US9216168B1 - Therapeutic compositions comprising imidazole and imidazolium compounds - Google Patents
Therapeutic compositions comprising imidazole and imidazolium compounds Download PDFInfo
- Publication number
- US9216168B1 US9216168B1 US14/540,333 US201414540333A US9216168B1 US 9216168 B1 US9216168 B1 US 9216168B1 US 201414540333 A US201414540333 A US 201414540333A US 9216168 B1 US9216168 B1 US 9216168B1
- Authority
- US
- United States
- Prior art keywords
- zoledronic acid
- amount
- compound
- dosage form
- administered
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title abstract description 87
- 230000001225 therapeutic effect Effects 0.000 title abstract description 4
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 title abstract 5
- 150000004693 imidazolium salts Chemical class 0.000 title abstract 2
- 208000002193 Pain Diseases 0.000 claims abstract description 57
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 21
- 201000011510 cancer Diseases 0.000 claims abstract description 14
- XRASPMIURGNCCH-UHFFFAOYSA-N zoledronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CN1C=CN=C1 XRASPMIURGNCCH-UHFFFAOYSA-N 0.000 claims description 222
- 229960004276 zoledronic acid Drugs 0.000 claims description 219
- 239000002552 dosage form Substances 0.000 claims description 89
- 238000000034 method Methods 0.000 claims description 74
- 241000124008 Mammalia Species 0.000 claims description 48
- 239000008194 pharmaceutical composition Substances 0.000 claims description 41
- 229940125782 compound 2 Drugs 0.000 claims description 38
- 229940125904 compound 1 Drugs 0.000 claims description 37
- 208000023890 Complex Regional Pain Syndromes Diseases 0.000 claims description 14
- 150000001875 compounds Chemical class 0.000 claims description 14
- 206010028391 Musculoskeletal Pain Diseases 0.000 claims description 6
- 206010002556 Ankylosing Spondylitis Diseases 0.000 claims description 5
- 229940126062 Compound A Drugs 0.000 claims description 5
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 claims description 5
- 230000002378 acidificating effect Effects 0.000 claims description 5
- 239000013543 active substance Substances 0.000 claims description 5
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 claims description 5
- 208000008035 Back Pain Diseases 0.000 claims description 4
- 125000000524 functional group Chemical group 0.000 claims description 4
- 208000006820 Arthralgia Diseases 0.000 claims 5
- 208000000112 Myalgia Diseases 0.000 claims 1
- 206010033425 Pain in extremity Diseases 0.000 claims 1
- 208000013465 muscle pain Diseases 0.000 claims 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 20
- 201000010099 disease Diseases 0.000 abstract description 19
- 210000000988 bone and bone Anatomy 0.000 abstract description 12
- 150000002460 imidazoles Chemical class 0.000 abstract description 2
- 239000006186 oral dosage form Substances 0.000 description 80
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 66
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 27
- 230000003442 weekly effect Effects 0.000 description 23
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 22
- 230000036470 plasma concentration Effects 0.000 description 21
- 150000003839 salts Chemical class 0.000 description 21
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 18
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 17
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 15
- 230000002459 sustained effect Effects 0.000 description 15
- 206010003246 arthritis Diseases 0.000 description 13
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 12
- 239000002904 solvent Substances 0.000 description 12
- 0 *C(*)(O)CN1C=CN(CC(*)(*)O)=C1.*C(*)(O)CN1C=CN(CC(=O)O)=C1.*C(C)(O)CN1C=CN=C1 Chemical compound *C(*)(O)CN1C=CN(CC(*)(*)O)=C1.*C(*)(O)CN1C=CN(CC(=O)O)=C1.*C(C)(O)CN1C=CN=C1 0.000 description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- 239000007787 solid Substances 0.000 description 9
- 206010065390 Inflammatory pain Diseases 0.000 description 8
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 8
- 229930000044 secondary metabolite Natural products 0.000 description 8
- -1 Na+ Chemical class 0.000 description 7
- 239000003814 drug Substances 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- HTFOYZBSKYAKDT-UHFFFAOYSA-N CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O Chemical compound CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O HTFOYZBSKYAKDT-UHFFFAOYSA-N 0.000 description 6
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 6
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 6
- MBXHKLIOSQNSCS-UHFFFAOYSA-N O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1 Chemical compound O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1 MBXHKLIOSQNSCS-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 6
- 201000008482 osteoarthritis Diseases 0.000 description 6
- 239000000825 pharmaceutical preparation Substances 0.000 description 6
- 229940127557 pharmaceutical product Drugs 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 208000001132 Osteoporosis Diseases 0.000 description 5
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 5
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000002425 crystallisation Methods 0.000 description 5
- 230000008025 crystallization Effects 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 230000004968 inflammatory condition Effects 0.000 description 5
- 159000000000 sodium salts Chemical group 0.000 description 5
- LFTTYJARUOVXJM-UHFFFAOYSA-N CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O.O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1 Chemical compound CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O.O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1 LFTTYJARUOVXJM-UHFFFAOYSA-N 0.000 description 4
- 206010058019 Cancer Pain Diseases 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- VLIVKFKWSNBCHL-UHFFFAOYSA-M methyl 2-[3-(2-methoxy-2-oxoethyl)imidazol-3-ium-1-yl]acetate;chloride Chemical compound [Cl-].COC(=O)CN1C=C[N+](CC(=O)OC)=C1 VLIVKFKWSNBCHL-UHFFFAOYSA-M 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- SWGASSWPIUTQKR-UHFFFAOYSA-N 2-[3-(carboxymethyl)imidazol-3-ium-1-yl]acetic acid;chloride Chemical compound [Cl-].OC(=O)CN1C=C[N+](CC(O)=O)=C1 SWGASSWPIUTQKR-UHFFFAOYSA-N 0.000 description 3
- 208000007815 Acquired Hyperostosis Syndrome Diseases 0.000 description 3
- 208000025705 Axial Spondyloarthritis Diseases 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 208000025962 Crush injury Diseases 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 208000012659 Joint disease Diseases 0.000 description 3
- 206010027476 Metastases Diseases 0.000 description 3
- 208000034578 Multiple myelomas Diseases 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 208000010191 Osteitis Deformans Diseases 0.000 description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 description 3
- 201000004854 SAPHO syndrome Diseases 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical class [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 201000002661 Spondylitis Diseases 0.000 description 3
- 235000013361 beverage Nutrition 0.000 description 3
- 201000010103 fibrous dysplasia Diseases 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 235000019359 magnesium stearate Nutrition 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000009401 metastasis Effects 0.000 description 3
- 208000004296 neuralgia Diseases 0.000 description 3
- 208000021722 neuropathic pain Diseases 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 230000001052 transient effect Effects 0.000 description 3
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 3
- GUBGYTABKSRVRQ-UHFFFAOYSA-N 2-(hydroxymethyl)-6-[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol Chemical compound OCC1OC(OC2C(O)C(O)C(O)OC2CO)C(O)C(O)C1O GUBGYTABKSRVRQ-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 208000036487 Arthropathies Diseases 0.000 description 2
- 229940122361 Bisphosphonate Drugs 0.000 description 2
- 206010064269 Bone marrow oedema syndrome Diseases 0.000 description 2
- 241000167854 Bourreria succulenta Species 0.000 description 2
- 208000001387 Causalgia Diseases 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- 208000002966 Giant Cell Tumor of Bone Diseases 0.000 description 2
- 206010020584 Hypercalcaemia of malignancy Diseases 0.000 description 2
- 208000003456 Juvenile Arthritis Diseases 0.000 description 2
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 2
- 208000008930 Low Back Pain Diseases 0.000 description 2
- 206010027452 Metastases to bone Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 208000027067 Paget disease of bone Diseases 0.000 description 2
- 208000014677 Periarticular disease Diseases 0.000 description 2
- 201000001947 Reflex Sympathetic Dystrophy Diseases 0.000 description 2
- 229910006069 SO3H Inorganic materials 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 150000001450 anions Chemical class 0.000 description 2
- 208000016738 bone Paget disease Diseases 0.000 description 2
- 201000011143 bone giant cell tumor Diseases 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 235000019693 cherries Nutrition 0.000 description 2
- 208000019069 chronic childhood arthritis Diseases 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 229910021485 fumed silica Inorganic materials 0.000 description 2
- 201000005787 hematologic cancer Diseases 0.000 description 2
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 2
- 208000008750 humoral hypercalcemia of malignancy Diseases 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000002981 neuropathic effect Effects 0.000 description 2
- 201000001119 neuropathy Diseases 0.000 description 2
- 230000007823 neuropathy Effects 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 208000033808 peripheral neuropathy Diseases 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 238000012746 preparative thin layer chromatography Methods 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000000859 sublimation Methods 0.000 description 2
- 230000008022 sublimation Effects 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 238000005084 2D-nuclear magnetic resonance Methods 0.000 description 1
- 229910002012 Aerosil® Inorganic materials 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- YESHYSMQBWACML-UHFFFAOYSA-M CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O.COC(=O)CCl.COC(=O)CN1C=C[N+](CC(=O)OC)=C1.C[Si](C)(C)N1C=CN=C1.O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1.O=C(O)CN1C=C[N+](CC(=O)O)=C1.[Cl-].[Cl-] Chemical compound CC(O)(CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1)P(=O)(O)O.COC(=O)CCl.COC(=O)CN1C=C[N+](CC(=O)OC)=C1.C[Si](C)(C)N1C=CN=C1.O=C(O)CN1=CN(CC(O)(P(=O)(O)O)P(=O)(O)O)C=C1.O=C(O)CN1C=C[N+](CC(=O)O)=C1.[Cl-].[Cl-] YESHYSMQBWACML-UHFFFAOYSA-M 0.000 description 1
- 208000000094 Chronic Pain Diseases 0.000 description 1
- 208000032131 Diabetic Neuropathies Diseases 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 208000007353 Hip Osteoarthritis Diseases 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 241000238367 Mya arenaria Species 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- YKFRUJSEPGHZFJ-UHFFFAOYSA-N N-trimethylsilylimidazole Chemical compound C[Si](C)(C)N1C=CN=C1 YKFRUJSEPGHZFJ-UHFFFAOYSA-N 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 208000031264 Nerve root compression Diseases 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 206010031243 Osteogenesis imperfecta Diseases 0.000 description 1
- 229910018828 PO3H2 Inorganic materials 0.000 description 1
- 208000027868 Paget disease Diseases 0.000 description 1
- 208000004983 Phantom Limb Diseases 0.000 description 1
- 206010056238 Phantom pain Diseases 0.000 description 1
- 206010036376 Postherpetic Neuralgia Diseases 0.000 description 1
- 208000004550 Postoperative Pain Diseases 0.000 description 1
- 206010037779 Radiculopathy Diseases 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 208000005298 acute pain Diseases 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000002567 autonomic effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000006189 buccal tablet Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 239000013626 chemical specie Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000007958 cherry flavor Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229940125773 compound 10 Drugs 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 208000016097 disease of metabolism Diseases 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 239000007888 film coating Substances 0.000 description 1
- 238000009501 film coating Methods 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007919 intrasynovial administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 208000027202 mammary Paget disease Diseases 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- QABLOFMHHSOFRJ-UHFFFAOYSA-N methyl 2-chloroacetate Chemical compound COC(=O)CCl QABLOFMHHSOFRJ-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000007968 orange flavor Substances 0.000 description 1
- XSXHWVKGUXMUQE-UHFFFAOYSA-N osmium dioxide Inorganic materials O=[Os]=O XSXHWVKGUXMUQE-UHFFFAOYSA-N 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical compound OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 208000020431 spinal cord injury Diseases 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 206010044652 trigeminal neuralgia Diseases 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 239000009637 wintergreen oil Substances 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/675—Phosphorus compounds having nitrogen as a ring hetero atom, e.g. pyridoxal phosphate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4172—Imidazole-alkanecarboxylic acids, e.g. histidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/56—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, attached to ring carbon atoms
- C07D233/60—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, attached to ring carbon atoms with hydrocarbon radicals, substituted by oxygen or sulfur atoms, attached to ring nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic System
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/645—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having two nitrogen atoms as the only ring hetero atoms
- C07F9/6503—Five-membered rings
- C07F9/6506—Five-membered rings having the nitrogen atoms in positions 1 and 3
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
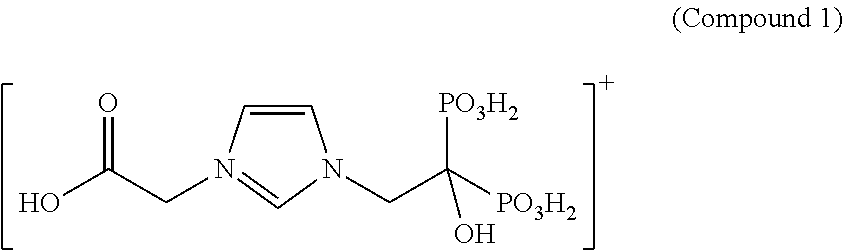
wherein each A is independently an acidic functional group, may be used for a number of medical purposes, such as treatment of undesirable conditions or diseases, including disease or conditions related to bone, cancer, and/or pain. In some embodiments, each A is CO2H, SO3H, OSO2, or PO3H2.
wherein X− is any suitable anion, e.g. F, Br, Cl−, I−, acetate, etc.; and M+ is any suitable cation, e.g. Na+, K+, NH4 +, etc.
wherein X− is any suitable anion, e.g. F−, Br−, Cl−, I−, acetate, etc.; and M+ is any suitable cation, e.g. Na+, K+, NH4 +, etc.
n d=(b a /b d)(n a)
wherein ba is the bioavailability of the diacid form, bd is the bioavailability of the disodium salt form, and na is the number of moles of the diacid that would be administered in a dosage form containing the diacid form of zoledronic acid. For example, if the diacid form has a bioavailability (ba) of 0.01 and the disodium salt form has a bioavailabity (bd) of 0.015, and a dosage form would normally contain 0.001 moles of the diacid, nd would be (0.01/0.015)(0.001 moles), or about 0.00067 moles. In some embodiments, the disodium salt is administered in an amount that has a value of about nd.
p f=1000(C t /C max)
wherein Cmax is the maximum plasma concentration of zoledronic acid after it is administered and Ct is the plasma concentration of zoledronic acid at the time of interest, such as 24 hours. For parenteral administration, the Cmax can be about the C0, or the concentration right after injection of the entire amount of the drug into the body. Sustained plasma level factors can also be obtained for other times, such as 48 hours, by using the plasma concentration of zoledronic acid for Ct in the equation above. For example, if the maximum plasma level of zoledronic acid after administration is 1000 ng/mL and the plasma level of zoledronic acid at 24 hours is 1 ng/mL, the 24 hour sustained plasma level factor is 1.
in an amount that is less than 0.1% w/w, and greater than 0% w/w, based upon the total weight of Compound A, Compound B, and Compound C; or
in an amount that is less than 0.1% w/w, and greater than 0% w/w, based upon the total weight of Compound A, Compound B, and Compound C;
-
- wherein each A is independently an acidic functional group.
is present in an amount that is less than 0.05% w/w, and greater than 0% w/w, based upon the total weight of Compound A, Compound B, and Compound C.
is present in an amount that is less than 0.05% w/w, and greater than 0% w/w, based upon the total weight of Compound A, Compound B, and Compound C.
in an amount that is less than 0.1% w/w, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2; or
in an amount that is less than 0.1% w/w, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
is present in an amount that is less than 0.08% w/w, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
is present in an amount that is less than 0.08% w/w, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
is present in an amount that is less than about 0.05%, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
is present in an amount that is less than about 0.02%, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
is present in an amount that is less than about 0.05% or about 0.02%, and greater than 0% w/w, based upon the total weight of zoledronic acid, Compound 1, and Compound 2.
Claims (21)
Priority Applications (38)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/540,333 US9216168B1 (en) | 2014-05-27 | 2014-11-13 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| AU2015267046A AU2015267046C1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| NZ726690A NZ726690A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| KR1020217009275A KR102409599B1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| EP15800666.8A EP3148534A4 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| CA2950443A CA2950443A1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| PCT/US2015/032739 WO2015184003A1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| CA3114271A CA3114271C (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| KR1020217038529A KR20210149861A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| SG10201706414SA SG10201706414SA (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| MYPI2016704377A MY185481A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| CA3194798A CA3194798A1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| KR1020167036115A KR101975645B1 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| CN201580033784.0A CN106456610A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| SG11201609767PA SG11201609767PA (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| JP2016569674A JP6457555B2 (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitor for pain |
| SG10201906609UA SG10201906609UA (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| MX2016015552A MX2016015552A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain. |
| KR1020197002069A KR20190010728A (en) | 2014-05-27 | 2015-05-27 | Osteoclast inhibitors for pain |
| US14/968,514 US9408862B2 (en) | 2014-05-27 | 2015-12-14 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US15/211,827 US9539268B2 (en) | 2014-05-27 | 2016-07-15 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US15/348,808 US9700570B2 (en) | 2014-05-27 | 2016-11-10 | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| IL249234A IL249234A0 (en) | 2014-05-27 | 2016-11-27 | Osteoclast inhibitors for pain |
| US15/378,939 US9827256B2 (en) | 2014-05-27 | 2016-12-14 | Compositions for administration of zoledronic acid or related compounds for treating lower back pain |
| US15/459,992 US20170182072A1 (en) | 2014-05-27 | 2017-03-15 | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US15/604,394 US9867840B2 (en) | 2014-05-27 | 2017-05-24 | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| AU2017213506A AU2017213506B2 (en) | 2014-05-27 | 2017-08-10 | Osteoclast inhibitors for pain |
| US15/702,616 US10004756B2 (en) | 2014-05-15 | 2017-09-12 | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US15/787,612 US9956238B2 (en) | 2014-05-15 | 2017-10-18 | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| AU2017272199A AU2017272199B2 (en) | 2014-05-27 | 2017-12-06 | Osteoclast inhibitors for pain |
| US15/952,017 US10335424B2 (en) | 2014-05-15 | 2018-04-12 | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US16/002,888 US10195223B2 (en) | 2014-05-15 | 2018-06-07 | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US16/208,413 US20190290664A9 (en) | 2012-05-14 | 2018-12-03 | Neridronic acid for treating complex regional pain syndrome |
| JP2018238424A JP2019069979A (en) | 2014-05-27 | 2018-12-20 | Osteoclast inhibitors for pain |
| US16/366,207 US20190216832A1 (en) | 2012-05-14 | 2019-03-27 | Neridronic acid and other bisphosphonates for treating complex regional pain syndrome and other diseases |
| US16/452,910 US11654152B2 (en) | 2012-05-14 | 2019-06-26 | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| AU2019204559A AU2019204559B2 (en) | 2014-05-27 | 2019-06-27 | Osteoclast inhibitors for pain |
| US18/298,122 US20240108641A1 (en) | 2012-05-14 | 2023-04-10 | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/288,241 US8901161B1 (en) | 2014-05-27 | 2014-05-27 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/288,720 US8865757B1 (en) | 2014-05-28 | 2014-05-28 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/481,097 US8962599B1 (en) | 2014-05-27 | 2014-09-09 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/540,333 US9216168B1 (en) | 2014-05-27 | 2014-11-13 | Therapeutic compositions comprising imidazole and imidazolium compounds |
Related Parent Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/288,241 Continuation US8901161B1 (en) | 2012-05-14 | 2014-05-27 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/481,097 Continuation US8962599B1 (en) | 2012-05-14 | 2014-09-09 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US15/348,808 Continuation US9700570B2 (en) | 2012-05-14 | 2016-11-10 | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US15/378,939 Continuation US9827256B2 (en) | 2012-05-14 | 2016-12-14 | Compositions for administration of zoledronic acid or related compounds for treating lower back pain |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2015/032739 Continuation WO2015184003A1 (en) | 2012-05-14 | 2015-05-27 | Osteoclast inhibitors for pain |
| US14/968,514 Continuation US9408862B2 (en) | 2012-05-14 | 2015-12-14 | Therapeutic compositions comprising imidazole and imidazolium compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20150344505A1 US20150344505A1 (en) | 2015-12-03 |
| US9216168B1 true US9216168B1 (en) | 2015-12-22 |
Family
ID=51702267
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/288,720 Active US8865757B1 (en) | 2012-05-14 | 2014-05-28 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/481,097 Active US8962599B1 (en) | 2012-05-14 | 2014-09-09 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/540,333 Active US9216168B1 (en) | 2012-05-14 | 2014-11-13 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/968,514 Active US9408862B2 (en) | 2012-05-14 | 2015-12-14 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US15/211,827 Active US9539268B2 (en) | 2012-05-14 | 2016-07-15 | Therapeutic compositions comprising imidazole and imidazolium compounds |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/288,720 Active US8865757B1 (en) | 2012-05-14 | 2014-05-28 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US14/481,097 Active US8962599B1 (en) | 2012-05-14 | 2014-09-09 | Therapeutic compositions comprising imidazole and imidazolium compounds |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/968,514 Active US9408862B2 (en) | 2012-05-14 | 2015-12-14 | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US15/211,827 Active US9539268B2 (en) | 2012-05-14 | 2016-07-15 | Therapeutic compositions comprising imidazole and imidazolium compounds |
Country Status (1)
| Country | Link |
|---|---|
| US (5) | US8865757B1 (en) |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9408861B2 (en) | 2012-05-14 | 2016-08-09 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9408860B2 (en) | 2012-05-14 | 2016-08-09 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating low back pain |
| US9408862B2 (en) * | 2014-05-27 | 2016-08-09 | Antecip Bioventures Ii Llc | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US9427403B2 (en) | 2012-05-14 | 2016-08-30 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US9493571B2 (en) | 2014-06-11 | 2016-11-15 | Antecip Bioventures Ii Llc | Compositions comprising RANK/RANKL antagonists and related compounds for treating pain |
| US9616078B2 (en) | 2012-05-14 | 2017-04-11 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9623036B2 (en) | 2014-08-08 | 2017-04-18 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US9655908B2 (en) | 2012-05-14 | 2017-05-23 | Antecip Bioventures Ii Llc | Neridronic acid molecular complex for treating complex regional pain syndrome |
| US9662343B2 (en) | 2012-05-14 | 2017-05-30 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9669040B2 (en) | 2012-05-14 | 2017-06-06 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9675626B2 (en) | 2012-05-14 | 2017-06-13 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9688765B2 (en) | 2014-06-11 | 2017-06-27 | Antecip Bioventures Ii Llc | Methods using RANK/RANKL antagonist antibodies for treating pain |
| US9694023B2 (en) | 2012-05-14 | 2017-07-04 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US9700570B2 (en) | 2014-05-27 | 2017-07-11 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9707245B2 (en) | 2012-05-14 | 2017-07-18 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9707247B2 (en) | 2012-05-14 | 2017-07-18 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9717747B2 (en) | 2012-05-14 | 2017-08-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9770457B2 (en) | 2012-05-14 | 2017-09-26 | Antecip Bioventures Ii Llc | Neridronic acid for treating bone marrow lesion |
| US9782421B1 (en) | 2012-05-14 | 2017-10-10 | Antecip Bioventures Ii Llc | Neridronic acid molecular complex for treating complex regional pain syndrome |
| US9789128B2 (en) | 2012-05-14 | 2017-10-17 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9795622B2 (en) | 2012-05-14 | 2017-10-24 | Antecip Bioventures Ii Llc | Neridronic acid for treating pain associated with a joint |
| US9820999B2 (en) | 2012-05-14 | 2017-11-21 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9827192B2 (en) | 2012-05-14 | 2017-11-28 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9827256B2 (en) | 2014-05-27 | 2017-11-28 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating lower back pain |
| US9844559B2 (en) | 2012-05-14 | 2017-12-19 | Antecip Bioventures Ii Llc | Neridronic acid for treating bone marrow lesions |
| US9861648B2 (en) | 2012-05-14 | 2018-01-09 | Antecip Boiventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9867839B2 (en) | 2012-05-14 | 2018-01-16 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for joint conditions |
| US9867840B2 (en) | 2014-05-27 | 2018-01-16 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9877977B2 (en) | 2012-05-14 | 2018-01-30 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9895383B2 (en) | 2012-05-14 | 2018-02-20 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9901589B2 (en) | 2012-05-14 | 2018-02-27 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9925203B2 (en) | 2012-05-14 | 2018-03-27 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9943531B2 (en) | 2014-08-08 | 2018-04-17 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US9949993B2 (en) | 2012-05-14 | 2018-04-24 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9956237B2 (en) | 2012-05-14 | 2018-05-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9956238B2 (en) | 2014-05-15 | 2018-05-01 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9956234B2 (en) | 2012-05-14 | 2018-05-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for joint conditions |
| US9999628B2 (en) | 2012-05-14 | 2018-06-19 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9999629B2 (en) | 2012-05-14 | 2018-06-19 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10004756B2 (en) * | 2014-05-15 | 2018-06-26 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10016446B2 (en) | 2012-05-14 | 2018-07-10 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating Paget's disease of bone |
| US10016445B2 (en) | 2012-05-14 | 2018-07-10 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10028969B2 (en) | 2012-05-14 | 2018-07-24 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10028908B2 (en) | 2012-05-14 | 2018-07-24 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10034890B2 (en) | 2012-05-14 | 2018-07-31 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10039773B2 (en) | 2012-05-14 | 2018-08-07 | Antecip Bioventures Ii Llc | Neridronic acid for treating arthritis |
| US10080765B2 (en) | 2012-05-14 | 2018-09-25 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10092581B2 (en) | 2014-05-15 | 2018-10-09 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US10111837B2 (en) | 2012-05-14 | 2018-10-30 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds |
| US10173986B2 (en) | 2012-05-14 | 2019-01-08 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US10350227B2 (en) | 2012-05-14 | 2019-07-16 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10413561B2 (en) | 2012-05-14 | 2019-09-17 | Antecip Bioventures Ii Llc | Neridronic acid and other bisphosphonates for treating complex regional pain syndrome and other diseases |
| US10413560B2 (en) | 2012-05-14 | 2019-09-17 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US10463682B2 (en) | 2012-05-14 | 2019-11-05 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating low back pain |
| US10493085B2 (en) | 2012-05-14 | 2019-12-03 | Antecip Bioventures Ii Llc | Neridronic acid and other bisphosphonates for treating complex regional pain syndrome and other diseases |
| US11654152B2 (en) | 2012-05-14 | 2023-05-23 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9079927B1 (en) | 2014-05-27 | 2015-07-14 | Antecip Bioventures Ii Llc | Substituted imidazolium compounds for treating disease |
| US11011254B2 (en) * | 2018-07-31 | 2021-05-18 | International Business Machines Corporation | Chemical formulation-aware cognitive search and analytics |
| CN114057648B (en) * | 2020-07-30 | 2024-02-20 | 常州方圆制药有限公司 | Preparation method of zoledronic acid intermediate impurity |
Citations (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4939130A (en) | 1986-11-21 | 1990-07-03 | Ciba-Geigy Corporation | Substituted alkanediphosphonic acids and pharmaceutical use |
| US5869471A (en) | 1992-06-30 | 1999-02-09 | The Proctor & Gamble Company | Methods for the treatment of arthritis using phosphonates and NSAIDS |
| US6015801A (en) | 1997-07-22 | 2000-01-18 | Merck & Co., Inc. | Method for inhibiting bone resorption |
| US20020033144A1 (en) * | 2000-07-21 | 2002-03-21 | Thompson Donald R. | Compositions and methods of preventing or reducing the risk or incidence of skeletal injuries in horses |
| US6419955B1 (en) | 1998-10-09 | 2002-07-16 | Hoffmann-La Roche Inc. | Process for making bisphosphonate compositions |
| WO2002087555A2 (en) | 2001-05-02 | 2002-11-07 | Novartis Ag | Use of bisphosphonates in the treatment of bone metastasis associated with prostate cancer |
| US20040063670A1 (en) | 2000-11-29 | 2004-04-01 | Alyson Fox | Use of bisphosphonates for pain treatment |
| WO2005063218A2 (en) | 2003-12-23 | 2005-07-14 | Novartis Ag | Pharmaceutical formulations of bisphosphonates |
| US6943155B2 (en) | 2000-04-07 | 2005-09-13 | Lenard M. Lichtenberger | Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity |
| WO2005107751A1 (en) | 2004-05-06 | 2005-11-17 | Merck & Co., Inc. | Methods for treating arthritic conditions in dogs |
| CN101259133A (en) | 2008-03-28 | 2008-09-10 | 王洪 | Zoledronic acid for preparing medicaments for preventing and treating osteoporosis |
| US20090281064A1 (en) | 2005-08-24 | 2009-11-12 | Emisphere Technologies, Inc. | Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers |
| US7658939B2 (en) | 2000-02-08 | 2010-02-09 | Purdue Pharma L.P. | Tamper-resistant oral opioid agonist formulations |
| US7704977B2 (en) | 2006-04-07 | 2010-04-27 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US20100215743A1 (en) | 2009-02-25 | 2010-08-26 | Leonard Thomas W | Composition and drug delivery of bisphosphonates |
| US20110028435A1 (en) | 2009-07-31 | 2011-02-03 | Thar Pharmaceuticals, Inc. | Crystallization method and bioavailability |
| US20110098252A1 (en) | 2002-07-24 | 2011-04-28 | New York University | Treatment of Spinal Mechanical Pain |
| US8053429B2 (en) | 1999-02-22 | 2011-11-08 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8119159B2 (en) | 1999-02-22 | 2012-02-21 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| WO2012071517A2 (en) | 2010-11-24 | 2012-05-31 | Thar Pharmaceuticals, Inc. | Novel crystalline forms |
| US20120190647A1 (en) | 2009-07-31 | 2012-07-26 | Mazen Hanna | Novel oral forms of a phosphonic acid derivative |
| US20130274282A1 (en) | 2012-04-16 | 2013-10-17 | Herriot Tabuteau | Compositions and methods comprising celecoxib or related compounds and dextromethorphan |
| US20130303487A1 (en) | 2012-05-14 | 2013-11-14 | Herriot Tabuteau | Compositions Comprising Zoledronic Acid or Related Compounds for Relieving Inflammatory Pain and Related Conditions |
| US20140051718A1 (en) | 2012-04-16 | 2014-02-20 | Antecip Bioventures Ii Llc | Compositions and Methods Comprising Celecoxib or Related Compounds and Dextromethorphan |
| US20140051669A1 (en) | 2012-05-14 | 2014-02-20 | Antecip Bioventures Ii Llc | Compositions for Oral Administration of Zoledronic Acid or Related Compounds for Treating Disease |
| US8859530B2 (en) | 2013-03-08 | 2014-10-14 | Voltarra Pharmaceuticals, Inc. | Co-administration of steroids and zoledronic acid to prevent and treat osteoarthritis |
| US8865757B1 (en) | 2014-05-28 | 2014-10-21 | Antecip Bioventures Ii Llp | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US8901162B1 (en) | 2014-01-30 | 2014-12-02 | Antecip Bioventures Ii Llc | Substituted imidazolium compounds for treating disease |
| US8901161B1 (en) | 2014-05-27 | 2014-12-02 | Antecip Bioventures Ii Llc | Therapeutic compositions comprising imidazole and imidazolium compounds |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FI109088B (en) | 1997-09-19 | 2002-05-31 | Leiras Oy | Tablet and process for its preparation |
| US20050272705A1 (en) | 2002-10-15 | 2005-12-08 | Victor Sloan | Method of adminstering bisphosphonates |
| EP1567533B1 (en) | 2003-07-03 | 2009-03-11 | Teva Pharmaceutical Industries Ltd. | Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation |
| US7652501B2 (en) | 2008-01-07 | 2010-01-26 | Unity Semiconductor Corporation | Programmable logic device structure using third dimensional memory |
| EP2192126B1 (en) | 2008-11-26 | 2013-03-27 | Synthon B.V. | Process for making zoledronic acid |
| US9169279B2 (en) | 2009-07-31 | 2015-10-27 | Thar Pharmaceuticals, Inc. | Crystallization method and bioavailability |
| US9211257B2 (en) | 2012-05-14 | 2015-12-15 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US20150051175A1 (en) | 2012-05-14 | 2015-02-19 | Antecip Bioventures Ii Llc | Co-Administration of Steroids and Zoledronic Acid to Prevent and Treat Pain |
| US9427403B2 (en) | 2012-05-14 | 2016-08-30 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US9289441B2 (en) | 2014-08-08 | 2016-03-22 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US9616078B2 (en) | 2012-05-14 | 2017-04-11 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US20150057250A1 (en) | 2012-05-14 | 2015-02-26 | Antecip Bioventures Ii Llp | Inhibitors of osteoclast activity for treating arthritis |
| US20140349974A1 (en) | 2014-08-12 | 2014-11-27 | Antecip Bioventures Ii Llc | Zoledronic acid dosage forms for the treatment of pain |
| US9127069B1 (en) | 2014-06-11 | 2015-09-08 | Antecip Bioventures LLC | Compositions comprising rank/rankl antagonists and related compounds for treating pain |
| US9079927B1 (en) | 2014-05-27 | 2015-07-14 | Antecip Bioventures Ii Llc | Substituted imidazolium compounds for treating disease |
| US20140348916A1 (en) | 2014-08-11 | 2014-11-27 | Antecip Bioventures Ii Llc | Treatment of Pain with Oral Dosage Forms Comprising Zoledronic Acid and An Enhancer |
-
2014
- 2014-05-28 US US14/288,720 patent/US8865757B1/en active Active
- 2014-09-09 US US14/481,097 patent/US8962599B1/en active Active
- 2014-11-13 US US14/540,333 patent/US9216168B1/en active Active
-
2015
- 2015-12-14 US US14/968,514 patent/US9408862B2/en active Active
-
2016
- 2016-07-15 US US15/211,827 patent/US9539268B2/en active Active
Patent Citations (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4939130A (en) | 1986-11-21 | 1990-07-03 | Ciba-Geigy Corporation | Substituted alkanediphosphonic acids and pharmaceutical use |
| US5869471A (en) | 1992-06-30 | 1999-02-09 | The Proctor & Gamble Company | Methods for the treatment of arthritis using phosphonates and NSAIDS |
| US6015801A (en) | 1997-07-22 | 2000-01-18 | Merck & Co., Inc. | Method for inhibiting bone resorption |
| US6419955B1 (en) | 1998-10-09 | 2002-07-16 | Hoffmann-La Roche Inc. | Process for making bisphosphonate compositions |
| US8828431B2 (en) | 1999-02-22 | 2014-09-09 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8053429B2 (en) | 1999-02-22 | 2011-11-08 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8323689B2 (en) | 1999-02-22 | 2012-12-04 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8323690B2 (en) | 1999-02-22 | 2012-12-04 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8119159B2 (en) | 1999-02-22 | 2012-02-21 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US7658939B2 (en) | 2000-02-08 | 2010-02-09 | Purdue Pharma L.P. | Tamper-resistant oral opioid agonist formulations |
| US6943155B2 (en) | 2000-04-07 | 2005-09-13 | Lenard M. Lichtenberger | Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity |
| US20020033144A1 (en) * | 2000-07-21 | 2002-03-21 | Thompson Donald R. | Compositions and methods of preventing or reducing the risk or incidence of skeletal injuries in horses |
| US20040063670A1 (en) | 2000-11-29 | 2004-04-01 | Alyson Fox | Use of bisphosphonates for pain treatment |
| WO2002087555A2 (en) | 2001-05-02 | 2002-11-07 | Novartis Ag | Use of bisphosphonates in the treatment of bone metastasis associated with prostate cancer |
| US8772267B2 (en) | 2002-07-24 | 2014-07-08 | New York University | Treatment of spinal mechanical pain |
| US20110098252A1 (en) | 2002-07-24 | 2011-04-28 | New York University | Treatment of Spinal Mechanical Pain |
| WO2005063218A2 (en) | 2003-12-23 | 2005-07-14 | Novartis Ag | Pharmaceutical formulations of bisphosphonates |
| WO2005107751A1 (en) | 2004-05-06 | 2005-11-17 | Merck & Co., Inc. | Methods for treating arthritic conditions in dogs |
| US20090281064A1 (en) | 2005-08-24 | 2009-11-12 | Emisphere Technologies, Inc. | Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers |
| US7704977B2 (en) | 2006-04-07 | 2010-04-27 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8883203B2 (en) | 2006-04-07 | 2014-11-11 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8883201B2 (en) | 2006-04-07 | 2014-11-11 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| CN101259133A (en) | 2008-03-28 | 2008-09-10 | 王洪 | Zoledronic acid for preparing medicaments for preventing and treating osteoporosis |
| US20100215743A1 (en) | 2009-02-25 | 2010-08-26 | Leonard Thomas W | Composition and drug delivery of bisphosphonates |
| US20120190647A1 (en) | 2009-07-31 | 2012-07-26 | Mazen Hanna | Novel oral forms of a phosphonic acid derivative |
| US20110028435A1 (en) | 2009-07-31 | 2011-02-03 | Thar Pharmaceuticals, Inc. | Crystallization method and bioavailability |
| US8399023B2 (en) | 2009-07-31 | 2013-03-19 | Thar Pharmaceuticals, Inc. | Crystallization method and bioavailability |
| US8933057B2 (en) | 2009-07-31 | 2015-01-13 | Thar Pharmaceuticals, Inc. | Crystallization method and bioavailability |
| WO2012071517A2 (en) | 2010-11-24 | 2012-05-31 | Thar Pharmaceuticals, Inc. | Novel crystalline forms |
| US20130274282A1 (en) | 2012-04-16 | 2013-10-17 | Herriot Tabuteau | Compositions and methods comprising celecoxib or related compounds and dextromethorphan |
| US20140051718A1 (en) | 2012-04-16 | 2014-02-20 | Antecip Bioventures Ii Llc | Compositions and Methods Comprising Celecoxib or Related Compounds and Dextromethorphan |
| US20140249108A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating musculoskeletal pain |
| US20140249112A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating low back pain |
| US20140051669A1 (en) | 2012-05-14 | 2014-02-20 | Antecip Bioventures Ii Llc | Compositions for Oral Administration of Zoledronic Acid or Related Compounds for Treating Disease |
| US20140249109A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating ankylosing spondylitis |
| US20140249107A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US20140249113A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral adminstration of zoledronic acid or related compounds for treating paget's disease of bone |
| US20140249317A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US20140249111A1 (en) | 2012-05-14 | 2014-09-04 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating multiple myeloma |
| US20130303488A1 (en) | 2012-05-14 | 2013-11-14 | Herriot Tabuteau | Compositions Comprising Zoledronic Acid or Related Compounds for Treatment of Complex Regional Pain Syndrome |
| US20130303487A1 (en) | 2012-05-14 | 2013-11-14 | Herriot Tabuteau | Compositions Comprising Zoledronic Acid or Related Compounds for Relieving Inflammatory Pain and Related Conditions |
| US20130303486A1 (en) | 2012-05-14 | 2013-11-14 | Herriot Tabuteau | Compositions Comprising Zoledronic Acid or Related Compounds for Relieving Pain Associated with Arthritis |
| US20130303485A1 (en) | 2012-05-14 | 2013-11-14 | Herriot Tabuteau | Compositions for Oral Administration of Zoledronic Acid or Related Compounds for Treating Disease |
| US8859530B2 (en) | 2013-03-08 | 2014-10-14 | Voltarra Pharmaceuticals, Inc. | Co-administration of steroids and zoledronic acid to prevent and treat osteoarthritis |
| US8901162B1 (en) | 2014-01-30 | 2014-12-02 | Antecip Bioventures Ii Llc | Substituted imidazolium compounds for treating disease |
| US8901161B1 (en) | 2014-05-27 | 2014-12-02 | Antecip Bioventures Ii Llc | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US8865757B1 (en) | 2014-05-28 | 2014-10-21 | Antecip Bioventures Ii Llp | Therapeutic compositions comprising imidazole and imidazolium compounds |
Non-Patent Citations (231)
| Title |
|---|
| Adami, S. et al., "Bisphosphonate therapy of reflex sympathetic dystrophy syndrome", Annals of Rheumatic Diseases, 56:201-204 (1997). |
| Allen, Ginger et al., "Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients", Pain® 80:539-544 (1999). |
| Alvarez-Lario, B. et al., "Acceptance of the different denominations for reflex sympathetic dystrophy", Ann Rheum Dis, 60:77-79 (2001). |
| Annual Mid-year Population Estimates, 2010, Office for National Statistics, Statistical Bulletin (2011). |
| Atkins, R.M. et al., "The Use of Dolorimetry in the Assessment of Post-Traumatic Algodystrophy of the Hand", British Journal of Rheumatology, 28:404-409 (1989). |
| Azari, Pari et al., "Efficacy and Safety of Ketamine in Patients with Complex Regional Pain Syndrome", CNS Drugs, 26(3):215-228 (2012). |
| Bertorelli et al., Nociceptin and the ORL-1 ligand [Phe1(CH2-NH)Gly2]nociceptin(1-13)NH2 exert anti-opioid effects in the Freund's adjuvant-induced arthritic rat model of chronic pain. British Journal of Pharmacology (1999) 128, 1252-1258. |
| Bickerstaff, D.R. et al., "Algodystrophy: An Under-Recognized Complication of Minor Trauma", British Journal of Rheumatology, 33:240-248 (1994). |
| Bickerstaff, D.R. et al., "Radiographic Changes in Algodystrophy of the Hand", Journal of Hand Surgery (British Volume) 16B:47-52 (1991). |
| Bingham III et al., Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medical compartment osteoarthritis of the knee. Arthritis & Rheumatism, vol. 54, No. 11, 2006, 3494-3507. |
| Birklein, F. MD, et al., "The important role of neuropeptides in complex regional pain syndrome", Neurology, 57:2179-2184 (2001). |
| Bodde, Marlies I. et al., "Therapy-Resistant Complex Regional Pain Syndrome Type I: To Amputate or Not?", J. Bone Joint Surg. Am, 93:1799-805 (2011). |
| Bonabello, A. et al., "Analgesic effect of bisphosphonates in mice", Pain® 91:269-275 (2001). |
| Breuer, Brenda et al., "An Open-label Pilot Trial of Ibandronate for Complex Regional Pain Syndrome", Clin J Pain, 24(8):685-689 (2008). |
| Bruehl, Stephen Ph.D., "An Update on the Pathophysiology of Complex Regional Pain Syndrome", Anesthesiology, 113(3):713-25 (2010). |
| Brunner, Florian et al., "Biphosphonates for the therapy of complex regional pain syndrome I-Systematic review", European Journal of Pain, 13:17-21 (2009). |
| Cantatore FP, Acquista CA, Pipitone V. Evaluation of bone turnover and osteoclastic cytokines in early rheumatoid arthritis treated with alendronate. J Rheumatol 1999; 26: 2318-2323. |
| Capello ZJ, et al., J Hand Surg Am. 2012; 37:288-296. |
| Cecchini, Marco G. et al., "Bisphosphonates in Vitro Specifically Inhibit, Amound the Hematopoietic Series, the Development of the Mouse Mononuclear Phagocyte Lineage", Journal of Bone and Mineral Research, 5 (10):1019-1027 (1990). |
| Chandler, Labeling of unit dose packages of drugs, Department of Pharmacy Policy, University of Kentucky Hospital, Chandler Medical Center, policy number: PH-04-06, 2009 (cited by examiner in co-pending U.S. Appl. No. 13/894,244). |
| Chauvineau et al. "What is the place of biphosphonates in the treatment of Complex Regional Pain Syndrome I?" Annales de readaptation de medecine physique 48 (2005) 150-157. |
| Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Second Edition. (2002). |
| Clunie, et al., Ann. Rheum. Dis., (Feb. 12, 2014). * |
| Commission Regulation (EC) No. 847/2000, Official Journal of the European Communities, (2000). |
| Committee for Orphan Medicinal Products (COMP) meeting report on the review of applications for orphan designation, European Medicines Agency, Sep. 2013. |
| Complex regional pain syndrome. NHS choices Feb. 27, 2012. |
| Complex Regional Pain Syndrome: Treatment Guidelines, Jun. 2006. Reflex Sympathetic Dystrophy Syndrome Association. Edited by R. Norman Harden. |
| Conte, et al., The Oncologist 2004:9(Supp 4):28-37. |
| Cortet et al., Treatment of Severe, Recalcitrant Reflex Sympathetic Dystrophy Assessment of Efficacy and Safety of the Second Generation Bisphosphonate Pamidronate, Clinical rheumatology, 1997, 16, 1:51-56. |
| Cremers, Serge C.L.M. et al., "Pharmacokinetics/Pharmacodynamics of Bisphosphonates", Clin Pharmacokinet, 44 (6):551-570 (2005). |
| CRPS Orphan Drug Designation, zoledronic acid, Axome Therapeutics, May 6, 2013. |
| CRPS: Biphosphonateshave yet to prove their usefulness. Douleurs Evaluation-Diagnostic-Traitement (2009) 10, 214-217. |
| Cullen et al., MER-101: A bioavailability study of various GIPET formulations in beagle dogs with intraduodenal cannulae. Poster Presentation, Nov. 2007. |
| De Castro et al., Zoledronic acid to treat complex regional pain syndrome type I in adult (case report). Rev. Dor. Sao Paulo, 2011, 12(1): 71-73. |
| de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: A population based study. Pain 2007; 129: 12-20. |
| de Mos M, Huygen FJPM, van der Hoeven-Borgman M, Dieleman JP, Stricker BHC, Sturkenboom MCJM. Outcome of the complex regional pain syndrome. Clin J Pain 2009; 25: 590-597. |
| Del Valle et al. "Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome." Brain, Behavior and Immunity 23 (2009) 85-91. |
| Devogelaer et al., Dramatic Improvement of Intractable Reflex Sympathetic Dystrophy Syndrome by Intravenous Infusions of the Second Generation Bisphosphonate APD., Poster presentation Jun. 1988, Tenth Annual Meeting of the American Society for Bone and Mineral Research Acadia Room, Bissonet Room and Exhibit Hall New Orleans Marriott Hotel Jun. 4-7, 1988. |
| Dielissen et al. "Amputation for Reflex Sympathetic Dystrophy." Jo Bone Joint Surg 1995:77-B: 270-3. |
| Drugs Orange Book Preface. Food and Drug Administration Center for Drug Evaluation and Research, 32nd Edition, accessed Mar. 6, 2014. |
| Drummond et al. "Reflex Sympathetic Dsytrophy: The Significance of Differing Plasma Catecholamine Concentrations in Affected and Unaffected Limbs." Brain (1991), 2025-2036. |
| Dubin, C., Drug Dev. Tech., 10:30 (2010). * |
| E.U. Summary of product characteristics for Zometa and Aclasta (zoledronic acid), (2010). |
| English, A life of pain: woman chooses amputation to deal with painful disorder. Http://www.katu.com/news/loca.A-life-of-pain-woman-chooses amputation-to-deal with . . . Nov. 18, 2013. |
| Epstein et al., Update of Monthly Oral Bisphosphanate Therapy for the Treatment of Osteoporosis: Focus on Ibandronate 150 mg and Risedronate 150 mg, Current Medical Research and Opinion Journal, 25(12): 2951-2960, 2009. |
| EU Product Label for Zometa, accessed 2013. |
| Fay A, Abinun M. Current management of hereditary angio-oedema (C'1 esterase inhibitor deficiency). J Clin Pathol 2002; 55: 266-270. |
| FDA Approved medication Guide-Reclast (zoledronic acid) injection, Aug. 2011. |
| FDA Commissioner Margaret A. Hamburg Statement on Prescription Opioid Abuse (Apr. 3, 2014), available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm391590.htm. |
| FDA Severe Pain with Osteoporosis drugs, Mar. 2008, FDA patient safety news: Show #73. |
| Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: A double-blind placebo-controlled trial of topical ketamine. Pain 2009; 146: 18-25. |
| Forouzanfar T, Koke AJ, van Kleef M, Weber WE. Treatment of complex regional pain syndrome type I. Eur J Pain 2002; 6: 105-122. |
| Gangji V, Appelboom T. Analgesic effect of intravenous pamidronate on chronic back pain due to osteoporotic vertebral fractures. Clin Rheumatol 1999;-18: 266-267. |
| Garth T. Whiteside et al., DiPOA ([8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1 ,3,8-triazaspiro[4.5]dec-3-yl]-acetic Acid), a Novel, Systemically Available, and Peripherally Restricted MU Opioid Agonist with Antihyperalgesic Activity: II. In Vivo D Pharmacological Characterization in the Rat, 310 J. Pharmacol. & Exp. Ther. 793 (2004)). |
| Geertzen JH, Dijkstra PU, van Sonderen EL, Groothoff JW, ten Duis HJ, Eisma WH. Relationship between impairments, disability and handicap in reflex sympathetic dystrophy patients: a long-term follow-up study. Clin Rehabil 1998; 12:402-412. |
| Giles, Risedronate not an Effective Diesease Modifier in Knee Osteoarthritis. Arthritis News (website) 2006. Accessed at http://www.hopkinsarthritis.org/arthritis-news/risedronate-not-an-effective-disease-modifier-in-knee-osteoarthritis. |
| Goebel A, Barker CH, Turner-Stokes L et al. Complex regional pain syndrome in adults: UK guidelines for diagnosis, referral and management in primary and secondary care. London: RCP, 2012. |
| Goebel A, Leite MI, Yang L, Deacon R, Cendan CM, Fox-Lewis A, Vincent A. The passive transfer of immunoglobulin G serum antibodies from patients with longstanding Complex Regional Pain Syndrome. Eur J Pain 2011; 15: 504. e1-504.e6. |
| Goebel A. Complex regional pain syndrome in adults. Rheumatology (Oxford) 2011; 50:1739-1750. |
| Green JR, Rogers MJ. Pharmacologic profile of zoledronic acid: a highly potent inhibitor of bone resorption. Drug Dev Res 2002; 55: 210-224. |
| Gremeaux et al., Complex Regional Pain Syndrome of the knee: early and good action of diphosphonates on pain and function, (English Abstract included) Annales de réadaptation et de médecine physique 50 (2007) 240-243. |
| Guideline Complex Regional Pain Syndrome type I, (2006). |
| Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another, Jul. 9, 2007. ENTR/6283/00 Rev 3. |
| Guo et al., Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain 108 (2004) 95-107. |
| Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005; 65: 9426-9435. |
| Harden RN, Brush! S, Perez RS, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Chont M, Vatine JJ. Validation of proposed diagnostic criteria (the "Budapest Criteria") for Complex Regional Pain Syndrome. Pain 2010; 150: 268-274. |
| Hendren et al., A review of the differences between normal and osteoarthrisis articular cartilage in human knee and ankle joints, The Foot, vol. 19, Issue 3, Sep. 2009, pp. 171-176. |
| Henry McQuay, Opioids in pain management, 353 Lancet 2229, Abstract and 2229 (1999). |
| Henson P, Bruehl S. Complex regional pain syndrome: state of the art update. Curr Treat Options Cardiovasc Med 2010; 12: 156-167. |
| http://cancerguide.org/drugdosing.html, Page Created: 2000, last Updated: Sep. 22, 2000. |
| Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm 2002; 11:47-51. |
| ICD-10-CM Tabular List of Diseases and Injuries 2012 p. 259-261,269-270. Source: The. National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention. |
| ISR & Written Opinion for PCT/US2014/050427, mailed Nov. 20, 2014. |
| Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnee CA, van den Wildenberg FA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 2000; 343:618-624. |
| Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Spinal cord stimulation for chronic reflex sympathetic dystrophy-five-year follow-up. N Engl J Med 2006; 354: 2394-2396. |
| Kemler MA, van de Vusse AC, van den Berg-loonen EM, Barendse GA, van Kleef M, Weber WE. HLA-001 associated with reflex sympathetic dystrophy. Neurology 1999; 53:1350-1351. |
| Kim et al, Analgesic effects of the non-nitrogen-containing bisphosphonates etidronate and clodronate, independent of anti-resorptive effects on bone, European Journal of Pharmacology, vol. 699, Issues 1-3, 15, Jan. 2013, pp. 14-22 (Available online: Nov. 28, 2012). |
| King, et al., Clinical Lung Cancer, 9:179 (2008). * |
| Kingery et al., A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain 104 (2003) 75-84. |
| Kirveskari E, Vartiainen NV, Gockel M, Forss N. Motor cortex dysfunction in complex regional pain syndrome. Clin Neurophysiol 2010; 121: 1085-1091. |
| Kohr D, Tschernatsch M, Schmitz K, Singh P, Kaps M, Schafer KH, Diener M, Mathies J, Matz 0, Kummer W, Maihofner C, FritzT, Birklein F, Blaes F. Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain. 2009; 143: 246-251. |
| Koman LA, Poehling GG, Smith BP, Smith TL, Chloros G. Chapter 59-Complex Regional Pain Syndrome. Green's Operative Hand Surgery, 6th ed.: Churchill Livingstone, 2010. |
| Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid. Arthritis Rheum 2004; 50: 2970-2973. |
| Kramer HH, Eberle T, Uceyler N, Wagner I, Klonschinsky T, Muller LP, Sommer C, Birklein F. TNF-alpha in CRPS and 'normal' trauma-significant differences between tissue and serum. Pain 2011; 152: 285-290. |
| Kretzchmar A, Wiegel T, Al-Batran S, Hinrichs HF, Kindler M, Steck T, Illiger HJ, Heinemann V, Schmidt K, Haus U, Kirner A, Ehninger G. Rapid and sustained influence of intravenous zoledronic acid on course of pain and analgesics consumption in patients with cancer with bone metastases: A multicenter open-label study over 1 year Supportive Cancer Therapy 2007; 4: 203-210. |
| Kubalek et al., Treatment of reflex sympathetic dystrophy with pamidronate: 29 cases, Rheumatology 2001; 40:1394-1397. |
| Laslett, Extended report: Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomized controlled trial. Ann. Rheum. Dis. 2012, 71: 1322-1328. |
| Leis et al. "Facilitated neurogenic inflammation in unaffected limbs of patients with complex regional pain syndrome." Neuroscience Letters 359 (2004) 163-166. |
| Leitha et al. "Pattern recognition in five-phase bone scintigraphy: diagnostic patterns of reflex sympathetic dystrophy in adults." European Journal of Nuclear Medicine vol. 23, No. 3, Mar. 1996. |
| Leonard et al., MER-101 Tablets: A pilot bioavailability study of a novel oral formulation of zoledronic acid. Poster Presentation, Oct. 2007. |
| Leonard et al., Safety Profile of Zoledronic acid in a novel oral formulation. Poster Presentation, Nov. 2009. |
| Leonard et al., Studies of bioavailability and food effects of MER-101 Zoledronic Acid Tablets in Postmenopausal Women. Poster Presentation, Oct. 2009. |
| Lewis et al. "Body perception disturbance: A contribution to pain in complex regional pain syndrome (CRPS)." Pain 133 (2007) 111-119. |
| Lipton, et al., Cancer Investigation, 20:45 (2002; Suppl. 2). * |
| Maihofner et al. "Cortical reorganization during recovery from complex regional pain syndrome." Neurology 2004; 63:693-701. |
| Maihofner et al. "Patterns of cortical reorganization in complex regional pain syndrome." Neurology 2003; 61:1707-1715. |
| Mailis-Gagnon et al. "Sympathectomy for neuropathic pain." Cochrane Database of Systematic Reviews 2002, Issue 1. Art CD002918, 2009. |
| Maillefert et al., Treatment of refractory reflex sympathetic dystrophy with pamidronate, letters to the editor 1995, Correspondence to: Dr J F Maillefert, Service de Rhumatologie, Hopital General, 3 rue du Fb Raines, 21000 Dijon, France. |
| Maksymowych et al. "A Six-Month Randomized, Controlled, Double-Blind, Dose-Response Comparison of Intravenous Pamidronate (60mg Versus 10mg) in the Treatment of Nonsteroidal Antiinflammatory Drug-Refractory Ankylosing Spondylitis." Arthritis & Rheumatism, vol. 46, No. 3, Mar. 2002, pp. 766-773. |
| Manicourt et al., Role of alendronate in therapy for postraumatic complex regional pain syndrome type 1 of the lower extremity arthirits rheum 2004.50:3690-3697. |
| Marinus et al. "Clinical features and pathophysiology of complex regional pain syndrome." Neurology vol. 10, Jul. 2011. |
| McHugh et al., MER-101-03, A multi center, phase II study to compare MER-101 20mg tablets to intravenous Zometa 4mg in prostate cancer patients. Poster Presentation, May 2009. |
| Merrion Pharmaceuticals press release, "Merrion finalises its Phase III development program for Orazol(TM) in the USA," (Nov. 16, 2010). * |
| Merrion Pharmaceuticals press release, "Merrion finalises its Phase III development program for Orazol™ in the USA," (Nov. 16, 2010). * |
| Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms, 2nd ed. Seattle, WA: IASP Press, 1994 p. 40-43. |
| Mos et al. "Medical history and the onset of complex regional pain syndrome (CRPS)." Pain 139 (2009) 458-466. |
| Mos et al. "Outcome of the Complex Regional Pain Syndrome." Clin J Pain, vol. 25, No. 7, Sep. 2009. |
| Mos et al. "The association between ACE inhibitors and the complex regional pain syndrome: Suggestions for a neuro-inflammatory pathogenesis of CRPS." Pain 142 (2009) 218-224. |
| Mos et al. "The incidence of complex regional pain syndrome: A population-based study."Pain 129 (2007) 12-20. |
| Nagae et al., Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J. Bone Miner. Metab. (2007) 25: 99-104. |
| Nagae et al., Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 39 (2006) 1107-1115. |
| Nagakura et al., Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. The Journal of Pharmacology and Experimental Therapeutics 306: 490-497, 2003. |
| Nath et al. "Reflex Sympathetic Dystrophy." Hand Surgery Update 1. Volume 23, No. 3, Jul. 1996. |
| National Health Service, United Kingdom. Complex regional pain syndrome. NHS Choices, www.nhs.uk. Last reviewed May 23, 2012. Accessed Jul. 27, 2012. |
| Olmsted County, Minnesota. Profile o General Demographic Characteristics: 2000. Source: U.S. Census Bureau, Census 2000-Summary File 1, Matrices P1, P3, P4, P8, P9, P12, P13, P17, P18, P19, P20, P23, P27, P28, P33, PCT5,PCT8, PCT11, PCT15, H1, H3, H4, H5, H11, and H12. |
| Opinion of the Committee for Orphan Medicinal Products on orphan medicinal product designation, European Medicines Agency, Sep. 2013, pp. 1-3. |
| Opree et al. "Involvement of the Proinflammatory Cytokines Tumor Necrosis Factor-a, IL-1B, and IL-6 But Not IL-8 in the Development of Heat Hyperalgesia: Effects on Heat Evoked Calcitonin Gene-Related Peptide Release from Rat Skin." The Journal of Neuroscience, Aug. 15, 2000, 20(16):6289-6293. |
| Orange Book: Active Ingredient Search. FDA/Center for Drug Evaluation and Research, Updated Feb. 28, 2014. |
| Orazol(R): Novel approach to adjuvant therapy for improving outcomes in breast cancer. Merrion Pharmaceuticals, accessed 2013. |
| Orcel "Response." Joint Bone Spine 2002; 69; 522-2. |
| Orcel et al. "Bisphosphonates in bone diseases other than osteoporosis." Joint Bone Spine 2002; 69; 19-27. |
| Otrock, et al., Ann. Hematol., 85:605 (Jul. 08, 2006). * |
| Pankaj et al. "Diagnosis of post-traumatic complex regional pain syndrome of the hand: current role of sympathetic skin response and three-phase bone schintigraphy." Journal of Orthopaedic Surgery 2006;14(3)284-90. |
| Pennanen et al. "Effect of Liposomal and Free Bisphosphonates on the IL-1B, IL-6 and TNFa Secretion from RAW 264 Cells in Vitro." Pharmaceutical Research, vol. 12, No. 6, 1995. |
| Perez et al. "Evidence based guideline for complex regional pain syndrome type 1." BMC Neurology 2010, 10:20. |
| Phase II Study, "Multi-Center Phase II Study to Compare MER-101 20mg Tablets to Intravenous Zometa 4mg in Male Bisphosphonate-Naïve Hormone Refractory Prostate Cancer Patients," NCT00636740 (Feb. 2009). * |
| Public summary of opinion on orphan designation. Zoledronic acid for the treatment of complex regional pain syndrome.European Medicines Agency, 2013. |
| Raj "Treatment of Fibrous Dysplasia with Zoledronic Acid Infusion Case Report, Review of Literature and Future Prospectives."WebMD Central, ISSN 2046-1690, Published Nov. 22, 2010. |
| Raja et al. Complex Regional Pain Syndrome I (Reflex Sympathetic Dystrophy).Anesthesiology 2002; 96:1254-60. |
| Reclast Label, Revised: Aug. 2011, Novartis Pharma Stein AG. |
| Reclast Medication Guide. Aug. 2011. |
| Recommendation on elements required to support the medical plausibility and the assumption of significant benefit for an orphan designation. EMA/COMP/15893/2009. Mar. 2, 2010. |
| Rehman et al, P36 Treatment of reflex sympathetic dystrophy with intravenous pamidronate, Abstracts from the Bone and Tooth Society Meeting, Apr. 1991, p. 116. |
| Reid et al. "Comparison of a Single Infusion of Zoledronic Acid with Risedronate for Paget's Disease." N Engl J Med 353:9, Sep. 1, 2005. |
| Reid et al., Intravenous Zoledronic Acid in Postmenopausal Women with Low Bone Mineral Density. N. Engl. J. Med., vol. 346, No. 9, Feb. 2002. |
| Rho et al. "Complex Regional Pain Syndrome." May Clin Proc. 2002; 77:174-180. |
| Ringe "Development of clinical utility of zoledronic acid and patient consideration in the treatment of osteoporosis." Patient Preference and Adherence 2010:4 231-245. |
| Ringe et al., A review of bone pain relief with ibandronate and other bisphosphonates in disorders of increased bone turnover. Clin. Exp. Rheumatol. 2007; 25: 766-774. |
| Ripamonti et al. "Decreases in pain at rest and movement-related pain during zoledronic acid treatment in patients with bone metastases due to breast or prostate cancer: a pilot study." Support Care Center (2007) 15:1177-1184. |
| Robinson et al, Efficacy of Pamidronate in Complex Regional Pain Syndrome Type I, American Academy of Pain Medicine 1526-2375, pp. 276-280,2004. |
| Robinson et al., Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 2004; 5:276-280. |
| Rooij et al. "Familial occurrence of complex regional pain syndrome." Eur J Pain 13 (2009) 171-177. |
| Rooij et al. "HLA-B62 and HLA-DQ8 are associated with Complex Regional Pain Syndrome with fixed dystonia." Pain 145 (2009) 82-85. |
| Rousselle et al. "Osteoclastic Acidifaction Pathways During Bone Resorption." Bone vol. 30, No. 4, Apr. 2002: 533-540. |
| Sandroni, Paola et al., "Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study", Pain® 103:199-207, (2003). |
| Sansoni, Paolo et al., "Inhibition of Antigen-Presenting Cell Function by Alendronate in Vitro", Journal of Bone and Mineral Research, 10(11):1719-1725 (1995). |
| Schinkel, Christian MD, et al., "Inflammatory Mediators are Altered in the Acute Phase of Posttraumatic Complex Regional Pain Syndrome", Clin J. Pain , 22(3):235-239 (2006). |
| Schott GO. Bisphosphonates for pain relief in reflex sympathetic dystrophy? Lancet1997;350: 1117. |
| Schwartzman, Robert J. et al., "The Natural History of Complex Regional Pain Syndrome", Clin J Pain, 25 (4):273-280 (2009). |
| Schwartzman, Robert J., et al., "Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study", Pain® 147:107-115 (2009). |
| Schwenkreis, P. et al., "Bilateral Motor Cortex Disinhibition in Complex Regional Pain Syndrome (CRPS) Type I of the Hand", American Academy of Neurology, 61:515-519 (2003). |
| Scientific Discussion. EMEA 2005, pp. 1-24. |
| Sebastian, Complex regional pain syndrome. Indian J. Past Surg., 44(2): 298-307 (2011). |
| Seok, Hyun et al., "Treatment of Transient Osteoporosis of the Hip with Intravenous Zoledronate", Ann Rehabil Med, 35:432-435 (2011). |
| Sevcik, Molly A. et al., "Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis", Pain® 111:169-180 (2004). |
| Sharma, Amit et al., "A Web-Based Cross-Sectional Epidemiological Survey of Complex Regional Pain Syndrome", Regional Anesthesia and Pain Medicine, 34(2):110-115 (2009). |
| Sharma, Amit et al., "Advances in treatment of complex regional pain syndrome: recent insights on a perplexing disease", Curr Opin Anaesthesiol, 19:566-572 (2006). |
| Sigtermans, Marnix J. et al., "Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type I", Pain® 145:304-311 (2009). |
| Siminoski et al., Intravenous Pamidronate for Treatment of Reflex Sympathetic Dystrophy During Breast Feeding, Journal of Bone and Mineral Research vol. 15, No. 10, 2000. |
| Simm et al., The successful use of pamidronate in an 11-year-old girl with complex regional pain syndrome: Response to treatment demonstrated by serial peripheral quantitative computerised tomographic scan, Bone 46 (2010) 885-888. |
| Sommer, Claudia et al., "Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia", Neurosience Letters, 361:184-187 (2004). |
| Sorbera et al., Zoledronate Disodium, Drugs of the Future 2000, 25(3):259-268. |
| St. Sauver, Jennifer L. et al., "Use of a Medical Records Linkage System to Enumerate a Dydamic Population Over Time: The Rochester Epidemiology Project", Am J. Epidemiol, 173(9):1059-1068 (2011). |
| Stanton-Hicks, Michael et al., "Complex Regional Pain Syndromes: Guidelines for Therapy", The Clinical Journal of Pain, 14:155-166 (1998). |
| Stevenson, P. Helen et al., "Cytotoxic and Migration Inhibitory Effects of Bisphosphonates on Macrophages", Calcif Tissue Int, 38:227-233 (1986). |
| Study: The Use of Zoledronic Acid to Complex Regional Pain Syndrome (Aclasta) sponsored by University of Sao Paulo General Hospital. 2012. Clinical Trials.gov. Accessed on Apr. 5, 2013 at http://clinicaltrials.gov/ct2/show/NCT01788176. |
| The University of Sheffield, Health & Economic impact of a new drug intervention for osteoporosis. http://www.sheffield.ac.uk/humanmetabolism/researchandyou/zoledronicacid, accessed Jun. 2014. |
| Tran, De Q.H., MD et al., "Treatment of complex regional pain syndrome: a review of the evidence-Traitement du syndrome de douleur regionale complexe: une revue des nonnees probantes", Can J. Anesth/J. Can Anesth, 57:149-166 (2010). |
| Transaction History page of related application U.S. Appl. No. 13/894,244 Oct. 2013. |
| Transaction History page of related application U.S. Appl. No. 13/894,252 Oct. 2013. |
| Transaction History page of related application U.S. Appl. No. 13/894,262 Oct. 2013. |
| Turner-Stokes, Lynne et al., "Complex regional pain syndrome in adults: concise guidance", Clinical Medicine, 11 (6):596-600 (2011). |
| U.S. Appl. No. 13/894,244, filed May 14, 2013, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 13/894,252, filed May 14, 2013, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 13/894,262, filed May 14, 2013, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 13/894,274, field May 14, 2013, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/106,291, filed Dec. 13, 2013, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,196, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,206, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,213, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,222, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,226, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,229, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,232, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,236, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/279,241, filed May 15, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/336,642, filed Jul. 21, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/456,939, filed Aug. 11, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/457,659, filed Aug. 12, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/481,097, filed Sep. 2014, Tabuteau, H. * |
| U.S. Appl. No. 14/481,097, filed Sep. 9, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/495,732, filed Jan. 26, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/530,556, filed Oct. 31, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/536,526, filed Jan. 28, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/538,709, filed Nov. 11, 2014, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/604,524, filed Jan. 23, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/605,822, filed Jan. 26, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/607,947, filed Jan. 28, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/607,985, filed Jan. 28, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/625,457, filed Feb. 18, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/635,857, filed Mar. 2, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| U.S. Appl. No. 14/639,013, filed Mar. 4, 2015, Herriot Tabuteau, Antecip Bioventures II LLC. |
| US Product Label for Zometa, revised 2012, Prescribing info. |
| US Product Label for Zometa, revised Sep. 2013, FDA Prescribing Information for Zometa (zoledronic acid) injection. |
| Van de Beek, Willem-Johan et al., "Susceptibility loci for complex regional pain syndrome", Pain® 103:93-97 (2003). |
| Van de Vusse, Anton C. et al., "Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type I [ISRCTN84121379]", BMC Neurology, 4:13: 1-9(2004). |
| Van der Laan, Lijckle MD, et al., "Severe Complications of Reflex Sympathetic Dystrophy: Infection, Ulcers, Chronic Edema, Dystonia, and Myoclonus", Arch Phys Med Rehabil, 79:424-429 (1998). |
| Van der Laan, Lycke, MD et al., "Reflex Sympathetic Dystrophy", Hand Clinics, 13(3):373-385 (1997). |
| Van Offel, J.F. et al., "Influence of cyclic intravenous pamidronate on proinflammatory monocytic cytokine profiles and bone density in rheumatoid arthritis treated with low dose prednisolone and methrotrexate", Clinical and Experimental Rheumatology, 19:13-20 (2001). |
| Varenna et al., Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 2000: 27: 1477-1483. |
| Varenna, Massimo et al., "Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study", Rheumatology, 1-9 (2012). |
| Veldman, Peter HJM et al., "Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients", The Lancet, 342:1012-1016 (1993). |
| Walker et al., Disease modifying and anti-nociceptive effects of the bisphosphonate, zoledronic acid in a model of bone cancer pain. Pain 100 (2002) 219-229. |
| Wasner, Gunnar et al., "Traumatic Neuralgias-Complex Regional Pain Syndromes (Reflex Sympathetic Dystrophy and Causalgia): Clinical Characteristics, Pathophysiologic Mechanisms and Therapy", Neurologic Clinics, 16 (4):851-868, (1998). |
| Wasner, Gunnar et al., "Vascular Abnormalities in Acute Reflex Sympathetic Dystrophy (CRPS I)", Arch Neurol, 56:613-620 (1999). |
| Weiss et al., Biodistribution and Plasma Protein Binding of Zoledronic Acid, Drug Metabolism and Disposition, vol. 36, No. 10, pp. 2043-2049, 2008. |
| Yanow, Jennifer et al., "Complex Regional Pain Syndrome (CRPS/RSD) and Neuropathic Pain: Role of Intravenous Bisphosphonates as Analgesics", TheScientificWorld Journal, 8:229-236 (2008). |
| Yasuhisa Abe et al., Improvement of Pain and Regional Osteoporotic Changes in the Foot and Ankle by Low-dose Bisphosphonate Therapy for Complex Regional Pain Syndrome Type I, J Med Case Reports. 2011;5(349). |
| Ying Wang et al., CXCL10 Controls Inflammatory Pain via Opioid Peptide-Containing Macrophages in Electroacupuncture, PLOS One, Apr. 2014, vol. 9, Issue 4, e94696. |
| Zaspel et al., Treatment of early stage CRPS I-cortisone (methylprednisolone) versus bisphosphonate (zoledronic acid). German Congress of Orthopedics and Traumatology. 71st Annual Meeting of the German Society of Trauma Surgery, 93rd Meeting of the German Society for Orthopedics and Orthopedic Surgery, 48th Meeting of the Professional Association of Specialists in Orthopedics. Berlin, Oct. 24-27, 2007. German Medical Science GMS Publishing House; 2007. |
| Zhang Y, et al., Eur Spine J. 2008; 17:1289-1299. |
| Zhang, et al., J. Med. Case Reports, 7:268 (Dec. 13, 2013). * |
| Zheng, Jack 2009, Formulation and Analytical development for low dose oral drug products, by John Wiley and Sons, Inc. |
| Zoledronate Disodium: Treatment of Tumor-Induced Hypercalcemia Angiogenesis Inhibitor, Drugs of the Future 2000, 25(3) 259-268. |
| Zometa FDA Pharmacology Review, part 1, accessed 2013. |
| Zometa FDA Pharmacology Review, part 2, accessed 2013. |
| Zometa FDA Pharmacology Review, part 3, accessed 2013. |
| Zometa FDA Pharmacology Review, part 4, accessed 2013. |
| Zometa FDA Pharmacology Review, part 5, accessed 2013. |
| Zometa Label, Revised: Mar. 2012, Novartis Pharma Stein AG. |
| Zyluk, A., "The Natural History of Post-Traumatic Reflex Sympathetic Dystrophy", Journal of Hand Surgery (British and European Volume), 23B: 1:20-23 (1998). |
Cited By (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9877977B2 (en) | 2012-05-14 | 2018-01-30 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9782421B1 (en) | 2012-05-14 | 2017-10-10 | Antecip Bioventures Ii Llc | Neridronic acid molecular complex for treating complex regional pain syndrome |
| US11654152B2 (en) | 2012-05-14 | 2023-05-23 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US9427403B2 (en) | 2012-05-14 | 2016-08-30 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US10493085B2 (en) | 2012-05-14 | 2019-12-03 | Antecip Bioventures Ii Llc | Neridronic acid and other bisphosphonates for treating complex regional pain syndrome and other diseases |
| US9884069B2 (en) | 2012-05-14 | 2018-02-06 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9517242B2 (en) | 2012-05-14 | 2016-12-13 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9522157B2 (en) | 2012-05-14 | 2016-12-20 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US10463682B2 (en) | 2012-05-14 | 2019-11-05 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating low back pain |
| US10420782B2 (en) | 2012-05-14 | 2019-09-24 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9585902B2 (en) | 2012-05-14 | 2017-03-07 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating Paget's disease of bone |
| US9585901B2 (en) | 2012-05-14 | 2017-03-07 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9610300B2 (en) | 2012-05-14 | 2017-04-04 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for joint conditions |
| US9616078B2 (en) | 2012-05-14 | 2017-04-11 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9616077B2 (en) | 2012-05-14 | 2017-04-11 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9623038B2 (en) | 2012-05-14 | 2017-04-18 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for bone marrow lesions |
| US10413560B2 (en) | 2012-05-14 | 2019-09-17 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9623037B2 (en) | 2012-05-14 | 2017-04-18 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9655908B2 (en) | 2012-05-14 | 2017-05-23 | Antecip Bioventures Ii Llc | Neridronic acid molecular complex for treating complex regional pain syndrome |
| US9662343B2 (en) | 2012-05-14 | 2017-05-30 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9669040B2 (en) | 2012-05-14 | 2017-06-06 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9675626B2 (en) | 2012-05-14 | 2017-06-13 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10413561B2 (en) | 2012-05-14 | 2019-09-17 | Antecip Bioventures Ii Llc | Neridronic acid and other bisphosphonates for treating complex regional pain syndrome and other diseases |
| US9694023B2 (en) | 2012-05-14 | 2017-07-04 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US9694022B2 (en) | 2012-05-14 | 2017-07-04 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US10350227B2 (en) | 2012-05-14 | 2019-07-16 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9707245B2 (en) | 2012-05-14 | 2017-07-18 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9707247B2 (en) | 2012-05-14 | 2017-07-18 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9717747B2 (en) | 2012-05-14 | 2017-08-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9770457B2 (en) | 2012-05-14 | 2017-09-26 | Antecip Bioventures Ii Llc | Neridronic acid for treating bone marrow lesion |
| US9408861B2 (en) | 2012-05-14 | 2016-08-09 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9789128B2 (en) | 2012-05-14 | 2017-10-17 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9795622B2 (en) | 2012-05-14 | 2017-10-24 | Antecip Bioventures Ii Llc | Neridronic acid for treating pain associated with a joint |
| US9820999B2 (en) | 2012-05-14 | 2017-11-21 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9827192B2 (en) | 2012-05-14 | 2017-11-28 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10265332B2 (en) | 2012-05-14 | 2019-04-23 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US9844559B2 (en) | 2012-05-14 | 2017-12-19 | Antecip Bioventures Ii Llc | Neridronic acid for treating bone marrow lesions |
| US9855213B2 (en) | 2012-05-14 | 2018-01-02 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9861648B2 (en) | 2012-05-14 | 2018-01-09 | Antecip Boiventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US9867839B2 (en) | 2012-05-14 | 2018-01-16 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for joint conditions |
| US10016445B2 (en) | 2012-05-14 | 2018-07-10 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9408860B2 (en) | 2012-05-14 | 2016-08-09 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating low back pain |
| US9511081B2 (en) | 2012-05-14 | 2016-12-06 | Antecip Bioventures II, LLC | Osteoclast inhibitors for knee conditions |
| US9895383B2 (en) | 2012-05-14 | 2018-02-20 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9901589B2 (en) | 2012-05-14 | 2018-02-27 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9925203B2 (en) | 2012-05-14 | 2018-03-27 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9931352B2 (en) | 2012-05-14 | 2018-04-03 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10238672B2 (en) | 2012-05-14 | 2019-03-26 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9949993B2 (en) | 2012-05-14 | 2018-04-24 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US9956237B2 (en) | 2012-05-14 | 2018-05-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for knee conditions |
| US10195141B2 (en) | 2012-05-14 | 2019-02-05 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9956234B2 (en) | 2012-05-14 | 2018-05-01 | Antecip Bioventures Ii Llc | Osteoclast inhibitors for joint conditions |
| US9999628B2 (en) | 2012-05-14 | 2018-06-19 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US9999629B2 (en) | 2012-05-14 | 2018-06-19 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10173986B2 (en) | 2012-05-14 | 2019-01-08 | Antecip Bioventures Ii Llc | Methods for the safe administration of imidazole or imidazolium compounds |
| US10016446B2 (en) | 2012-05-14 | 2018-07-10 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating Paget's disease of bone |
| US10137139B2 (en) | 2012-05-14 | 2018-11-27 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US10028969B2 (en) | 2012-05-14 | 2018-07-24 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10028908B2 (en) | 2012-05-14 | 2018-07-24 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10034890B2 (en) | 2012-05-14 | 2018-07-31 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10039774B2 (en) | 2012-05-14 | 2018-08-07 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10039773B2 (en) | 2012-05-14 | 2018-08-07 | Antecip Bioventures Ii Llc | Neridronic acid for treating arthritis |
| US10052338B2 (en) | 2012-05-14 | 2018-08-21 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10080765B2 (en) | 2012-05-14 | 2018-09-25 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10117880B2 (en) | 2012-05-14 | 2018-11-06 | Antecip Bioventures Ii Llc | Neridronic acid for treating complex regional pain syndrome |
| US10111891B2 (en) | 2012-05-14 | 2018-10-30 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US10111837B2 (en) | 2012-05-14 | 2018-10-30 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds |
| US10111894B2 (en) | 2012-05-14 | 2018-10-30 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US10004756B2 (en) * | 2014-05-15 | 2018-06-26 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US10335424B2 (en) | 2014-05-15 | 2019-07-02 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating disease |
| US9956238B2 (en) | 2014-05-15 | 2018-05-01 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating low back pain |
| US10092581B2 (en) | 2014-05-15 | 2018-10-09 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US10195223B2 (en) | 2014-05-15 | 2019-02-05 | Antecip Bioventures Ii Llc | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease |
| US9539268B2 (en) * | 2014-05-27 | 2017-01-10 | Antecip Bioventures Ii Llc | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US9867840B2 (en) | 2014-05-27 | 2018-01-16 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9408862B2 (en) * | 2014-05-27 | 2016-08-09 | Antecip Bioventures Ii Llc | Therapeutic compositions comprising imidazole and imidazolium compounds |
| US9700570B2 (en) | 2014-05-27 | 2017-07-11 | Antecip Bioventures Ii Llc | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome |
| US9827256B2 (en) | 2014-05-27 | 2017-11-28 | Antecip Bioventures Ii Llc | Compositions for administration of zoledronic acid or related compounds for treating lower back pain |
| US9688765B2 (en) | 2014-06-11 | 2017-06-27 | Antecip Bioventures Ii Llc | Methods using RANK/RANKL antagonist antibodies for treating pain |
| US9579323B2 (en) | 2014-06-11 | 2017-02-28 | Antecip Bioventures Ii Llc | Compositions comprising RANK/RANKL antagonists and related compounds for treating pain |
| US9493571B2 (en) | 2014-06-11 | 2016-11-15 | Antecip Bioventures Ii Llc | Compositions comprising RANK/RANKL antagonists and related compounds for treating pain |
| US9623036B2 (en) | 2014-08-08 | 2017-04-18 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
| US9943531B2 (en) | 2014-08-08 | 2018-04-17 | Antecip Bioventures Ii Llc | Osteoclast inhibitors such as zoledronic acid for low back pain treatment |
Also Published As
| Publication number | Publication date |
|---|---|
| US20160324882A1 (en) | 2016-11-10 |
| US9539268B2 (en) | 2017-01-10 |
| US20150344505A1 (en) | 2015-12-03 |
| US8865757B1 (en) | 2014-10-21 |
| US9408862B2 (en) | 2016-08-09 |
| US8962599B1 (en) | 2015-02-24 |
| US20160095872A1 (en) | 2016-04-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9216168B1 (en) | Therapeutic compositions comprising imidazole and imidazolium compounds | |
| US8901161B1 (en) | Therapeutic compositions comprising imidazole and imidazolium compounds | |
| US9079927B1 (en) | Substituted imidazolium compounds for treating disease | |
| US8835650B1 (en) | Substituted imidazolium compounds for treating disease | |
| US9662343B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US9669040B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US9717747B2 (en) | Osteoclast inhibitors for knee conditions | |
| US9700570B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US9770457B2 (en) | Neridronic acid for treating bone marrow lesion | |
| US10265332B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating disease | |
| US9895383B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US9707245B2 (en) | Neridronic acid for treating complex regional pain syndrome | |
| US9675626B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US9694023B2 (en) | Methods for the safe administration of imidazole or imidazolium compounds | |
| US10039774B2 (en) | Neridronic acid for treating complex regional pain syndrome | |
| US9795622B2 (en) | Neridronic acid for treating pain associated with a joint | |
| US20170079996A1 (en) | Osteoclast Inhibitors for Knee Conditions | |
| US9827256B2 (en) | Compositions for administration of zoledronic acid or related compounds for treating lower back pain | |
| US9999629B2 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US20170071960A1 (en) | Osteoclast inhibitors for knee conditions | |
| US20170079995A1 (en) | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease | |
| US20170065625A1 (en) | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease | |
| US20170065622A1 (en) | Compositions for oral administration of zoledronic acid or related compounds for treating complex regional pain syndrome | |
| US20170056427A1 (en) | Dosage forms for oral administration of zoledronic acid or related compounds for treating disease | |
| US20180064734A1 (en) | Neridronic acid for treating complex regional pain syndrome |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ANTECIP BIOVENTURES II LLC, NEW YORK Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TABUTEAU, HERRIOT;REEL/FRAME:034547/0531 Effective date: 20140515 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| AS | Assignment |
Owner name: ANTECIP BIOVENTURES II LLC, NEW YORK Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TABUTEAU, HERRIOT;REEL/FRAME:039076/0377 Effective date: 20160614 |
|
| CC | Certificate of correction | ||
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 4TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2551); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment: 4 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2552); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment: 8 |
|
| FEPP | Fee payment procedure |
Free format text: ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: BIG.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |