. Nos. 4,231,759; 5,514,190; 5,634,951; 5,697,988; 5,725,612; and 5,876,468, the disclosures of which are incorporated herein by reference.
- In some embodiments, the additive concentrates may comprise a fuel soluble carrier. Such carriers may be of various types, such as liquids or solids, e.g., waxes. Examples of liquid carriers include, but are not limited to, mineral oil and oxygenates, such as liquid polyalkoxylated ethers (also known as polyalkylene glycols or polyalkylene ethers), liquid polyalkoxylated phenols, liquid polyalkoxylated esters, liquid polyalkoxylated amines, and mixtures thereof. Examples of the oxygenate carriers may be found in U.S. Pat. No. 5,752,989, issued May 19, 1998 to Henly et. al., the description of which carriers is herein incorporated by reference in its entirety. Additional examples of oxygenate carriers include alkyl-substituted aryl polyalkoxylates described in U.S. Patent Publication No. 2003/0131527, published Jul. 17, 2003 to Colucci et. al., the description of which is herein incorporated by reference in its entirety.
- Suitable carriers can be of various types, such as for example liquid poly-alpha-olefin oligomers, mineral oils, liquid poly(oxyalkylene) compounds, liquid alcohols or polyols, polyalkenes, liquid esters, and similar liquid carriers. Mixtures of two or more such carriers can be employed.
- Suitable liquid carriers may include 1) a mineral oil or a blend of mineral oils that have a viscosity index of less than about 120, 2) one or more poly-alpha-olefin oligomers, 3) one or more poly(oxyalkylene) compounds having an average molecular weight in the range of about 500 to about 3000, 4) polyalkenes, 5) polyalkyl-substituted hydroxyaromatic compounds, or 6) mixtures thereof. The mineral oil carrier fluids that can be used include paraffinic, naphthenic, and asphaltic oils, and can be derived from various petroleum crude oils and processed in any suitable manner. For example, the mineral oils may be solvent extracted or hydrotreated oils. Reclaimed mineral oils can also be used. The mineral oil used may have a viscosity at 40° C. of less than about 1600 SUS, and as another example between about 300 and 1500 SUS at 40° C. Paraffinic mineral oils may have viscosities at 40° C. in the range of about 475 SUS to about 700 SUS. In some embodiments, the mineral oil may have a viscosity index of less than about 100, as a further example, less than about 70, and, as an even further example, in the range of from about 30 to about 60.
- When the carrier fluids are used in combination with the amine detergents, the ratio (wt/wt) of detergent to carrier fluid(s) is typically in the range of from 1:0.1 to 1:3.
- In other aspects, compositions of the present application may not contain a carrier. For example, some compositions of the present application may not contain mineral oil or oxygenates, such as those oxygenates described above.
- In some aspects of the disclosed embodiments, organic nitrate ignition accelerators that include aliphatic or cycloaliphatic nitrates in which the aliphatic or cycloaliphatic group is saturated, and that contain up to about 12 carbons may be used. Examples of organic nitrate ignition accelerators that may be used are methyl nitrate, ethyl nitrate, propyl nitrate, isopropyl nitrate, allyl nitrate, butyl nitrate, isobutyl nitrate, sec-butyl nitrate, tert-butyl nitrate, amyl nitrate, isoamyl nitrate, 2-amyl nitrate, 3-amyl nitrate, hexyl nitrate, heptyl nitrate, 2-heptyl nitrate, octyl nitrate, isooctyl nitrate, 2-ethylhexyl nitrate, nonyl nitrate, decyl nitrate, undecyl nitrate, dodecyl nitrate, cyclopentyl nitrate, cyclohexyl nitrate, methylcyclohexyl nitrate, cyclododecyl nitrate, 2-ethoxyethyl nitrate, 2-(2-ethoxyethoxy)ethyl nitrate, tetrahydrofuranyl nitrate, and the like. Mixtures of such materials may also be used.
- Examples of suitable optional metal deactivators useful in the compositions of the present application are disclosed in U.S. Pat. No. 4,482,357, issued Nov. 13, 1984, the disclosure of which is herein incorporated by reference in its entirety. Such metal deactivators include, for example, salicylideneaminophenol, disalicylidene ethylenediamine, disalicylidene propylenediamine, and N,N′-disalicylidene-1,2-diaminopropane.
- Suitable optional cyclomatic manganese tricarbonyl compounds which may be employed in the compositions of the present application include, for example, cyclopentadienyl manganese tricarbonyl, methylcyclopentadienyl manganese tricarbonyl, indenyl manganese tricarbonyl, and ethylcyclopentadienyl manganese tricarbonyl. Yet other examples of suitable cyclomatic manganese tricarbonyl compounds are disclosed in U.S. Pat. No. 5,575,823, issued Nov. 19, 1996, and U.S. Pat. No. 3,015,668, issued Jan. 2, 1962, both of which disclosures are herein incorporated by reference in their entirety.
- The base fuels suitable for use in formulating the fuel compositions of embodiments described herein may include any base fuels suitable for use in the operation of direct injection engine such as leaded or unleaded motor gasolines, and so-called reformulated gasolines which typically contain both hydrocarbons of the gasoline boiling range and fuel-soluble oxygenated blending agents (“oxygenates”), such as alcohols, ethers, and other suitable oxygen-containing organic compounds. The fuel may be a mixture of hydrocarbons boiling in the gasoline boiling range. The fuel may consist of straight chain or branch chain paraffins, cycloparaffins, olefins, aromatic hydrocarbons, or any mixture of these. The fuel may be a gasoline derived from straight run naphtha, polymer gasoline, natural gasoline or from catalytically reformed stocks boiling in the range from about 80 to about 450° F. The octane level of the gasoline is not critical and any conventional gasoline may be employed. In embodiments where the fuel comprises a diesel fuel, the diesel fuel may be applicable to the operation of both stationary diesel engines (e.g., engines used in electrical power generation installations, in pumping stations, etc.) and ambulatory diesel engines (e.g., engines used as prime movers in automobiles, trucks, road-grading equipment, military vehicles, etc.).
- Suitable fuels may include any known hydrocarbon fuel or mixture thereof. Suitable fuels include, but are not limited to, any and all middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no. 6), low sulfur fuels, synthetic fuels, such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil. “Biorenewable fuels” as used herein is understood to mean any fuel which is derived from resources other than petroleum. Such resources include, but are not limited to, corn, maize, soybeans and other crops; grasses, such as switchgrass, miscanthus, and hybrid grasses; algae, seaweed, vegetable oils; natural fats; and mixtures thereof. In an aspect, the biorenewable fuel can comprise monohydroxy alcohols, such as those comprising from 1 to about 5 carbon atoms. Non-limiting examples of suitable monohydroxy alcohols include methanol, ethanol, bioethanol, biobutanol, propanol, n-butanol, isobutanol, t-butyl alcohol, amyl alcohol, and isoamyl alcohol.
- Suitable oxygenates include methanol, ethanol, isopropanol, t-butanol, mixed C1 to C5 alcohols, methyl tertiary butyl ether, tertiary amyl methyl ether, ethyl tertiary butyl ether, and mixed ethers. Oxygenates, when used, will normally be present in the base fuel in an amount below about 30% by volume, and in an amount that provides an oxygen content in the overall fuel in the range of about 0.5 to about 5 percent by volume.
- When formulating the fuel compositions of this application, the additives may be employed in amounts sufficient to remove, reduce, inhibit, and/or prevent deposit formation in an injection engine. In some aspects, the fuels may contain minor amounts of the above described hexahydro triazine compound that removes, reduces, inhibits, or prevents the formation of engine deposits, for example injector deposits. For example, the fuels of this application may contain, on an active ingredient basis, an amount of the hexahydro triazine compound in the range of about 1 ppm to about 100 ppm in a fuel. As a further example, a fuel may contain from about 5 ppm to about 20 ppm of the hexahydro triazine.
- The additives of the present application, including the hexahydro triazine compound described above, and optional additives used in formulating the fuels of this disclosure may be blended into the base fuel individually or in various sub-combinations. In some embodiments, the additive components of the present application may be blended into the fuel concurrently using an additive concentrate, as this takes advantage of the mutual compatibility and convenience afforded by the combination of ingredients when in the form of an additive concentrate. Also, use of a concentrate may reduce blending time and lessen the possibility of blending errors. In some embodiments, the hexahydrotriazine may be combined with one or more additives, such as a dispersant, to form a top treat. The top treat may be added to an additive concentrate, which is then added to a fuel. Alternatively, the top treat may be added directly to a fuel that either does or does not contain a separate additive concentrate. The top treat or after-market additive may be added to a fuel in the vehicle or to a fuel storage facility.
- Accordingly, aspects of the present application are directed to methods for removing, reducing, inhibiting, and/or preventing the occurrence of and/or amount of injector deposits in an injection engine. In another aspect, the improvements may also be observed in direct fuel injectors. In some aspects, the methods comprise injecting a hydrocarbon-based compression ignition fuel comprising the hexahydro triazine compound additive of the present application, through the injectors of the engine. In some aspects, the method may also comprise mixing into the fuel at least one of the optional additional ingredients described above. In some embodiments the additive concentrate may be provided as a top treat, or after-market additive package, that may be added to a fuel in the vehicle, at a fuel storage facility, to a fuel storage receptacle, or the like.
- The following examples are illustrative of exemplary embodiments of the disclosure. In these examples as well as elsewhere in this application, all parts and percentages are by weight unless otherwise indicated. It is intended that these examples are being presented for the purpose of illustration only and are not intended to limit the scope of the invention disclosed herein.
- Inventive and comparative samples were prepared and tested to demonstrate the effectiveness of the inventive additive concentrations in reducing deposits in injection engines. Tests were conducted in a Mitsubishi 4G93 GDI engine. The vehicle engine specifications are as follows:
- Engine name: Mitsubishi 4G93 GDI
- Cylinder arrangement: In-line 4 cylinder
- Induction capacity (Displacement): 1834 cc
- Valve train: DOHC 4 valves (2 intake and 2 exhaust valves) per cylinder
- Bore X stroke: 81.0×89.0 mm
- The test method and details of the vehicle test cycle are disclosed in the test procedure, “The Evaluation of Deposits in a Direct Injection Gasoline Engine,” developed by CEC, OACIS, and CRC. Each cycle lasted about 100 minutes, and each test had 30 cycles. A complete test takes about 2 days. Injectors are cleaned and re-used after each test. All test conditions are defined by this procedure except the test cycle. A Quad-4 cycle was used in the disclosed tests.
- In the following Table, results from samples run in the above-described test are shown. The treat rate of the additive package and added component(s) is given in PPM (weight based) added to the base fuel. In the 6th column, the treat rate (PPM, wt) of the chemical structure or comparative structure is used in addition to the treat rate of the additive package. The chemical structure numerical reference is the same numerical reference disclosed herein. The plugging average (%) is a measurement of injector flow loss compared to that of a new, unused (and, consequently, unplugged) injector. It is provided as an average of 4 injectors plugged due to deposit during the test.
- The data in the following table illustrates the advantage in performance of the inventive examples (Tests 3 and 5) versus unadditized fuel (Test 1), versus fuel containing a dispersant package (Test 2), and versus a comparative triazine (Test 4). As shown in Test 1, a base fuel without any additive caused injector plugging at an average of 7.5%. A fluid containing only an additive package at 292 ppm (wt) resulted in injector plugging of 9%, as shown in Test 2. However, when the presently disclosed chemical structures were added at only 11.4 ppm (wt) on top of the additive package in the fuel, injector plugging dropped to 0.75% and 0.24%, as shown in Tests 3 and 5, respectively. A comparative chemical structure added to the fuel at the same treat rate led to injector plugging of 1.93%, which was less effective than the presently disclosed chemical structures.
- The comparative example demonstrates less than desirable performance when relatively hindered triazines, with branching only one carbon away from the ring-nitrogen, are used. That feature appears to negatively impact performance, as the nitrogen of the ring is not exposed and therefore less available to keep clean or clean injectors. Further, it seems that the hindrance of the triazine ring overrides the polarity effect.
-
PPM (wt) PPM (wt) Additive additive Chemical Chemical Chemical Plugging Test package* package Structure name structure average, % 1 no 7.5 2 Yes 292 9.00 3 Yes 292 Structure I Dodecyl hexahydro 11.4 0.75 triazine 4 Yes 292 Comparative Ethyl-hexyl hexa 11.4 1.93 Structure I hydro triazine 5 Yes 292 Structure II Octyl hexahydro 11.4 0.24 triazine *additive package is a dibutyl amine polyisobutylene cresol Mannich - It is noted that, as used in this specification and the appended claims, the singular forms “a,” “an,” and “the,” include plural referents unless expressly and unequivocally limited to one referent. Thus, for example, reference to “a dispersant” includes one, two, or more different dispersants. As used herein, the term “include” and its grammatical variants are intended to be non-limiting, such that recitation of items in a list is not to the exclusion of other like items that can be substituted or added to the listed items.
- For the purposes of this specification and appended claims, unless otherwise indicated, all numbers expressing quantities, percentages or proportions, and other numerical values used in the specification and claims, are to be understood as being modified in all instances by the term “about.” Accordingly, unless indicated to the contrary, the numerical parameters set forth in the following specification and attached claims are approximations that can vary depending upon the desired properties sought to be obtained by the present disclosure. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques.
- While particular embodiments have been described, alternatives, modifications, variations, improvements, and substantial equivalents that are or can be presently unforeseen can arise to applicants or others skilled in the art. Accordingly, the appended claims as filed and as they can be amended are intended to embrace all such alternatives, modifications, variations, improvements, and substantial equivalents.
Claims (34)
1. A fuel suitable for use in a fuel injection engine comprising:
a major amount of a fuel; and
a minor amount of an additive concentrate comprising a hexahydro triazine.
2. The fuel of claim 1 , wherein an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring.
3. The fuel of claim 2 , wherein the alkyl group is linear.
4. The fuel of claim 2 , wherein the alkyl group is branched at a position after the beta position to the at least one nitrogen.
5. The fuel of claim 1 , wherein the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
6. The fuel of claim 1 , wherein the hexahydro triazine has a molecular weight of from about 100 to about 700.
7. The fuel of claim 1 , wherein the hexahydro triazine has a molecular weight of from about 400 to about 600.
8. The fuel of claim 1 , wherein the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
10. The fuel of claim 1 , wherein the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
11. The fuel of claim 1 , wherein the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm.
12. The fuel of claim 1 , wherein the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
13. The fuel of claim 1 , wherein the additive concentrate further comprises a dispersant/detergent.
14. The fuel of claim 13 , wherein the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, a polyisobutylene amine dispersant, or a Mannich base dispersant.
15. The fuel of claim 1 , wherein the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no. 6), low sulfur fuels, synthetic fuels, such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
16. A method for removing and/or preventing the occurrence of deposits in a fuel injector of a fuel injection engine comprising:
adding to and operating in an injection engine having fuel injectors a fuel composition comprising (a) a major amount of fuel and (b) a minor amount of an additive concentrate comprising a hexahydro triazine.
17. The method of claim 16 , wherein an alkyl group is attached to at least one nitrogen in the hexahydro triazine ring.
18. The method of claim 17 , wherein the alkyl group is linear.
19. The method of claim 17 , wherein the alkyl group is branched at a position after the beta position to the at least one nitrogen.
20. The method of claim 16 , wherein the hexahydro triazine comprises the product combining, mixing, admixing, or contacting one or more primary amine(s) and a formaldehyde.
21. The method of claim 16 , wherein the hexahydro triazine has a molecular weight of from about 100 to about 700.
22. The method of claim 16 , wherein the hexahydro triazine has a molecular weight of from about 400 to about 600.
23. The method of claim 16 , wherein the hexahydro triazine is selected from the group consisting of at least one of dodecylamine hexahydro triazine and octyl hexahydro triazine.
25. The method of claim 16 , wherein the hexahydro triazine is present in an amount sufficient to remove and/or prevent the occurrence of deposits in a fuel injector of an injection engine.
26. The method of claim 16 , wherein the hexahydro triazine is present in the fuel in an amount from about 1 ppm to about 100 ppm.
27. The method of claim 16 , wherein the hexahydro triazine is present in the fuel in an amount from about 5 ppm to about 20 ppm.
28. The method of claim 16 , wherein the additive concentrate further comprises a dispersant/detergent.
29. The method of claim 28 , wherein the dispersant/detergent comprises at least one of an amine dispersant/detergent, an alkenyl succinimide dispersant, an alkenyl succinic acid ester dispersant, an alkenyl succinic ester-amide dispersant, polyisobutylene amine dispersant, or a Mannich base dispersant.
30. The method of claim 16 , wherein the fuel is selected from the group consisting of middle distillate fuels, diesel fuels, gasolines, biorenewable fuels, biodiesel fuels, gas-to-liquid (GTL) fuels, jet fuels, aviation fuels, marine fuels, burner fuels, alcohols, ethers, esters, kerosene, home heating oils (for example, home heating oil no. 6), low sulfur fuels, synthetic fuels, such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to liquid (CTL) fuels, biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal (natural, cleaned, and petcoke), genetically engineered biofuels and crops and extracts therefrom, natural gas, a Group I base oil, a Group II base oil, a Group III base oil, and a Group IV base oil.
31. The method of claim 16 , wherein the injection engine comprises a direct injection engine.
32. A fuel additive concentrate for removing and/or preventing the occurrence of deposits in a fuel injector of an injection engine comprising a hexahydro triazine.
33. The additive concentrate of claim 32 , wherein the hexahydro-triazine is a member of the group consisting of octyl hexahydro triazine and dodecylamine hexahydro triazine.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/262,420 US20100107476A1 (en) | 2008-10-31 | 2008-10-31 | Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/262,420 US20100107476A1 (en) | 2008-10-31 | 2008-10-31 | Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20100107476A1 true US20100107476A1 (en) | 2010-05-06 |
Family
ID=42129721
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/262,420 Abandoned US20100107476A1 (en) | 2008-10-31 | 2008-10-31 | Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US20100107476A1 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103710060A (en) * | 2013-12-25 | 2014-04-09 | 济南开发区星火科学技术研究院 | Preparation method for compound diesel cleansing agent |
| US20150113858A1 (en) * | 2013-10-24 | 2015-04-30 | Shell Oil Company | Liquid fuel compositions |
| US9120897B1 (en) | 2014-05-27 | 2015-09-01 | International Business Machines Corporation | Preparation of thioether polymers |
| US9120899B1 (en) | 2014-06-02 | 2015-09-01 | International Business Machines Corporation | Preparation of functional polysulfones |
| US9228034B1 (en) | 2014-10-09 | 2016-01-05 | International Business Machines Corporation | Hexahydrotriazine, dithiazine, and thioether functionalized materials |
| US9243107B2 (en) | 2013-10-10 | 2016-01-26 | International Business Machines Corporation | Methods of preparing polyhemiaminals and polyhexahydrotriazines |
| US9255172B1 (en) | 2014-08-05 | 2016-02-09 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9271498B2 (en) | 2014-06-19 | 2016-03-01 | International Business Machines Corporation | Antimicrobial PHT coatings |
| US9296863B2 (en) | 2014-06-16 | 2016-03-29 | International Business Machines Corporation | Preparation of poly(octatriazacane) |
| US9303186B1 (en) * | 2014-10-16 | 2016-04-05 | International Business Machines Corporation | PHT powder coating materials |
| US9352045B2 (en) | 2014-06-20 | 2016-05-31 | International Business Machines Corporation | Methods and materials for therapeutic delivery |
| US9389181B2 (en) | 2014-06-06 | 2016-07-12 | International Business Machines Corporation | Methods and apparatus for detecting metals in liquids |
| US9453108B2 (en) | 2014-08-22 | 2016-09-27 | International Business Machines Corporation | Flame retardant PHT compositions |
| WO2016160187A1 (en) * | 2015-03-31 | 2016-10-06 | Baker Hughes Incorporated | Methods of manufacturing poly(hexahydrotriazine)s via suspension or emulsion polymerization |
| US9469660B2 (en) | 2014-06-03 | 2016-10-18 | International Business Machines Corporation | Sulfur scavenging materials comprising hexahydrotriazine-modified particle |
| US9512332B2 (en) | 2014-06-27 | 2016-12-06 | International Busuiness Machines Corporation | Soluble, processable polyhemiaminals and polyhexahydrotriazines |
| US9522977B2 (en) | 2014-07-24 | 2016-12-20 | International Business Machines Corporation | Thermoplastic toughening of PHT's |
| US9592470B2 (en) | 2014-05-27 | 2017-03-14 | International Business Machines Corporation | Sulfur scavenging materials for filters and coatings |
| CN106565618A (en) * | 2016-10-10 | 2017-04-19 | 常州大学 | Catalyst ligand for directly synthesizing lubricating oil base oil from alpha-olefin, and coordination compound thereof, and preparation methods and applications of catalyst ligand and coordination compound |
| US9656239B2 (en) | 2014-06-16 | 2017-05-23 | International Business Machines Corporation | Apparatus for controlling metals in liquids |
| US9676891B2 (en) | 2014-08-22 | 2017-06-13 | International Business Machines Corporation | Synthesis of dynamic covalent 3D constructs |
| US9758620B2 (en) | 2015-09-03 | 2017-09-12 | International Business Machines Corporation | Tailorable viscoelastic properties of PEG-hemiaminal organogel networks |
| US9765188B2 (en) | 2015-11-02 | 2017-09-19 | International Business Machines Corporation | High molecular weight polythioaminals from a single monomer |
| US9777116B2 (en) | 2014-10-16 | 2017-10-03 | International Business Machines Corporation | Porous/nanoporous PHT |
| US9828467B2 (en) | 2016-02-05 | 2017-11-28 | International Business Machines Corporation | Photoresponsive hexahydrotriazine polymers |
| US9862802B2 (en) | 2015-11-30 | 2018-01-09 | International Business Machines Corporation | Poly(thioaminal) probe based lithography |
| US9873766B2 (en) | 2015-11-24 | 2018-01-23 | International Business Machines Corporation | Systems chemistry approach to polyhexahydrotriazine polymeric structures |
| US9879118B2 (en) | 2015-10-05 | 2018-01-30 | International Business Machines Corporation | Polymers from stabilized imines |
| US9957345B2 (en) | 2014-08-18 | 2018-05-01 | International Business Machines Corporation | 3D printing with PHT based materials |
| US10023735B2 (en) | 2014-08-18 | 2018-07-17 | International Business Machines Corporation | 3D printing with PHT/PHA based materials and polymerizable monomers |
| US20180238229A1 (en) * | 2014-10-08 | 2018-08-23 | Bernie C. Thompson | Compositions for Engine Carbon Removal and Methods and Apparatus for Removing Carbon |
| US10080806B2 (en) | 2015-08-19 | 2018-09-25 | International Business Machines Corporation | Sulfur-containing polymers from hexahydrotriazine and dithiol precursors as a carrier for active agents |
| CN114292682A (en) * | 2021-12-01 | 2022-04-08 | 江苏大学 | Green triazine lubricating oil additive, preparation method and application |
Citations (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3015668A (en) * | 1959-11-24 | 1962-01-02 | Ethyl Corp | Process for producing cyclomatic manganese tricarbonyl compounds |
| US4152499A (en) * | 1977-01-22 | 1979-05-01 | Basf Aktiengesellschaft | Polyisobutenes |
| US4231759A (en) * | 1973-03-12 | 1980-11-04 | Standard Oil Company (Indiana) | Liquid hydrocarbon fuels containing high molecular weight Mannich bases |
| US4482357A (en) * | 1983-12-30 | 1984-11-13 | Ethyl Corporation | Fuel Compositions |
| US4605808A (en) * | 1983-11-01 | 1986-08-12 | Bp Chemicals Limited | Cationic polymerization of 1-olefins |
| US5162049A (en) * | 1991-09-09 | 1992-11-10 | Ethyl Petroleum Additives | Middle distillate fuels and additives therefor |
| US5322528A (en) * | 1992-03-30 | 1994-06-21 | Texaco Inc. | Composition of matter for high temperature phenolphthalein-, phenolphmalide-, fluorene-, xanthane-, and anthrone-s-triazines that are soluble in diesel fuel |
| US5354453A (en) * | 1993-04-13 | 1994-10-11 | Exxon Chemical Patents Inc. | Removal of H2 S hydrocarbon liquid |
| US5480860A (en) * | 1988-12-23 | 1996-01-02 | Petrolite Corporation | Methods for reducing sulfides in sewage gas |
| US5514190A (en) * | 1994-12-08 | 1996-05-07 | Ethyl Corporation | Fuel compositions and additives therefor |
| US5575823A (en) * | 1989-12-22 | 1996-11-19 | Ethyl Petroleum Additives Limited | Diesel fuel compositions |
| US5634951A (en) * | 1996-06-07 | 1997-06-03 | Ethyl Corporation | Additives for minimizing intake valve deposits, and their use |
| US5697988A (en) * | 1991-11-18 | 1997-12-16 | Ethyl Corporation | Fuel compositions |
| US5725612A (en) * | 1996-06-07 | 1998-03-10 | Ethyl Corporation | Additives for minimizing intake valve deposits, and their use |
| US5746783A (en) * | 1994-03-30 | 1998-05-05 | Martin Marietta Energy Systems, Inc. | Low emissions diesel fuel |
| US5752989A (en) * | 1996-11-21 | 1998-05-19 | Ethyl Corporation | Diesel fuel and dispersant compositions and methods for making and using same |
| US5830243A (en) * | 1997-09-11 | 1998-11-03 | The Lubrizol Corporation | Fuel compositions containing N-substituted perahydro-s triazines |
| US5876468A (en) * | 1996-09-05 | 1999-03-02 | Lubrizol Adibis Holdings (Uk) Limited | Detergents for hydrocarbon fuels |
| US20020020107A1 (en) * | 1999-07-02 | 2002-02-21 | Bailey Brent K. | Low molecular weight compression ignition fuel |
| US20030172583A1 (en) * | 2001-10-16 | 2003-09-18 | Kitchen George H. | Fuel additive |
| US20040123515A1 (en) * | 1999-11-23 | 2004-07-01 | International Fuel Technology, Inc. | Fuel additive, additive-containing fuel compositions and method of manufacture |
| US20050044778A1 (en) * | 1997-12-08 | 2005-03-03 | Orr William C. | Fuel compositions employing catalyst combustion structure |
| US20050066572A1 (en) * | 2003-09-25 | 2005-03-31 | Colucci William J. | Fuels compositions and methods for using same |
| US20050215441A1 (en) * | 2002-03-28 | 2005-09-29 | Mackney Derek W | Method of operating internal combustion engine by introducing detergent into combustion chamber |
| US7112230B2 (en) * | 2001-09-14 | 2006-09-26 | Afton Chemical Intangibles Llc | Fuels compositions for direct injection gasoline engines |
| US7374588B2 (en) * | 2000-05-02 | 2008-05-20 | Interfacial Technologies (Uk) Limited | Fuel combustion |
-
2008
- 2008-10-31 US US12/262,420 patent/US20100107476A1/en not_active Abandoned
Patent Citations (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3015668A (en) * | 1959-11-24 | 1962-01-02 | Ethyl Corp | Process for producing cyclomatic manganese tricarbonyl compounds |
| US4231759A (en) * | 1973-03-12 | 1980-11-04 | Standard Oil Company (Indiana) | Liquid hydrocarbon fuels containing high molecular weight Mannich bases |
| US4152499A (en) * | 1977-01-22 | 1979-05-01 | Basf Aktiengesellschaft | Polyisobutenes |
| US4605808A (en) * | 1983-11-01 | 1986-08-12 | Bp Chemicals Limited | Cationic polymerization of 1-olefins |
| US4482357A (en) * | 1983-12-30 | 1984-11-13 | Ethyl Corporation | Fuel Compositions |
| US5480860A (en) * | 1988-12-23 | 1996-01-02 | Petrolite Corporation | Methods for reducing sulfides in sewage gas |
| US5575823A (en) * | 1989-12-22 | 1996-11-19 | Ethyl Petroleum Additives Limited | Diesel fuel compositions |
| US5162049A (en) * | 1991-09-09 | 1992-11-10 | Ethyl Petroleum Additives | Middle distillate fuels and additives therefor |
| US5697988A (en) * | 1991-11-18 | 1997-12-16 | Ethyl Corporation | Fuel compositions |
| US5322528A (en) * | 1992-03-30 | 1994-06-21 | Texaco Inc. | Composition of matter for high temperature phenolphthalein-, phenolphmalide-, fluorene-, xanthane-, and anthrone-s-triazines that are soluble in diesel fuel |
| US5354453A (en) * | 1993-04-13 | 1994-10-11 | Exxon Chemical Patents Inc. | Removal of H2 S hydrocarbon liquid |
| US5746783A (en) * | 1994-03-30 | 1998-05-05 | Martin Marietta Energy Systems, Inc. | Low emissions diesel fuel |
| US5514190A (en) * | 1994-12-08 | 1996-05-07 | Ethyl Corporation | Fuel compositions and additives therefor |
| US5634951A (en) * | 1996-06-07 | 1997-06-03 | Ethyl Corporation | Additives for minimizing intake valve deposits, and their use |
| US5725612A (en) * | 1996-06-07 | 1998-03-10 | Ethyl Corporation | Additives for minimizing intake valve deposits, and their use |
| US5876468A (en) * | 1996-09-05 | 1999-03-02 | Lubrizol Adibis Holdings (Uk) Limited | Detergents for hydrocarbon fuels |
| US5752989A (en) * | 1996-11-21 | 1998-05-19 | Ethyl Corporation | Diesel fuel and dispersant compositions and methods for making and using same |
| US5830243A (en) * | 1997-09-11 | 1998-11-03 | The Lubrizol Corporation | Fuel compositions containing N-substituted perahydro-s triazines |
| US20050044778A1 (en) * | 1997-12-08 | 2005-03-03 | Orr William C. | Fuel compositions employing catalyst combustion structure |
| US20020020107A1 (en) * | 1999-07-02 | 2002-02-21 | Bailey Brent K. | Low molecular weight compression ignition fuel |
| US20040123515A1 (en) * | 1999-11-23 | 2004-07-01 | International Fuel Technology, Inc. | Fuel additive, additive-containing fuel compositions and method of manufacture |
| US7374588B2 (en) * | 2000-05-02 | 2008-05-20 | Interfacial Technologies (Uk) Limited | Fuel combustion |
| US7112230B2 (en) * | 2001-09-14 | 2006-09-26 | Afton Chemical Intangibles Llc | Fuels compositions for direct injection gasoline engines |
| US20060242893A1 (en) * | 2001-09-14 | 2006-11-02 | Malfer Dennis J | Fuels compositions for direct injection gasoline engines |
| US20030172583A1 (en) * | 2001-10-16 | 2003-09-18 | Kitchen George H. | Fuel additive |
| US20050215441A1 (en) * | 2002-03-28 | 2005-09-29 | Mackney Derek W | Method of operating internal combustion engine by introducing detergent into combustion chamber |
| US20050066572A1 (en) * | 2003-09-25 | 2005-03-31 | Colucci William J. | Fuels compositions and methods for using same |
| US20060168876A1 (en) * | 2003-09-25 | 2006-08-03 | Afton Chemical Corporation | Fuels compositions and methods for using same |
Cited By (78)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9243107B2 (en) | 2013-10-10 | 2016-01-26 | International Business Machines Corporation | Methods of preparing polyhemiaminals and polyhexahydrotriazines |
| US9951184B2 (en) | 2013-10-10 | 2018-04-24 | International Business Machines Corporation | Methods of preparing polyhemiaminals and polyhexahydrotriazines |
| US20150113858A1 (en) * | 2013-10-24 | 2015-04-30 | Shell Oil Company | Liquid fuel compositions |
| US9663735B2 (en) * | 2013-10-24 | 2017-05-30 | Shell Oil Company | Liquid fuel compositions |
| EP3060633A1 (en) * | 2013-10-24 | 2016-08-31 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| CN105658774A (en) * | 2013-10-24 | 2016-06-08 | 国际壳牌研究有限公司 | Liquid fuel compositions |
| CN103710060A (en) * | 2013-12-25 | 2014-04-09 | 济南开发区星火科学技术研究院 | Preparation method for compound diesel cleansing agent |
| US9388281B2 (en) | 2014-05-27 | 2016-07-12 | International Business Machines Corporation | Preparation of thioether polymers |
| US9592470B2 (en) | 2014-05-27 | 2017-03-14 | International Business Machines Corporation | Sulfur scavenging materials for filters and coatings |
| US9610535B2 (en) | 2014-05-27 | 2017-04-04 | International Business Machines Corporation | Sulfur scavenging materials for filters and coatings |
| US9228059B2 (en) | 2014-05-27 | 2016-01-05 | International Business Machines Corporation | Preparation of thioether polymers |
| US9120897B1 (en) | 2014-05-27 | 2015-09-01 | International Business Machines Corporation | Preparation of thioether polymers |
| US9206289B1 (en) | 2014-06-02 | 2015-12-08 | International Business Machines Corporation | Preparation of functional polysulfones |
| US9120899B1 (en) | 2014-06-02 | 2015-09-01 | International Business Machines Corporation | Preparation of functional polysulfones |
| US9139698B1 (en) | 2014-06-02 | 2015-09-22 | International Business Machines Corporation | Preparation of functional polysulfones |
| US10604539B2 (en) | 2014-06-03 | 2020-03-31 | International Business Machines Corporation | Sulfur scavenging materials |
| US11447507B2 (en) | 2014-06-03 | 2022-09-20 | International Business Machines Corporation | Sulfur scavenging materials |
| US9469660B2 (en) | 2014-06-03 | 2016-10-18 | International Business Machines Corporation | Sulfur scavenging materials comprising hexahydrotriazine-modified particle |
| US9599560B2 (en) | 2014-06-06 | 2017-03-21 | International Business Machines Corporation | Methods and apparatus for detecting metals in liquids |
| US9389181B2 (en) | 2014-06-06 | 2016-07-12 | International Business Machines Corporation | Methods and apparatus for detecting metals in liquids |
| US10525442B2 (en) | 2014-06-16 | 2020-01-07 | International Business Machines Corporation | Methods and apparatus for controlling metals in liquids |
| US10328415B2 (en) | 2014-06-16 | 2019-06-25 | International Business Machines Corporation | Methods and apparatus for controlling metals in liquids |
| US9656239B2 (en) | 2014-06-16 | 2017-05-23 | International Business Machines Corporation | Apparatus for controlling metals in liquids |
| US9770702B2 (en) | 2014-06-16 | 2017-09-26 | International Business Machines Corporation | Methods and apparatus for controlling metals in liquids |
| US9296862B2 (en) | 2014-06-16 | 2016-03-29 | International Business Machines Corporation | Preparation of poly(octatriazacane) |
| US9296863B2 (en) | 2014-06-16 | 2016-03-29 | International Business Machines Corporation | Preparation of poly(octatriazacane) |
| US10015970B2 (en) | 2014-06-19 | 2018-07-10 | International Business Machines Corporation | Antimicrobial PHT coatings |
| US9271498B2 (en) | 2014-06-19 | 2016-03-01 | International Business Machines Corporation | Antimicrobial PHT coatings |
| US9352045B2 (en) | 2014-06-20 | 2016-05-31 | International Business Machines Corporation | Methods and materials for therapeutic delivery |
| US9931409B2 (en) | 2014-06-20 | 2018-04-03 | International Business Machines Corporation | Methods and materials for therapeutic delivery |
| US10071161B2 (en) | 2014-06-20 | 2018-09-11 | International Business Machines Corporation | Methods and materials for therapeutic delivery |
| US9598608B2 (en) | 2014-06-27 | 2017-03-21 | International Business Machines Corporation | Soluble, processable polyhemiaminals and polyhexahydrotriazines |
| US9512332B2 (en) | 2014-06-27 | 2016-12-06 | International Busuiness Machines Corporation | Soluble, processable polyhemiaminals and polyhexahydrotriazines |
| US9587073B2 (en) | 2014-07-24 | 2017-03-07 | International Business Machines Corporation | Thermoplastic toughening of PHT's |
| US9522977B2 (en) | 2014-07-24 | 2016-12-20 | International Business Machines Corporation | Thermoplastic toughening of PHT's |
| US9695282B2 (en) | 2014-08-05 | 2017-07-04 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9586824B2 (en) | 2014-08-05 | 2017-03-07 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9403945B2 (en) | 2014-08-05 | 2016-08-02 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9255172B1 (en) | 2014-08-05 | 2016-02-09 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9598284B2 (en) | 2014-08-05 | 2017-03-21 | International Business Machines Corporation | High-performance, filler-reinforced, recyclable composite materials |
| US9957345B2 (en) | 2014-08-18 | 2018-05-01 | International Business Machines Corporation | 3D printing with PHT based materials |
| US10023735B2 (en) | 2014-08-18 | 2018-07-17 | International Business Machines Corporation | 3D printing with PHT/PHA based materials and polymerizable monomers |
| US9676891B2 (en) | 2014-08-22 | 2017-06-13 | International Business Machines Corporation | Synthesis of dynamic covalent 3D constructs |
| US9809673B2 (en) | 2014-08-22 | 2017-11-07 | International Business Machines Corporation | Synthesis of dynamic covalent 3D constructs |
| US10035882B2 (en) | 2014-08-22 | 2018-07-31 | International Business Machines Corporation | Flame retardant PHT compositions |
| US9453108B2 (en) | 2014-08-22 | 2016-09-27 | International Business Machines Corporation | Flame retardant PHT compositions |
| US11788463B2 (en) * | 2014-10-08 | 2023-10-17 | Ats Chemical, Llc | Compositions for engine carbon removal and methods and apparatus for removing carbon |
| US20180238229A1 (en) * | 2014-10-08 | 2018-08-23 | Bernie C. Thompson | Compositions for Engine Carbon Removal and Methods and Apparatus for Removing Carbon |
| US9228034B1 (en) | 2014-10-09 | 2016-01-05 | International Business Machines Corporation | Hexahydrotriazine, dithiazine, and thioether functionalized materials |
| US9422378B2 (en) | 2014-10-09 | 2016-08-23 | International Business Machines Corporation | Hexahydrotriazine, dithiazine, and thioether functionalized materials |
| US10364326B2 (en) | 2014-10-16 | 2019-07-30 | International Business Machines Corporation | Porous/nanoporous PHT |
| US9644065B2 (en) | 2014-10-16 | 2017-05-09 | International Business Machines Corporation | PHT powder coating materials |
| US9890248B2 (en) | 2014-10-16 | 2018-02-13 | International Business Machines Corporation | Porous/nanoporous PHT |
| US9777116B2 (en) | 2014-10-16 | 2017-10-03 | International Business Machines Corporation | Porous/nanoporous PHT |
| US11098159B2 (en) | 2014-10-16 | 2021-08-24 | International Business Machines Corporation | Porous/nanoporous PHT |
| US10767013B2 (en) | 2014-10-16 | 2020-09-08 | International Business Machines Corporation | Porous/nanoporous PHT |
| US10308765B2 (en) | 2014-10-16 | 2019-06-04 | International Business Machines Corporation | Porous/nanoporous PHT |
| US9303186B1 (en) * | 2014-10-16 | 2016-04-05 | International Business Machines Corporation | PHT powder coating materials |
| US10556990B2 (en) | 2014-10-16 | 2020-02-11 | International Business Machines Corporation | Porous/nanoporous PHT |
| WO2016160187A1 (en) * | 2015-03-31 | 2016-10-06 | Baker Hughes Incorporated | Methods of manufacturing poly(hexahydrotriazine)s via suspension or emulsion polymerization |
| US9505885B2 (en) | 2015-03-31 | 2016-11-29 | Baker Hughes Incorporated | Methods of manufacturing poly(hexahydrotriazine)s via suspension or emulsion polymerization |
| US10080806B2 (en) | 2015-08-19 | 2018-09-25 | International Business Machines Corporation | Sulfur-containing polymers from hexahydrotriazine and dithiol precursors as a carrier for active agents |
| US10702610B2 (en) | 2015-08-19 | 2020-07-07 | International Business Machines Corporation | Method of making sulfur-containing polymers from hexahydrotriazine and dithiol precursors |
| US10336897B2 (en) | 2015-09-03 | 2019-07-02 | International Business Machines Corporation | Tailorable viscoelastic properties of PEG-hemiaminal organogel networks |
| US9758620B2 (en) | 2015-09-03 | 2017-09-12 | International Business Machines Corporation | Tailorable viscoelastic properties of PEG-hemiaminal organogel networks |
| US10113034B2 (en) | 2015-10-05 | 2018-10-30 | International Business Machines Corporation | Polymers from stabilized imines |
| US9879118B2 (en) | 2015-10-05 | 2018-01-30 | International Business Machines Corporation | Polymers from stabilized imines |
| US9765188B2 (en) | 2015-11-02 | 2017-09-19 | International Business Machines Corporation | High molecular weight polythioaminals from a single monomer |
| US10118993B2 (en) | 2015-11-24 | 2018-11-06 | International Business Machines Corporation | Systems chemistry approach to polyhexahydrotriazine polymeric structures |
| US10975201B2 (en) | 2015-11-24 | 2021-04-13 | International Business Machines Corporation | Systems chemistry approach to polyhexahydrotriazine polymeric structures |
| US9873766B2 (en) | 2015-11-24 | 2018-01-23 | International Business Machines Corporation | Systems chemistry approach to polyhexahydrotriazine polymeric structures |
| US9862802B2 (en) | 2015-11-30 | 2018-01-09 | International Business Machines Corporation | Poly(thioaminal) probe based lithography |
| US10006936B2 (en) | 2015-11-30 | 2018-06-26 | International Business Machines Corporation | Poly(thioaminal) probe based lithography |
| US10301429B2 (en) | 2016-02-05 | 2019-05-28 | International Business Machines Corporation | Photoresponsive hexahydrotriazine polymers |
| US9828467B2 (en) | 2016-02-05 | 2017-11-28 | International Business Machines Corporation | Photoresponsive hexahydrotriazine polymers |
| US11066519B2 (en) | 2016-02-05 | 2021-07-20 | International Business Machines Corporation | Photoresponsive hexahydrotriazine polymers |
| CN106565618A (en) * | 2016-10-10 | 2017-04-19 | 常州大学 | Catalyst ligand for directly synthesizing lubricating oil base oil from alpha-olefin, and coordination compound thereof, and preparation methods and applications of catalyst ligand and coordination compound |
| CN114292682A (en) * | 2021-12-01 | 2022-04-08 | 江苏大学 | Green triazine lubricating oil additive, preparation method and application |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20100107476A1 (en) | Compositions and Methods Including Hexahydrotriazines Useful as Direct Injection Fuel Additives | |
| US8231695B2 (en) | Fuel compositions comprising hydrocarbon oil carriers and methods for using the same | |
| US7112230B2 (en) | Fuels compositions for direct injection gasoline engines | |
| US7491248B2 (en) | Fuels compositions and methods for using same | |
| CA2929233C (en) | Mixed detergent composition for intake valve deposit control | |
| JP3554261B2 (en) | Fuel dispersant with enhanced lubricity | |
| US20110010985A1 (en) | Fuel Additive to Control Deposit Formation | |
| AU2014314324B2 (en) | Methods and uses for controlling deposits on valves in direct-injection spark-ignition engines | |
| AU2014314325B2 (en) | Methods and uses for intake-valve and direct-injector deposit clean-up. | |
| EP3375848B1 (en) | Polyol carrier fluids and fuel compositions including polyol carrier fluids | |
| US11884890B1 (en) | Gasoline additive composition for improved engine performance | |
| EP3191568B1 (en) | Uses of controlling particulate emissions in an internal combustion engine | |
| EP4345151A1 (en) | Gasoline additive composition for improved engine performance | |
| KR20240046009A (en) | Gasoline additive composition for improved engine performance | |
| CN117801852A (en) | Gasoline additive composition for improving engine performance | |
| KR20240046010A (en) | Gasoline additive composition for improved engine performance | |
| US20170253822A1 (en) | Methods and uses of controlling piston varnish formation in an internal combustion engine | |
| US20170260470A1 (en) | Methods and uses for controlling sludge in engines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: AFTON CHEMICAL CORPORATION,VIRGINIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:COSIMBESCU, LELIA;REEL/FRAME:021873/0889 Effective date: 20081108 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |