WO1997041844A1 - Combinations of angiostatic compounds - Google Patents
Combinations of angiostatic compounds Download PDFInfo
- Publication number
- WO1997041844A1 WO1997041844A1 PCT/US1997/005574 US9705574W WO9741844A1 WO 1997041844 A1 WO1997041844 A1 WO 1997041844A1 US 9705574 W US9705574 W US 9705574W WO 9741844 A1 WO9741844 A1 WO 9741844A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compounds
- compound
- angiostatic
- alkyl
- composition
- Prior art date
Links
- 0 **C(*)(C1(CCCC1)C1)Oc2c1c(*)c(**)c(*=C)c2*=C Chemical compound **C(*)(C1(CCCC1)C1)Oc2c1c(*)c(**)c(*=C)c2*=C 0.000 description 2
- ICTXHFFSOAJUMG-CEVCPLMDSA-N CC(CC1)(C(CC2)C(CC3)C1C(CC1)=C3CC1=O)[C@@]2(C#C)O Chemical compound CC(CC1)(C(CC2)C(CC3)C1C(CC1)=C3CC1=O)[C@@]2(C#C)O ICTXHFFSOAJUMG-CEVCPLMDSA-N 0.000 description 1
- RUYZTDOMKYWHFC-UHFFFAOYSA-N CC(CCO)(CCc1c2C)Oc1c(C)c(C)c2O Chemical compound CC(CCO)(CCc1c2C)Oc1c(C)c(C)c2O RUYZTDOMKYWHFC-UHFFFAOYSA-N 0.000 description 1
- YUWPMEXLKGOSBF-SQIVGGFUSA-N C[C@]1(C(CC2)C(CCC([C@]3(C)CC4)=CC4=O)C3=CC1)[C@]2(C(COC(C)=O)=O)O Chemical compound C[C@]1(C(CC2)C(CCC([C@]3(C)CC4)=CC4=O)C3=CC1)[C@]2(C(COC(C)=O)=O)O YUWPMEXLKGOSBF-SQIVGGFUSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/34—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide
- A61K31/343—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide condensed with a carbocyclic ring, e.g. coumaran, bufuralol, befunolol, clobenfurol, amiodarone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
Definitions
- the present invention relates to certain compounds useful in preventing and
- compositions are directed to compositions
- Angiogenesis is a term used to describe the development of new blood vessels or
- vitreoretinopathies psoriasis, arthritis and solid tumor development.
- angiogenesis occurs in several phases which include: elaboration of the angiogenic
- Tumor growth is dependent on neovascularization.
- Angiogenesis is also associated with important diseases of ocular tissue especially
- Neovascularization can
- the most threatening ocular neovascular diseases are those which involve the retina.
- Retinal neovascularization is often treated with multiple laser burns to the
- neovascularization and the progress of the overall disease. In addition, they can cause
- angiostatic steroids functioning to
- Glucocorticoids as mentioned above, have also been shown to inhibit
- Antiestrogens have been shown to alter the activity of a number of
- Vitamin D 3 analogs (Oikawa et al., Inhibition of Angiogenesis by
- Vitamin D 3 Analogues European Journal of Pharmacology, volume 178, pages 247-250 (1990).
- the use of a variety of pharmaceutical proteins has also been proposed for
- Such therapies have included: monoclonal antibodies directed to
- fibroblast growth factor disclosed in WO 91/06668
- platelet factor 4 disclosed in WO
- the present invention involves the angiostatic therapy of a combination of two or
- the present invention is directed to methods of using combinations of angiostatic compounds for the
- compositions containing combinations of angiostatic agents are also directed to compositions containing combinations of angiostatic agents.
- compositions are useful for controlling ocular neovascularization.
- the combination therapy of the present invention is believed to have the
- compositions and methods of the present invention involve various combinations
- Table 1 contains a list of different angiostatic compounds
- Matrix metalloproteinase inhibitors (betimastat, BB-2516, TIMPs, minocycline, GM6001 )
- Urokinase receptor antagonists Platelet factor 4 and analogs Heparinases
- TSP-1 Thrombospondin and related analogs
- Anti-sense oligonucleotides (specific for bFGF and VEGF)
- VEGF and bFGF antagonists VEGF receptor-chimeric proteins
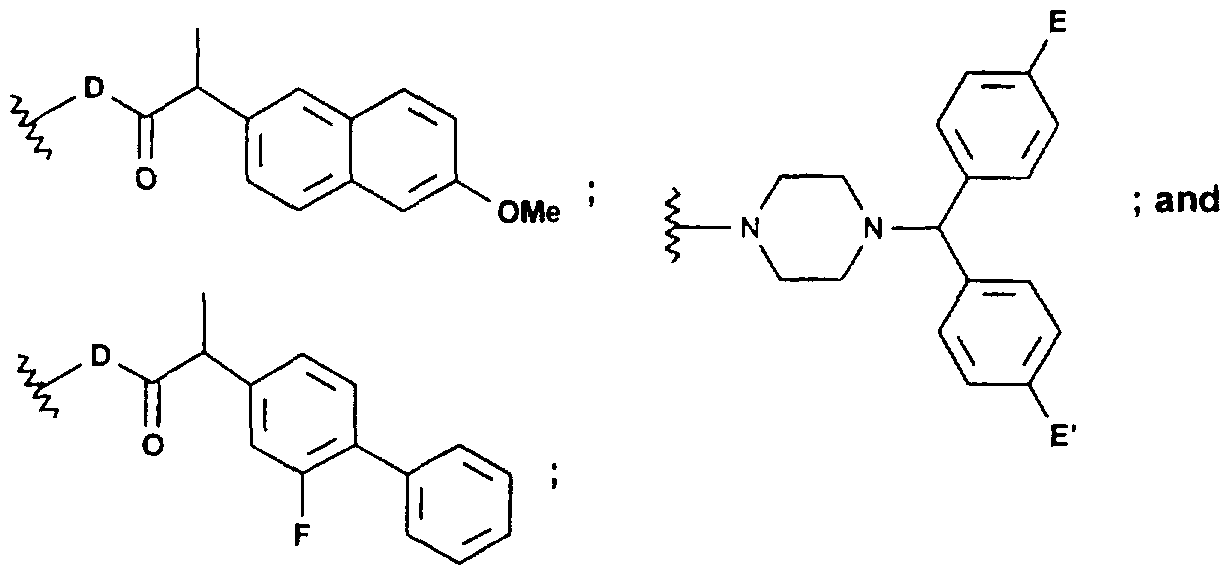
- n 1 or 2;
- R is H, C,-C 6 alkyl or C 3 -C 6 cycloalkyl
- Y is H, C,-C 6 alkyl, C 3 -C 6 cycloalkyl, O, NR, C(R) 2 , CH(OH) or S(O) n .
- n' is 0 to 2;
- R" is H or C,-C 6 alkyl
- R' can not be H, when R is q is 1 to 10;
- Z if present, is H, C r C 6 alkyl, C 3 -C 6 cycloalkyl, or selected from the group consisting of:
- D is O or NR
- E and E' are independently H, F or Cl.
- the compounds ofthe present invention also include pharmaceutically acceptable salts of
- neovascularization is similar in all tissues regardless ofthe associated disease; see, Furcht,
- angiostatic agents work by inhibiting one or more steps in the process of
- angiogenesis is brought about through a number of biochemical
- This process generally consists of the following steps:
- endothelial cells become “activated” and release proteases and other degradative enzymes which dissolve the basement membranes surrounding the cells.
- the endothelial cells can
- antibodies to growth factors such as bFGF (Hori et al., Suppression oj solid
- VEGF (Kim et al., Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo, Nature, volume 362, pages 841-844 (1993)) can
- fumagillin-type and suramin-type compounds inhibit
- Plasminogen Activator Activity Via Stimulation of Plasminogen Activator Inhibitor
- the present invention in preventing neovascularization than either drug alone. Therefore, the present invention
- angiostatic agent or compound refers to any compound which inhibits one or more
- Vitamin E succinate (VES) ("Compound G")
- Angiostatic steroids are compounds containing the 6-6-6-5-ring steroid
- angiostatic steriods are: 21 -Nor-5 ⁇ -pregnan-3c ⁇ , 17 ⁇ ,20-triol
- suramin-type compounds examples are: suramin,
- the most preferred suramin-type compounds include: suramin and 4,4 ' -bis[[4-(o- hydroxyanilino)-6-(m-sulfoanilino)-s-triazin-2-yl]amino]-2,2 ' stilbenedisulfonic acid.
- "Fumagillin-type compounds” are oxaspiro[2,5]octane derivatives, such as those described in European Patent Application Nos. 0 354 787 A 1 , 0 386 667 A 1 and 0 387 650 Al , the entire contents of these three publications are inco ⁇ orated herein by reference to the extent they disclose angiostatic fumagillin-type compounds.
- Anti -estrogen compounds are those compounds which at least partially bind estrogen receptors. A number of anti-estrogens have been shown to inhibit angiogenesis. Anti-estrogen compounds of the present invention include: clomiphene, tamoxifen, nafoxidine, ICI 164,984 and ICI 182,780.
- angiostatic compounds contained in the Table 1 are known in the art to possess angiostatic activity. Of those classes listed, 5-flurouracil, mitomycin-C, taxol, 2- methoxyestradiol, betimastat, BB-2516, TIMPs, minocycline, GM6001 , PF4, CDI, TSP- 1 , TNP-470, SU101 , anti-endoglin, ⁇ -IFN and VEGF receptor-chimeric proteins are preferred for use in combinations of angiostatic compounds ofthe present invention.
- angiostatic compounds include compounds of formula (I), anti-mitotics, angiostatic steroids, fumagillin- type compounds, suramin-type compounds, estrogen metabolites, matrix metalloproteinase inhibitors and thalidomide.
- a compound of formula (I) and an angiostatic steroid 2 A compound of formula (I) and a suramin-type compound
- pathological neovascularization refers to those conditions where the formation of blood
- neovascularization dependent diseases include: head trauma, spinal trauma, systemic or
- angiofibroma angiofibroma, arteriovenous malformations, corneal graft neovascularization,
- hemophilic joints hypertrophic scars, ocular neovascularization, nonunion fractures,
- compositions of the present invention are useful in preventing
- retinal diseases including, but not limited to: retinal diseases
- retinoblastoma retinoblastoma, pseudoglioma and melanoma
- Fuchs' heterochromic iridocyclitis retinoblastoma, pseudoglioma and melanoma
- neovascular glaucoma corneal neovascularization (inflammatory, transplantation and
- angiostatic agents are useful in treating
- hyperkeratosis hyperkeratosis, cheloid formation and polyp formation.
- compositions of the present invention to ameliorate complications
- Glaucoma filtration surgery involves the surgical creation of a fistula with a
- conjuctival flap which allows the direct drainage of aqueous humor from the anterior
- fibroblasts may feed the fibroblasts which migrate, and proliferate, and block the bleb, or the
- vascuiarization itself may also result in physical blockage of the bleb. It is therefore
- the angiostatic compounds may be contained in various types of pharmaceutical
- compositions either together as a single composition or in separate compositions, in
- the compounds may be included in tablets, capsules, solutions, suspensions and other
- dosage forms adapted for oral administration; solutions and suspensions adapted for
- solutions, suspensions or gels for topical ocular administration solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions, suspensions or gels for topical ocular administration; solutions
- Solutions, suspensions and other dosage forms adapted for topical application to the involved tissues, such as tissue irrigating solutions, are particularly preferred for treatment
- the present invention is particularly directed to the provision of compositions
- Aqueous solutions are generally preferred,
- compositions by means of instilling one to two drops of the solutions in the affected eyes.
- compositions such as suspensions, viscous or semi-viscous gels or other
- Suspensions may be preferred for compounds having the types of solid or semi-solid compositions. Suspensions may be preferred for compounds having the types of solid or semi-solid compositions. Suspensions may be preferred for compounds having the types of solid or semi-solid compositions. Suspensions may be preferred for compounds having the types of solid or semi-solid compositions. Suspensions may be preferred for compounds having the types of solid or semi-solid compositions. Suspensions may be preferred for compounds
- compositions ofthe present invention may also include various other ingredients, such as
- An appropriate buffer system e.g., sodium phosphate, sodium acetate or sodium
- borate may be added to prevent pH drift under storage conditions.
- Ophthalmic products are typically packaged in multidose form. Preservatives are
- Such preservatives are typically employed at a
- the compounds may also be used as an adjunct to ophthalmic surgery, such as
- the compounds may also be any compound that chronically, especially in the case of degenerative disease.
- the compounds may also be any compound that chronically, especially in the case of degenerative disease.
- the compounds may also be any compound that chronically, especially in the case of degenerative disease.
- the compounds may also be any compound that chronically, especially in the case of degenerative disease.
- the compounds may also be any compound that chronically, especially in the case of degenerative disease.
- the compounds may also be any other neuropeptide
- electrolytes such as sodium, potassium, calcium, magnesium and/or
- Intraocular Irrigating Solution (Alcon Laboratories, Inc., Fort Worth, Texas, USA) are examples of Intraocular Irrigating Solution.
- the compound or its salt being used, the dosage frequency, and the disease being treated.
- Topical aqueous solutions, suspensions, ointments, creams and gels are the preferred
- Topical ophthalmic formulations are suitable for preventing glaucoma
- concentrations range from about 0.1 to about 5.0 weight/percent.
- these formulations are delivered to the disease site one to six times a day,
- Tablets containing 10- 1000 mg of a compound can be taken 2-3 times per day
- compositions of the present invention are further illustrated by the following
- angiostatic compound refers to any compound of the present
- Topical combination compositions useful for controlling ocular neovascularization are:
- a preferred topical composition useful for controlling neovascularzation is a topical composition useful for controlling neovascularzation:
- the above formulation is prepared by first placing a portion of the purified water
- HPMC hydroxypropylmethylcellulose
- HPMC HPMC is dispersed. The resulting mixture is then allowed to cool while undergoing mixing
- the angiostatic compounds are sterilized by either dry heat or ethylene oxide. If
- ethylene oxide sterilization is selected, aeration for at least 72 hours at 50°C. is necessary.
- the sterilized angiogenic compound is weighed aseptically and placed into a pressurized
- the container are milled aseptically at 225 ⁇ m for 16 hours, or until all particles are in the
- a preferred formulation for oral administration is a preferred formulation for oral administration:
- Cream 1 mg/g each of two angiostatic compounds in cream base of purifed water, emulsifying wax, propylene glycol, stearic acid, isopropyl palmitate, synthetic beeswax, polysorbate 60, potassium sorbate, sorbic acid, propyl gallate, citric acid, and sodium hydroxide.
- Ointment 1 mg/g each of two angiostatic compounds in base of mineral oil and polyethylene.
- Some of the compounds of the present invention may contain a nonsteroidal anti-
- NSAIA inflammatory agent
- flunarizine calcium channel blocker
- V L C1, BR, I, OMs, OTs I
- the conversion of the carboxylic acid containing nonsteroidal anti-inflammatory agents (II) to esters or amides (I) may be carried out by the following methods:
- carboxylic acids (II) may be reacted with the appropriate amine or alcohol derivative (III) in the presence of a coupling reagent, such as dicyclohexylcarbodiimide or l -(3-dimethylaminopropyl)-3-ethyl carbodiimide HCl, and 4-dimethylamine pyridine or 1 -hydroxybenzotriazole, in an inert organic solvent, such as acetonitrile or tetrahydrofuran, and at a temperature from 0°C to 50°C.
- a coupling reagent such as dicyclohexylcarbodiimide or l -(3-dimethylaminopropyl)-3-ethyl carbodiimide HCl, and 4-dimethylamine pyridine or 1 -hydroxybenzotriazole
- carboxylic acids (II) may be converted to acid chlorides (IV) by reacting them with a reagent such as thionyl chloride or oxalyl chloride, in the presence of an inert solid or neat, at a temperature from 0°C to 80°C.
- a reagent such as thionyl chloride or oxalyl chloride
- the resulting acid chloride (IV) may be reacted with the desired amine or alcohol (III) in an inert solvent such as tetrahydrofuran, in the presence of pyridine or a tertiary amine, such as triethylamine.
- esters (I) may be formed by reacting carboxylate anions (V), formed by reacting the carboxylic acid (II) with a base such as sodium hydride, with a halide (iodide, bromide, chloride) or sulfonate (mesylate, tosylate) (VI), in a solvent such as acetonitrile or dimethylformamide, at a temperature from 0°C to
- amides (I) may be prepared by reacting carboxylate anions (V), formed by reacting carboxylic acid (II) with a base such as sodium hydride, with ethyl bromoacetate.
- the resulting ester (VII) is reacted with the desired amine (VIII), neat or in an inert solvent, such as acetonitrile or dimethylformamide, at a temperature from 0°C to 100°C.
- the nitrile (IX) can be reduced using a reagent such as lithium aluminum
- W is (CH 2 ) p -Q; p is 0-1;
- Q is CH 2 OH or CO 2 H
- R' is H, C(O)R, C(O)NR 2 , PO 3 " , or SO 3 " ;
- R" is H or C,-C 6 alkyl.
- the alcohols (XI a . b ) may be resolved by forming esters with optically active carboxylic acids, separating the diastereomers, and then hydrolyzing the resolved diastereomers.
- the corresponding carboxylic acids (XI a . b ) may be resolved by forming an ester with an optically active alcohol, separating the diastereomers, and then hydrolyzing the resolved diastereomers.
- the carboxylic acids (XI a-b ) may be resolved by forming an amine salt with an optically active amine. Separation by recrystallization and neutralization of the resolved carboxylic acid salt may be utilized to provide the resolved carboxylic acid.
- Resolution of the esters and amides (I) may also be effected using chromatographic techniques known to those skilled in the art.
- the amines of formula (I), where Y is NR, may be converted to amine salts by reacting the amine with acids of sufficient strength to produce an organic or inorganic salt.
- the pharmaceutically acceptable anions include: acetate, bromide, chloride, citrate, maleate, fumarate, mesylate, phosphate, sulfate and tartrate.
- the white solid was recrystallized from an ethyl acetate- hexanes mixture to give 0.60 g (33.1% yield) of 2-(6-hydroxy-2,5,7,8-tetramethyl-3,4- dihydro-2H-benzo[ 1 ,2-b]pyran-2yl)ethyl 2-(6-methoxy-2-naphthyl)propionate, a mixture of diastereomers, as a white solid.
Abstract
The present invention is directed to compositions containing combinations of angiostatic compounds and methods for their use in preventing pathological neovascularization.
Description
COMBINATIONS OF ANGIOSTATIC COMPOUNDS
Background ofthe invention
The present invention relates to certain compounds useful in preventing and
treating neovascularization. Specifically, the invention is directed to compositions
containing two or more combinations of angiostatic agents and methods of using
combinations of these angiostatic agents to treat neovascularization.
Angiogenesis is a term used to describe the development of new blood vessels or
neovascularization (L. Diaz-Flores et al., Angiogenesis: an Update, Histology and
Histopathology. volume 9, pages 807-843 (1994)). Though angiogenesis is a normal
process for the development or maintenance of the vasculature, pathological conditions
(i.e.. angiogenesis dependent diseases) arise where blood vessel growth is actually
harmful. Such pathologies include diabetic retinopathies, proliferative
vitreoretinopathies, psoriasis, arthritis and solid tumor development. The progression of
angiogenesis occurs in several phases which include: elaboration of the angiogenic
signal; dissolution of the blood vessel basement membrane; endothelial cell proliferation;
endothelial cell migration; and formation and differentiation of capillary tubules and
loops. Each of these phases is a potential target for pharmacological intervention.
Tumor growth is dependent on neovascularization. For solid tumors to grow
beyond the size of a pea, they must become vascularized. They do so by secreting their
own angiogenic factor(s) which recruit new blood vessels to provide essential nutrients
and oxygen.
Angiogenesis is also associated with important diseases of ocular tissue especially
in older patients and diabetics. Any abnormal growth of blood vessels in the eye can
scatter and block the incident light prior to reaching the retina. Neovascularization can
occur at almost any site in the eye and significantly alter ocular tissue function. Some of
the most threatening ocular neovascular diseases are those which involve the retina. For
example, many diabetic patients develop a retinopathy which is characterized by the
formation of leaky, new blood vessels on the anterior surface of the retina and in the
vitreous causing proliferative vitreoretinopathy. A subset of patients with age related
macular degeneration develop subretinal neovascularization which leads to their eventual
blindness.
Current therapy for the treatment of ocular neovascular disease is not very
effective. Retinal neovascularization is often treated with multiple laser burns to the
retina to remove the pathological vasculature. Panretinal photocoagulation, however,
destroys normal retinal tissue. Patients with neovascular diseases of the anterior chamber
(e.g. corneal neovascularization, iritis rubeosis) are treated with potent topical ocular
glucocorticoids. These therapies are only partially effective and generally only slow
neovascularization and the progress of the overall disease. In addition, they can cause
severe side effects if used over a relatively long period of time.
Other attempts have been made to provide therapies for the prevention or
treatment of pathological angiogenesis. For example, angiostatic steroids functioning to
inhibit angiogenesis in the presence of heparin or specific heparin fragments in the
chicken embryo model of neovascularization are disclosed in Crum, et al., A New Class of
Steroids Inhibits Angiogenesis in the Presence of Heparin or a Heparin Fragment,
Science, volume 230, pages 1375-1378 (1985). Other groups of angiostatic steroids
useful in inhibiting angiogenesis are disclosed in commonly assigned WIPO Publication
No. WO 93/10141 (Clark et al.) and United States Patent No. 5,371,078 (Clark et al.), as
well as WO 95/18621 (Prioa et al.).
Glucocorticoids, as mentioned above, have also been shown to inhibit
angiogenesis. However, the use of glucocorticoid therapy in general is complicated by
the inherent problems associated with steroid applications. Such problems include
elevated intraocular pressure (Kitazawa, Increased Intraocular Pressure Induced by
Corticosteroids, American Journal of Ophthalmology, volume 82, pages 492-495 (1976)),
and the development of posterior subcapsular cataracts.
Suramin, a complex molecule, has been described as a growth factor antagonist
and possessing angiosuppressive action (Takano, Angiosuppressive and Antiproliferative
Actions of Suramin: A Growth Factor Antagonist, Growth Factors. Peptides and
Receptors. Ed. T.W. Moody, Plenum Press, New York, pages 255-264 (1993)). Suramin
and its derivatives have also been disclosed in WIPO Publication No. WO 90/15816, as
inhibitors of fibroblast growth factor (an angiogenesis factor) as well as apparent
angiostatic agents.
Fumagillin and analogs of fumagillin have been reported to possess angiostatic
properties (Ingber et al., Synthetic Analogues of Fumagillin that Inhibit Angiogenesis and
Suppress Tumor Growth, Nature, volume 348, pages 555-557 (1990)). Several European
patent applications have disclosed fumagillin analogs including: European Patent
Application Nos. 0 354 787 Al, 0 386 667 Al and 0 470 569 Al, all assigned to Takeda
Chemical (Japan) or Fujisawa Pharmaceutical (Japan).
Anti-estrogens, or estrogen antagonists, have also been reported to possess
angiostatic activity. Antiestrogens have been shown to alter the activity of a number of
growth factors that are important in the control of cellular proliferation (Freiss et al.
Antisteroidal and anti-growth factor activities of antiestrogens, Journal of Steroid
Biochemistry and Molecular Biology, volume 37, pages 777-781 (1990)). They are
known to affect biochemical functions and to inhibit angiogenesis in a dose dependent
manner (Gagliardi et al., Inhibition of Angiogenesis by Antiestrogens, Cancer Research.
volume 53, pages 533-535 (1993)).
Still other therapies have been proposed, including, the use of protamine (S.
Taylor, Protamine is an Inhibitor of Angiogenesis, Nature, volume 297, pages 307-312
(1982)), and the use of Vitamin D3 analogs (Oikawa et al., Inhibition of Angiogenesis by
Vitamin D3 Analogues, European Journal of Pharmacology, volume 178, pages 247-250 (1990)). The use of a variety of pharmaceutical proteins has also been proposed for
treating angiogenesis. Such therapies have included: monoclonal antibodies directed to
fibroblast growth factor, disclosed in WO 91/06668; platelet factor 4, disclosed in WO
93/02192; and thrombospondin fragments, disclosed in WO 93/16716.
Summary ofthe Invention
The present invention involves the angiostatic therapy of a combination of two or
more molecules selected from a set of angiostatic compounds. As such, the present
invention is directed to methods of using combinations of angiostatic compounds for the
prevention and/or treatment of neovascularization in human patients. The present
invention is also directed to compositions containing combinations of angiostatic
compounds. In particular, the compositions are useful for controlling ocular neovascularization.
The combination therapy of the present invention is believed to have the
advantage of providing effective, multi-mechanistic angiostatic therapy which is more
efficacious with fewer side effects.
Detailed Description of the Invention
The compositions and methods of the present invention involve various
combinations of angiostatic compounds. Table 1 contains a list of different angiostatic
compounds useful in the present invention:
Table 1
Classes of Angiostatic Agents (examples where specified)
Anti-mitotics (5-flurouracil, mytomycin-C, taxol) Estrogen metabolites (2-methoxyestradiol)
Matrix metalloproteinase inhibitors (betimastat, BB-2516, TIMPs, minocycline, GM6001 )
Plasminogen activator/urokinase inhibitors
Urokinase receptor antagonists Platelet factor 4 and analogs
Heparinases
Cartilage-derived inhibitor of angiogenesis
Thrombospondin and related analogs (TSP-1)
Angiostatin, vasculostatin
Proliferin-related protein
Fumagillin analogs (TNP-470)
Tecogalan
Pentosan polysulfate
Thalidomide and related analogs
CM101
Tyrosine kinase inhibitors (SU101)
Anti-sense oligonucleotides (specific for bFGF and VEGF)
Suramin and suramin analogs
Angiostatic steriods αvB3 and αvβ5 integrin antagonists
Cytotoxic antibodies against endothelial cell antigens (anti-endoglin)
Interferon (α-IFN)
VEGF and bFGF antagonists (VEGF receptor-chimeric proteins) flk-1 and fit- 1 antagonists
IL- 1 , and TNF antagonists
Additionally, compounds of formula (I) below are also useful angiostatic compounds of
the present invention:
n is 1 or 2;
R is H, C,-C6 alkyl or C3-C6 cycloalkyl;
Y is H, C,-C6 alkyl, C3-C6 cycloalkyl, O, NR, C(R)2 , CH(OH) or S(O)n. n' is 0 to 2;
R' is H, C(O)R, C(O)N(R)2, PO3 ' , SO3 " , or HO2C(CH2)2(C=O)-~ ;
R" is H or C,-C6 alkyl;
R3 is H, C,-C6 alkyl, (CH2)q(OH), — (C=O)O(CH2)qCH3 or
Z, if present, is H, CrC6 alkyl, C3-C6 cycloalkyl, or selected from the group consisting of:
wherein:
D is O or NR; and
E and E' are independently H, F or Cl.
The compounds ofthe present invention also include pharmaceutically acceptable salts of
the compounds of formula (I) and the compounds included in the angiostatic classes listed on Table 1.
The initiation of new blood vessel formation may arise quite differently in various
tissues or as a result of different diseases. Many substances have been found to induce
neovascularization; see, Folkman, et al., Angiogenic Factors, Science, volume 235, pages 442-447 (1987). It is believed, however, that once initiated, the process of
neovascularization is similar in all tissues regardless ofthe associated disease; see, Furcht,
Critical Factors Controlling Angiogenesis: Cell Products, Cell Matrix, and Growth
Factors, Laboratory Investigation, volume 55, No. 5, pages 505-509 (1986).
There are many theories associated with the cause of neovascularization, and there
may be different inducers depending on the disease or surgery involved; see, BenEzra,
Neovasculogenic Ability of Prostaglandins, Growth Factors, and Synthetic Chemoattractants, American Journal of Ophthalmology, volume 86, No. 4, pages 455-
461, (October, 1978). Regardless of the cause or the associated disease, it is believed that
angiostatic agents work by inhibiting one or more steps in the process of
neovascularization.
As stated above, angiogenesis is brought about through a number of biochemical
and cellular mechanisms involving several steps, each of which is a potential target for
pharmacological intervention. This process generally consists of the following steps:
breakdown of blood vessel basement membranes, endothelial cell activation, migration,
proliferation and formation of capillary tubules. In response to an angiogenic signal,
endothelial cells become "activated" and release proteases and other degradative enzymes
which dissolve the basement membranes surrounding the cells. The endothelial cells can
now migrate towards the stimulus elongating and aligning themselves to create a sprout.
Rapid proliferation lengthens the columns of cells and branches will appear at the top of
the column Branches from adjacent columns fuse and in this way a network of new
capillaries is formed. Thus, inhibition of this complex process of angiogenesis is possible
at several stages These include interception of the activation signal, inhibition of
basement membrane breakdown, inhibition of cell migration, inhibition of cell
proliferation as well as interference with the formation of capillary tubules.
Different compounds may affect different stages of the angiogenic process. For
example, antibodies to growth factors such as bFGF (Hori et al., Suppression oj solid
tumor growth by immunoneutralizing monoclonal antibody against human basic
fibroblast growth factor, Cancer Research, volume 51 , pages 6180-6184 (1991)) and
VEGF (Kim et al., Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo, Nature, volume 362, pages 841-844 (1993)) can
interfere with the activation signal; fumagillin-type and suramin-type compounds inhibit
endothelial cell migration; chroman deπvatives and fumagillin-type compounds (Ingber et
al., Synthetic analogs of fumagillin that inhibit angiogenesis and suppress tumour growth, Nature, volume 348, pages 555-557 (1990)) and suramin-type compounds (C Stein,
Suramin A novel antineoplastic agent with multiple potential mechanisms of action,
Cancer Research, volume 53, pages 2239-2248 (1993)) inhibit cell proliferation, and
angiostatic steroids are currently thought to exert their anti-angiogenic effects by
inhibiting basement membrane breakdown (Ashino-Fuse et al., Medroxyprogesterone
Acetate, An Anti-Cancer And Anti-Angiogemc Steroid, fnhibits The Plasminogen
Activator In Bovine Endothelial Cells, volume 44, pages 859-864 (1989)) or by inhibiting
PAI-1 synthesis (Blei et al., Mechanism of Action of Angiostatic Steriods: Suppression of
Plasminogen Activator Activity Via Stimulation of Plasminogen Activator Inhibitor
Synthesis, Journal of Cellular Physiology, volume 155, pages 568-578 (1993)). Therefore, therapeutic intervention is possible at several points in the process.
While applicants do not wish to be bound by any theory, it is believed that the
inhibition of multiple cellular/biological mechanisms associated with angiogenesis will
more effectively inhibit neovascularization. Therefore, the use of combinations of
compounds affecting different mechanisms of angiogenesis would be more effective in
preventing neovascularization than a single therapeutic approach. For example,
combinations of anti-proliferative compounds such as chroman derivatives and angiostatic steroids which affect basement membrane breakdown would be more effective
in preventing neovascularization than either drug alone. Therefore, the present invention
sets forth the use of combinations of different angiostatic agents to provide a more
effective therapeutic approach to inhibiting neovascularization. As used herein, the term
"angiostatic agent or compound" refers to any compound which inhibits one or more
processes of angiogenesis such that angiogenesis is inhibited or retarded.
Preferred compounds of formula (I), also known as "chroman derivatives,"
include the following:
2-(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[l,2-b]pyran-2-yl)ethyl 2-(6- methoxy-2-naphthyl)propionate ("Compound A");
2-(5-hydroxy-2,4,6,7-tetramethyl-2,3-dihydro-benzo[l,2-b]furan-2-yl)methyl 2-(6- methoxy-2-naphthyl)propionate ("Compound B");
N-(2-(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[l,2-b]pyran-2-yl)methyl) 2- (6-methoxy-2-naphthyl)propionamide ("Compound C");
2-(5-hydroxy-2,4,6,7-tetramethyl-2,3-dihydro-benzo[l ,2-b]furan-2-yl)ethyl 2-(6- methoxy-2-naphthyl)propionate ("Compound D");
l-[2-(5-hydroxy-2,4,6,7-tetramethyl-2,3-dihydro-benzo[l,2-b]furan-2-yl)2-ethyl]-4-[4,4'- fluorobenzhydryljpiperzine ("Compound E")
2-(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[l,2-b]pyran-2-yl)ethanol ("Compound F")
Vitamin E succinate (VES) ("Compound G")
2-(6-hydroxy-2,5,7,8-tetramethyl-2,3-dihydro-2H-benzo[l,2-b]pyran-2-yl)ethyl 2-(3- fluoro-4-phenyl-phenyl)propionate ("Compound H").
"Angiostatic steroids" are compounds containing the 6-6-6-5-ring steroid
backbone and possessing angiostatic activity. Preferred angiostatic steroids of the present
invention have been disclosed in United States Patent No. 5,371 ,078 (Clark et al.) and
WIPO Publication WO 93/10141 (Clark et al.); the entire contents of these publications
are incorporated herein by reference. Preferred angiostatic steriods are:
21 -Nor-5β-pregnan-3c<, 17α,20-triol
-Nor-5β-pregn- 17(20)en-3α, 16-diol-3-acetate- 16-(O-methyl)malonate
21 -Nor-5β-pregnan-3α, 17α,20-triol-3 -acetate
21-Nor-5α-pregnan-3α,17α,20-triol-3-phosphate
4-Androsten-3-one-17β-carboxylic acid
10
21 -Nor-5β-pregn- 17(20)en-3α, 16-diol
15
21 -Nor-5β-pregnan-3α, 17 β,20-triol
20-Acetamido-21-nor-5β-pregnan-3α,17α-diol-3-acetate
10
3β-Azido-5β- pregnan -1 1 β,17α,21-triol-20- one-21 -acetate
21 -Nor-5α-pregnan-3α, 17β,20-triol
α-Methyl-5β-pregnan-3α, 11 β, 17α,21 -tetrol-20-one-21 -methyl ether
4,9( 11 )-Pregnadien- 17α,21 -diol-3,20-dione-21 -acetate
4,9( 1 1 )-Pregnadien- 17α,21 -diol-3 ,20-dione
"Suramin-type compounds" are compounds which mimic the anti-angiogenic
action of suramin. Suramin and suramin-type compounds are known to those skilled in
the art. Examples of suramin-type compounds are:
suramin,
3-hydroxy-2,7-naphthalenesulfonic acid, 4,5-dihydroxy-2, 7-naphthalenedi sulfonic acid,
2,2'-[(l,8-dihydroxy-3,6-disulfo-2,7-napthylene)bis(azo]di-benzenearsonic acid, 5 4,4'-bis[[4-(o-hydroxyanilino)-6-(m-sulfoanilino)-s-triazin-2-yl]amino]-
2,2 'stilbenedisulfonic acid,
4,5-dihydroxy-3-[(p-nitrophenyl)azo]-2,7-naphthalenedisulfonic acid, 4,5-dihydroxy-3,6-bis[(4-sulfo-l -naphthyl)azo]-2,7-naphthalene-disulfonic acid, 3-[(5-chloro-2-hydroxyphenyl)azo]-4,5-dihydroxy-2,7-naphthalene-disulfonic I o acid,
4,5 '-dihydroxy-3,6 ' [3,3 '-dimethoxy-4,4 '-biphenylylene)bis(azo)-di- 1 - naphthalenesulfonic acid,
3,6-[(2,3-dimethyl-5-oxo-l-phenyl-3-pyrazolin-4-yl)azo]-4,5-dihydroxy-2,7- naphthalenedisulfonic acid, 15 5,5 '-[ureylenebis[2-sulfo-p-phenylene_azo]bis[6-amino-4-hydroxy-2- naphthalenesulfonic acid,
4-[(o-arsonophenyl)azo]3-hydroxy-2,7-naphthalenedisulfonic acid, 4.5-dihydroxy-3-(phenylazo)-2,7-naphthaienedisulfonic acid, 4-acetamido-5-hydroxy-6-(phenylazo)- 1 ,7-naphthaIenedisulfonic acid, 20 2-[p-f(l -hydroxy-4-sulfo-2-naphthyl)azo]phenyl]-6-methyl-7- benzothiazolesulfonic acid,
4-[(2,4-dimethyiphenyl)azo]-3-hydroxy-2,7-napthalenedisulfonic acid, 3-[(4-Sulfophenyl)azo]-4,5-dihydroxy-2,7-naphthalenedisulfonic acid, 3-[(4-nitrophenyl)azo]-4-amino-5-hydroxy-2,7-naphthalene-disulfonic acid, 25 l-nitro-4,6,8-naphthalenetrisulfonic acid, l -amino-4,6,8-naphthalenetrisulfonic acid and pharmaceutically acceptable salts thereof.
The most preferred suramin-type compounds include: suramin and 4,4'-bis[[4-(o- hydroxyanilino)-6-(m-sulfoanilino)-s-triazin-2-yl]amino]-2,2'stilbenedisulfonic acid. 30 "Fumagillin-type compounds" are oxaspiro[2,5]octane derivatives, such as those described in European Patent Application Nos. 0 354 787 A 1 , 0 386 667 A 1 and 0 387
650 Al , the entire contents of these three publications are incoφorated herein by reference to the extent they disclose angiostatic fumagillin-type compounds.
"Anti -estrogen compounds" are those compounds which at least partially bind estrogen receptors. A number of anti-estrogens have been shown to inhibit angiogenesis. Anti-estrogen compounds of the present invention include: clomiphene, tamoxifen, nafoxidine, ICI 164,984 and ICI 182,780.
Other angiostatic compounds contained in the Table 1 are known in the art to possess angiostatic activity. Of those classes listed, 5-flurouracil, mitomycin-C, taxol, 2- methoxyestradiol, betimastat, BB-2516, TIMPs, minocycline, GM6001 , PF4, CDI, TSP- 1 , TNP-470, SU101 , anti-endoglin, α-IFN and VEGF receptor-chimeric proteins are preferred for use in combinations of angiostatic compounds ofthe present invention.
The following are the most preferred types of angiostatic compounds to be included in combinations of angiostatic compounds ofthe present invention: compounds of formula (I), anti-mitotics, angiostatic steroids, fumagillin- type compounds, suramin-type compounds, estrogen metabolites, matrix metalloproteinase inhibitors and thalidomide.
The following are the most preferred combinations of compounds to be used in compositions and methods ofthe present invention:
1 ) A compound of formula (I) and an angiostatic steroid 2) A compound of formula (I) and a suramin-type compound
3) A compound of formula (I) and a fumagillin-type compound
4) A compound of formula (1) and an anti-mitotic
5) An angiostatic steroid and a fumagillin-type compound
6) An anti-mitotic and an angiostatic steriod 7) A compound of formula (I) and an estrogen derivative
8) An angiostatic steroid and an estrogen metabolite
9) A compound of formula (I) and a matrix metalloprotease inhibitor
The combination of angiostatic compounds of the present invention are useful in
inhibiting pathological neovascularization in human patients. As used herein, the term
"pathological neovascularization" refers to those conditions where the formation of blood
vessels (neovascularization) is harmful to the patient. Examples of pathological
neovascularization dependent diseases include: head trauma, spinal trauma, systemic or
traumatic shock, stroke, hemorrhagic shock, cancer, arthritis, arteriosclerosis,
angiofibroma, arteriovenous malformations, corneal graft neovascularization,
inappropriate wound healing, diabetic retinopathy, granulations, burns, hemangioma,
hemophilic joints, hypertrophic scars, ocular neovascularization, nonunion fractures,
Osier-Weber Syndrome, psoriasis, pyogenic granuloma, retrolental fibroplasia,
scleroderma, trachoma, vascular adhesions, and solid tumor growth.
In particular, the compositions of the present invention are useful in preventing
and treating any ocular neovascularization, including, but not limited to: retinal diseases
(diabetic retinopathy, chronic glaucoma, retinal detachment, sickle cell retinopathy and
subretinal neovascularization due to age related macular degeneration); rubeosis iritis;
proliferative vitreoretinopathy; inflammatory diseases; chronic uveitis; neoplasms
(retinoblastoma, pseudoglioma and melanoma); Fuchs' heterochromic iridocyclitis; neovascular glaucoma; corneal neovascularization (inflammatory, transplantation and
developmental hypoplasia of the iris); neovascularization following a combined
vitrectomy and lensectomy; vascular diseases (retinal ischemia, choroidal vascular
insufficiency, choroidal thrombosis and carotid artery ischemia); neovascularization of
the optic nerve; and neovascularization due to penetration of the eye or contusive ocular
injury.
Additionally the combinations of angiostatic agents are useful in treating
pterygium (primary and recurrent), glaucoma filtration surgery bleb failure,
hyperkeratosis, cheloid formation and polyp formation.
The use of the compositions of the present invention to ameliorate complications
arising from glaucoma filtration surgery is a particularly important aspect of the
invention. Glaucoma filtration surgery involves the surgical creation of a fistula with a
conjuctival flap which allows the direct drainage of aqueous humor from the anterior
chamber into the conjuctival tissue thereby lowering the elevated intraocular pressure
associated with glaucoma. However, in many patients, the filtration "bleb" becomes
scarred or healed over so that aqueous drainage can no longer occur. It has been noted
that failing filtration blebs may become vascularized prior to failure. This vascuiarization
may feed the fibroblasts which migrate, and proliferate, and block the bleb, or the
vascuiarization itself may also result in physical blockage of the bleb. It is therefore
likely that inhibition of filtration bleb neovascularization may inhibit filtration bleb
failure.
The angiostatic compounds may be contained in various types of pharmaceutical
compositions, either together as a single composition or in separate compositions, in
accordance with formulation techniques known to those skilled in the art. For example,
the compounds may be included in tablets, capsules, solutions, suspensions and other
dosage forms adapted for oral administration; solutions and suspensions adapted for
parenteral use; solutions, suspensions or gels for topical ocular administration; solutions
and suspensions adapted for intra-vitreal or intra-cameral use; and suppositories for rectal
use. Solutions, suspensions and other dosage forms adapted for topical application to the
involved tissues, such as tissue irrigating solutions, are particularly preferred for treatment
of acute conditions associated with surgery or other forms of trauma.
The present invention is particularly directed to the provision of compositions
adapted for treatment of ophthalmic tissues. Various types of vehicles may be used. The
vehicles will generally be aqueous in nature. Aqueous solutions are generally preferred,
based on ease of formulation, as well as a patient's ability to easily administer such
compositions by means of instilling one to two drops of the solutions in the affected eyes.
However, the compounds of the present invention may also be readily incorporated into
other types of compositions, such as suspensions, viscous or semi-viscous gels or other
types of solid or semi-solid compositions. Suspensions may be preferred for compounds
of the present invention which are relatively insoluble in water. The ophthalmic
compositions ofthe present invention may also include various other ingredients, such as
buffers, preservatives, co-solvents and viscosity building agents.
An appropriate buffer system (e.g., sodium phosphate, sodium acetate or sodium
borate) may be added to prevent pH drift under storage conditions.
Ophthalmic products are typically packaged in multidose form. Preservatives are
thus required to prevent microbial contamination during use. Suitable preservatives
include: benzalkonium chloride, thimerosal, chlorobutanol, methyl paraben, propyl
paraben, phenylethyl alcohol, edetate disodium, sorbic acid, polyquaternium-l , or other
agents known to those skilled in the art. Such preservatives are typically employed at a
level of from 0.001 to 1.0 percent by weight, based on the total weight ofthe composition
(wt.%).
The route of administration (e.g., topical, parenteral or oral) and the dosage
regimen will be determined by skilled clinicians, based on factors such as the exact nature
of the condition being treated, the severity of the condition, the age and general physical
condition of the patient, and so on.
As indicated above, use of compounds of the present invention to prevent or
reduce angiogenesis in ophthalmic tissues is a particularly important aspect of the present
invention. The compounds may also be used as an adjunct to ophthalmic surgery, such as
by vitreal or subconjunctival injection following ophthalmic surgery. The compounds
may be used for acute treatment of temporary conditions, or may be administered
chronically, especially in the case of degenerative disease. The compounds may also be
used prophylactically, especially prior to ocular surgery or noninvasive ophthalmic
procedures, or other types of surgery.
The use of physiologically balanced irrigating solutions as pharmaceutical
vehicles for the angiostic compounds is preferred when the compositions are administered
intraocularly. As used herein, the term "physiologically balanced irrigating solution"
means a solution which is adapted to maintain the physical structure and function of
tissues during invasive or noninvasive medical procedures. This type of solution will
typically contain electrolytes, such as sodium, potassium, calcium, magnesium and/or
chloride; an energy source, such as dextrose; and a buffer to maintain the pH of the
solution at or near physiological levels. Various solutions of this type are known (e.g.,
Lactated Ringers Solution). BSS® Sterile Irrigating Solution and BSS Plus® Sterile
Intraocular Irrigating Solution (Alcon Laboratories, Inc., Fort Worth, Texas, USA) are
examples of physiologically balanced intraocular irrigating solutions. The latter type of
solution is described in United States Patent No. 4,550,022 (Garabedian, et al.), the entire
contents of which are hereby incoφorated in the present specification by reference.
The specific type of formulation selected will depend on various factors, such as
the compound or its salt being used, the dosage frequency, and the disease being treated.
Topical aqueous solutions, suspensions, ointments, creams and gels are the preferred
dosage forms for the treatment of pterygium, hyperkeratosis, and cheloid and polyp
formation. Topical ophthalmic formulations are suitable for preventing glaucoma
filtration bleb failure or scar formation associated with ophthalmic surgery.
In general, the doses used for the above described puφoses will vary, but will be
in an effective amount to inhibit or reduce neovascularization. As used herein, the term
"pharmaceutically effective amount" to inhibit or reduce neovascularization, is that
amount of a combination of two or more compounds of the present invention which inhibits formation of new blood vessels or reduces the number of blood vessels which are
involved in the pathological condition. The compounds will normally be contained in
these formulations in an amount from about 0.01 to about 10.0 weight/percent. Preferable
concentrations range from about 0.1 to about 5.0 weight/percent. Thus, for topical
administration, these formulations are delivered to the disease site one to six times a day,
depending on the routine discretion of the skilled clinician. Systemic administration, for
example, in the form of tablets or suppositories is useful for the treatment of polyp
formation. Tablets containing 10- 1000 mg of a compound can be taken 2-3 times per day
depending on the discretion of the skilled clinician.
The compositions of the present invention are further illustrated by the following
examples. The term "angiostatic compound" refers to any compound of the present
invention, as described above.
Example 1
Topical combination compositions useful for controlling ocular neovascularization:
Example 2
A preferred topical composition useful for controlling neovascularzation:
The above formulation is prepared by first placing a portion of the purified water
into a beaker and heating to 90°C. The hydroxypropylmethylcellulose (HPMC) is then
added to the heated water and mixed by means of vigorous vortex stirring until all of the
HPMC is dispersed. The resulting mixture is then allowed to cool while undergoing mixing
in order to hydrate the HPMC. The resulting solution is then sterilized by means of
autoclaving in a vessel having a liquid inlet and a hydrophobic, sterile air vent filter.
The sodium chloride and the edetate disodium are then added to a second portion of
the purified water and dissolved. The benzalkonium chloride is then added to the solution,
and the pH of the solution is adjusted to 7.4 with 0.1M NaOH/HCl. The solution is then
sterilized by means of filtration.
The angiostatic compounds are sterilized by either dry heat or ethylene oxide. If
ethylene oxide sterilization is selected, aeration for at least 72 hours at 50°C. is necessary.
The sterilized angiogenic compound is weighed aseptically and placed into a pressurized
ballmill container. The tyloxapol, in sterilized aqueous solution form, is then added to the
ballmill container. Sterilized glass balls are then added to the container and the contents of
the container are milled aseptically at 225 φm for 16 hours, or until all particles are in the
range of approximately 5 microns.
Under aseptic conditions, the micronized drug suspension formed by means of the
preceding step is then poured into the HPMC solution with mixing. The ballmill container
and balls contained therein are then rinsed with a portion of the solution containing the
sodium chloride, the edetate disodium and benzalkonium chloride. The rinse is then added
aseptically to the HPMC solution. The final volume of the solution is then adjusted with
purified water and, if necessary, the pH of the solution is adjusted to pH 7.4 with
NaOH/HCl.
Example 3
Formulation for oral administration:
Tablet:
10-1000 mg of two angiostatic compounds with inactive ingredients such as starch, lactose and magnesium stearate can be formulated according to procedures known to those skilled in the art of tablet formulation.
Example 4
Formulation for sterile intraocular injection:
Example 5
Preferred formulation for a topical ocular solution:
Example fr
A preferred formulation for oral administration:
Tablet:
5-100 mg of Compound A and 10-1000 mg of another Angiostatic Compound with inactive ingredients such as starch, lactose and magnesium stearate can be formulated according to procedures known to those skilled in the art of tablet formulation.
Example 7
Formulations for topical dermatological use:
Cream: 1 mg/g each of two angiostatic compounds in cream base of purifed water, emulsifying wax, propylene glycol, stearic acid, isopropyl palmitate, synthetic beeswax, polysorbate 60, potassium sorbate, sorbic acid, propyl gallate, citric acid, and sodium hydroxide.
Ointment: 1 mg/g each of two angiostatic compounds in base of mineral oil and polyethylene.
Example 8
Formulation for suppository:
10-500 mg each of two angiostatic compounds with the following inactive ingredients: glycerin, butylateal hydroxytoluene, butylated hydroxy anisole, edetic acid, polyethylene glycol, and sodium chloride.
Some of the compounds of the present invention may contain a nonsteroidal anti-
inflammatory agent (NSAIA) component or a calcium channel blocker (flunarizine)
component. These individual moieties may add additional pharmaceutical benefit to the
angiostatic efficacy of compounds of formula (I).
The compounds of formula (I) are synthesized by known methods in the art.
Compounds containing a non-steroidal anti-inflammatory agent (flurbiprofen or
naproxen) can be made by methods illustrated in Scheme 1 and 2, and Examples 11-14.
Compounds containing a flunarizine moiety may be made by methods disclosed in
commonly assigned PCT Patent Publication No. WO/9515958, the entire contents of
which are hereby incoφorated by reference. Other compounds of formula (I) are
commercially available from: Sigma Chemical Co. (St. Louis, Missouri) and Aldrich
Chemical Co. (Milwaukee, Wisconsin).
Scheme 1
A-OH + H-X-(CH2)n-Y-(CH2)m-Z — > A-X-(CH2)„-Y-(CH2)m-Z (eq 1 )
II III I
A-OH — > A-CI + H-X-(CH2)„-Y-(CH2)m-Z — >* A-X-(CH2)„-Y-(CH2)n,-Z (eq.2) II IV III I
A-OH — > A-0"M+ + L-(CH2)„-Y-(CH2)m-Z — >A-0-(CH2)n-Y-(CH2)m-Z (eq. 3)
II V L=C1, BR, I, OMs, OTs I
VI
A-OH — > A-0"M+ + Br-CH2-C(0)OEt — ^ A-0-CH,-C(0)OEt + II V VII
H-NR-(CH2)„-Y-(CH2)m-Z —> A-NR-(CH2)„-Y-(CH2)τn-Z (eq 4)
VIII I
The conversion of the carboxylic acid containing nonsteroidal anti-inflammatory agents (II) to esters or amides (I) may be carried out by the following methods:
(i) As illustrated in equation 1 above, carboxylic acids (II) may be reacted with the appropriate amine or alcohol derivative (III) in the presence of a coupling reagent, such as dicyclohexylcarbodiimide or l -(3-dimethylaminopropyl)-3-ethyl carbodiimide HCl, and 4-dimethylamine pyridine or 1 -hydroxybenzotriazole, in an inert organic solvent, such as acetonitrile or tetrahydrofuran, and at a temperature from 0°C to 50°C.
(ii) As illustrated in equation 2 above, carboxylic acids (II) may be converted to acid chlorides (IV) by reacting them with a reagent such as thionyl chloride or oxalyl chloride, in the presence of an inert solid or neat, at a temperature from 0°C to 80°C. The resulting acid chloride (IV) may be reacted with the desired amine or alcohol (III) in an inert solvent such as tetrahydrofuran, in the presence of pyridine or a tertiary amine, such as triethylamine.
(iii) As illustrated in equation 3 above, esters (I) may be formed by reacting carboxylate anions (V), formed by reacting the carboxylic acid (II) with a base such as sodium hydride, with a halide (iodide, bromide, chloride) or sulfonate (mesylate, tosylate) (VI), in a solvent such as acetonitrile or dimethylformamide, at a temperature from 0°C to
100°C.
(iv) As illustrated in equation 4 above, amides (I) may be prepared by reacting carboxylate anions (V), formed by reacting carboxylic acid (II) with a base such as sodium hydride, with ethyl bromoacetate. The resulting ester (VII) is reacted with the desired amine (VIII), neat or in an inert solvent, such as acetonitrile or dimethylformamide, at a temperature from 0°C to 100°C.
The intermediate compounds (X) of Scheme 2 below, which can be used as
compounds (III) and (VIII), were prepared using the general methods described in Cohen,
et al., Lewis Acid Mediated Nucleophilic Substitution Reactions of 2-Alkoxy-3,4-dihydro-
2H-l-ben∑opyrans: Regiochemistry and Utility in the Synthesis of 3,4-Dihydro-2H-l -
benzopyran-2-carboxylic Acids, Journal of Organic Chemistry, volume 54, pages 3282-
3292, (1989). The nitrile (IX) can be reduced using a reagent such as lithium aluminum
hydride to afford the amine (X), which may be isolated as the hydrochloride salt.
The use of certain protecting groups and deprotection steps may be necessary, as
will be appreciated by those skilled in the art.
Scheme 2
Compounds of formula (I) may exist as mixtures of stereoisomers. The
preparation of the individual stereoisomers may be effected by preparing and resolving
the acids (II), by known methods, and then using a single stereoisomer as starting
material. Compounds (III), (VI) and (VIII) may be prepared as single stereoisomers from
compounds of formula (XIa.b), shown in Table 5 below, using known methods:
Table 5
wherein:
W is (CH2)p-Q; p is 0-1;
Q is CH2OH or CO2H;
R' is H, C(O)R, C(O)NR2, PO3 " , or SO3 " ; and
R" is H or C,-C6 alkyl.
The alcohols (XIa.b) may be resolved by forming esters with optically active carboxylic acids, separating the diastereomers, and then hydrolyzing the resolved diastereomers. The corresponding carboxylic acids (XIa.b) may be resolved by forming an ester with an optically active alcohol, separating the diastereomers, and then hydrolyzing the resolved diastereomers. Or, the carboxylic acids (XIa-b) may be resolved by forming an amine salt with an optically active amine. Separation by recrystallization and neutralization of the resolved carboxylic acid salt may be utilized to provide the resolved carboxylic acid. Resolution of the esters and amides (I) may also be effected using chromatographic techniques known to those skilled in the art. The amines of formula (I), where Y is NR, may be converted to amine salts by reacting the amine with acids of sufficient strength to produce an organic or inorganic salt. The pharmaceutically acceptable anions include: acetate, bromide, chloride, citrate, maleate, fumarate, mesylate, phosphate, sulfate and tartrate.
Methods of synthesizing the compounds formula (I) are further illustrated by the
following examples:
Example 11
Synthesis of N-[(6-hydroxv-2.5.7.8-tetramethvl-3.4-dihvdro-2H-l -benzo[1.2-b]pyran-2- vnmethyl] 2-(6-methoxv-2-naphthvnpropionamide
The intermediate, (6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H- 1 -benzo[ 1 ,2-b]pyran-2- yl)methylamine, was first synthesized:
A 1 molar (M) ethereal solution of lithium aluminum hydride (Aldrich, 32.4 mL, 32.43 mmol) was added slowly over a 5 minute period to a chilled, (4-6°C) stirring solution of (2-cyano-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-l-benzo[l,2-b]pyran in tetrahydrofuran (50 mL). After 2 hours, the reaction mixture was quenched by the slow sequential addition of 10% aqueous tetrahydrofuran (30 mL), 15% sodium hydroxide (10 mL) and then water (20 mL), while stirring. The resulting suspension was filtered through celite, and the celite pad was washed with ethyl ether (400 mL). The organic
layer was separated, dried (Na2SO4), and concentrated in vacuo. resulting in a residue. A 1 M ethereal solution of hydrochloride was then added to a solution of the residue in ethyl ether (100 mL), a solid formed, and the solid was then collected by filtration and washed with ethyl ether to give 2.31 g (65.4% yield) of a white solid. The product was used crude in the next reaction.
1H-NMR (DMSO-d6/TMS): 1.15 (s, 3H), 1.75 (t, 2H), 1.99 (s, 6H), 2.01 (s, 3H), 2.54 (t, 2H), 2.98 (s, 2H).
MS (CI): 236 (m+l).
The hydrochloride salt of (6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-l-benzo[l,2- b]pyran-2-yl)mefhylamine (0.30 g, 1.10 mmole) and 6-methoxy-α-methyl naphthaleneacetic acid (Aldrich, 0.28 g, 1.21 mmole) were stirred in the presence of dimethylaminopyridine (Aldrich, 0.26 g, 2.20 mmole) and l-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride (Janssen Chimica-Spectrum, 0.21 g, 1.10 mmole), in tetrahydrofuran (4.0 mL) under an atmosphere of nitrogen. After stirring 17 hours at ambient temperature, the reaction mixture was diluted with ethyl acetate (70 mL), washed with water (2x 15 mL), followed by brine (15 mL) and then dried (sodium sulfate). The mixture was concentrated in vacuo and the residue subjected to flash chromatography
(silica gel, 100-50:0-50, v:v, hexanes:ethyl acetate). The appropriate fractions were concentrated in vacuo. and the resulting crystalline foam suspension was then washed in hexanes to give 0.28 g (58.3% yield) of N-[(5-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-
2H-l-benzopyran-2-yl)methyl]-2-(6-methoxy-2-naphthyl)propionamide as a white amoφhous solid.
Η-NMR (CDC13) d 1.03-1.08 (d,3H), 1.57-1.64 (m, 6H), 1.70 (t, 2H,), 2.04-2.05 (m, 6H,), 2.48-2.51 (m, 2H), 3.16-3.58 (m, 2H), 3.74 (q, IH), 3.91 (s, 3H), 4.91 (br s, IH), 5.751 (t, IH), 7.01-7.19 (m, 2H), 7.29-7.40 (t, IH), 7.52-7.81 (m, 3H).
Elemental Analysis: Calculated for C28H33NO4 Calculated: C, 75.14; H, 7.43; N, 3.13. Found: C, 75.04; H, 7.50; N, 2.97.
Melting point: 67-70°C.
Example 12
Synthesis of 2-(6-hvdroxv-2.5.7.8-tetramethyl-3.4-dihvdro-2H-henzo[L2-h]pyran- 2ynethyl 2-(6-methoxv-2-naphthvnpropionate
A solution of 1 ,3-dicyclohexylcarbodiimide (Aldrich, 0.89 g, 4.31 mmol) in acetonitrile (25 mL), was added dropwise to a stirring slurry of (+)-6-methoxy-a-methyl-2- naphthaleneacetic acid (Aldrich, 0.90 g, 3.91 mmol), 2-hydroxy-2,5,7,8-tetramethyl-3,4- dihydro-2H-benzo[l,2-b]pyran-2yl)ethanol (0.98 g, 3.91 mmol, USP 5,266,709 column 45) and 1 -hydroxybenzotriazole hydrate (Aldrich, 0.59 g, 4.31 mmol), in acetonitrile (50 mL). After stirring for 18 hours, the reaction mixture was concentrated in vacuo. The residue was partitioned between water (30 mL) and methylene chloride (30 mL). The layers were separated, and the aqueous layer was extracted with methylene chloride (2 x 20 mL). The combined organic extracts were washed with water (20 mL), then dried (magnesium sulfate) and concentrated in vacuo. Flash chromatography (silica gel, 2:8, v:v, ethyl acetate: hexanes) of the residue afforded a white solid upon the concentration of the appropriate fractions. The white solid was recrystallized from an ethyl acetate- hexanes mixture to give 0.60 g (33.1% yield) of 2-(6-hydroxy-2,5,7,8-tetramethyl-3,4- dihydro-2H-benzo[ 1 ,2-b]pyran-2yl)ethyl 2-(6-methoxy-2-naphthyl)propionate, a mixture of diastereomers, as a white solid.
1H NMR (CDC13) d 1.1 (d, 3H), 1.6-1.5 (m, 3H), 1.6 (m, 2H), 1.9 (m,2H). 2.0 (s, 6H), 2.1 (s, 3H), 2.4 (t, 2H), 3.8 (q, 2H), 3.9 (s, 3H), 4.2 (s, IH), 4.1-4.4 (m, 2H), 7.1-7.7 (m,6H).
Elemental Analysis: Calculated for C29H34O5 Calculated: C, 75.30; H, 7.41. Found: C, 75.24; H, 7.46. Melting Point: 99.5-101.5°C.
Example 13
Synthesis of 2-(5-hvdroxy-2.4.6.7-tetramethyl-3.4-dihydro-benzo[1.2-h]furan-2ynethyl 2- (6-methoxy-2-naphthynpropionate
A solution of 2-(5-hydroxy-2,4,6,7-tetramethyl-2,3-dihydrobenzo[l,2-b]furan-2- yl)ethanol (1.30 g, 5.51 mmol) and 6-methoxy-α-methyl naphthaleneacetic acid (Aldrich,
1.39 g, 6.06 mmol) was stirred in the presence of dimethylaminopyridine (0.67 g, 5.51 mmol) and l-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (1.06 g, 5.51 mmol), in tetrahydrofuran (25 mL). The reaction mixture was stirred at ambient temperature under nitrogen for 24 hours, diluted with ethyl acetate (150 mL), washed with water (2x 40 mL) and then brine (30 mL). The organic extract was dried (sodium sulfate) and concentrated in vacuo. The residue was subjected to flash chromatography
(silica gel, 100-50:0-50, v:v, hexanes:ethyl acetate), and the appropriate fractions were combined to give 1.84 g (74.5% yield) of a foam residue. Fractional crystallization and recrystallization from methylene chloride-hexanes gave 0.40 g (13.0% yield) of white solid.
Η-NMR (CDCLJ: 1.34 (s, 3H), 1.54-1.57 (d, 3), 1.99 (t, 2H), 2.01 (s, 3H), 2.05 (s, 3H), 2.10 (s, 3), 2.73-2.81 (d, 1 ), 2.90-2.97 (d, 1), 3.77-3.89 (q, IH), 3.91 (s, 3H), 4.102 (s, IH, 4.165-4.29 (m, 2H), 7.10-7.16 (m, 2H), 7.35-7.40 (m, IH), 7.64-7.70 (m, 2H).
Elemental Analysis: Calculated for C28H32O5 0.1 mole CH2C12. Calculated: C, 73.84; H, 7.10. Found: C, 73.85, 73.83; H, 7.12. Melting point: 129.5- 131°C.
Example 14
Synthesis of2-(6-hydroxv-2.5.7.8-tetramethvl-3.4-dihydro-2H-benzo [1.2-b]pyran- 2yhethyl 2-('3-fIuoro-4-phenyl-phenvnpropionate
The intermediate, 2-(6-benzyloxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[l ,2-b]pyran- 2yl)ethyl 2-(3-fluoro-4-phenyl-phenyl)propionate, was first synthesized:
A solution of flubiprofen (Sigma, 2.0 g, 8.2 mmol), 2-(6-benzyloxy-2,5,7,8-tetramethyl- 3,4-dihydro-2H-benzo[l,2-b]pyran-2-yl)ethanol (2.4 g, 8.2 mmol) 1- hydroxybenzotriazole hydrate (Aldrich, 2.4 g, 13.9 mmol) and l-(3- dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (Aldrich, 2.8 g, 12.3 mmol), in acetonitrile (40 ml), was stirred at ambient temperature. After 72 hours, the reaction mixture was concentrated in vacuo and the residue partitioned between water and methylene chloride. A solid formed which was removed by filtration and discarded. The layers were separated and the aqueous layer was extracted with methylene chloride (2 x 25 ml). The combined organic extracts were then dried (magnesium sulfate) and concentrated in vacuo. The residue was chromatographed (silica gel, 2:8, v:v, ethyl acetate:hexane). Concentration of the appropriate fractions afforded 3.0 g (64% yield, mixture of stereoisomers) ofthe product as a clear oil.
1H NMR (CDCLJ d: 1.23-1.27 (m, 3H), 1.53-1.57 (m, 3H), 1.75 (m, 2H), 1.95 (m, 2H), 2.08 (s, 3H), 2.14 (s, 3H), 2.21 (s, 3H), 2.55 (t, 3H), 3.75 (m, 2H), 4.3 (m, IH), 4.65 (s, 2H), 7.1-7.7 (m, 13H).
A solution of 2-(6-benzyloxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo
[l ,2-b]pyran-2yl)ethyl 2-(3-fluoro-4-phenyl-phenyl)propionate in ethyl acetate was treated with 10% palladium on charcoal (Aldrich, 0.5 g). The resulting mixture was hydrogenated on a Parr Apparatus [initial pressure 60 pounds/inch (psi)]. After 18 hours, the reaction mixture was filtered, and the resulting solution concentrated in vacuo. The residue was subjected to flash chromatography (silica gel, 2:8, v:v, ethyl acetate:hexane). Concentration of the appropriate fractions afforded a clear oil. Hexane was added to the oil and a white solid formed upon standing. The white solid was collected by filtration to
afford 0.91 g (36% yield) of 2-6-hydroxy-2,5,7,8-tetra methyl -,4-dihydro-H-benzo[ 1,2- b]pyran-yl)ethyl 2-(3-fluoro-4-phenyl-phenyl) propionate as a mixture of stereoisomers.
Η NMR (CDClj d: 1.22-1.23 (m, 3H), 1.51-1.55 (m, 3H), 1.65-1.8 (m, 2H), 1.85-2.00 (m, 2H), 2.08 (s, 6H), 2.14 (s, 3H), 2.57 (t, 2H), 3.75 (q, IH), 4.1-4.5 (m, 2H), 7.10-7.65 (m, 8H).
Elemental Analysis: Calculated for C30H33FO4. Calculated: C,75.60; H, 6.98. Found: C,75.69; H.,7.01. Melting point: 85-87°C.
Other angiostatic compounds of the present invention are known to those skilled
in the art. These compounds may be obtained by commercial sources, or synthesized by
methods described in the respective publications incoφorated herein or listed above.
The invention in its broader aspects is not limited to the specific details shown and
described above. Departures may be made from such details within the scope of the
accompanying claims without departing from the principles of the invention and without
sacrificing its advantages.
Claims
What is claimed is:
1. A method of treating pathological neovascularization which comprises
administering to a human a pharmaceutically effective amount of a combination of two or
more angiostatic compounds.
2. A method according to Claim 1 wherein the angiostatic compounds are selected from the group consisting of: anti-mitotics, estrogen metabolites, matrix
metalloproteinase inhibitors, plasminogen activator/urokinase inhibitors, urokinase
receptor antagonists, platelet factor 4 and analogs, heparinases, cartilage-derived inhibitor
of angiogenesis, thrombospondin and related analogs, angiostatin, vasculostatin, proliferin-related protein, fumagillin-type compounds, tecogalan, pentosan polysulfate,
thalidomide and related analogs, CM101 , tyrosine kinase inhibitors, anti-sense
oligonucleotides, suramin-type compounds, angiostatic steriods, αvβ3 and αvβ5 integrin
antagonists, cytotoxic antibodies against endothelial cell antigens, interferon, VEGF and
bFGF antagonists, flk-1 and flt-1 antagonists, IL-1 and TFN antagonists, and a compound
according to formula (I):
wherein:
n is 1 or 2;
R is H, C,-C6 alkyl or C3-C6 cycloalkyl;
Y is H, C,-C6 alkyl, C3-C6 cycloalkyl, O, NR, C(R)2 , CH(OH) or S(O)n. ; n' is 0 to 2;
R' is H, C(O)R, C(O)N(R)2, PO3 " , SO3 ' or HO2C(CH2)2(C=O)— ;
R" is H or C,-C6 alkyl;
R3 is H, C,-C6 alkyl, (CH2)q(OH), — (C=O)O(CH2)qCH3 or
Z, if present, is H, CrC6 alkyl, C3-C6 cycloalkyl, or selected from the group consisting of:
wherein: D is O or NR; and
E and E' are independently H, F or Cl; and pharmaceutically acceptable salts thereof.
3. A method according to Claim 1 , wherein: R is H, R' is H; R" is CH3; R3 is CH3; and Y is C,-C2 alkyl.
4. A method according to Claim 1, wherein one of the compounds is selected from the group consisting of:
5. A method according to Claim 1, wherein the compounds are combined in a single composition comprising a topical ophthalmic formulation.
6. A method according to Claim 2, wherein the compounds are combined in a single composition comprising a topical ophthalmic formulation.
7. A method according to Claim 1, wherein the compounds are combined in a single composition comprising a surgical irrigating solution.
8. A method according to Claim 2, wherein one ofthe compounds are combined in a single composition comprising a surgical irrigating solution.
9. A method according to Claim 1 , wherein the compounds comprise an angiostatic steroid and a compound of formula (I).
10. A method according to Claim 1 , wherein the compounds comprise a suramin-type compound and a compound of formula (I).
11. A method according to Claim 1 , wherein the compounds comprise a fumagillin-type compound and a compound of formula (I).
12. A method according to Claim 1 , wherein the compounds comprise an angiostatic steroid and a fumagillin-type compound.
13. A method according to Claim 1 , wherein the compounds comprise an angiostatic steroid and a suramin-type compound.
14. A method according to Claim 1 , wherein the compounds comprise an anti-
mitotic and compound of formula (I).
15. A composition for treating pathological neovascularization which
comprises a pharmaceutically effective amount of a combination of two or more
angiostatic compounds in a pharmaceutically acceptable vehicle.
16. A composition according to Claim 15 wherein the angiostatic compounds
are selected from the group consisting of: anti-mitotics, estrogen metabolites, matrix
metalloproteinase inhibitors, plasminogen activator/urokinase inhibitors, urokinase
receptor antagonists, platelet factor 4 and analogs, heparinases, cartilage-derived inhibitor of angiogenesis, thrombospondin and related analogs, angiostatin, vasculostatin,
proliferin-related protein, fumagillin-type compounds, tecogalan, pentosan polysulfate,
thalidomide and related analogs, CM101 , tyrosine kinase inhibitors, anti-sense
oligonucleotides, suramin-type compounds, angiostatic steriods, αvβ3 and α5β3 integrin antagonists, cytotoxic antibodies against endothelial cell antigens, interferon, VEGF and
bFGF antagonists, flk-1 and flt-1 antagonists, IL-1 and TFN antagonists, and a compound
according to formula (I):
wherein:
n is 1 or 2;
R is H, CrC6 alkyl or C3-C6 cycloalkyl;
Y is H, C,-C6 alkyl, C3-C6 cycloalkyl, O, NR, C(R)2 , CH(OH) or S(O)n. ; n' is 0 to 2;
R' is H, C(O)R, C(O)N(R)2, PO3 " , SO3 ' or HO2C(CH2)2(C=O)~;
R" is H or C,-C6 alkyl;
R >3J i •s H, C,-C6 alkyl, (CH2),(OH), — (C=O)O(CH2)qCH3 or
Z, if present, is H, C,-C6 alkyl, C3-C6 cycloalkyl, or selected from the group consisting of:
wherein:
D is O or NR; and
E and E' are independently H, F or Cl; and pharmaceutically acceptable salts thereof.
17. A composition according to Claim 15, wherein: R is H, R' is H; R" is CH3;
R , 3J is CH3; and Y is C,-C2 alkyl.
18. A composition according to Claim 15, wherein one ofthe compounds is selected from the group consisting of:
19. A composition according to Claim 15, wherein the composition is a topical ophthalmic formulation.
20. A composition according to Claim 16, wherein the composition is a topical ophthalmic formulation.
21. A composition according to Claim 15, wherein the composition is a surgical irrigating solution.
22. A composition according to Claim 16, wherein the composition is a surgical irrigating solution.
23. A composition according to Claim 15, wherein the compounds comprise an angiostatic steroid and a compound of formula (I).
24. A composition according to Claim 15, wherein the compounds comprise a suramin-type compound and a compound of formula (I).
25. A composition according to Claim 15, wherein the compounds comprise a fumagillin-type compound and a compound of formula (I).
26. A composition according to Claim 15, wherein the compounds comprise an angiostatic steroid and a fumagillin-type compound.
27. A composition according to Claim 15, wherein the compounds comprise an angiostatic steroid and a suramin-type compound.
29. A composition according to Claim 15, wherein the compounds comprise
an anti-mitotic and compound of formula (I).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU24382/97A AU2438297A (en) | 1996-05-09 | 1997-04-03 | Combinations of angiostatic compounds |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1709696P | 1996-05-09 | 1996-05-09 | |
| US60/017,096 | 1996-05-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1997041844A1 true WO1997041844A1 (en) | 1997-11-13 |
Family
ID=21780695
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1997/005574 WO1997041844A1 (en) | 1996-05-09 | 1997-04-03 | Combinations of angiostatic compounds |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU2438297A (en) |
| WO (1) | WO1997041844A1 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998032453A1 (en) * | 1997-01-29 | 1998-07-30 | Vanderbilt University | Facilitation of wound healing with cm101/gbs toxin |
| WO1998032452A1 (en) * | 1997-01-29 | 1998-07-30 | Vanderbilt University | Treatment of chronic inflammatory diseases with cm101/gbs toxin |
| EP0930067A2 (en) * | 1997-12-19 | 1999-07-21 | Pfizer Products Inc. | Mmp inhibitors for the treatment of ocular angiogenesis |
| US5981508A (en) * | 1997-01-29 | 1999-11-09 | Vanderbilt University | Facilitation of repair of neural injury with CM101/GBS toxin |
| WO1999062925A1 (en) * | 1998-06-02 | 1999-12-09 | Eli Lilly And Company | Angiopoietin related gene sequence scarface 1 |
| WO2000007565A2 (en) * | 1998-08-03 | 2000-02-17 | Insite Vision, Incorporated | Methods of ophthalmic administration |
| WO2000015244A2 (en) * | 1998-09-16 | 2000-03-23 | Merck Patent Gmbh | Pharmaceutical preparation containing a cyclopeptide and a chemotherapeutic agent or an angiogenesis inhibitor |
| EP0995437A1 (en) * | 1997-05-23 | 2000-04-26 | Chugai Seiyaku Kabushiki Kaisha | 2,3-dihydrobenzofuran derivatives |
| WO2000035420A2 (en) * | 1998-12-17 | 2000-06-22 | Alcon Laboratories, Inc. | Stable surgical irrigating solutions |
| WO2000038719A1 (en) * | 1998-12-23 | 2000-07-06 | G.D. Searle & Co. | Use of a matrix metalloproteinase inhibitor and an integrin antagonist in the treatment of neoplasia |
| US6093748A (en) * | 1995-02-28 | 2000-07-25 | Ahluwalia; Gurpreet S. | Inhibition of hair growth |
| DE19957342A1 (en) * | 1999-11-29 | 2001-05-31 | Gruenenthal Gmbh | Treatment of interleukin (IL)-12 mediated illnesses comprises simultaneous administration of thalidomide or analogue and antiinflammatory cytokine |
| WO2001041781A2 (en) * | 1999-12-09 | 2001-06-14 | Alcon Universal Ltd. | Id protein inhibitors for treating ocular diseases |
| WO2001068053A2 (en) * | 2000-03-10 | 2001-09-20 | Insite Vision Incorporated | Methods and compositions for treating and preventing posterior segment ophthalmic disorders |
| US6309374B1 (en) | 1998-08-03 | 2001-10-30 | Insite Vision Incorporated | Injection apparatus and method of using same |
| EP1252895A1 (en) * | 2000-01-31 | 2002-10-30 | Santen Pharmaceutical Co., Ltd. | Remedies for ophthalmic diseases |
| WO2003051347A1 (en) * | 2001-12-19 | 2003-06-26 | Vlaams Interuniversitair Instituut Voor Biotechnologie Vzw | Use of urokinase receptor antagonists to modulate ischemiareperfusion injury |
| WO2003092691A1 (en) * | 2002-04-30 | 2003-11-13 | Pharmacia Coporation | Combination of cyclooxygenase-2 inhibitors and thalidomide for the treatment of neoplasia |
| US6670337B1 (en) | 1998-01-29 | 2003-12-30 | Yeda Reaearch And Development Co., Ltd. | Facilitation of wound healing with CM101/GBS toxin |
| WO2004037286A2 (en) * | 2002-10-23 | 2004-05-06 | F. Hoffmann-La Roche Ag | Combination of a trioxopyrimidine compound inhibitor and an anti-tumor agent, and uses thereof |
| US6753321B2 (en) | 2000-09-15 | 2004-06-22 | Genvec, Inc. | Method of modulating neovascularization |
| US6803448B1 (en) | 1998-07-22 | 2004-10-12 | Vanderbilt University | GBS toxin receptor |
| EP1473043A1 (en) * | 2003-04-29 | 2004-11-03 | Boehringer Ingelheim Pharma GmbH & Co.KG | Pharmaceutical combination for the treatment of diseases involving cell proliferation, migration or apotosis of myeloma cells, or angiogenesis |
| EP1481678A1 (en) * | 2002-03-05 | 2004-12-01 | Eisai Co., Ltd. | Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with angiogenesis inhibitor |
| WO2004096224A3 (en) * | 2003-04-29 | 2004-12-16 | Boehringer Ingelheim Int | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells, or angiogenesis |
| US6833373B1 (en) | 1998-12-23 | 2004-12-21 | G.D. Searle & Co. | Method of using an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| US7141607B1 (en) | 2000-03-10 | 2006-11-28 | Insite Vision Incorporated | Methods and compositions for treating and inhibiting retinal neovascularization |
| WO2007084670A2 (en) | 2006-01-18 | 2007-07-26 | Merck Patent Gmbh | Specific therapy using integrin ligands for treating cancer |
| WO2008087025A2 (en) | 2007-01-18 | 2008-07-24 | Merck Patent Gmbh | Specific therapy and medicament using integrin ligands for treating cancer |
| WO2010136168A2 (en) | 2009-05-25 | 2010-12-02 | Merck Patent Gmbh | Continuous administration of integrin ligands for treating cancer |
| EP2292251A1 (en) | 2001-04-24 | 2011-03-09 | Merck Patent GmbH | Combination therapy using anti-angiogenic agents and TNF-alpha |
| ITMI20101030A1 (en) * | 2010-06-09 | 2011-12-10 | Sergio Capaccioli | USE OF ANTISENSE OLIGONUCLEOTIDS FOR THE TREATMENT OF RETINAL DEGENERATIONS AND NEOPLASES |
| US8609614B2 (en) | 1998-07-22 | 2013-12-17 | Vanderbilt University | GBS toxin receptor compositions and methods of use |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0325199A2 (en) * | 1988-01-19 | 1989-07-26 | Takeda Chemical Industries, Ltd. | Fumagillin as angiostatic agent |
| WO1990015816A1 (en) * | 1989-06-16 | 1990-12-27 | The Upjohn Company | Suramin type compounds and angiostatic steroids to inhibit angiogenesis |
| WO1992002240A2 (en) * | 1990-07-27 | 1992-02-20 | Repligen Corporation | Novel methods and compositions for treatment of angiogenic diseases |
| JPH05331070A (en) * | 1992-02-07 | 1993-12-14 | Takeda Chem Ind Ltd | Antineoplastic agent containing both tnp and interleukin |
| JPH06157344A (en) * | 1992-02-07 | 1994-06-03 | Childrens Medical Center Corp:The | Preparation for blocking new formation of blood vessel and method of blocking new formation of blood vessel |
| WO1994026278A1 (en) * | 1993-05-17 | 1994-11-24 | University Of Kentucky Research Foundation | Aurintricarboxylic acid fractions and analogues with anti-angiogenic activity and methods of use |
| US5424321A (en) * | 1993-12-08 | 1995-06-13 | Alcon Laboratories, Inc. | Compounds having both potent calcium antagonist and antioxidant activity and use thereof as cytoprotective agents |
-
1997
- 1997-04-03 WO PCT/US1997/005574 patent/WO1997041844A1/en active Application Filing
- 1997-04-03 AU AU24382/97A patent/AU2438297A/en not_active Abandoned
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0325199A2 (en) * | 1988-01-19 | 1989-07-26 | Takeda Chemical Industries, Ltd. | Fumagillin as angiostatic agent |
| WO1990015816A1 (en) * | 1989-06-16 | 1990-12-27 | The Upjohn Company | Suramin type compounds and angiostatic steroids to inhibit angiogenesis |
| WO1992002240A2 (en) * | 1990-07-27 | 1992-02-20 | Repligen Corporation | Novel methods and compositions for treatment of angiogenic diseases |
| JPH05331070A (en) * | 1992-02-07 | 1993-12-14 | Takeda Chem Ind Ltd | Antineoplastic agent containing both tnp and interleukin |
| JPH06157344A (en) * | 1992-02-07 | 1994-06-03 | Childrens Medical Center Corp:The | Preparation for blocking new formation of blood vessel and method of blocking new formation of blood vessel |
| WO1994026278A1 (en) * | 1993-05-17 | 1994-11-24 | University Of Kentucky Research Foundation | Aurintricarboxylic acid fractions and analogues with anti-angiogenic activity and methods of use |
| US5424321A (en) * | 1993-12-08 | 1995-06-13 | Alcon Laboratories, Inc. | Compounds having both potent calcium antagonist and antioxidant activity and use thereof as cytoprotective agents |
Non-Patent Citations (2)
| Title |
|---|
| DATABASE WPI Week 9403, Derwent World Patents Index; AN 94-022828, XP002034658 * |
| DATABASE WPI Week 9430, Derwent World Patents Index; AN 94-242963, XP002034659 * |
Cited By (74)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6093748A (en) * | 1995-02-28 | 2000-07-25 | Ahluwalia; Gurpreet S. | Inhibition of hair growth |
| WO1998032452A1 (en) * | 1997-01-29 | 1998-07-30 | Vanderbilt University | Treatment of chronic inflammatory diseases with cm101/gbs toxin |
| US5858991A (en) * | 1997-01-29 | 1999-01-12 | Vanderbilt University | Facilitation of wound healing with CM101/GBS toxin |
| US6476001B1 (en) | 1997-01-29 | 2002-11-05 | Vanderbilt University | Facilitation of repair of neural injury with CM101/GBS toxin |
| US6476002B1 (en) | 1997-01-29 | 2002-11-05 | Vanderbilt University | Treatment of chronic inflammatory diseases with CM101/GBS toxin |
| US5981508A (en) * | 1997-01-29 | 1999-11-09 | Vanderbilt University | Facilitation of repair of neural injury with CM101/GBS toxin |
| US6569838B1 (en) | 1997-01-29 | 2003-05-27 | Vanderbilt University | Facilitation of keloid healing with CM101/GBS toxin |
| WO1998032453A1 (en) * | 1997-01-29 | 1998-07-30 | Vanderbilt University | Facilitation of wound healing with cm101/gbs toxin |
| US6028060A (en) * | 1997-01-29 | 2000-02-22 | Vanderbilt University | Treatment of chronic inflammatory diseases with CM101/GBS toxin |
| EP0995437A4 (en) * | 1997-05-23 | 2002-01-30 | Chugai Pharmaceutical Co Ltd | 2,3-dihydrobenzofuran derivatives |
| EP0995437A1 (en) * | 1997-05-23 | 2000-04-26 | Chugai Seiyaku Kabushiki Kaisha | 2,3-dihydrobenzofuran derivatives |
| US6686389B2 (en) | 1997-05-23 | 2004-02-03 | Chugai Seiyaku Kabushiki Kaisha | 2,3-dihydrobenzofuran derivatives |
| US6403639B1 (en) | 1997-05-23 | 2002-06-11 | Chugai Seiyaku Kabushiki Kaisha | 2,3-dihydrobenzofuran derivatives |
| EP0930067A3 (en) * | 1997-12-19 | 1999-09-15 | Pfizer Products Inc. | MMP inhibitors for the treatment of ocular angiogenesis |
| EP0930067A2 (en) * | 1997-12-19 | 1999-07-21 | Pfizer Products Inc. | Mmp inhibitors for the treatment of ocular angiogenesis |
| US6670337B1 (en) | 1998-01-29 | 2003-12-30 | Yeda Reaearch And Development Co., Ltd. | Facilitation of wound healing with CM101/GBS toxin |
| WO1999062925A1 (en) * | 1998-06-02 | 1999-12-09 | Eli Lilly And Company | Angiopoietin related gene sequence scarface 1 |
| US7410640B2 (en) | 1998-07-22 | 2008-08-12 | Vanderbilt University | GBS toxin receptor antibodies |
| US6803448B1 (en) | 1998-07-22 | 2004-10-12 | Vanderbilt University | GBS toxin receptor |
| US8609614B2 (en) | 1998-07-22 | 2013-12-17 | Vanderbilt University | GBS toxin receptor compositions and methods of use |
| WO2000007565A3 (en) * | 1998-08-03 | 2000-05-11 | Insite Vision Inc | Methods of ophthalmic administration |
| WO2000007565A2 (en) * | 1998-08-03 | 2000-02-17 | Insite Vision, Incorporated | Methods of ophthalmic administration |
| US6397849B1 (en) | 1998-08-03 | 2002-06-04 | Insite Vision Incorporated | Methods of ophthalmic administration |
| US6378526B1 (en) | 1998-08-03 | 2002-04-30 | Insite Vision, Incorporated | Methods of ophthalmic administration |
| US6309374B1 (en) | 1998-08-03 | 2001-10-30 | Insite Vision Incorporated | Injection apparatus and method of using same |
| JP2002524526A (en) * | 1998-09-16 | 2002-08-06 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフトング | Pharmaceutical formulations containing cyclopeptides and chemotherapeutic or angiogenesis inhibitors |
| WO2000015244A3 (en) * | 1998-09-16 | 2000-06-22 | Merck Patent Gmbh | Pharmaceutical preparation containing a cyclopeptide and a chemotherapeutic agent or an angiogenesis inhibitor |
| EP1466615A1 (en) | 1998-09-16 | 2004-10-13 | MERCK PATENT GmbH | Pharmaceutical composition |
| WO2000015244A2 (en) * | 1998-09-16 | 2000-03-23 | Merck Patent Gmbh | Pharmaceutical preparation containing a cyclopeptide and a chemotherapeutic agent or an angiogenesis inhibitor |
| US6683051B1 (en) | 1998-09-16 | 2004-01-27 | Merck Patent Gmbh | Pharmaceutical preparation containing a cyclopeptide and a chemotherapeutic agent or an angiogenesis inhibitor |
| EP1466615B1 (en) * | 1998-09-16 | 2009-06-03 | MERCK PATENT GmbH | Pharmaceutical composition |
| CN100352494C (en) * | 1998-09-16 | 2007-12-05 | 默克专利股份公司 | Pharmaceutical preparation containing cyclopeptide and chemotherapeutic agent or angiogenesis inhibitor |
| WO2000035420A2 (en) * | 1998-12-17 | 2000-06-22 | Alcon Laboratories, Inc. | Stable surgical irrigating solutions |
| WO2000035420A3 (en) * | 1998-12-17 | 2000-10-19 | Alcon Lab Inc | Stable surgical irrigating solutions |
| WO2000038715A2 (en) * | 1998-12-23 | 2000-07-06 | G.D. Searle & Co. | Use of an integrin antagonist and radiation in the treatment of neoplasia |
| WO2000038719A1 (en) * | 1998-12-23 | 2000-07-06 | G.D. Searle & Co. | Use of a matrix metalloproteinase inhibitor and an integrin antagonist in the treatment of neoplasia |
| WO2000038665A2 (en) * | 1998-12-23 | 2000-07-06 | G.D. Searle & Co. | Use of an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| US6833373B1 (en) | 1998-12-23 | 2004-12-21 | G.D. Searle & Co. | Method of using an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| WO2000038665A3 (en) * | 1998-12-23 | 2000-11-16 | Searle & Co | Use of an integrin antagonist and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia |
| WO2000038715A3 (en) * | 1998-12-23 | 2001-01-04 | Searle & Co | Use of an integrin antagonist and radiation in the treatment of neoplasia |
| DE19957342A1 (en) * | 1999-11-29 | 2001-05-31 | Gruenenthal Gmbh | Treatment of interleukin (IL)-12 mediated illnesses comprises simultaneous administration of thalidomide or analogue and antiinflammatory cytokine |
| WO2001041781A3 (en) * | 1999-12-09 | 2002-04-25 | Abbot F Clark | Id protein inhibitors for treating ocular diseases |
| WO2001041781A2 (en) * | 1999-12-09 | 2001-06-14 | Alcon Universal Ltd. | Id protein inhibitors for treating ocular diseases |
| EP1252895A4 (en) * | 2000-01-31 | 2003-04-16 | Santen Pharmaceutical Co Ltd | Remedies for ophthalmic diseases |
| EP1252895A1 (en) * | 2000-01-31 | 2002-10-30 | Santen Pharmaceutical Co., Ltd. | Remedies for ophthalmic diseases |
| WO2001068053A3 (en) * | 2000-03-10 | 2002-08-29 | Insite Vision Inc | Methods and compositions for treating and preventing posterior segment ophthalmic disorders |
| WO2001068053A2 (en) * | 2000-03-10 | 2001-09-20 | Insite Vision Incorporated | Methods and compositions for treating and preventing posterior segment ophthalmic disorders |
| US7141607B1 (en) | 2000-03-10 | 2006-11-28 | Insite Vision Incorporated | Methods and compositions for treating and inhibiting retinal neovascularization |
| AU785285B2 (en) * | 2000-03-10 | 2006-12-21 | Sun Pharma Global Fze | Methods and compositions for treating and preventing posterior segment ophthalmic disorders |
| EP1938799A1 (en) * | 2000-03-10 | 2008-07-02 | Insite Vision Incorporated | Methods and compositions for treating and preventing posterior segment ophthalmic disorders |
| US6753321B2 (en) | 2000-09-15 | 2004-06-22 | Genvec, Inc. | Method of modulating neovascularization |
| EP2292251A1 (en) | 2001-04-24 | 2011-03-09 | Merck Patent GmbH | Combination therapy using anti-angiogenic agents and TNF-alpha |
| WO2003051347A1 (en) * | 2001-12-19 | 2003-06-26 | Vlaams Interuniversitair Instituut Voor Biotechnologie Vzw | Use of urokinase receptor antagonists to modulate ischemiareperfusion injury |
| EP1481678A1 (en) * | 2002-03-05 | 2004-12-01 | Eisai Co., Ltd. | Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with angiogenesis inhibitor |
| EP1481678A4 (en) * | 2002-03-05 | 2009-12-30 | Eisai R&D Man Co Ltd | Antitumor agent comprising combination of sulfonamide-containing heterocyclic compound with angiogenesis inhibitor |
| WO2003092691A1 (en) * | 2002-04-30 | 2003-11-13 | Pharmacia Coporation | Combination of cyclooxygenase-2 inhibitors and thalidomide for the treatment of neoplasia |
| WO2004037286A3 (en) * | 2002-10-23 | 2004-06-24 | Hoffmann La Roche | Combination of a trioxopyrimidine compound inhibitor and an anti-tumor agent, and uses thereof |
| WO2004037286A2 (en) * | 2002-10-23 | 2004-05-06 | F. Hoffmann-La Roche Ag | Combination of a trioxopyrimidine compound inhibitor and an anti-tumor agent, and uses thereof |
| EP2361626A1 (en) * | 2003-04-29 | 2011-08-31 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells, or angiogenesis |
| EP2359829A1 (en) * | 2003-04-29 | 2011-08-24 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells, or angiogenesis |
| WO2004096224A3 (en) * | 2003-04-29 | 2004-12-16 | Boehringer Ingelheim Int | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells, or angiogenesis |
| EP2826480A1 (en) * | 2003-04-29 | 2015-01-21 | Boehringer Ingelheim International GmbH | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells, or angiogenesis |
| US7846936B2 (en) | 2003-04-29 | 2010-12-07 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells or angiogenesis |
| EA011888B1 (en) * | 2003-04-29 | 2009-06-30 | Бёрингер Ингельхайм Интернациональ Гмбх | Combinations for the treatment of diseases |
| EP2409705A1 (en) * | 2003-04-29 | 2012-01-25 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation, migration or apoptosis of myeloma cells or angiogenesis |
| EP1473043A1 (en) * | 2003-04-29 | 2004-11-03 | Boehringer Ingelheim Pharma GmbH & Co.KG | Pharmaceutical combination for the treatment of diseases involving cell proliferation, migration or apotosis of myeloma cells, or angiogenesis |
| EP2338518A1 (en) | 2006-01-18 | 2011-06-29 | Merck Patent GmbH | Specific therapy using integrin ligands for treating cancer |
| WO2007084670A2 (en) | 2006-01-18 | 2007-07-26 | Merck Patent Gmbh | Specific therapy using integrin ligands for treating cancer |
| EP2335733A1 (en) | 2006-01-18 | 2011-06-22 | Merck Patent GmbH | Specific therapy using integrin ligands for treating cancer |
| EP2441464A1 (en) | 2007-01-18 | 2012-04-18 | Merck Patent GmbH | Specific therapy and medicament using integrin ligands for treating cancer |
| WO2008087025A2 (en) | 2007-01-18 | 2008-07-24 | Merck Patent Gmbh | Specific therapy and medicament using integrin ligands for treating cancer |
| EP2578225A1 (en) | 2007-07-18 | 2013-04-10 | Merck Patent GmbH | Specific Therapy and Medicament Using Integrin Ligands for Treating Cancer |
| WO2010136168A2 (en) | 2009-05-25 | 2010-12-02 | Merck Patent Gmbh | Continuous administration of integrin ligands for treating cancer |
| ITMI20101030A1 (en) * | 2010-06-09 | 2011-12-10 | Sergio Capaccioli | USE OF ANTISENSE OLIGONUCLEOTIDS FOR THE TREATMENT OF RETINAL DEGENERATIONS AND NEOPLASES |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2438297A (en) | 1997-11-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO1997041844A1 (en) | Combinations of angiostatic compounds | |
| EP0614463B1 (en) | Angiostatic steroids | |
| EP0799219B1 (en) | Esters and amides of non-steroidal anti-inflammatory carboxylic acids which may be used as anti-oxidants, 5-lipoxygenase inhibitors and non-steroidal anti-inflammatory products | |
| US5798356A (en) | Angiostatic compounds | |
| US6011023A (en) | Angiostatic steroids | |
| US6242480B1 (en) | Ophthalmic viscoelastic compositions | |
| US20100227797A1 (en) | Use of cyclolignans for the treatment of type 2 diabetes and as contraceptives | |
| US5998465A (en) | Esters of non-steroidal anti-flammatory carboxylic acids | |
| US20020193444A1 (en) | Composition | |
| US5686621A (en) | Substituted hydrindanes for the treatment of angiogenesis-dependent diseases | |
| US5929111A (en) | A-seco steroids effective at treating ophthalmic pathological neovascularization and controlling intraocular pressure | |
| JP5484353B2 (en) | Pharmaceutical composition for the treatment and prevention of glaucoma | |
| WO2009116076A2 (en) | Sterile opthalmic preparations and a process for preparation thereof | |
| US5719167A (en) | Angiostatic compounds | |
| WO2007083145A1 (en) | The treatment of ocular conditions and the systemic side-effects of glucocorticoids | |
| US5925673A (en) | Benzofurans and benzopyrans as cytoprotective agents | |
| ES2267707T3 (en) | PHARMACEUTICAL COMPOSITIONS CONTAINING STEROID STRUCTURES AND ITS USE. | |
| US20050239760A1 (en) | Angiostatic agents and methods and compositions for controlling ocular hypertension | |
| JP2985007B2 (en) | Angiogenesis inhibitor | |
| US20040142915A1 (en) | 11beta-short chain substituted estradiol analogs and their use in the treatment of menopausal symptoms and estrogen sensitive cancer | |
| WO1994004143A1 (en) | Substituted hydrindanes for the treatment of angiogenesis-dependent diseases | |
| KR20040027920A (en) | Remedial agent for cardiac failure | |
| CARBOXYLIC | Hellberg et ai. | |
| KR20040019106A (en) | Remedial agent for arthrosis deformans |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU CA JP US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| NENP | Non-entry into the national phase |
Ref country code: JP Ref document number: 97539328 Format of ref document f/p: F |
|
| NENP | Non-entry into the national phase |
Ref country code: CA |
|
| 122 | Ep: pct application non-entry in european phase |