WO1995011898A1 - Condensed pyridine type mevalonolactone intermediate and process for its production - Google Patents
Condensed pyridine type mevalonolactone intermediate and process for its production Download PDFInfo
- Publication number
- WO1995011898A1 WO1995011898A1 PCT/JP1993/001551 JP9301551W WO9511898A1 WO 1995011898 A1 WO1995011898 A1 WO 1995011898A1 JP 9301551 W JP9301551 W JP 9301551W WO 9511898 A1 WO9511898 A1 WO 9511898A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- chloro
- hydrogen
- alkyl
- butyl
- Prior art date
Links
- 0 CC(CC(C1)*#C)OC1=O Chemical compound CC(CC(C1)*#C)OC1=O 0.000 description 3
- OJBONMQCZOSSMB-FPYGCLRLSA-N C=[O]C(CC(CC/C=C/c1c(-c(cc2)ccc2F)c(cccc2)c2nc1C1CC1)O)=O Chemical compound C=[O]C(CC(CC/C=C/c1c(-c(cc2)ccc2F)c(cccc2)c2nc1C1CC1)O)=O OJBONMQCZOSSMB-FPYGCLRLSA-N 0.000 description 1
- IOBOLJSHLCWRQV-CWCINCBISA-N CC(C)(O[C@H](CC(OC)=O)C1)O[C@H]1/C=C/c1ccccc1 Chemical compound CC(C)(O[C@H](CC(OC)=O)C1)O[C@H]1/C=C/c1ccccc1 IOBOLJSHLCWRQV-CWCINCBISA-N 0.000 description 1
- VCNWJEZPTFGKLI-FVWNJDPFSA-N COC(C[C@H](C[C@H](/C=C/c1ccccc1)O)O)=O Chemical compound COC(C[C@H](C[C@H](/C=C/c1ccccc1)O)O)=O VCNWJEZPTFGKLI-FVWNJDPFSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/06—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic System
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/553—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having one nitrogen atom as the only ring hetero atom
- C07F9/576—Six-membered rings
- C07F9/60—Quinoline or hydrogenated quinoline ring systems
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Definitions
- the present invention relates to a novel intermediate for a condensed pyridine type mevalonolactone derivative which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent, and a process for its production as well as a novel condensed pyridine derivative useful as the starting material thereof.
- This method provides a relatively good yield in each step, but has drawbacks such that it is cumbersome including many steps, special conditions (an extremely low temperature, a borane reactant) are reguired to control the steric configuration of two hydroxyl groups (a syn-form is highly active), since the side chain is stepwisely extended, and a highly sophisticated asymmetrical synthetic method is reguired or an inefficient optical resolution has to be carried out to obtain an optically active substance (a (3R,5S)-form is highly active) .
- the present invention provides a novel process developed to solve such problems of the conventional method and a novel intermediate useful for the process.
- ring X is a benzene ring, a substituted benzene ring or a substituted 5- or 6-membered heteroaromatic ring
- each of R 1 and R 2 which are independent of each other is hydrogen, C ⁇ g alkyl, C 3 _ 7 cycloalkyl, C 1-3 alkoxy, butoxy, i-butoxy, sec-butoxy, tert-butoxy, R 20 R 2i N _
- R 3 is hydrogen, ⁇ _ Q alkyl, C 2 _ 6 alkenyl, C 3 _ 7
- R 7 is hydrogen, C- ⁇ . g alkyl, C ⁇ g alkoxy, ⁇ _ 3 alkylthio, chloro, bromo, fluoro, chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or
- R 9a and R 9b represents a hydroxyl-protecting group and is independently methoxymethyl, 2- methoxyethoxymethyl, tetrahydropyranyl, 4- methoxytetrahydropyranyl, 1-ethoxyethyl, 1-methyl-l- methoxyethyl, allyl, benzyl, p-methoxybenzyl, triphenylmethyl, trimethylsilyl, tert-butyldimethylsilyl or tert-butyldiphenylsilyl, or R 9a and R 9b together form isopropylidene, cyclopentylidene, cyclohexylidene or benzylidene; and
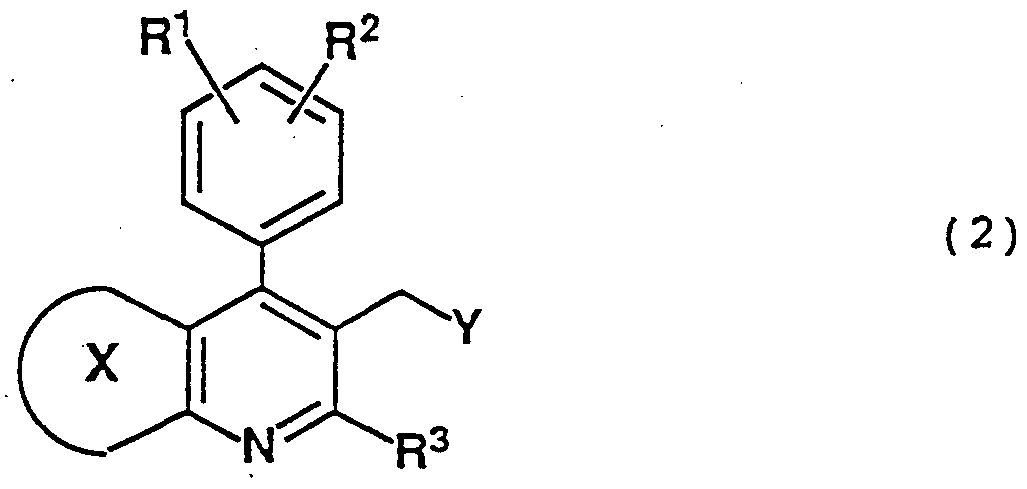
- R 10 is methyl, ethyl, propyl, isopropyl, tert-butyl, tetrahydropyranyl, allyl, benzyl, triphenylmethyl, trimethylsilyl or tert-butyldimethylsilyl), can be produced by reacting a condensed pyridine derivative of the formula (2) :
- Y is P + R 1;L R 12 R 13 Hal ⁇ or P( )R 14 R 15 (wherein each of R 11 , R 12 and R 13 which are independent of one another, is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2-trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl or benzyl, each of R 14 and R 15 which are independent of each other, is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2- trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl, benzyl, methoxy, ethoxy, propoxy, isopropoxy, butoxy, 2-chloroethoxy, 2,2,2- trifluoroethoxy
- R 14 and R 15 together form a 5- or 6-membered ring, Hal is chlorine, bromine or iodine, and is O or S, with a base to form an anion, which is then condensed with a compound of the formula (3):
- R 9a , R 9b and R 10 are as defined above.
- the intermediate of the formula (1) is a novel compound, and it is a useful intermediate which can easily be led to a condensed pyridine type mevalonolactone derivative (I) which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent, stepwisely or in a single step by hydrolyzing R 9a and R 9b which are hydroxyl-protecting groups, and R 10 which is an ester.
- a condensed pyridine type mevalonolactone derivative (I) which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent, stepwisely or in a single step by hydrolyzing R 9a and R 9b which are hydroxyl-protecting groups, and R 10 which is an ester.
- (I-l) represents a condensed pyridine type mevalonic acid ester
- (1-2) represents a condensed pyridine type mevalonic acid
- (1-5) represents a pharmaceutically acceptable salt of the condensed pyridine type mevalonic acid
- (1-3) represents a condensed pyridine type mevalonolactone.
- the compound of the formula (3) can be synthesized by the method of Scheme 5.
- the condensation reaction of the condensed pyridine derivative of the formula (2) with the aldehyde compound of the formula (3) is carried out by withdrawing a hydrogen atom adjacent to Y in the formula (2) by means of a base in an anhydrous inert solvent to form an anion, which is then reacted with the aldehyde compound of the formula (3) .
- the inert solvent may, for example, be an aliphatic hydrocarbon, an aromatic hydrocarbon or an ether type solvent.
- an ether type solvent such as diethyl ether, 1,2-diethoxyethane, 1,2-dimethoxyethane or tetrahydrofuran.
- a polar solvent such as hexamethylphosphoric acid triamide, dimethylsulfoxide or dimethylimidazolidone, may be employed, as the case requires.
- the base may, for example, be a sodium compound such as sodium hydride or sodium amide, a potassium compound such as tert-butoxypotassium, a lithium compound such as butyl lithium or phenyl lithium, or an amide lithium compound such as 2,2,6,6-tetramethylpiperidide lithium.
- a sodium compound such as sodium hydride or sodium amide
- a potassium compound such as tert-butoxypotassium
- a lithium compound such as butyl lithium or phenyl lithium
- an amide lithium compound such as 2,2,6,6-tetramethylpiperidide lithium.
- the reaction temperature varies to some extent depending upon the substrate. However, it is from -78 to . 30°C at the time of the addition of the base, and the reaction with the aldehyde is conducted at a temperature of from -70°C to the refluxing temperature of the solvent.
- the compound of the formula (1) thus synthesized can readily be led to a condensed pyridine type mevalonolactone derivative (I) by the method of the above-mentioned Scheme 3.
- the condensed pyridine type mevalonolactone intermediate of the formula (1) as the compound of the present invention includes compounds of the formulas (la), (lb) and (lc):
- condensed pyridine type mevalonolactone intermediate of the formula (2) as the compound of the present invention includes compounds of the formulas (2a), (2b) and (2c):
- substituents of the above compounds the following substituents may be mentioned.
- substituents R 1 , R 2 , R 3 , Z and Y are as defined above.
- Each of R 1 and R 2 is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert- butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl, cyclobutyl, cyclohexyl, methoxy, ethoxy, propoxy, i-propoxy, butoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or hydroxymethyl.
- R 3 is preferably hydrogen, methyl, ethyl, propyl, i- propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i- pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1-propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl- 1-propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-d
- each of R 4a , R 5a and R 6a which are independent of one another is hydrogen, C- ⁇ g alkyl, C 3 _ 7 cycloalkyl, C ⁇ _ 3 alkoxy, butoxy, i-butoxy, sec-butoxy, R 26 R 27 N- (wherein R 26 and R 27 which are independent of each other, s hydrogen or C ⁇ _ 3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or - 0(CH 2 ) m OR 28 (wherein R 28 is hydrogen or C ⁇ _ 3 alkyl, and m is 1, 2 or 3) ; or
- R 4a and R 5a together form -OC(R 29 ) (R 30 )O- (wherein each of R 29 and R 30 which are independent of each other, is hydrogen or C 1-3 alkyl) when they are at the o- position to each other.
- R a is preferably hydrogen, 5-fluoro, 6-fluoro, 7-fluoro, 8- fluoro, 5-chloro, 6-chloro, 7-chloro, 8-chloro, 5-bromo, 6-bromo, 7-bromo, 8-bromo, 5-methyl, 6-methyl, 7-methyl, 8-methyl, 5-methoxy, 6-methoxy, 7-methoxy, 8-methoxy, 5- trifluoromethyl, 6-trifluoromethyl, 7-trifluoromethyl, 8- trifluoromethyl, 6-trifluoromethox , 6-difluoromethoxy, 8-hydroxyethyl, 5-hydroxy, 6-hydroxy, 7-hydroxy, 8- hydroxy, 6-ethyl, 6-butyl or 7-dimethylamino.
- R a and R 5a may together represent 6-chloro-8-methyl, 6-bromo-7-methoxy, 6-methyl- 7-chloro, 6-chloro-8-hydroxy, 5-methyl-2-hydroxy, 6- methoxy-7-chloro, 6-chloro-7-methox , 6-hydroxy-7 ⁇ chloro, 6-chloro-7-hydroxy, 6-chloro-8-bromo, 5-chloro-6-hydroxy, 6-bromo-8-chloro, 6-bromo-8-hydrox , 5-methyl-8-chloro, 7-hydroxy-8-chloro, 6-bromo-8-hydroxy, 6-methoxy-7- methyl, 6-chloro-8-bromo, 6-methyl-8-bromo, 6,7-difluoro, 6,8-difluoro, 6,7-methylenedioxy, 6 ,8-dichloro, 5,8- dimethyl, 6,8-dimethyl, 6,7-dimethox , 6,7-diethoxy, 6,7
- R 4a , R 5a and R 6a may together represent 5,7- dimethoxy-8-hydroxy, 5,8-dichloro-6-hydroxy, 6,7,8- trimethoxy, 6,7,8-trimethy1, 6,7,8-trichloro, 5-fluoro- 6,8-dibromo or 5-chloro-6,8-dibromo.
- R b is hydrogen, C 1-8 alkyl, C 1-6 alkoxy, C 3 _ 7 cycloalkyl, C 2 _ 6 alkenyl, a- or ⁇ -naphthyl, 2-, 3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3-furyl, fluoro, chloro, bromo,
- each of R 6b , R 7b and R 8b which are independent of one another, is hydrogen, C- ⁇ g alkyl, C ⁇ g alkoxy, C 1-3 alkylthio, chloro, bromo, fluoro, -NR 31 R 32 (wherein each of R 31 and R 32 which are independent of each other, is C ⁇ _ 3 alkyl), chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH 2 ) n OR 33 (wherein R 33 is hydrogen or C ⁇ _ 3 alkyl, and n is 1, 2 or 3), or when R 8b is hydrogen, R 6b and R 7b together form -OC(R 34 ) (R 35 )0- when they are at the o-
- R 5b is bonded to nitrogen atom at the 1- or 2- position of the pyrazolopyridine ring, and such R 5b is hydrogen, ⁇ _ Q alkyl, C 1-3 alkyl substituted by from one to three fluorine atoms, C 3 _ 7 cycloalkyl, a- or ⁇ - naphthyl, 2-, 3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3- furyl or
- C 1 _ 3 alkyl substituted by one member selected f rom C 1 _ 3 alkoxy , hydroxy , naphthyl and
- R 6b , R 7b and R 8 b are as def ined above
- R 6b , R 7b and R 8 b are as def ined above
- R 1 , R 2 , R 3 and Z are as defined with respect to the formula (1) .
- R 4b is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, cyclopropyl, cyclohexyl, phenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4- bromophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- methoxyphenyl, 2-, 3- or 4-trifluoromethylphenyl, 2-, 3- or 4-chloromethylphenyl, 3- or 4-ethoxyphenyl, 4-(2- methylbutyl)phenyl, 4-heptylphenyl, 4-octylphenyl, 4- pentylphenyl, 4-hexylphenyl, 4-propylphenyl, 4- butylphenyl, 4-tert-butylphenyl, 4-butoxyphenyl, 4-
- R 5b is preferably a group bonded to the nitrogen atom at the 1-position of the pyrazolopyridine ring and is methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec- butyl, tert-butyl, 2,2,2-trifluoroethyl, 2-hydroxyethyl, cyclohexyl, benzyl, 2-chlorobenzyl, 2-hydroxybenzyl, 3- trifluoro ethylbenzyl, 2-phenylethyl, phenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- trifluoromethylphenyl, 3- or 4-methoxyphenyl, 2- hydroxyphenyl, 4-isopropylphenyl, 4-tert-butylphenyl, 4- trifluorometh
- each of R 4c and R 5c which are independent of each other is hydrogen, C 1 _ 8 alkyl, C 2 _ 6 alkenyl, C 3 _ 7 cycloalkyl, C ⁇ _ 6 alkoxy, fluoro, chloro, bromo,
- each of R 6c , R 7c and R 8c which are independent of one another, is hydrogen, C 1 _ 4 alkyl, C 1 _ 3 alkoxy, C 3 _ 7 cycloalkyl, trifluoromethyl, fluoro, chloro and bromo), 2-, 3- or 4-pyridyl, 2- or 5- pyrimidyl, 2- or 3-thienyl, 2- or 3-furyl,
- R 4c and R 5c together form C 2 _ 6 alkylene substituted by from zero to three members selected from C 1-4 alkyl, C 3 _ 7 cycloalkyl, fluoro, chloro and bromo and by zero or one member selected from
- R 6c and j are as defined above
- each of R 39 and R 40 which are independent of each other is hydrogen or C 1-4 alkyl
- the following substituents may be mentioned as preferred substituents for R c and R 5c .
- each of R 4c and R 5c which are independent of each other is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl cyclobutyl, cyclopentyl, cyclohexyl, 2- methylcyclohexyl, cycloheptyl, cyclopropylmethyl, vinyl, 1-methylvinyl, 1-propenyl, allyl, 1-methyl-l-propenyl, 1- methyl-2-propenyl, 2-methyl-2-propenyl, 2-butenyl, 1- ethylvinyl, 1,2-dimethyl-l-propenyl, l,2-dimethyl-2- propenyl, 1-ethyl-l-
- R 4c and R 5c together form ethylene, trimethylene, tetramethylene, pentamethylene, methyltetramethylene, chlorotetramethylene or phenyltetramethylene.
- Thienopyridine compounds of the formula (lc) and (2c) wherein R 1 is p-fluoro, R 2 is hydrogen, R c is ethyl, R 5c is methyl and R 3 is cyclopropyl, are preferred.
- Methyl (3R * ,5S * ,6E)-7 ⁇ phenyl-3,5-isopropylidenedioxy- 6-heptenoate (340 mg, 1.17 mmol) obtained in Reference Example 2 was dissolved in ethanol (50 ml), and the solution was cooled to -78°C. A gas mixture of ozone and oxygen supplied from an ozone-generator, was introduced, and the introduction was continued until the reaction solution turned blue. Then, nitrogen gas was introduced to remove excess ozone gas. Then, dimethylsulfide (1.0 ml) was added thereto. The reaction mixture was stirred at room temperature for 12 hours and concentrated.
- Methyl (3S,5R,6E)-7-phen l-3,5-isopropylidenedioxy-6- heptenoate (120 mg, 0.41 mmol) obtained in Reference Example 5 was dissolved in methanol (20 ml), and the solution was cooled to -78°C. A gas mixture of ozone and oxygen supplied from an ozone generator, was introduced, and the introduction was continued until the reaction solution turned blue. Then, nitrogen gas was introduced to remove excess ozone gas, and dimethyl sulfide (0.5 ml) was added thereto. The reaction mixture was stirred at room temperature for 12 hours and concentrated.
- Triphenylphosphine (2.81 g, 10.7 mmol) was added to a toluene solution (50 ml) of 3-bromomethyl-2-cyclopropyl- 4-(4-fluorophenyl)-quinoline (4.00 g, 10.2 mmol) obtained in Reference Example 7, and the mixture was refluxed under heating for 5 hours.
- the formed solid was collected by filtration, washed with toluene and then dried to obtain ⁇ 2-cyclopropyl-4-(4-fluorophenyl)- quinolin-3-yl ⁇ methyltriphenylphosphonium bromide (6.80 g, quantitative yield) as white powder. Melting point 245°C (decomposed)
- IR(CHC1 3 ) 3300, 3050, 1600, 1520, 1495, 1440, 1320, 1220, 1150, 920, 840 cm “1 .
- IR(CHC1 3 ) 3000, 1730, 1600, 1510, 1490, 1380, 1230, 1160, 1090, 840 cm" 1 .
- a THF solution (2.0 ml) of methyl (3R*,5S*)-6-oxo-3,5- isopropylidenedioxy-6-heptenoate (50 mg, 0.23 mmol) obtained in Reference Example 3 was stirred at -78°C for 4 hours, and then the temperature was raised to room temperature overnight with stirring.
- Butyl lithium (0.42 ml, 0.67 mmol) was added at -70°C to a THF solution (15 ml) of ⁇ 2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl ⁇ methyltriphenylphosphonium bromide obtained in Example 1, and the mixture was stirred at -78°C for 30 minutes.
- Butyl lithium (1.24 ml, 2.0 mmol) was added at 0°C to a THF solution (5 ml) of 2,2,6,6-tetramethylpiperidine (161 mg, 1.1 mmol), and the mixture was stirred for 15 minutes. The mixture was cooled to -78°C. Then, a THF solution (5 ml) of diphenyl ⁇ 2-cyclopropyl-4-(4- fluorophenyl)isoquinolin-3-yl ⁇ methylphosphine oxide (419 mg, 0.86 mmol) obtained in Example 3, was added thereto, and the mixture was stirred for 30 minutes.
- a methyl chloride solution (2 ml) of trifluoroacetic acid (170 mg, 1.5 mmol) was added to a methylene chloride solution (5 ml) of methyl (3R * ,5S * ,6E)-7- ⁇ 2-cyclopropyl- 4-( -fluorophenyl)quinolin-3-yl ⁇ -3,5-isopropylidenedioxy- 6-heptenoate (48 mg, 0.1 mmol) obtained in Example 5, and the mixture was stirred at room temperature for 24 hours.
- the reaction solution was cooled with ice, and then a 5% sodium hydrogencarbonate aqueous solution was added, and the mixture was extracted with methyl chloride.
- the present invention provides a novel intermediate for a condensed pyridine type mevalonolactone derivative which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent and a process for its production as well as a novel condensed pyridine derivative useful as a starting material therefor.
Abstract
The present invention provides a synthetic condensed pyridine type mevalonolactone intermediate of formula (1), wherein Z is (a) or (b), each of R?9a and R9b¿ is a hydroxyl-protecting group, and R10 is methyl, ethyl, propyl, isopropyl, tert-butyl, tetrahydropyranyl, allyl, benzyl, triphenylmethyl, trimethylsilyl or tert-butyldimethylsilyl. The intermediate is useful for producing a 7-position substituted (E, 3R, 5S)-3,5-dihydroxy-6-heptenoic acid or its 1,5-lactone or its enantiomer which has an activity as an HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent.
Description
SPECIFICATION CONDENSED PYRIDINE TYPE MEVALONOLACTONE INTERMEDIATE AND PROCESS FOR ITS PRODUCTION TECHNICAL FIELD
The present invention relates to a novel intermediate for a condensed pyridine type mevalonolactone derivative which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent, and a process for its production as well as a novel condensed pyridine derivative useful as the starting material thereof.
BACKGROUND ART Heretofore, a condensed pyridine type mevalonolactone derivative has been synthesized by stepwisely eκrending the side chain of a condensed pyridine ring moiety, as disclosed in European Patent No. 535548 or Japanese Patent Application No. 257870/1991. (Scheme 1) Scheme 1
acceptable salt)
(1-4)
This method provides a relatively good yield in each step, but has drawbacks such that it is cumbersome including many steps, special conditions (an extremely low temperature, a borane reactant) are reguired to control the steric configuration of two hydroxyl groups (a syn-form is highly active), since the side chain is stepwisely extended, and a highly sophisticated asymmetrical synthetic method is reguired or an inefficient optical resolution has to be carried out to obtain an optically active substance (a (3R,5S)-form is highly active) .
DISCLOSURE OF THE INVENTION
The present invention provides a novel process developed to solve such problems of the conventional method and a novel intermediate useful for the process.
A condensed pyridine type mevalonolactone intermediate of the formula (1)
(1)
wherein ring X is a benzene ring, a substituted benzene ring or a substituted 5- or 6-membered heteroaromatic ring, each of R1 and R2 which are independent of each other, is hydrogen, C^g alkyl, C3_7 cycloalkyl, C1-3 alkoxy, butoxy, i-butoxy, sec-butoxy, tert-butoxy, R 20 R 2i N_ (Wherein each of R20 and R21 which are independent of each other, is hydrogen or C1-3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2)eOR22 (wherein R22 is hydrogen or C1-3 alkyl, and e is 1, 2, or 3); or R1 and R2 together form -CH=CH-CH=CH- or methylenedioxy, when they are at the o-position to each other;
R3 is hydrogen, λ_Q alkyl, C2_6 alkenyl, C3_7
_ 'R? cycloalkyl, C5_7 cycloalkenyl or —<>Λ (wherein R7 is hydrogen, C-^.g alkyl, C^g alkoxy, λ_3 alkylthio, chloro, bromo, fluoro, chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or
(each of R9a and R9b represents a hydroxyl-protecting group and is independently methoxymethyl, 2- methoxyethoxymethyl, tetrahydropyranyl, 4- methoxytetrahydropyranyl, 1-ethoxyethyl, 1-methyl-l- methoxyethyl, allyl, benzyl, p-methoxybenzyl, triphenylmethyl, trimethylsilyl, tert-butyldimethylsilyl or tert-butyldiphenylsilyl, or R9a and R9b together form isopropylidene, cyclopentylidene, cyclohexylidene or benzylidene; and
R10 is methyl, ethyl, propyl, isopropyl, tert-butyl, tetrahydropyranyl, allyl, benzyl, triphenylmethyl, trimethylsilyl or tert-butyldimethylsilyl), can be produced by reacting a condensed pyridine derivative of the formula (2) :
Y is P+R1;LR12R13Hal~ or P( )R14R15 (wherein each of R11, R12 and R13 which are independent of one another, is
methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2-trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl or benzyl, each of R14 and R15 which are independent of each other, is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2- trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl, benzyl, methoxy, ethoxy, propoxy, isopropoxy, butoxy, 2-chloroethoxy, 2,2,2- trifluoroethoxy, phenoxy, methoxyphenyloxy, methylphenyloxy, pentafluorophenyloxy or benzyloxy, or
R14 and R15 together form a 5- or 6-membered ring, Hal is chlorine, bromine or iodine, and is O or S, with a base to form an anion, which is then condensed with a compound of the formula (3):
wherein R9a, R9b and R10 are as defined above.
Especially when Y in the formula (2) is P(0)Ph2, the yield of the condensation reaction and the stereo selectivity (trans-selectivity) will be excellent, and the compound of the formula (1) can be obtained in good yield and with a high purity. (Scheme 2)
Scheme 2
.(2) (vm< (l)
The intermediate of the formula (1) is a novel compound, and it is a useful intermediate which can easily be led to a condensed pyridine type mevalonolactone derivative (I) which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent, stepwisely or in a single step by hydrolyzing R9a and R9b which are hydroxyl-protecting groups, and R10 which is an ester. (In the formulas, (I-l) represents a condensed pyridine type mevalonic acid ester, (1-2) represents a condensed pyridine type mevalonic acid, (1-5) represents a pharmaceutically acceptable salt of the condensed pyridine type mevalonic acid, and (1-3) represents a condensed pyridine type mevalonolactone.) (Scheme 3)
Scheme 3
(1) (I-l)
hydrolysis
C 1-5 ) (R =pharmaceutically acceptable salt)
lactonization
These compounds respectively have four stereoisomerε depending upon the steric configurations of hydroxyl groups of the compound of the formula (3) to be used, and they can be produced by the process of the present invention.
The compound of the formula (2) is also novel, and it can be synthesized from a conventional intermediate in accordance with Scheme 4. Scheme 4
( NI ) (DO
(2)
The hydroxyl group of the compound of the formula (VI) is treated by a halogenating agent such as PBr3 to obtain a halogenated compound of the formula (IX). When the halogenated compound is reacted with PR11R12R13 (wherein R11, R12 and R13 are as defined above), a phosphonium salt (a compound of the formula (2) wherein Y is P+R11R12R13Hal_) can be obtained. When the halogenated compound is subjected to an Arbusow reaction with PR14R15(WR16) (wherein R14, R15 and W are as defined above, and R16 is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2-trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl or benzyl), or the above-mentioned phosphonium salt is hydrolyzed, a compound of the formula (2) wherein Y is P(W)R1 R15 (wherein R1 , R15 and W are as defined above) can be prepared.
The compound of the formula (3) can be synthesized by the method of Scheme 5.
Scheme 5
protection O?
O O Y reduction transesterification
0-. — ( 3 ) (Optical isomer)
The condensation reaction of the condensed pyridine derivative of the formula (2) with the aldehyde compound of the formula (3) is carried out by withdrawing a hydrogen atom adjacent to Y in the formula (2) by means of a base in an anhydrous inert solvent to form an anion, which is then reacted with the aldehyde compound of the formula (3) .
The inert solvent may, for example, be an aliphatic
hydrocarbon, an aromatic hydrocarbon or an ether type solvent. Preferred is an ether type solvent such as diethyl ether, 1,2-diethoxyethane, 1,2-dimethoxyethane or tetrahydrofuran. Further, as a stabilizer for the anion, a polar solvent such as hexamethylphosphoric acid triamide, dimethylsulfoxide or dimethylimidazolidone, may be employed, as the case requires.
The base may, for example, be a sodium compound such as sodium hydride or sodium amide, a potassium compound such as tert-butoxypotassium, a lithium compound such as butyl lithium or phenyl lithium, or an amide lithium compound such as 2,2,6,6-tetramethylpiperidide lithium.
The reaction temperature varies to some extent depending upon the substrate. However, it is from -78 to. 30°C at the time of the addition of the base, and the reaction with the aldehyde is conducted at a temperature of from -70°C to the refluxing temperature of the solvent.
The compound of the formula (1) thus synthesized, can readily be led to a condensed pyridine type mevalonolactone derivative (I) by the method of the above-mentioned Scheme 3. The condensed pyridine type mevalonolactone intermediate of the formula (1) as the compound of the present invention includes compounds of the formulas (la), (lb) and (lc):
Further, the condensed pyridine type mevalonolactone intermediate of the formula (2) as the compound of the present invention includes compounds of the formulas (2a), (2b) and (2c):
(2a7 (2b; (2c)
As the substituents of the above compounds, the following substituents may be mentioned. In any compounds, substituents R1, R2, R3, Z and Y are as defined above.
Each of R1 and R2 is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert- butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl, cyclobutyl, cyclohexyl, methoxy, ethoxy, propoxy, i-propoxy, butoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or
hydroxymethyl.
R3 is preferably hydrogen, methyl, ethyl, propyl, i- propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i- pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1-propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl- 1-propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- clorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2-phenethyl or 1-methylbenzyl. In the quinoline type compounds of the formula (la) and (2a), each of R4a, R5a and R6a which are independent of one another, is hydrogen, C-^g alkyl, C3_7 cycloalkyl, Cλ_3 alkoxy, butoxy, i-butoxy, sec-butoxy, R26R27N- (wherein R26 and R27 which are independent of each other, s hydrogen or Cλ_3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or - 0(CH2)mOR28 (wherein R28 is hydrogen or Cλ_3 alkyl, and m is 1, 2 or 3) ; or
R a and R5a together form -CH=CH-CH=CH-; or
R4a and R5a together form -OC(R29) (R30)O- (wherein
each of R29 and R30 which are independent of each other, is hydrogen or C1-3 alkyl) when they are at the o- position to each other.
The following substituents may be mentioned as preferred substituents for R4a, R5a and R6a.
Namely, when both R5a and R6a are hydrogen, R a is preferably hydrogen, 5-fluoro, 6-fluoro, 7-fluoro, 8- fluoro, 5-chloro, 6-chloro, 7-chloro, 8-chloro, 5-bromo, 6-bromo, 7-bromo, 8-bromo, 5-methyl, 6-methyl, 7-methyl, 8-methyl, 5-methoxy, 6-methoxy, 7-methoxy, 8-methoxy, 5- trifluoromethyl, 6-trifluoromethyl, 7-trifluoromethyl, 8- trifluoromethyl, 6-trifluoromethox , 6-difluoromethoxy, 8-hydroxyethyl, 5-hydroxy, 6-hydroxy, 7-hydroxy, 8- hydroxy, 6-ethyl, 6-butyl or 7-dimethylamino. When R6a is hydrogen, R a and R5a may together represent 6-chloro-8-methyl, 6-bromo-7-methoxy, 6-methyl- 7-chloro, 6-chloro-8-hydroxy, 5-methyl-2-hydroxy, 6- methoxy-7-chloro, 6-chloro-7-methox , 6-hydroxy-7~chloro, 6-chloro-7-hydroxy, 6-chloro-8-bromo, 5-chloro-6-hydroxy, 6-bromo-8-chloro, 6-bromo-8-hydrox , 5-methyl-8-chloro, 7-hydroxy-8-chloro, 6-bromo-8-hydroxy, 6-methoxy-7- methyl, 6-chloro-8-bromo, 6-methyl-8-bromo, 6,7-difluoro, 6,8-difluoro, 6,7-methylenedioxy, 6 ,8-dichloro, 5,8- dimethyl, 6,8-dimethyl, 6,7-dimethox , 6,7-diethoxy, 6,7- dibromo or 6,8-dibromo.
Further, R4a, R5a and R6a may together represent 5,7- dimethoxy-8-hydroxy, 5,8-dichloro-6-hydroxy, 6,7,8-
trimethoxy, 6,7,8-trimethy1, 6,7,8-trichloro, 5-fluoro- 6,8-dibromo or 5-chloro-6,8-dibromo.
Quinoline compounds of the formulas (la) and (2a) wherein R1 is p-fluoro, each of R2, R a, R5a and R6a is hydrogen, and R3 is cyclopropyl, are preferred.
In the pyrazolopyridine type compounds of the formulas (lb) and (2b), R b is hydrogen, C1-8 alkyl, C1-6 alkoxy, C3_7 cycloalkyl, C2_6 alkenyl, a- or ^-naphthyl, 2-, 3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3-furyl, fluoro, chloro, bromo,
(wherein each of R6b, R7b and R8b
which are independent of one another, is hydrogen, C-^g alkyl, C^g alkoxy, C1-3 alkylthio, chloro, bromo, fluoro, -NR31R32 (wherein each of R31 and R32 which are independent of each other, is Cλ_3 alkyl), chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2)nOR33 (wherein R33 is hydrogen or Cλ_3 alkyl, and n is 1, 2 or 3), or when R8b is hydrogen, R6b and R7b together form -OC(R34) (R35)0- when they are at the o-position to each other (wherein each of R34 and R35 which are independent of each other, is hydrogen or C1-3 alkyl group), or when R7b and R8b are simultaneously hydrogen, R6b is
(wherein R,3J6b is hydrogen, C1_4 alkyl,
Cλ_3 alkoxy, trifluoromethyl, chloro, bromo or fluoro), phenyl-C2_3 alkenyl wherein the phenyl group may be substituted by C1_4 alkyl, Cλ_3 alkoxy, fluorine, chlorine or bromine, or C1_3 alkyl substituted by one member selected from C1_3 alkoxy, naphthyl and
R5b is bonded to nitrogen atom at the 1- or 2- position of the pyrazolopyridine ring, and such R5b is hydrogen, ±_Q alkyl, C1-3 alkyl substituted by from one to three fluorine atoms, C3_7 cycloalkyl, a- or β- naphthyl, 2-, 3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3- furyl or
C1_3 alkyl substituted by one member selected f rom C1_3 alkoxy , hydroxy , naphthyl and
R1, R2, R3 and Z are as defined with respect to the formula (1) .
The following substituents may be mentioned as preferred substituents for R b and R5b.
Namely, R4b is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, cyclopropyl, cyclohexyl, phenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4- bromophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- methoxyphenyl, 2-, 3- or 4-trifluoromethylphenyl, 2-, 3- or 4-chloromethylphenyl, 3- or 4-ethoxyphenyl, 4-(2- methylbutyl)phenyl, 4-heptylphenyl, 4-octylphenyl, 4- pentylphenyl, 4-hexylphenyl, 4-propylphenyl, 4- butylphenyl, 4-tert-butylphenyl, 4-butoxyphenyl, 4- pentyloxyphenyl, 4-hexyloxyphenyl, 4-heptyloxyphenyl, 4- octyloxyphenyl, 4-phenoxyphenyl, 4-biphenyl, 4- trichloro ethoxyphenyl, 2,4-difluorophenyl, 2,6- difluorophenyl, 2,3-difluorophenyl, 3,5-difluorophenyl, 2,5-difluorophenyl, 3,4-difluorophenyl, 2,4- dichlorophenyl, 2,6-dichlorophenyl, 2,3-dichlorophenyl, 2,5-dichlorophenyl, 3,5-dichlorophenyl, 3,4- dichlorophenyl, 2,3-dimethylphenyl, 2,5-dimethylphenyl, 2,6-dimethylphenyl, 3,4-dimethylphenyl, 2,5- dimethoxyphenyl, 2,6-dimethoxyphenyl, 2,4- dimethoxyphenyl, 3,4-dimethoxyphenyl, 3,5-
dimethoxyphenyl, 3,5-bis(trifluoromethyl)phenyl, 3,4- methylenedioxyphenyl, 2,4,6-trimethoxyphenyl, 3,4,5- trimethylphenyl or 2,4,6-triisopropylphenyl.
R5b is preferably a group bonded to the nitrogen atom at the 1-position of the pyrazolopyridine ring and is methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec- butyl, tert-butyl, 2,2,2-trifluoroethyl, 2-hydroxyethyl, cyclohexyl, benzyl, 2-chlorobenzyl, 2-hydroxybenzyl, 3- trifluoro ethylbenzyl, 2-phenylethyl, phenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- trifluoromethylphenyl, 3- or 4-methoxyphenyl, 2- hydroxyphenyl, 4-isopropylphenyl, 4-tert-butylphenyl, 4- trifluoromethoxyphenyl, 2,3-dichlorophenyl, 2,4- dichlorophenyl, 2,5-dichlorophenyl, 2,6-dichlorophenyl, 3,4-dichlorophenyl, 3,5-dichlorophenyl, 2,4,6- trichlorophenyl, 2,3,4-trichlorophenyl, 2,4- difluorophenyl, 3,5-bis(trifluoromethylJphenyl, 3-chloro- 4-tolyl, 3-chloro-6-tolyl, 4-chloro-2-tolyl, 2-chloro-6- tolyl, 2-chloro-6-fluorophenyl, 2-chloro-5- trifluoromethylphenyl, 3-chloro-4-fluorophenyl, 4-bromo- 3-chlorophenyl, 2-chloro-4-trifluoromethylphenyl, 3- fluoro-6-tolyl, α-naphthyl, 2-pyridyl, 3-methyl-5- trifluoromethyl-2-pyridyl, 4-pyridyl or 2,6-dichloro-4- pyridyl.
Pyrazolopyridine compounds of the formulas (lb) and (2b) wherein R1 is p-fluoro, R2 is hydrogen, each of R4b
and R5b is methyl and R3 is cyclopropyl, are preferred.
In the pyrazolopyridine type compounds of the formulas (lc) and (2c), each of R4c and R5c which are independent of each other, is hydrogen, C1_8 alkyl, C2_6 alkenyl, C3_7 cycloalkyl, Cχ_6 alkoxy, fluoro, chloro, bromo,
(wherein each of R6c, R7c and R8c which
are independent of one another, is hydrogen, C1_4 alkyl, C1_3 alkoxy, C3_7 cycloalkyl, trifluoromethyl, fluoro, chloro and bromo), 2-, 3- or 4-pyridyl, 2- or 5- pyrimidyl, 2- or 3-thienyl, 2- or 3-furyl,
-NR37R38 (wherein each of R37 and R38 which are independent of each other, is hydrogen, C1_ alkyl,
(wherein j is 1, 2 or 3, and R6c
is as defined above), or R37 and R38 together form -(CH2)k- (wherein k is 3, 4 or 5)), C1_3 alkyl substituted by R6c
— (s (wherein R6c is as defined above), and by
zero, one or two Cα_3 alkyl, or a- or /3-naphthyl; or
R4c and R5c together form C2_6 alkylene substituted by from zero to three members selected from C1-4 alkyl, C3_7 cycloalkyl, fluoro, chloro and bromo and by zero or one member selected from
-(CHR39)p-A-(CHR 0)g- (wherein each of p and q is 0, 1, 2 or 3, A is -C(R41)=C(R42)- (wherein each of R41 and R42 is hydrogen or Cχ_3 alkyl), -0-, -S- or -N(R43)- (wherein R43 is hydrogen, C1-4 alkyl, or
(wherein R6c and j are as defined
above)), and each of R39 and R40 which are independent of each other, is hydrogen or C1-4 alkyl) or -CH=CH-CH=CH-. The following substituents may be mentioned as preferred substituents for R c and R5c.
Namely, each of R4c and R5c which are independent of each other, is preferably hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl cyclobutyl, cyclopentyl, cyclohexyl, 2- methylcyclohexyl, cycloheptyl, cyclopropylmethyl, vinyl, 1-methylvinyl, 1-propenyl, allyl, 1-methyl-l-propenyl, 1- methyl-2-propenyl, 2-methyl-2-propenyl, 2-butenyl, 1- ethylvinyl, 1,2-dimethyl-l-propenyl, l,2-dimethyl-2- propenyl, 1-ethyl-l-propenyl, l-ethyl-2-
propenyl, 1-methyl-l-butenyl, l-methyl-2-butenyl, 2- methyl-1-butenyl, 1-i-propylvinyl, 1-methyl-l-pentenyl or phenyl; or
R4c and R5c together form ethylene, trimethylene, tetramethylene, pentamethylene, methyltetramethylene, chlorotetramethylene or phenyltetramethylene.
Thienopyridine compounds of the formula (lc) and (2c) wherein R1 is p-fluoro, R2 is hydrogen, R c is ethyl, R5c is methyl and R3 is cyclopropyl, are preferred. BEST MODE FOR CARRYING OUT THE INVENTION
REFERENCE EXAMPLE 1
Preparation of methyl (3R* ,5S*,6E)-7~phenyl-3,5- dihydroxy-6-heptenoate
Diethylmethoxyborane (1.07 ml, 8.13 mmol) was added at -78°C to a THF (20 ml)/methanol (5.0 ml) solution of methyl (E)-7-phenyl-3,5-dioxo-6-heptenoate (2.00 g, 8.12 mmol), and the mixture was stirred for 15 minutes to room temperature. The mixture was again cooled to -78°C, and sodium borohydride (1.54 g, 40.7 mmol) was added thereto. Then, the reaction mixture was stirred at -78°C for 4 hours and from -78°C to room temperature for 8 hours. Acetic acid (2.0 ml) was added to terminate the reaction, and the reaction mixture was poured into a saturated sodium hydrogencarbonate aqueous solution and extracted
with diethyl ether. The organic layer was washed with a saturated sodium chloride aqueous solution, then dried over sodium sulfate and concentrated. The residue was dissolved in methanol (10 ml) and then concentrated. This operation was repeated 10 times, and the organic boron compound was decomposed and distilled off. The product was purified by column chromatography (hexane:ethyl acetate = 2:1) to obtain methyl (3R*,5S*,6E)-7-phenyl-3,5-dihydroxy-6-heptenoate (1.16 g, 56%).
Rf = 0.08 (hexane:ethyl acetate = 2:1)
IR(CHC13): 3475, 3005, 1720, 1490, 1435, 1205, 1110, 1070, 1030, 775,
730 cm"1.
1H-NMR(CDC13): δ 7.38(d, J=7.2 Hz, 2H), 7.31(t, J=7.2 Hz, 2H), 7.24(π,
J=7.2, 1.3 Hz, IH), 6.62(d, J=15.7 Hz, IH), 6.21(dd, J=15.7, 6.4 Hz, IH),
4.59(m,lH), 4.43(m, IH), 3.74(s, IH), 3.72(s, 3H), 3.24(s, IH), 2.54(dd,
J=19.8, 16.5 Hz, IH), 2.52(dd, J=17.4, 16.5 Hz, IH), 1.80(dt, J=14.3, 9.4
Hz, IH), 1.73(dt, J=14.3, 3.1 Hz, IH).
MS (m/z) 250(M+, 2.5), 232(M+-H20, 3.5), 218(4), 215(4), 200(15),
158(60), 104(100).
REFERENCE EXAMPLE 2
Preparation of methyl (3R*,5S*,6E)-7-phenyl-3,5- isopropylidenedioxy-6-heptenoate
Methyl ( 3R* , 5S* , 6E ) -7-phenyl-3 , 5-dihydroxy-6- heptenoate ( 1 . 10 g , 4 . 39 mmol ) obtained in Reference
Example 1 and p-toluenesulfonic acid (50 mg, catalytic amount) were dissolved in acetone dimethylacetal (10.0 ml), and the reaction mixture was stirred at room temperature for 2 hours. The mixture was diluted with diethyl ether, and the organic layer was washed with a saturated sodium hydrogencarbonate aqueous solution and a sodium chloride aqueous solution, then dried over anhydrous magnesium sulfate and concentrated. The product was purified by column chromatography (hexane:ethyl acetate = 10:1) to obtain methyl
(3R*,5S*,6E)-7-phenyl-3,5-isopropylidenedioxy-6- heptenoate (1.17 g, 92%) as colorless oil. Rf = 0.78 (hexane:ethyl acetate = 2:1)
IR(CHC13): 3000, 1735, 1440, 1380, 1200, 1160, 10S5, 1030, 770, 740 cm"1.
1H-NMR(CDC13): δ 7.37(d, J=7.2 Hz, 2H), 7.29(t, J=7.2 Hz, 2H), 7.24(tt, J=7.2, 1.3 Hz, IH), 6.60(d, J=15.9 Hz, IH), 6.16(dd, J=15.9, 6.2 Hz, IH), 4.57(m,lH), 4.40(m, IH), 3.70(s, 3H), 2.60(dd, J=15.6, 6.9 Hz, IH), 2.52(dd, J=15.6, 6.2 Hz, IH), 1.74(dt, J=12.3, 2.5 Hz, IH), 1.54(s, 3H), 1.45(s, 3H), 1.40(dd, J=11.4, 10.2 Hz, IH).
MS (m/z) 290(M+, 3), 232(M+-C09Me, 4), 215(15), 158(50), 104(100).
REFERENCE EXAMPLE 3
Preparation of methyl (3R* ,5S*)-6-oxo-3, 5- isopropylidenedioxy-6-heptenoate
Methyl (3R*,5S*,6E)-7~phenyl-3,5-isopropylidenedioxy- 6-heptenoate (340 mg, 1.17 mmol) obtained in Reference Example 2 was dissolved in ethanol (50 ml), and the solution was cooled to -78°C. A gas mixture of ozone and oxygen supplied from an ozone-generator, was introduced, and the introduction was continued until the reaction solution turned blue. Then, nitrogen gas was introduced to remove excess ozone gas. Then, dimethylsulfide (1.0 ml) was added thereto. The reaction mixture was stirred at room temperature for 12 hours and concentrated. The product was purified by column chromatography (hexane:ethyl acetate = 3:1) to obtain methyl (3R",5SX)- 6-0x0-3,5-isopropylidenedioxy-6-heptenoate (210 mc, 83%) as colorless crystals. Rf = 0.14 (hexane:ethyl acetate = 2:1)
IR(CHC13):2950, 1735, 1435, 1380, 1080, 1030, 775, 730 cm.-1 . 1H-NMR(CDC13): S 9.58(s, IH), 4.38(m, IH), 4.33(m, IH), 3.70(s, 3H), 2.58(dd, J=15.8, 7.0 Hz, IH), 2.44(dd, J=15.8, 6.0 Hz, IH), 1.86(dt, J=12.9, 2.7 Hz, IH), 1.50(s, 3H), 1.46(s, 3H), 1.35(dt, J=12.0, 12.0 Hz, IH).
MS (m/z) 201(M+-Me, 24), 129(31), 97(36), 59(100). REFERENCE EXAMPLE 4
Preparation of methyl (3S, 5R, 6E)-7-phenyl-3 , 5-dihvdroxy- 6-heotenoate
(4S)-4,7,7-trimethyl-3-exo-(l- naphthyl)bicyclo[2.2.1]heptan-2-exo-yl (3S,5R,6E)-7- phenyl-3,5-dihydroxy-6-heptenoate (210 mg, 0.42 mmol) prepared in accordance with a literature [J. Org. Chem. , 56_, 5752 (1991)] was dissolved in methanol, and a IM sodium hydroxide aqueous solution (0.2 ml) was added thereto. The mixture was stirred at room temperature for 12 hours. Then, methanol was removed under reduced pressure. The residue was diluted with water, and (4S)- 4,7,7-trimethy1-3-exo-(1-naphthyl)bicyclo[ 2.2.1]heptan-2- exo-ol was extracted with diethyl ether. The aqueous layer was acidified with hydrochloric acid, and the carboxylic acid was extracted with diethyl ether. The organic layer was treated with a diethyl ether solution of diazomethane to form a methyl ester. Acetic acid was added to consume excess diazomethane, and the organic layer was washed with a saturated sodium hydrogencarbonate aqueous solution, then dried over anhydrous magnesium sulfate and concentrated. The product was purified by column chromatography
(hexane:ethyl acetate = 2:1) to obtain methyl (3S,5R,6E)- 7-phenyl-3,5-dihydroxy-6-heptenoate (92 mg, 87%) as colorless oil. Rf = 0.08 (hexane:ethyl acetate = 2:1)
IR(CHC13): 3475, 3005, 1720, 1490, 1435, 1205, 1110, 1070, 1030, 775, 730 cm"1. IH- MR(CDC13): δ 7.38(d, J=7.2 Hz, 2H), 7.31(t, J=7.2 Hz, 2H), 7.24(tt,
J=7.2 Hz, 1.3, IH), 6.62(d, J = 15.7 Hz, IH), 6.21(dd, J=15.7, 6.4 Hz, IH), 4.59(m,lH), 4.43(m, IH), 3.74(s, IH), 3.72(s, 3H), 3.24(s, IH), 2.54(dd, J=19.8, 16.5 Hz, IH), 2.52(dd, J=17.4, 16.5 Hz, IH), l.S0(dt, J=14.3, 9.4 Hz, IH), 1.73(dt, J=14.3, 3.1 Hz, IH). MS (m/z) 250(M+, 2.5), 232(M÷-H20, 3.5), 218(4), 215(4), 200(15), 158(60), 104(100).
HRMS Calcd. for C14Hlg04; M+ 250.1222, found m/z 250.1224. [*]D 20+8.23° (c 1.19, CHC13)
REFERENCE EXAMPLE 5 Preparation of methyl (3S,5R,6E)-7-phenyl-3,5- isopropylidenedioxy-6-heptenoate
Methyl (3S,5R,6E)-7-phenyl-3,5-dihydroxy-6-heptenoate (90 mg, 0.36 mmol) obtained in Reference Example 4 and p- toluenesulfonic acid (5 mg, catalytic amount) were dissolved in acetone dimethylacetal (1.0 ml). The reaction mixture was stirred at room temperature for 6 hours. Then, the mixture was diluted with diethyl ether, and the organic layer was washed with a saturated sodium hydrogencarbonate aqueous solution and a sodium chloride aqueous solution, then dried over anhydrous magnesium sulfate and concentrated. The product was purified by column chromatography (hexane:ethyl acetate = 10:1) to obtain methyl (3S,5R,6E)-7-phenyl-3,5- isopropylidenedioxy-6-heptenoate (97 mg, 92%) as
colorless oil.
Rf = 0.78 (hexane:ethyl acetate = 2:1)
IR(CHC13): 3000, 1735, 1440, 1380, 1200, 1160, 1085, 1030, 770, 740 cm"1. 1H-NMR(CDC13): δ 7.37(d, J=7.2 Hz, 2H), 7.29(t, J=7.2 Hz, 2H), 7.24(tt, J=7.2, 1.3 Hz, IH), 6.60(d, J=15.9 Hz, IH), 6.16(dd, J = 15.9, 6.2 Hz, IH), 4.57(m,lH), 4.40(m, IH), 3.70(s, 3H), 2.60(dd, J=15.6, 6.9 Hz, IH), 2.52(dd, J=15.6, 6.2 Hz, IH), 1.74(dt, J=12.3, 2.5 Hz, IH), 1.54(s, 3H), 1.45(s, 3H), 1.40(dd, J=l 1.4, 10.2 Hz, IH).
MS (m/z) 290(M+, 3), 232(M+-C02Me, 4), 215(15), 158(50), 104(100). Milli MS Calcd. for C17H2204 M+; 290.1498, found m/z; 290.1496. [ ]D 20 +6.66* (c 1.11, CHC13)
REFERENCE EXAMPLE 6
Preparation of methyl (3S,5R)-6-oxo-3,5- isopropylidenedioxy-6-heptenoate
Methyl (3S,5R,6E)-7-phen l-3,5-isopropylidenedioxy-6- heptenoate (120 mg, 0.41 mmol) obtained in Reference Example 5 was dissolved in methanol (20 ml), and the solution was cooled to -78°C. A gas mixture of ozone and oxygen supplied from an ozone generator, was introduced, and the introduction was continued until the reaction solution turned blue. Then, nitrogen gas was introduced to remove excess ozone gas, and dimethyl sulfide (0.5 ml) was added thereto. The reaction mixture was stirred at
room temperature for 12 hours and concentrated. The product was purified column chromatography (hexane:ethyl acetate = 3:1) to obtain methyl (3S,5R)-6-oxo-3,5- isopropylidenedioxy-6-heptenoate (49 mg, 90%) as colorless oil. Rf = 0.14 (hexane:ethyl acetate = 2:1)
IR(CHC13): 2950, 1735, 1435, 1380, 1080, 1030, 775, 730 cm'1. 1H-NMR(CDC13): δ 9.58(s, IH), 4.38(m,lH), 4.33(m, IH), 3.70(s, 3H), 2.58(dd, J=15.8, 7.0, IH), 2.44(dd, J=15.8, 6.0, IH), 1.86(dt, J=12.9, 2.7, IH), 1.50(s, 3H), 1.46(s, 3H), 1.35(dt, J=12.0. 12.0, IH). MS (m/z) 201(M+-Mε, 24), 129(31), 97(36), 59(100). [ a ]D 20=20.0θ' (c 1.03, CHC13)
REFERENCE EXAMPLE 7
Preparation of 3-bromomethyl-2-cyclopropyl-4-(4- fluorophenyl)quinoline
Phosphorus tribromide (4.0 ml, 42.1 mmol) was added at room temperature to a toluene (40 ml)-methylene chloride (20 ml) solution of 2-cyclopropyl-3- hydroxymethyl-4-(4-fluorophenyl)quinoline (6.0 g, 20.5 mmol). The reaction mixture was stirred at room temperature for 3 hours and then poured into an aqueous sodium hydrogencarbonate solution to terminate the
reaction. The mixture was extracted with ethyl acetate, and the organic layer was dried over anhydrous magnesium sulfate and concentrated. The residue was purified by column chromatography (hexane:chloroform = 3:1) to obtain the desired product (6.54 g, 89%) as white crystals. Melting point: 140°C
!H-NMR(CDC13): δ 7.97(d, J=8.9, IH), 7.62(dd, J=6.8. 1.6 Hz, IH), 7.40(m, 6H), 4.59(s, 2H), 2.5l(m, IH), 1.41-1.37(m, 2H), 1.16-1.12(m, 2H).
Triphenylphosphine (2.81 g, 10.7 mmol) was added to a toluene solution (50 ml) of 3-bromomethyl-2-cyclopropyl- 4-(4-fluorophenyl)-quinoline (4.00 g, 10.2 mmol) obtained in Reference Example 7, and the mixture was refluxed under heating for 5 hours. The formed solid was collected by filtration, washed with toluene and then dried to obtain {2-cyclopropyl-4-(4-fluorophenyl)- quinolin-3-yl}methyltriphenylphosphonium bromide (6.80 g, quantitative yield) as white powder. Melting point 245°C (decomposed)
IR(CHC13): 3300, 3050, 1600, 1520, 1495, 1440, 1320, 1220, 1150, 920, 840 cm"1.
1H-NMR(CDC13):^ 0.3-0.7 (m, 2H), 1.2-0.9 ( , 2H), 2.5-2.0 ( , IH), 5.5(d, 2H, 14.4Hz), 8.0-6.8(m, 23H).
EXAMPLE 2
Preparation of diethyl {2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}methylphosphonate
Triethyl phosphite (3.50 ml, 20.4 mmol) was added to a toluene solution (30 ml) of 3-bromomethyl-2- cyclopropyl-4-(4-fluorophenyl)quinoline (4.00 g, 10.2 mmol) obtained in Reference Example 7, and the reaction mixture was refluxed under heating for 12 hours. The solvent was removed under reduced pressure, and the product was purified by column chromatography (hexane:ethyl acetate = 2:1) to obtain diethyl {2- cyclopropyl-4-(4-fluorophenyl-quinolin-3- yl}methylphosphonate (4.14 g, quantitative yield) as colorless crystals. Melting point: 89°C Rf = 0.09 (hexane:ethyl acetate = 5:1)
IR(CHC13) 2950, 1600, 1510, 1490, 1435, 1240, 1145, 1020, 970, 830 cm"1 1H-NMR(CDC13): δ 8.95(d, J=8.4 Hz, IH), 7.59(dt, 7.0, 1.2 Hz, IH), 7.35-7.17(m, 6H), 4.01-3.84(m, 4H), 3.43(d, J=22.5 Hz, 2H), 2.67- 2.61(m, IH), 1.33-1.29(m, 2H), 1.19(t, J=7.0 Hz, 6H), 1.09(dd, J=8.0, 3.1
Hz, 2H).
MS (m/z) 413 (M+, 63 ), 385 (4), 356(4), 328 (5) , 276( 100) .
EXAMPLE 3
Preparation of diphenyl {2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}methylphosphine oxide
Diphenylethoxyphosphorane (1.30 g, 5.65 mmol) was added to a toluene solution (20 ml) of 3-bromomethyl-2- cyclopropyl-4-(4-fluorophenyl)quinoline (1.00 g, 2.80 mmol) obtained in Reference Example 7, and the reaction mixture was refluxed under heating for 12 hours. The solvent was removed under reduced pressure, and the product was purified by column chromatography (hexane:ethyl acetate = 1:1) to obtain diphenyl {2- cyclopropyl-4-(4-fluorophenyl)quinolin-3- yljmethylphosphine oxide (1.38 g, quantitative yield) as colorless crystals. Melting point: 170°C Rf = 0.11 (hexane:ethyl acetate = 2:1)
IR(CHC13) 2950, 1605, 1510, 1490, 1435, 1210, 1110, 1025, 830 cm'1 Η-NMR(CDC13): δ 7.95(d, J=S.3 Hz, IH), 7.57(t, J=7.2 Hz, IH), 7.51- 7.33(m, IH), 7.24(td, J=7.0. 1.2 Hz, IH), 7.05(d, J=S.4 Hz, IH), 6.99(d,
J=8.7 Hz, IH), 6.97(d, J=8.7 Hz, IH), 6.80(d, J=5.5 Hz, IH), 6.78(d,
J=5.5 Hz, IH), 4.04(d, J=14.0 Hz, 2H), 2.61-2.55(m, IH), 1.24-1.20(m,
2H), 0.89(dd, J=8.8, 3.1 Hz, 2H).
MS (m/z) 477(M+, 3), 449(0.1), 352(4), 246(8), 201(50), 124(25), 77(100).
EXAMPLE 4
Preparation of methyl (3R*,5S*)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
6Z
t-Butyl lithium (0.15 ml, 1.60M pentane solution, 0.24 mmol) was added at -78°C to a THF solution (3.0 ml) of diethyl {2-cyclopropyl-4-(4-fluorophenyl)quinolin-3- yl}methylphosphonate (100 mg, 0.24 mmol) obtained in Example 2, and the mixture was stirred at -78°C for 30 minutes. A THF solution (2.0 ml) of methyl (3R*,5S*)-6- oxo-3,5-isopropylidenedioxy-6-heptenoate (50 mg, 0.23 mmol) obtained in Reference Example 3, was added at -78°C, and reaction mixture was stirred for 3 hours from
-78°C to 0°C and further stirred for 2 hours at room temperature. A saturated sodium hydrogencarbonate aqueous solution was added to terminate the reaction, and the reaction mixture was extracted with diethyl ether. The organic layer was washed with a sodium chloride aqueous solution, then dried over anhydrous magnesium sulfate and concentrated. The residue was purified by column chromatography (hexane:ethyl acetate = 10:1) to obtain methyl (3R*,5S*,6Z)-7-{2-cyclopropyl-4-(4- luorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate (14 mg, 12%) as colorless crystals and (6E)- form (31 mg, 29%) as colorless crystals. 6Z-form
Rf = 0.40 (hexane:ethyl acetate = 5:1)
IR(CHC13) : 3000, 1730, 1600, 1510, 1490, 1380, 1230, 1160, 1090, 840 cm"1.
1H-NMR(CDC13):5 7.96(d, J=8.2 Hz, IH), 7.62(dd, J=6.7, 1.5 Hz, IH),
7.37-7.15(m, 6H), 6.42(d, J=11.4 Hz, IH), 5.61(dd, J=11.4, 8.2 Hz, IH),
'4.38-4.30(m, IH), 4.13-4.06(m, IH), 3.64(s, 3H), 2.48(dd, J=15.5, 6.8
Hz, IH), 2.46-2.41(m, IH), 2.29(dd, J=15.5, 6.3 Hz, IH), 1.46(s, 3H),
1.40-1.35(m, 4H), 1.37(s, 3H), 1.31-1.25(m, 2H), 1.04(dd, J=8.1, 3.3 Hz,
2H).
MS (m/z) 475(M+, 6), 416(8), 400(5), 344(21), 288(100), 275(43)
6E-form Melting point: 133°C
Rf = 0.33 (hexane:ethyl acetate = 5:1)
IR(CHC ) : 3000, 1730, 1605, 1510, 1490, 1380, 1230, 1160, 1090, 840 cm
1H-NMR(CDC13): δ 7.95(d, J=8.4 Hz, IH), 7.58(dd, J=6.6, 1.6 Hz, IH), 7.37-7.15(m, 6H), 6.55(dd, J=16.3, 1.2 Hz, IH), 5.57(dd, J=16.3, 6.1 Hz, IH), 4.38-4.33(m, IH), 4.32-4.25(m, IH), 3.71(s, 3H), 2.54(dd, J=15.6, 6.7 Hz, IH), 2.43(m, IH), 2.35(dd, J=15.6. 6.4 Hz, IH), 1.46(s. 3H), 1.40-1.35(m, 4H), 1.37(s, 3H), 1.31-1.25(m, 2H), 1.04(dd, J=8.1, 3.3 Hz,
2H).
MS (m/z): 475(M+, 6), 416(8), 400(5), 344(21), 288(100), 275(43).
EXAMPLE 5
Preparation of methyl (3R*,5S*,6E)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
A THF solution (6 ml) of diphenyl {2-cyclopropyl-4- (4-fluorophenyl-3-yl}methylphosphine oxide (115 mg, 0.24 mmol) obtained in Example 3, was cooled to -78°C. Then, butyl lithium (0.16 ml, 0.26 mmol) was added thereto, and the mixture was stirred for 30 minutes. A THF solution (2.0 ml) of methyl (3R*,5S*)-6-oxo-3,5- isopropylidenedioxy-6-heptenoate (50 mg, 0.23 mmol) obtained in Reference Example 3 was stirred at -78°C for 4 hours, and then the temperature was raised to room temperature overnight with stirring. The mixture was
treated in the same manner as in Example 4 to obtain methyl (3R*,5S*,6E)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate (71 mg, 64%) as colorless crystals. The E/Z ratio was 99:1 as measured by 1H-NMR.
The melting point, Rf, IR, ^-NM and MS agreed to 6E-form of Example 4. EXAMPLE 6
Preparation of methyl (3R*,5S*,6E)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
Butyl lithium (0.16 ml, 1.62M hexane solution, 0.26 mmol) was added at -78°C to a THF solution (2.0 ml) of 2,2,6,6-tetramethylpiperidine (37 mg, 0.26 mmol), and the mixture was stirred at -78°C for 15 minutes. A THF solution (4.0 ml) of diphenyl {2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}methylphosphine oxide (115 mg, 0.24 mmol) obtained in Example 3, was added thereto at -78°C, and the mixture was stirred at room temperature for 30 minutes. A THF solution (2.0 ml) of methyl
(3R*,5S*)-6-OXO-3,5-isopropylidenedioxy-6-heptenoate (50 mg, 0.23 mmol) obtained in Reference Example 3, was added
thereto at room temperature, and the reaction mixture was stirred at room temperature for 3 hours. The mixture was treated in the same manner as in Example 4 to obtain methyl (6E)-7-{2-cyclopropyl-4-(4-fluorophenyl)quinolin- 3-yl}-3,5-isopropylidenedioxy-6-heptenoate (84 mg, 76%) as colorless crystals. The E/Z ratio was 98:2 as measured by ^- MR.
The melting point, Rf, IR, 1H-NMR and MS agreed to 6E-form of Example 4. EXAMPLES 7 to 10
Using the following amines instead of 2,2,6,6- tetramethylpiperidine in Example 6, the reaction was conducted in the same manner to obtain the following results.
Yield of
Examples Amines 6E-form (%) E/Z
7 Diisopropylamine 54 98:2
8 Bistri ethylsilylamine 47 98:2
9 Dicyclohexylamine 60 97:3
10 Isopropylcyclohexylamine 64 95:5
EXAMPLE 11
Preparation of methyl (3S,5R,6E)-7-{2-cyclopropyl-4-(4- fluorophenyl)cτuinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
Butyl lithium (0.125 ml, 1.62M hexane solution, 0.20 mmol) was added at -78°C to a THF solution (2.0 ml) of 2,2,6,6-tetramethylpiperidine (29 mg, 0.20 mmol), and the mixture was stirred at -78°C for 15 minutes. A THF solution (4.0 ml) of diphenyl {2-cyclopropyl-4-(4- fluorophenyl)isoquinolin-3-yl}-methylphosphine oxide (98 mg, 0.20 mmol) obtained in Example 3, was added thereto at -78°C, and the mixture was stirred at room temperature for 30 minutes. Then, a THF solution (2.0 ml) of methyl (3S,5R)-6-0x0-3,5-isopropylidenedioxy-6-heptenoate (38 mg, 0.18 mmol) obtained in Reference Example 6, was added thereto at room temperature, and the reaction mixture was stirred at room temperature for 3 hours. Then, a saturated sodium hydrogencarbonate aqueous solution was added to terminate the reaction, and the mixture was extracted with diethyl ether. The organic layer was washed with a sodium chloride aqueous solution, then dried over anhydrous magnesium sulfate and concentrated. The product was purified by column chromatography (hexanerethyl acetate = 5:1) to obtain methyl (3S,5R,6E)- 7-{2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl}-3,5- isopropylidenedioxy-6-heptenoate (62 mg, 74%) as colorless oil. The E/Z ratio was 98:2 as confirmed by
LH-NMR .
[α]D 20 = 19.16° (c 0.96, CHC13)
Rf = 0.33 (hexane:ethyl acetate = 5:1)
IR(CHC13): 3000, 1730,1605, 1510, 1490, 1380, 1230, 1160, 1090, 840 cm"1.
IH-NMR(CDCl3):^7.95(d, J=S.4Hz, IH), 7.58(dd, J=6.6, 1.6 Hz, IH),
7.37-7.15(m, 6H), 6.55(dd, J=16.3, 1.2 Hz, IH), 5.57(dd, J=16.3, 6.1 Hz,
IH), 4.38-4.33(m,lH), 4.32-4.25(m, IH), 3.71(s, 3H), 2.54(dd, J=15.6, 6.7Hz, IH), 2.43(m,lH), 2.35(dd, J=15.6, 6.4Hz, IH), 1.46(s,3H), 1.40- 1.35(m,4H), 1.37(s,3H), 1.3 l-1.25(m,2H), 1.04(dd, J=8.1, 3.3Hz, 2H). MS (m/z) 475(M+, 6), 416(8), 400(5), 344(21), 288(100), 275(43).' HRMS(Calcd. for C29H30O4NF; M+ 475.21^9, found m/z 475.2157.
EXAMPLE 12
6Z
Preparation of t-butyl ( 3R,5S)-7-{2-cyclopropyl-4-( 4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
Butyl lithium (0.42 ml, 0.67 mmol) was added at -70°C
to a THF solution (15 ml) of {2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}methyltriphenylphosphonium bromide obtained in Example 1, and the mixture was stirred at -78°C for 30 minutes. A THF solution (5 ml) of t-butyl (3R,5S)-6-oxo-3,5-isopropylidenedioxy-6- heptenoate (208 mg, 0.78 mmol) separately prepared, was added thereto at -78°C, and the mixture was stirred for 2 hours at -78°C and further overnight while raising the temperature to room temperature. The mixture was treated and purified in the same manner as in Example 4 to obtain t-butyl (3R,5S,6Z)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate (140 mg, 40%) as colorless oil and 6E-form (200 mg, 58%) as colorless crystals. 6Z-form
Rf = 0.40 (hexane:ethyl acetate = 5:1)
IR(CHC13): 3450, 3000, 1720, 1595, 1560, 1510, 1490, 1380, 1310, 1260, 1220, 1200, 1160, 1100, 1030, 970, 950, 920, 840 cm"1
1H- MR(CDC13):<? 7.0-8. l(m, 8H), 6.4(d, J=llHz, IH), 5.6(dd, J=ll, 8Hz, IH), 3.7-4.2(m,2H), 2. l-2.7(m,3H), 0.8-1.7( , 21H).
MS (m/z) 518(M+H, 100), 462, 404, 386, 344, 316, 288, 274, 262, 220, 173, 154, 136.
6E-form
Melting point: 46°C D 25 = +10.4° (c 1.0, CHC13)
Rf = 0.33 (hexane:ethyl acetate = 5:1)
IR(-KBr): 3450, 3000, 1720, 1600, 1560, 1510, 1490, 1380, 1310, 1260, 1220, 1200, 1160, 1100, 1030, 970, 950, 920, 840 cm"1.
1H-NMR(CDCl3):60MHz, 57-8(m,8H), 6.5(d, J=16Hz, IH), 5.5(dd, J=16, 6Hz, IH), 4.0-4.5(m, 2H), 2.2-2.6(m, 3H), 0.85-1.7(m, 21H). MS (m/z) 518(M+H, 100), 462, 404, 386, 344, 316, 288, 274, 262, 220, 173, 154, 136.
EXAMPLE 13
Preparation of t-butyl (3R,5S,6E)-7-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy-6- heptenoate
Butyl lithium (1.24 ml, 2.0 mmol) was added at 0°C to a THF solution (5 ml) of 2,2,6,6-tetramethylpiperidine (161 mg, 1.1 mmol), and the mixture was stirred for 15 minutes. The mixture was cooled to -78°C. Then, a THF solution (5 ml) of diphenyl {2-cyclopropyl-4-(4- fluorophenyl)isoquinolin-3-yl}methylphosphine oxide (419 mg, 0.86 mmol) obtained in Example 3, was added thereto, and the mixture was stirred for 30 minutes. Then, a THF solution (5 ml) of t-butyl (3R,5S)-6-oxo-3,5- isopropylidenedioxy-6-heptenoate (294 mg, 1.1 mmol) separately prepared, was added thereto, and the mixture was stirred for 4 hours at -78°C and then overnight to room temperature. The mixture was treated in the same
manner as in Example 6 to obtain t-butyl (3R,5S,6E)-7-{2- cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl}-3,5- isopropylidenedioxy-6-heptenoate (378 mg, 83%) as white crystals. The E/Z ratio was 95:5 as confirmed by high performance liquid chromatography.
The melting point, ! ]D 25, Rf, IR, 1H-NMR and MS agreed to 6E-form of Example 12.
REFERENCE EXAMPLE 8
Preparation of (4R*,6S*)-6-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl-E-ethenyl}-4-hydroxy- ,4,5,6- tetrahydro-2H-pyran-2-one
A methyl chloride solution (2 ml) of trifluoroacetic acid (170 mg, 1.5 mmol) was added to a methylene chloride solution (5 ml) of methyl (3R*,5S*,6E)-7-{2-cyclopropyl- 4-( -fluorophenyl)quinolin-3-yl}-3,5-isopropylidenedioxy- 6-heptenoate (48 mg, 0.1 mmol) obtained in Example 5, and the mixture was stirred at room temperature for 24 hours. The reaction solution was cooled with ice, and then a 5% sodium hydrogencarbonate aqueous solution was added, and the mixture was extracted with methyl chloride. Then, the organic layer was washed with a saturated sodium chloride aqueous solution and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced
pressure, and the residue was purified by column chromatography (hexane:ethyl acetate = 5:1) to obtain (4R*,6S*)-6-{2-cyclopropyl-4-(4-fluorophenyl)quinolin-3- yl-E-ethenyl}-4-hydroxy-3,4,5,6-tetrahydro-2H-pyran-2-one (30 mg, 75%) as white crystals. Melting point: 201°C
IR(CHC13): 3440, 3005, 1730, 1600, 1560, 1510, 1490, 1410, 1230, 1155, 1060, 970, 830, 730 cm"1.
1H-NMR(CDC13):5 1.03-1.08(m,2H), 1.30-1.40( , 2H), 1.56-1.60(m, IH), 1.78(m, IH), 2.38(m, IH), 2.60(ddd, J=7.4, 4.0, 1.5Hz, IH), 2.70(dd, J=13.0, 4.8 Hz, IH), 4.25(m, IH), 5.18 and 4.66(m, IH, ratio 64:36), 5.62(dd, J=16.1, 6.2Hz, IH), 6.72(dd, J=16.1, 1.4Hz, IH), 7.17-7.25(m, 4H), 7.30-7.37(m, 2H), 7.61(dd, 3=6. 1 , 2.1Hz, IH), 7.96(d, J=8.3Hz, IH). MS (m/z) 403(M+, 9), 316(11), 288(100), 274(12).
REFERENCE EXAMPLE 9
Preparation of (4R,6S)-6-{2-cyclopropyl-4-(4- fluorophenyl)quinolin-3-yl-E-ethenyl)-4-hydroxy-3,4,5,6- tetrahydro-2H-pyran-2-one
t-Butyl (3R,5S,6E)-7-{2-cycloropyl-4-(4- fluorophenyl)quinolin-3-yl}-3,5-isopropylideneoxy-6- heptenoate (259 mg, 0.5 mmol) obtained in Example 13, was reacted in the same manner as in Reference Example 8 to obtain (4R,6S)-6-{2-cyclopropyl-4-(4-
fluorophenyl )quinolin-3-yl-E-ethenyl}-4-hydroxy-3 ,4,5,6- tetrahydro-2H-pyran-2-one (151 mg, 75%) as white crystals.
Melting point: 139°C [α]D 20 = +9.0° (c 1.0, CHC13)
Rf = 0.19 (hexane: ethyl acetate = 2:1)
IR(CHC13):3440, 3005, 1730, 1600, 1560, 1510, 1490, 1410, 1230, 1155, 1060, 970, 830, 730 cm"1.
1H-NMR(CDC13):5 1.03-1.08(m,2H), 1.30-1.40(m, 2H), 1.56-1.60(m, IH), 1.78(m, IH), 2.38(m, IH), 2.60(ddd, J=7.4, 4.0, 1.5Hz, IH), 2.70(dd, 1=13.0, 4.8 Hz, IH), 4.25(m, IH), 5.18 and 4.66(m, IH, ratio 64:36), 5.62(dd, J=16.1, 6.2Hz, IH), 6.72(dd, J=16.1, 1.4Hz, IH), 7.17-7.25(m, 4H), 7.30-7.37(m, 2H), 7.61(dd, J=6.1, 2.1Hz, IH), 7.96(d, J=8.3Hz, IH). MS (m/z) 403(M+, 9), 316(11), 288(100), 274(12).
INDUSTRIAL APPLICABILITY
The present invention provides a novel intermediate for a condensed pyridine type mevalonolactone derivative which is a HMG-CoA reductase inhibitor and which is useful as a hypercholesterolemia therapeutic agent or as an arteriosclerosis therapeutic agent and a process for its production as well as a novel condensed pyridine derivative useful as a starting material therefor.
Claims
1. A condensed pyridine type mevalonolactone intermediate of the formula (1):
wherein ring X is a benzene ring, a substituted benzene ring or a substituted 5- or 6-membered heteroaromatic ring, each of R1 and R2 which are independent of each other, is hydrogen, λ_8 alkyl, C3_7 cycloalkyl, C1-3 alkoxy, butoxy, i-butoxy, sec-butoxy, tert-butoxy, R20R21N- (wherein each of R20 and R21 which are independent of each other, is hydrogen or C1-3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2)€OR22 (wherein R22 is hydrogen or Cχ_3 alkyl, and is 1, 2, or 3); or R1 and R2 together form -CH=CH-CH=CH- or methylenedioxy, when they are at the o-position to each other;
R3 is hydrogen, C^g alkyl, C2_6 alkenyl, C3_7
hydrogen, C-^g alkyl, C1_8 alkoxy, Cλ_3 alkylthio, chloro, bromo, fluoro, chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethox , difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or
(wherein R7 is as defined above) and zero, one or two
C1-3 alkyl;
Z is
(each of R9a and R9b represents a hydroxyl-protecting group and is independently methoxymethyl, 2- methoxyethoxymethyl, tetrahydropyranyl, 4- methoxytetrahydropyranyl, 1-ethoxyethyl, 1-methyl-l- methoxyethyl, allyl, benzyl, p-methoxybenzyl, triphenylmethyl, trimethylsilyl, tert-butyldimethylsilyl or tert-butyldiphenylsilyl, or R9a and R9b together form isopropylidene, cyclopentylidene, cyclohexylidene or benzylidene; and
R10 is methyl, ethyl, propyl, isopropyl, tert-butyl, tetrahydropyranyl, allyl, benzyl, triphenylmethyl, trimethylsilyl or tert-butyldimethylsilyl).
2. The intermediate according to Claim 1, which is a guinoline type mevalonolactone intermediate of the formula (la)
wherein each of R a, R5a and R6a which are independent of one another, is hydrogen, C2_6 alkyl, C3_7 cycloalkyl, C1-3 alkoxy, butoxy, i-butoxy, sec-butoxy, R26R27N-
( herein each of R26 and R27 which are independent of each other, is hydrogen or C1-3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or
-0(CH2)mOR28 (wherein R28 is hydrogen or Cχ_3 alkyl, and m is 1, 2, or 3) ; or
R4a and R5a together form -CH=CH-CH=CH-; or R4a and R5a together form -OC(R29) (R30)O- when they are at the o-position to each other (wherein each of R29 and R30 which are independent of each other, is hydrogen or C1-3 alkyl); and
R1, R2, R3 and Z are as defined with respect to the formula (1) . 3. The intermediate according to Claim 1, which is a pyrazolopyridine type mevalonolactone intermediate of the formula (lb):
wherein R4b is hydrogen, λ_8 alkyl, C1-6 alkoxy, C3_7 cycloalkyl, C2_6 alkenyl, a- or /?-naphthyl, 2-, 3- or 4- pyridyl, 2- or 3-thienyl, 2- or 3-furyl, fluoro, chloro,
bromo , ( wherein each of R6b , R7b and R8b which are independent of one another, is hydrogen, C±_8 alkyl, _8 alkoxy, C1_3 alkylthio, chloro, bromo, fluoro, -NR31R32 (wherein each of R31 and R32 which are independent of each other, is Cχ_3 alkyl), chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2)nOR33 (wherein R33 is hydrogen or C1_3 alkyl, and n is 1, 2 or 3), or when R8b is hydrogen, R6b and R7b together form -OC(R34) (R35)0- when they are at the o-position to each other (wherein each of R34 and R35 which are independent of each other, is hydrogen or C1_3 alkyl group), or when R7b and R8b are simultaneously hydrogen, R6b is (wherein R36 is hydrogen, C1-4 alkyl,
C _3 alkoxy, trifluoromethyl, chloro, bromo or fluoro), phenyl-C2_3 alkenyl wherein the phenyl group may be substituted by Cχ_4 alkyl, C1_3 alkoxy, fluorine, chlorine or bromine, or Cλ_3 alkyl substituted by one member selected from C1_3 alkoxy, naphthyl and
R5b is bonded to nitrogen atom at the 1- or 2- position of the pyrazolopyridine ring, and such R5b is hydrogen, C1_8 alkyl, C1_3 alkyl substituted by from one to three fluorine atoms, C3_7 cycloalkyl, a- or β- naphthyl, 2-,
3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3- furyl or
C1_3 alkyl substituted by one member selected from C1_3 alkoxy, hydroxy, naphthyl and (wherein R6b, R7b and R8b are as defined above) and zero, one or two C _8 alkyl; and
R1, R2, R3 and Z are as defined with respect to the formula (1) .
4. The intermediate according to Claim 1, which is a thienopyridine type mevalonolactone intermediate of the formula (lc) :
wherein each of R4c and R5c which are independent of each other, is hydrogen, C1_8 alkyl, C2_5 alkenyl, C3_7 cycloalkyl, C-^g alkoxy, fluoro, chloro, bromo,
(wherein each of R6c, R7c and R8c which are independent of one another, is hydrogen, C1_ alkyl, C1_3 alkoxy, C3_7 cycloalkyl, trifluoromethyl, fluoro, chloro and bromo), 2-, 3- or 4-pyridyl, 2- or 5- pyrimidyl, 2- or 3-thienyl, 2- or 3-furyl, (wherein R6c is as defined above),
-NR37R38 (wherein each of R37 and R38 which are independent of each other, is hydrogen, C1_4 alkyl,
(wherein j is 1, 2 or 3, and R6c is as defined above), or R37 and R38 together form -(CH2)k- (wherein k is 3, 4 or 5)), C1_3 alkyl substituted by
R4c and R5c together form C2_6 alkylene substituted by from zero to three members selected from C1-4 alkyl, C3_7 cycloalkyl, fluoro, chloro and bromo and zero or one member selected from
(CHR ? J399)p-_Aa-_(/CpHWRR 4400)'q- (wherein each of p and q is 0, 1, 2 or 3, A is -C(R 1)=C(R42)- (wherein each of R41 and R42 is hydrogen or C1-3 alkyl), -0-, -S- or -N(R43)- (wherein R43 is hydrogen, C1_4 alkyl, or
-(CH2) (wherein R6c and j are as defined above)), and each of R39 and R40 which are independent of each other, is hydrogen or Cχ_4 alkyl) or -CH=CH-CH=CH-; and
R1, R2, R3 and Z are as defined with respect to the formula (I) .
5. The quinoline type mevalonolactone intermediate according to Claim 2, wherein in the formula (la),
R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1, 2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl; when R5a and R6a are simultaneously hydrogen, R4a is hydrogen, 5-fluoro, 6-fluoro, 7-fluoro, 8-fluoro, 5- chloro, 6-chloro, 7-chloro, 8-chloro, 5-bromo, 6-bromo, 7-bromo, 8-bromo, 5-methyl, 6-methyl, 7-methyl, 8-methyl, 5-methoxy, 6-methoxy, 7-methoxy, 8-methoxy, 5- trifluoromethyl, 6-trifluoromethyl, 7-trifluoromethyl, 8- trifluoromethyl, 6-trifluoromethoxy, 6-difluoromethoxy, 8-hydroxyethyl, 5-hydroxy, 6-hydroxy, 7-hydroxy, 8- hydroxy, 6-ethyl, 6-butyl or 7-dimethylamino; or when R6a is hydrogen, R4a and R5a together represent 6-chloro-8-methyl, 6-bromo-7-methoxy, 6-methyl-7-chloro, 6-chloro-8-hydroxy, 5-methyl-2-hydroxy, 6-methoxy-7- chloro, 6-chloro-7-methoxy, 6-hydroxy-7-chloro, 6-chloro- 7-hydroxy, 6-chloro-8-bromo, 5-chloro-6-hydroxy, 6-bromo- 8-chloro, 6-bromo-8-hydroxy, 5-methyl-8-chloro, 7- hydroxy-8-chloro, 6-bromo-8-hydroxy, 6-methoxy-7-methyl, 6-chloro-8-bromo, 6-methyl-8-bromo, 6,7-difluoro, 6,8- difluoro, 6,7-methylenedioxy, 6,8-dichloro, 5,8-dimethyl, 6,8-dimethyl, 6,7-dimethoxy, 6,7-diethoxy, 6,7-dibromo or 6,8-dibromo; or
R4 , R5a and R6a together represent 5,7-dimethoxy-8- hydroxy, 5 8-dichloro-6-hydroxy, 6,7,8-trimethoxy, 6,7,8- trimethyl, 6,7,8-trichloro, 5-fluoro-6,8-dibromo or 5- chloro-6,8-dibromo.
6. The pyrazolopyridine type mevalonolactone intermediate according to Claim 3, wherein in the formula (lb),
R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl;
R b is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, cyclopropyl, cyclohexyl, phenyl, 2-, 3- or 4-fluorophenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- tolyl, 2-, 3- or 4-methoxyphenyl, 2-, 3- or 4- trifluoromethylphenyl, 2-, 3- or 4-chloromethylphenyl, 3- or 4-ethoxyphenyl, 4-(2-methylbutyl)phenyl, 4- heptylphenyl, 4-octylphenyl, 4-pentylphenyl, 4- hexylphenyl, 4-propylphenyl, 4-butylphenyl, 4-tert- butylphenyl, 4-butoxyphenyl, 4-pentyloxyphenyl, 4- hexyloxyphenyl, 4-heptyloxyphenyl, 4-octyloxyphenyl, 4-phenoxyphenyl, 4-biphenyl, 4-trichloromethoxyphenyl, 2,4-difluorophenyl, 2,6-difluorophenyl, 2,3- difluorophenyl, 3,5-difluorophenyl, 2,5-difluorophenyl, 3,4-difluorophenyl, 2,4-dichlorophenyl, 2,6- dichlorophenyl, 2,3-dichlorophenyl, 2,5-dichlorophenyl, 3,5-dichlorophenyl, 3,4-dichlorophenyl, 2,3- di ethylphenyl, 2,5-dimethylphenyl, 2,6-dimethylphenyl, 3,4-dimethylphenyl, 2,5-dimethoxyphenyl, 2,6- dimethoxyphenyl, 2,4-dimethoxyphenyl, 3,4- dimethoxyphenyl, 3,5-dimethoxyphenyl, 3,5- bis(trifluoromethyl)phenyl, 3,4-methylenedioxyphenyl, 2,4,6-trimethoxyphenyl, 3,4,5-trimethylphenyl or 2,4,6- triisopropylphenyl;
R5b is a group bonded to the nitrogen atom at the 1- position of the pyrazolopyridine ring and is methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert- butyl, 2,2,2-trifluoroethyl, 2-hydroxyethyl, cyclohexyl, benzyl, 2-chlorobenzyl, 2-hydroxybenzyl, 3- trifluoro ethylbenzyl, 2-phenylethyl, phenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- trifluoromethylphenyl, 3- or 4-methoxyphenyl, 2- hydroxyphenyl, 4-isopropylphenyl, 4-tert-butylphenyl, 4- trifluoromethoxyphenyl, 2,3-dichlorophenyl, 2,4- dichlorophenyl, 2,5-dichlorophenyl, 2,6-dichlorophenyl, 3,4-dichlorophenyl, 3,5-dichlorophenyl, 2,4,6- trichlorophenyl, 2,3,4-trichlorophenyl, 2,4- difluorophenyl, 3,5-bis(trifluoromethyl)phenyl, 3-chloro- 4-tolyl, 3-chloro-6-tolyl, 4-chloro-2-tolyl, 2-chloro-6- tolyl, 2-chloro-6-fluorophenyl, 2-chloro-5- trifluoromethylphenyl, 3-chloro-4-fluorophenyl, 4-bromo- 3-chlorophenyl, 2-chloro-4-trifluoromethylphenyl, 3- fluoro-6-tolyl, c-naphthyl, 2-pyridyl, 3-methyl-5- trifluoromethyl-2-pyridyl, 4-pyridyl or 2,6-dichloro-4- pyridyl.
7. The thienopyridine type mevalonolactone intermediate according to Claim 4, wherein in the formula (lc), R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl; each of R4c and R5c which are independent of each other, is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1,2- dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl cyclobutyl, cyclopentyl, cyclohexyl, 2-methylcyclohexyl, cycloheptyl, cyclopropylmethyl, vinyl, 1-methylvinyl, 1- propenyl, allyl, 1-methyl-l-propenyl, l-methyl-2- propenyl, 2-methyl-2-propenyl, 2-butenyl, 1-ethylvinyl, 1,2-dimethyl-l-propenyl, l,2-dimethyl-2-propenyl, 1- ethyl-1-propenyl, l-ethyl-2-propenyl, 1-methyl-l-butenyl, l-methyl-2-butenyl, 2-methyl-l-butenyl, 1-i-propylvinyl, 1-methyl-l-pentenyl or phenyl; or
R c and R5c together form ethylene, trimethylene, tetramethylene, pentamethylene, methyltetramethylene, chlorotetramethylene or phenyltetramethylene.
8. The quinoline type mevalonolactone intermediate according to Claim 2, wherein in the formula (la), R1 is p-fluoro, each of R2, R4a, R5a and R6a is hydrogen, and R3 is cyclopropyl.
9. The pyrazolopyridine type mevalonolactone intermediate according to Claim 3, wherein in the formula (lb), R1 is p-fluoro, R2 is hydrogen, each of R4b and R5b is methyl, and R3 is cyclopropyl.
10. The thienopyridine type mevalonolactone intermediate according to Claim, 4, wherein in the formula (lc), R1 is p-fluoro, R2 is hydrogen, R c is ethyl, R5c is methyl, and R3 is cyclopropyl.
11. A condensed pyridine derivative of the formula (2):
wherein ring X is a benzene ring, a substituted benzene ring or a substituted 5- or 6-membered heteroaromatic ring, each of R1 and R2 which are independent of each other, is hydrogen, Cλ_8 alkyl, C3_7 cycloalkyl, C1-3 alkoxy, butoxy, i-butoxy, sec-butoxy, tert-butoxy, R 20 R 2i N_ (Wherein each of R20 and R21 which are independent of each other, is hydrogen or C-^-j alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2) OR22 (wherein R22 is hydrogen or C±-3 alkyl, and £ is 1, 2, or 3); or R1 and R2 together form -CH=CH-CH=CH- or methylenedioxy, when they are at the o-position to each other;
R3 is hydrogen, Cλ_8 alkyl, C2_6 alkenyl, C3_7
cycloalkyl hydrogen, bromo, fluoro, chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethoxy, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy or
(wherein R7 is as defined above) and zero, one or two C1_3 alkyl;
Y is P+R11R12R13Hal" or P(W)R14R15 (wherein each of R11, R12 and R13 which are independent of one another, is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2-trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl or benzyl, each of R14 and R15 which are independent of each other, is methyl, ethyl, propyl, isopropyl, butyl, 2-chloroethyl, 2,2,2- trifluoroethyl, phenyl, methoxyphenyl, methylphenyl, pentafluorophenyl, benzyl, methoxy, ethoxy, propoxy, isopropoxy, butoxy, 2-chloroethoxy, 2,2,2- trifluoroethoxy, phenoxy, methoxyphenyloxy, methylphenyloxy, pentafluorophenyloxy or benzyloxy, or R14 and R15 together form a 5- or 6-membered ring, Hal is chlorine, bromine or iodine, and is 0 or S.
12. The derivative according to Claim 11, which is a guinoline derivative of the formula (2a):
wherein each of R4a, R5a and R6a which are independent of one another, is hydrogen, C1-6 alkyl, C3_7 cycloalkyl, Cλ_3 alkoxy, butoxy, i-butoxy, sec-butoxy, R26R27N- ( herein each of R26 and R27 which are independent of each other, is hydrogen or Cj__3 alkyl), trifluoromethyl, trifluoromethoxy, difluoromethoxy, fluoro, chloro, bromo, phenyl, phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0 ( R2 ) mOR2 (wherein R28 is hydrogen or C1-3 alkyl, and m is 1, 2, or 3) ; or
R4a and R5a together form -CH=CH-CH=CH-; or R a and R5a together form -OC(R29) (R30)O- when they are at the o-position to each other (wherein each of R29 and R30 which are independent of each other, is hydrogen or Cχ_3 alkyl); and
R1, R2, R3 and Y are as defined with respect to the formula ( 2) .
13. The derivative according to Claim 11, which is a pyrazolopyridine derivative of the formula (2b):
wherein R is hydrogen, C-^g alkyl, Cλ_6 alkoxy, C3_7 cycloalkyl, C2_6 alkenyl, a- or /?-naphthyl, 2-, 3- or 4- pyridyl, 2- or 3-thienyl, 2- or 3-furyl, fluoro, chloro,
bromo, (wherein each of R° 6Db, R ,7/Db and R ,8b which are independent of one another, is hydrogen, C-^g alkyl, Cj,_8 alkoxy, C1_3 alkylthio, chloro, bromo, fluoro, -NR31R32 (wherein each of R31 and R32 which are independent of each other, is C1_3 alkyl), chloromethyl, trichloromethyl, trifluoromethyl, trifluoromethoxy, trichloromethoxy, difluoromethox , phenoxy, benzyloxy, hydroxy, trimethylsilyloxy, diphenyl-tert-butylsilyloxy, hydroxymethyl or -0(CH2)nOR33 (wherein R33 is hydrogen or
'1-3 alkyl, and n is 1, 2 or 3), or when R8b is hydrogen, R6b and R7b together form -OC(R3 ) (R35)0- when they are at the o-position to each other (wherein each of R34 and R35 which are independent of each other, is hydrogen or C1_3 alkyl group), or when R7b and R8b are simultaneously hydrogen, R6b is
Cλ_3 alkoxy, trifluoromethyl, chloro, bromo or fluoro), phenyl-C2_3 alkenyl wherein the phenyl group may be substituted by Cχ_4 alkyl, C1_3 alkoxy, fluorine, chlorine or bromine, or C1_3 alkyl substituted by one member selected from C1_3 alkoxy, naphthyl and
R 5b is bonded to nitrogen atom at the 1- or 2- position of the pyrazolopyridine ring, and such R5b is hydrogen, C-^g alkyl, C1_3 alkyl substituted by from one to three fluorine atoms, C3_7 cycloalkyl, a- or β- naphthyl, 2-, 3- or 4-pyridyl, 2- or 3-thienyl, 2- or 3- furyl or
Cχ_3 alkyl substituted by one member selected from C1_3 alkoxy, hydroxy, naphthyl and
R1, R2, R3 and Y are as defined with respect to the formula (2) .
14. The derivative according to Claim 11, which is a thienopyridine derivative of the formula (2c):
wherein each of R4c and R5c which are independent of each other, is hydrogen, C-^g alkyl, C2_6 alkenyl, C3_7 cycloalkyl, C1_6 alkoxy, fluoro, chloro, bromo,
(wherein each of R6c, R7c and R8c which are independent of one another, is hydrogen, C1_4 alkyl, C1_3 alkoxy, C3_7 cycloalkyl, trifluoromethyl, fluoro, chloro and bromo), 2-, 3- or 4-pyridyl, 2- or 5- pyrimidyl, 2- or 3-thienyl, 2- or 3-furyl, (wherein R6c is as defined above),
-NR37R38 (wherein each of R37 and R38 which are independent of each other, is hydrogen, C1_4 alkyl,
-(CH2)j (wherein j is 1, 2 or 3, and R6c is as defined above), or R37 and R38 together form -(CH2)k- (wherein k is 3, 4 or 5)), C1_3 alkyl substituted by
R c and R5c together form C2_6 alkylene substituted by from zero to three members selected from C1_4 alkyl, C3_7 cycloalkyl, fluoro, chloro and bromo and zero or one member selected from
-(CHR39)p-A-(CHR40)q- (wherein each of p and q is 0, 1, 2 or 3, A is -C(R 1)=C(R42)- (wherein each of R41 and R42 is hydrogen or Cχ_3 alkyl), -0-, -S- or -N(R43)- (wherein R43 is hydrogen, C1_4 alkyl, or τ?6c
-(CH2)-—C. /) (wherein R6c and j are as defined above)), and each of R39 and R40 which are independent of each other, is hydrogen or C1-4 alkyl) or -CH=CH-CH=CH-; and
R1, R2, R3 and Y are as defined with respect to the formula (2) .
15. The quinoline derivative according to Claim 12, wherein in the formula (2a),
R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1, 2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl; when R5a and R6a are simultaneously hydrogen, R a is hydrogen, 5-fluoro, 6-fluoro, 7-fluoro, 8-fluoro, 5- chloro, 6-chloro, 7-chloro, 8-chloro, 5-bromo, 6-bromo, 7-bromo, 8-bromo, 5-methyl, 6-methyl, 7-methyl, 8-methyl, 5-methoxy, 6-methoxy, 7-methoxy, 8-methoxy, 5- trifluoromethyl, 6-trifluoromethyl, 7-trifluoromethyl, 8- trifluoromethyl, 6-trifluoromethoxy, 6-difluoromethoxy, 8-hydroxyethyl, 5-hydroxy, 6-hydroxy, 7-hydroxy, 8- hydroxy, 6-ethyl, 6-butyl or 7-dimethylamino; or when R6a is hydrogen, R4a and R5a together represent 6-chloro-8-methyl, 6-bromo-7-methoxy, 6-methyl-7-chloro, 6-chloro-8-hydroxy, 5-methyl-2-hydroxy, 6-methoxy-7- chloro, 6-chloro-7-methoxy, 6-hydroxy-7-chloro, 6-chloro- 7-hydroxy, 6-chloro-8-bromo, 5-chloro-6-hydroxy, 6-bromo- 8-chloro, 6-bromo-8-hydroxy, 5-methyl-8-chloro, 7- hydroxy-8-chloro, 6-bromo-8-hydroxy, 6-methoxy-7-methyl, 6-chloro-8-bromo, 6-methyl-8-bromo, 6,7-difluoro, 6,8- difluoro, 6,7-methylenedioxy, 6,8-dichloro, 5,8-dimethyl, 6,8-dimethyl, 6,7-dimethoxy, 6,7-diethoxy, 6,7-dibromo or 6,8-dibromo; or
R4a, R5a and R6a together represent 5,7-dimethoxy-8- hydroxy, 5,8-dichloro-6-hydroxy, 6,7,8-trimethoxy, 6,7,8- trimethyl, 6,7,8-trichloro, 5-fluoro-6,8-dibromo or 5- chloro-6,8-dibromo.
16. The pyrazolopyridine derivative according to Claim 13, wherein in the formula (2b), R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl;
R4b is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, cyclopropyl, cyclohexyl, phenyl, 2-, 3- or 4-fluorophenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- tolyl, 2-, 3- or 4-methoxyphenyl, 2-, 3- or 4- trifluoromethylphenyl, 2-, 3- or 4-chloromethylphenyl, 3- or 4-ethoxyphenyl, 4-(2-methylbutyl)phenyl, 4- heptylphenyl, 4-octylphenyl, 4-pentylphenyl, 4- hexylphenyl, 4-propylphenyl, 4-butylphenyl, 4-tert- butylphenyl, 4-butoxyphenyl, 4-pentyloxyphenyl, 4- hexyloxyphenyl, 4-heptyloxyphenyl, 4-octyloxyphenyl, 4-phenoxyphenyl, 4-biphenyl, 4-trichloromethoxyphenyl, 2,4-difluorophenyl, 2,6-difluorophenyl, 2,3- difluorophenyl, 3,5-difluorophenyl, 2,5-difluorophenyl, 3,4-difluorophenyl, 2,4-dichlorophenyl, 2,6- dichlorophenyl, 2,3-dichlorophenyl, 2,5-dichlorophenyl, 3,5-dichlorophenyl, 3,4-dichlorophenyl, 2,3- dimethylphenyl, 2,5-dimethylphenyl, 2,6-dimethylphenyl, 3,4-dimethylphenyl, 2,5-dimethoxyphenyl, 2,6- dimethoxyphenyl, 2,4-dimethoxyphenyl, 3,4- dimethoxyphenyl, 3,5-dimethoxyphenyl, 3,5- bis(trifluoromethyl)phenyl, 3,4-methylenedioxyphenyl, 2,4,6-trimethoxyphenyl, 3,4,5-trimethylphenyl or 2,4,6- triisopropylphenyl;
R5b is a group bonded to the nitrogen atom at the 1- position of the pyrazolopyridine ring and is methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert- butyl, 2,2,2-trifluoroethyl, 2-hydroxyethyl, cyclohexyl, benzyl, 2-chlorobenzyl, 2-hydroxybenzyl, 3- trifluoromethylbenzyl, 2-phenylethyl, phenyl, 2-, 3- or 4-chlorophenyl, 2-, 3- or 4-bromophenyl, 2-, 3- or 4- fluorophenyl, 2-, 3- or 4-tolyl, 2-, 3- or 4- trifluoromethylphenyl, 3- or 4-methoxyphenyl, 2- hydroxyphenyl, 4-isopropylphenyl, 4-tert-butylphenyl, 4- trifluoromethoxyphenyl, 2,3-dichlorophenyl, 2,4- dichlorophenyl, 2,5-dichlorophenyl, 2,6-dichlorophenyl, 3,4-dichlorophenyl, 3,5-dichlorophenyl, 2,4,6- trichlorophenyl, 2,3,4-trichlorophenyl, 2,4- difluorophenyl, 3,5-bis( rifluoromethyl)phenyl, 3-chloro- 4-tolyl, 3-chloro-6-tolyl, 4-chloro-2-tolyl, 2-chloro-6- tolyl, 2-chloro-6-fluorophenyl, 2-chloro-5- trifluoromethylphenyl, 3-chloro-4-fluorophenyl, 4-bromo- 3-chlorophenyl, 2-chloro-4-trifluoromethylphenyl, 3- fluoro-6-tolyl, α-naphthyl, 2-pyridyl, 3-methyl-5- trifluoromethyl-2-pyridyl, 4-pyridyl or 2,6-dichloro-4- pyridyl.
17. The thienopyridine derivative according to Claim 14, wherein in the formula (2c),
R3 is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, i-pentyl, 1,2-dimethylpentyl, hexyl, heptyl, octyl, vinyl, 1- propenyl, 1-methylvinyl, 1-methyl-l-propenyl, 2-methyl-l- propenyl, 1,2-dimethyl-l-propenyl, cyclopropyl, cyclobutyl, cyclohexyl, 1-methylcyclopropyl, 2- methylcyclopropyl, phenyl, 2-methylphenyl, 3- methylphenyl, 4-methylphenyl, 2-chlorophenyl, 3- chlorophenyl, 4-chlorophenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 3,4-dimethylphenyl, 3,4- dichlorophenyl, 3-trifluoromethylphenyl, benzyl, 4- chlorobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 2- phenethyl or 1-methylbenzyl; each of R4c and R5c which are independent of each other, is hydrogen, methyl, ethyl, propyl, i-propyl, butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1,2- dimethylpentyl, hexyl, heptyl, octyl, cyclopropyl cyclobutyl, cyclopentyl, cyclohexyl, 2-methylcyclohexyl, cycloheptyl, cyclopropylmethyl, vinyl, 1-methylvinyl, 1- propenyl, allyl, 1-methyl-l-propenyl, l-methyl-2- propenyl, 2-methyl-2-propenyl, 2-butenyl, 1-ethylvinyl, 1,2-dimethyl-l-propenyl, l,2-dimethyl-2-propenyl, 1- ethyl-1-propenyl, l-ethyl-2-propenyl, 1-methyl-l-butenyl, l-methyl-2-butenyl, 2-methyl-l-butenyl, 1-i-propylvinyl, 1-methyl-l-pentenyl or phenyl; or R4c and R5c together form ethylene, trimethylene, tetramethylene, pentamethylene, methyltetramethylene, chlorotetramethylene or phenyltetramethylene.
18. The quinoline derivative according to Claim 12, wherein in the formula (2a), R1 is p-fluoro, each of R2, R a, R5a and R6a is hydrogen, and R3 is cylcopropyl.
19. The pyrazolopyridine derivative according to Claim 13, wherein in the formula (2b), R1 is p-fluoro, R2 is hydrogen, each of R b and R5b is methyl, and R3 is cyclopropyl.
20. The thienopyridine derivative according to Claim 14, wherein in the formula (2c), R1 is p-fluoro, R2 is hydrogen, R c is ethyl, R5c is methyl, and R3 is cylcopropyl.
21. A process for producing a compound of the formula (1) as defined in Claim 1, which comprises reacting a compound of the formula (2) as defined in Claim 11 with a base to form an anion, which is then condensed with a compound of the formula (3):
(3). wherein each R9a and R9b is a hydroxyl-protecting group, and is independently methoxymethyl, 2- methoxyethoxymethyl, tetrahydropyranyl, 4- methoxytetrahydropyranyl, 1-ethoxyethyl, 1-methyl-l- methoxyethyl, allyl, benzyl, p-methoxybenzyl, triphenylmethyl, trimethylsilyl, tert-butyldimethylsilyl or tert-butyldiphenylsilyl, or R9a and R9b together form isopropylidene, cyclopentylidene, cyclohexylidene or benzylidene; and
R10 is methyl, ethyl, propyl, isopropyl, tert-butyl, tetrahydropyranyl, allyl, benzyl, triphenylmethyl, trimethylsilyl or tert-butyldimethylsilyl.
22. A process for producing a compound of the formula (la) as defined in Claim 2, which comprises reacting a compound of the formula (2a) as defined in Claim 12, with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
23. A process for producing a compound of the formula (lb) as defined in Claim 3, which comprises reacting a compound of the formula (2b) as defined in Claim 13 with. a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
24. A process for producing a compound of the formula (lc) as defined in Claim 4, which comprises reacting a compound of the formula (2c) as defined in Claim 14 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
25. A process for producing a quinoline type mevalonolactone intermediate as defined in Claim 5, which comprises reacting a quinoline derivative as defined in Claim 15 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
26. A process for producing a pyrazolopyridine type mevalonolactone intermediate as defined in Claim 6, which comprises reacting a pyrazolopyridine derivative as defined in Claim 16 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
27. A process for producing a thienopyridine type mevalonolactone intermediate as defined in Claim 7, which comprises reacting a thienopyridine derivative as defined in Claim 17 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
28. A process for producing a quinoline type mevalonolactone intermediate as defined in Claim 8, which comprises reacting a quinoline derivative as defined in Claim 18 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
29. A process for producing a pyrazolopyridine type mevalonolactone intermediate as defined in Claim 9, which comprises reacting a pyrazolopyridine derivative as defined in Claim 19 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
30. A process for producing a thienopyridine type mevalonolactone intermediate as defined in Claim 10, which comprises reacting a thienopyridine derivative as defined in Claim 20 with a base to form an anion, which is then condensed with a compound of the formula (3) as defined in Claim 21.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP4118881A JPH05310700A (en) | 1992-05-12 | 1992-05-12 | Condensed pyridine-based mevalonolactone intermediate and its production |
| AU53443/94A AU5344394A (en) | 1992-05-12 | 1993-10-27 | Condensed pyridine type mevalonolactone intermediate and process for its production |
| PCT/JP1993/001551 WO1995011898A1 (en) | 1992-05-12 | 1993-10-27 | Condensed pyridine type mevalonolactone intermediate and process for its production |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP4118881A JPH05310700A (en) | 1992-05-12 | 1992-05-12 | Condensed pyridine-based mevalonolactone intermediate and its production |
| PCT/JP1993/001551 WO1995011898A1 (en) | 1992-05-12 | 1993-10-27 | Condensed pyridine type mevalonolactone intermediate and process for its production |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1995011898A1 true WO1995011898A1 (en) | 1995-05-04 |
Family
ID=26434449
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP1993/001551 WO1995011898A1 (en) | 1992-05-12 | 1993-10-27 | Condensed pyridine type mevalonolactone intermediate and process for its production |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO1995011898A1 (en) |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2751540A1 (en) * | 1996-07-26 | 1998-01-30 | Sanofi Sa | ANTITHROMBOTIC PHARMACEUTICAL COMPOSITION |
| EP0778271A3 (en) * | 1995-12-08 | 2000-03-22 | F. Hoffmann-La Roche Ag | Aminoalkyl-substituted benzo-heterocyclic compounds |
| WO2007132482A2 (en) * | 2006-05-17 | 2007-11-22 | Manne Satyanarayana Reddy | Novel process for the preparation of pitavastatin and its pharmaceutically acceptable salts |
| WO2011089623A2 (en) | 2010-01-20 | 2011-07-28 | Cadila Healthcare Limited | Process for preparing pitavastatin and pharmaceutically acceptable salts thereof |
| WO2012013325A1 (en) | 2010-07-26 | 2012-02-02 | Lek Pharmaceuticals D.D. | Process for the preparation of key intermediates for the synthesis of statins or pharmaceutically acceptable salts thereof |
| WO2012025939A1 (en) | 2010-08-25 | 2012-03-01 | Cadila Healthcare Limited | Pitavastatin calcium and process for its preparation |
| WO2012140490A2 (en) | 2011-04-11 | 2012-10-18 | Aurobindo Pharma Limited | Process for preparing quinoline derivative |
| US8404841B2 (en) | 2006-10-09 | 2013-03-26 | Msn Laboratories Limited | Process for the preparation of statins and their pharmaceutically acceptable salts thereof |
| US8455640B2 (en) | 2006-05-03 | 2013-06-04 | Msn Laboratories Limited | Process for statins and its pharmaceutically acceptable salts thereof |
| US8487105B2 (en) | 2009-01-19 | 2013-07-16 | Msn Laboratories Limited | Process for preparing pitavastatin, intermediates and pharmaceuctically acceptable salts thereof |
| US20130296561A1 (en) * | 2011-01-18 | 2013-11-07 | Dsm Sinochem Pharmaceuticals Netherlands B.V. | Process for the preparation of statins in the presence of base |
| US8987444B2 (en) | 2010-01-18 | 2015-03-24 | Msn Laboratories Private Limited | Process for the preparation of amide intermediates and their use thereof |
| US9156845B2 (en) | 2012-06-29 | 2015-10-13 | Pfizer Inc. | 4-(substituted amino)-7H-pyrrolo[2,3-d] pyrimidines as LRRK2 inhibitors |
| US9695171B2 (en) | 2013-12-17 | 2017-07-04 | Pfizer Inc. | 3,4-disubstituted-1 H-pyrrolo[2,3-b]pyridines and 4,5-disubstituted-7H-pyrrolo[2,3-c]pyridazines as LRRK2 inhibitors |
| US10039753B2 (en) | 2015-09-14 | 2018-08-07 | Pfizer Inc. | Imidazo[4,5-c]quinoline and imidazo[4,5-c][1,5]naphthyridine derivatives as LRRK2 inhibitors |
| CN108822090A (en) * | 2018-08-17 | 2018-11-16 | 杭州乐敦科技有限公司 | A kind of preparation method of statins drug midbody |
| US10676441B2 (en) | 2015-08-05 | 2020-06-09 | Api Corporation | Method for producing pitavastatin calcium |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4625039A (en) * | 1983-12-21 | 1986-11-25 | Sandoz Pharm. Corp. | 4-trisubstituted silyl protected hydroxy-6-oxo-tetrahydropyran-2-yl-aldehyde intermediates |
| US4822799A (en) * | 1988-01-27 | 1989-04-18 | Sandoz Pharm. Corp. | Pyrazolopyridine analogs of mevalonolactone and derivatives thereof useful for inhibiting cholesterol biosynthesis in mammals |
| US4870199A (en) * | 1986-04-30 | 1989-09-26 | Sandoz Pharm. Corp. | Processes for the synthesis of diprotected R[R*,S*]-3,5-dihydroxy-6-oxohexanoate esters |
| US4939159A (en) * | 1986-09-10 | 1990-07-03 | Sandoz Pharm. Corp. | Azaindole derivatives useful as cholesterol biosynthesis inhibitors |
| EP0535548A1 (en) * | 1991-10-04 | 1993-04-07 | Nissan Chemical Industries Ltd. | Inhibitor of atherosclerotic intimal thickening |
| JPH05310700A (en) * | 1992-05-12 | 1993-11-22 | Sagami Chem Res Center | Condensed pyridine-based mevalonolactone intermediate and its production |
-
1993
- 1993-10-27 WO PCT/JP1993/001551 patent/WO1995011898A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4625039A (en) * | 1983-12-21 | 1986-11-25 | Sandoz Pharm. Corp. | 4-trisubstituted silyl protected hydroxy-6-oxo-tetrahydropyran-2-yl-aldehyde intermediates |
| US4870199A (en) * | 1986-04-30 | 1989-09-26 | Sandoz Pharm. Corp. | Processes for the synthesis of diprotected R[R*,S*]-3,5-dihydroxy-6-oxohexanoate esters |
| US4939159A (en) * | 1986-09-10 | 1990-07-03 | Sandoz Pharm. Corp. | Azaindole derivatives useful as cholesterol biosynthesis inhibitors |
| US4822799A (en) * | 1988-01-27 | 1989-04-18 | Sandoz Pharm. Corp. | Pyrazolopyridine analogs of mevalonolactone and derivatives thereof useful for inhibiting cholesterol biosynthesis in mammals |
| EP0535548A1 (en) * | 1991-10-04 | 1993-04-07 | Nissan Chemical Industries Ltd. | Inhibitor of atherosclerotic intimal thickening |
| JPH05310700A (en) * | 1992-05-12 | 1993-11-22 | Sagami Chem Res Center | Condensed pyridine-based mevalonolactone intermediate and its production |
Non-Patent Citations (4)
| Title |
|---|
| CHEMICAL ABSTRACTS, vol. 120, 9 May 1994, Columbus, Ohio, US; abstract no. 244704V, HYAMA, TAMEJIRO ET AL: "Preparation of pyridine-type mevalonolactone intermediates" * |
| G. WESS ET AL.: "Stereoselective synthesis of HR 780 , a new highly potent HMG- CoA reductase inhibitor", TETRAHEDRON LETTERS., vol. 31, no. 18, 1990, OXFORD GB, pages 2545 - 2548 * |
| G.T. LEE ET AL.: "A general method for the synthesis of syn-(E) -3,5-dihydroxy-6-heptenoates", SYNLETT, vol. 9, 1990, STUTTGART DE, pages 508 * |
| TATSUYA MINAMI ET AL.: "A novel enantioselective synthesis of HMG Co-A reductase inhibitor NK-104 and a related compound.", TETRAHEDRON LETTERS, vol. 33, no. 49, 1992, OXFORD GB, pages 7525 - 7526 * |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0778271A3 (en) * | 1995-12-08 | 2000-03-22 | F. Hoffmann-La Roche Ag | Aminoalkyl-substituted benzo-heterocyclic compounds |
| FR2751540A1 (en) * | 1996-07-26 | 1998-01-30 | Sanofi Sa | ANTITHROMBOTIC PHARMACEUTICAL COMPOSITION |
| WO1998004259A1 (en) * | 1996-07-26 | 1998-02-05 | Sanofi | ANTITHROMBOTIC AND ANTIATHEROGENIC PHARMACEUTICAL COMPOSITION INCLUDING A THIENOPYRIDINE DERIVATIVE AND AN HMG-CoA-REDUCTASE INHIBITOR |
| AU725949B2 (en) * | 1996-07-26 | 2000-10-26 | Sanofi-Aventis | Antithrombotic antiatherogenic pharmaceutical composition comprising a thienopyridine derivative and an HMG-CoA-reductase inhibitor |
| US6218403B1 (en) | 1996-07-26 | 2001-04-17 | Sanofi-Synthelabo | Antithrombotic and antiatherogenic pharmaceutical composition including a thienopyridine derivative and an HMG-CoA-reductase inhibitor |
| US8455640B2 (en) | 2006-05-03 | 2013-06-04 | Msn Laboratories Limited | Process for statins and its pharmaceutically acceptable salts thereof |
| WO2007132482A2 (en) * | 2006-05-17 | 2007-11-22 | Manne Satyanarayana Reddy | Novel process for the preparation of pitavastatin and its pharmaceutically acceptable salts |
| WO2007132482A3 (en) * | 2006-05-17 | 2008-04-10 | Reddy Manne Satyanarayana | Novel process for the preparation of pitavastatin and its pharmaceutically acceptable salts |
| US8404841B2 (en) | 2006-10-09 | 2013-03-26 | Msn Laboratories Limited | Process for the preparation of statins and their pharmaceutically acceptable salts thereof |
| US8487105B2 (en) | 2009-01-19 | 2013-07-16 | Msn Laboratories Limited | Process for preparing pitavastatin, intermediates and pharmaceuctically acceptable salts thereof |
| US8987444B2 (en) | 2010-01-18 | 2015-03-24 | Msn Laboratories Private Limited | Process for the preparation of amide intermediates and their use thereof |
| WO2011089623A2 (en) | 2010-01-20 | 2011-07-28 | Cadila Healthcare Limited | Process for preparing pitavastatin and pharmaceutically acceptable salts thereof |
| US20120022102A1 (en) * | 2010-01-20 | 2012-01-26 | Cadila Healthcare Limited | Method for preparation of pitavastatin and its pharmaceutical acceptable salts thereof |
| US9085538B2 (en) | 2010-07-26 | 2015-07-21 | Lek Pharmaceuticals D.D. | Process for the preparation of key intermediates for the synthesis of statins or pharmaceutically acceptable salts thereof |
| EP2423195A1 (en) | 2010-07-26 | 2012-02-29 | LEK Pharmaceuticals d.d. | Process for the preparation of key intermediates for the synthesis of statins or pharmaceutically acceptable salts thereof |
| WO2012013325A1 (en) | 2010-07-26 | 2012-02-02 | Lek Pharmaceuticals D.D. | Process for the preparation of key intermediates for the synthesis of statins or pharmaceutically acceptable salts thereof |
| WO2012025939A1 (en) | 2010-08-25 | 2012-03-01 | Cadila Healthcare Limited | Pitavastatin calcium and process for its preparation |
| US20130296561A1 (en) * | 2011-01-18 | 2013-11-07 | Dsm Sinochem Pharmaceuticals Netherlands B.V. | Process for the preparation of statins in the presence of base |
| US9126975B2 (en) * | 2011-01-18 | 2015-09-08 | Dsm Sinochem Pharmaceuticals Netherlands B.V. | Process for the preparation of statins in the presence of base |
| WO2012140490A2 (en) | 2011-04-11 | 2012-10-18 | Aurobindo Pharma Limited | Process for preparing quinoline derivative |
| US9156845B2 (en) | 2012-06-29 | 2015-10-13 | Pfizer Inc. | 4-(substituted amino)-7H-pyrrolo[2,3-d] pyrimidines as LRRK2 inhibitors |
| US9642855B2 (en) | 2012-06-29 | 2017-05-09 | Pfizer Inc. | Substituted pyrrolo[2,3-d]pyrimidines as LRRK2 inhibitors |
| US9695171B2 (en) | 2013-12-17 | 2017-07-04 | Pfizer Inc. | 3,4-disubstituted-1 H-pyrrolo[2,3-b]pyridines and 4,5-disubstituted-7H-pyrrolo[2,3-c]pyridazines as LRRK2 inhibitors |
| US10676441B2 (en) | 2015-08-05 | 2020-06-09 | Api Corporation | Method for producing pitavastatin calcium |
| US10815201B2 (en) | 2015-08-05 | 2020-10-27 | Api Corporation | Method for producing pitavastatin calcium |
| US10039753B2 (en) | 2015-09-14 | 2018-08-07 | Pfizer Inc. | Imidazo[4,5-c]quinoline and imidazo[4,5-c][1,5]naphthyridine derivatives as LRRK2 inhibitors |
| CN108822090A (en) * | 2018-08-17 | 2018-11-16 | 杭州乐敦科技有限公司 | A kind of preparation method of statins drug midbody |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO1995011898A1 (en) | Condensed pyridine type mevalonolactone intermediate and process for its production | |
| KR100423627B1 (en) | Novel intermediates and methods for the preparation of camptothecin derivatives (capiti-11) and related compounds | |
| JP5285594B2 (en) | Substituted chromanol derivatives and their use | |
| CA2000553C (en) | Process for the preparation of 7-substituted-hept-6-enoic and -heptanoic acids and derivatives and intermediates thereof | |
| CA2046734A1 (en) | Substituted pyrrolo-pyridines pharmaceuticals | |
| US5003087A (en) | Process for preparing a naphthalene derivative and a synthetic intermediate thereof | |
| US5958970A (en) | Tricyclic and tetracyclic pyrones | |
| EP0312269B1 (en) | 3,5-dihydroxy-6,8-nonadienoic acids and derivatives as hypocholesterolemic agents | |
| JPH05310700A (en) | Condensed pyridine-based mevalonolactone intermediate and its production | |
| AU693588B2 (en) | Novel process for the preparation of diisopinocampheylchloroborane | |
| CA2452105A1 (en) | Dibenzocycloheptene compound | |
| US5807866A (en) | Process for preparing N-benzyl indoles | |
| US5459269A (en) | 14-halo-camptothecins | |
| WO1996036608A1 (en) | α,β-UNSATURATED KETONE DERIVATIVES | |
| KR101528359B1 (en) | Novel boronate ether intermediates for preparation of statin compounds, preparation method thereof and preparation method of statin compounds using said boronate ether intermediates | |
| KR100302346B1 (en) | A method of preparing 6-hydroxy-2-oxo-1,2,3,4-tetrahydroquinoline | |
| US5428166A (en) | Method of making asymmetric de ring intermediates for the synthesis of camptothecin and camptothecin analogs | |
| RU2045527C1 (en) | Quinoline salt and a method of its synthesis | |
| US6160120A (en) | Process for preparing n-benzyl indoles | |
| KR100396431B1 (en) | Method for producing 2-sulfonylpyridine derivatives and method for producing 2-[((2-pyridyl)methyl)thio]-1h-benzimidazole derivatives | |
| JPH06128205A (en) | Preparation of pyridine and quinoline derivatives | |
| RU2051907C1 (en) | Method for production of 7-substituted heptene-6-oic acid | |
| JP2001097970A (en) | Method for producing isocoumarin derivative | |
| JPH03173883A (en) | Indole derivative | |
| SI8911973A (en) | Process for the preparation of 7-substituted-hept-6-enoic and heptanoic acids and their derivatives and intermediates thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU CA CZ FI HU KR NO NZ RO RU UA US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |