WO1999004794A1 - Cyclic amine modulators of chemokine receptor activity - Google Patents
Cyclic amine modulators of chemokine receptor activity Download PDFInfo
- Publication number
- WO1999004794A1 WO1999004794A1 PCT/US1998/014990 US9814990W WO9904794A1 WO 1999004794 A1 WO1999004794 A1 WO 1999004794A1 US 9814990 W US9814990 W US 9814990W WO 9904794 A1 WO9904794 A1 WO 9904794A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methylbenzenesulfonamide
- phenyl
- alkyl
- substituted
- phenylpiperidin
- Prior art date
Links
- 0 CCN(C1CCN(CC[C@@](C)(C*(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccccc1)=O Chemical compound CCN(C1CCN(CC[C@@](C)(C*(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccccc1)=O 0.000 description 8
- WQFFWKZRMSUQAL-UHFFFAOYSA-N CC(CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OC)=O)CC(C)(C)C Chemical compound CC(CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OC)=O)CC(C)(C)C WQFFWKZRMSUQAL-UHFFFAOYSA-N 0.000 description 1
- ZEJXLTXHGQJODD-MILIPEGGSA-N CCC(CCC1CCN(CC[C@](C)(CC)c2ccccc2)CC1)C(OCc1ccccc1)=O Chemical compound CCC(CCC1CCN(CC[C@](C)(CC)c2ccccc2)CC1)C(OCc1ccccc1)=O ZEJXLTXHGQJODD-MILIPEGGSA-N 0.000 description 1
- YLHGXPVRHCKKIX-UHFFFAOYSA-N CCCCCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccccc1)=O Chemical compound CCCCCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccccc1)=O YLHGXPVRHCKKIX-UHFFFAOYSA-N 0.000 description 1
- MBAQWZPBZUGQNO-UHFFFAOYSA-N CCCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccc(C)cc1)=O Chemical compound CCCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCc1ccc(C)cc1)=O MBAQWZPBZUGQNO-UHFFFAOYSA-N 0.000 description 1
- RDGFEFQGOMPJRP-UHFFFAOYSA-N CCCOC(N(CC1CCCCC1)C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)=O Chemical compound CCCOC(N(CC1CCCCC1)C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)=O RDGFEFQGOMPJRP-UHFFFAOYSA-N 0.000 description 1
- DXVIZQRYEHUENT-UHFFFAOYSA-N CCN(C1CCN(CCC(CC(C)OCc2ccccc2)c2ccccc2)CC1)C(OCc1ccccc1)=O Chemical compound CCN(C1CCN(CCC(CC(C)OCc2ccccc2)c2ccccc2)CC1)C(OCc1ccccc1)=O DXVIZQRYEHUENT-UHFFFAOYSA-N 0.000 description 1
- KQUMJWICVOFFEQ-UHFFFAOYSA-N CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OC(C)(C)C)=O Chemical compound CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OC(C)(C)C)=O KQUMJWICVOFFEQ-UHFFFAOYSA-N 0.000 description 1
- FUUQBKMKRDQXBE-UHFFFAOYSA-N CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCC)=O Chemical compound CCN(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)C(OCC)=O FUUQBKMKRDQXBE-UHFFFAOYSA-N 0.000 description 1
- UANRWBRDDQPTMZ-UHFFFAOYSA-N CCN(CC)C(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)=O Chemical compound CCN(CC)C(C1CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)=O UANRWBRDDQPTMZ-UHFFFAOYSA-N 0.000 description 1
- ZYDCCASEMPEUGD-AREMUKBSSA-N CCN(C[C@@H](CCN(CC1)CCC1c1ccccc1)c(cc1Cl)ccc1Cl)S(c1ccccc1)(=O)=O Chemical compound CCN(C[C@@H](CCN(CC1)CCC1c1ccccc1)c(cc1Cl)ccc1Cl)S(c1ccccc1)(=O)=O ZYDCCASEMPEUGD-AREMUKBSSA-N 0.000 description 1
- NUSLPQVXOIFRQB-MUUNZHRXSA-N CCN(C[C@@H](CCN(CC1)CCC1c1ccccc1)c1ccc(C)c(Cl)c1)S(c1ccccc1)(=O)=O Chemical compound CCN(C[C@@H](CCN(CC1)CCC1c1ccccc1)c1ccc(C)c(Cl)c1)S(c1ccccc1)(=O)=O NUSLPQVXOIFRQB-MUUNZHRXSA-N 0.000 description 1
- FTWQRKWRMDEMFJ-UHFFFAOYSA-N CN(CC#CCN(CC1)CCC1c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC#CCN(CC1)CCC1c1ccccc1)S(c1ccccc1)(=O)=O FTWQRKWRMDEMFJ-UHFFFAOYSA-N 0.000 description 1
- FPTDJHBGTSPZED-UHFFFAOYSA-N CN(CC(CCN(CC1)CC=C1N(C1CCCCC1)C(OC)=O)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CC=C1N(C1CCCCC1)C(OC)=O)c1ccccc1)S(c1ccccc1)(=O)=O FPTDJHBGTSPZED-UHFFFAOYSA-N 0.000 description 1
- RRAJRRCBTUGQHF-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1(C(N)=O)c1ccccc1)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1(C(N)=O)c1ccccc1)c1ccccc1)S(c1ccccc1)(=O)=O RRAJRRCBTUGQHF-UHFFFAOYSA-N 0.000 description 1
- RAHZPTRRMBXSFG-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1(c1ccccc1)O)c(cc1Cl)ccc1OCc1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1(c1ccccc1)O)c(cc1Cl)ccc1OCc1ccccc1)S(c1ccccc1)(=O)=O RAHZPTRRMBXSFG-UHFFFAOYSA-N 0.000 description 1
- UNXXYTGACLKVSG-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1N(CC1CC1)C(OCc1ccccc1)=O)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1N(CC1CC1)C(OCc1ccccc1)=O)c1ccccc1)S(c1ccccc1)(=O)=O UNXXYTGACLKVSG-UHFFFAOYSA-N 0.000 description 1
- DCZWOGGQUQFEII-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1N(Cc1ccccc1)C(OC)=O)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1N(Cc1ccccc1)C(OC)=O)c1ccccc1)S(c1ccccc1)(=O)=O DCZWOGGQUQFEII-UHFFFAOYSA-N 0.000 description 1
- JQGCUAAPERHLCG-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1NC(N)=O)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1NC(N)=O)c1ccccc1)S(c1ccccc1)(=O)=O JQGCUAAPERHLCG-UHFFFAOYSA-N 0.000 description 1
- PARVCFLGHKTKBO-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1NS(C)(=O)=O)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1NS(C)(=O)=O)c1ccccc1)S(c1ccccc1)(=O)=O PARVCFLGHKTKBO-UHFFFAOYSA-N 0.000 description 1
- RJQZUKFOHCVBIK-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1c1ccccc1)c(cc1F)ccc1F)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1c1ccccc1)c(cc1F)ccc1F)S(c1ccccc1)(=O)=O RJQZUKFOHCVBIK-UHFFFAOYSA-N 0.000 description 1
- UETIGXLCWMKVCO-UHFFFAOYSA-N CN(CC(CCN(CC1)CCC1c1ccccc1)c1ccccc1)S(c1ccccc1)(=O)=O Chemical compound CN(CC(CCN(CC1)CCC1c1ccccc1)c1ccccc1)S(c1ccccc1)(=O)=O UETIGXLCWMKVCO-UHFFFAOYSA-N 0.000 description 1
- ZNJNNPMHLRVTBO-UHFFFAOYSA-N CN(CC(CN1CCC(Cc2ccccc2)CC1)c1ccccc1)[S+](c1ccccc1)(=O)=O Chemical compound CN(CC(CN1CCC(Cc2ccccc2)CC1)c1ccccc1)[S+](c1ccccc1)(=O)=O ZNJNNPMHLRVTBO-UHFFFAOYSA-N 0.000 description 1
- XZBJJSAJTDAWIL-HSZRJFAPSA-N CN(C[C@@H](CCN(CC1)CCC1N(c(cccc1)c1N1)C1=O)c1cccc(Cl)c1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CC1)CCC1N(c(cccc1)c1N1)C1=O)c1cccc(Cl)c1)S(c1ccccc1)(=O)=O XZBJJSAJTDAWIL-HSZRJFAPSA-N 0.000 description 1
- ONZDPJCRJPUTPJ-XMMPIXPASA-N CN(C[C@@H](CCN(CC1)CCC1N(c1c(CO2)cccc1)C2=O)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CC1)CCC1N(c1c(CO2)cccc1)C2=O)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O ONZDPJCRJPUTPJ-XMMPIXPASA-N 0.000 description 1
- CSTXXZIYGUONDI-XMMPIXPASA-N CN(C[C@@H](CCN(CC1)CCC1N1c(cccc2)c2OCC1=O)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CC1)CCC1N1c(cccc2)c2OCC1=O)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O CSTXXZIYGUONDI-XMMPIXPASA-N 0.000 description 1
- HQINIQYECXZZQZ-RUZDIDTESA-N CN(C[C@@H](CCN(CC1)CCC1c1ccccc1OC)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CC1)CCC1c1ccccc1OC)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O HQINIQYECXZZQZ-RUZDIDTESA-N 0.000 description 1
- LDWLNIJRXLVNRV-HHHXNRCGSA-N CN(C[C@@H](CCN(CC1)CCC1c1nnn[n]1Cc1ccccc1)c1cccc(Cl)c1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CC1)CCC1c1nnn[n]1Cc1ccccc1)c1cccc(Cl)c1)S(c1ccccc1)(=O)=O LDWLNIJRXLVNRV-HHHXNRCGSA-N 0.000 description 1
- BSYOLLNMELLWPP-FXDYGKIASA-N CN(C[C@@H](CCN(CCC1)CC1c1ccccc1)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O Chemical compound CN(C[C@@H](CCN(CCC1)CC1c1ccccc1)c1cc(Cl)ccc1)S(c1ccccc1)(=O)=O BSYOLLNMELLWPP-FXDYGKIASA-N 0.000 description 1
- YJMPVOHDCPCHAL-UHFFFAOYSA-N CNC(CC1(CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)c1ccccc1)=O Chemical compound CNC(CC1(CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)c1ccccc1)=O YJMPVOHDCPCHAL-UHFFFAOYSA-N 0.000 description 1
- AEHBCHVVDMYQIT-UHFFFAOYSA-N CNC(NCC1(CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)c1ccccc1)=O Chemical compound CNC(NCC1(CCN(CCC(CN(C)S(c2ccccc2)(=O)=O)c2ccccc2)CC1)c1ccccc1)=O AEHBCHVVDMYQIT-UHFFFAOYSA-N 0.000 description 1
- NLMHENJRNZNGGT-UHFFFAOYSA-N Cc1ccc(C(CCN(CC2)CCC2c2ccccc2)CN(C)S(c2ccccc2)(=O)=O)cc1F Chemical compound Cc1ccc(C(CCN(CC2)CCC2c2ccccc2)CN(C)S(c2ccccc2)(=O)=O)cc1F NLMHENJRNZNGGT-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/10—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with radicals containing only carbon and hydrogen atoms attached to ring carbon atoms
- C07D211/14—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with radicals containing only carbon and hydrogen atoms attached to ring carbon atoms with hydrocarbon or substituted hydrocarbon radicals attached to the ring nitrogen atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/18—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D211/20—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms
- C07D211/22—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by singly bound oxygen or sulphur atoms by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/40—Oxygen atoms
- C07D211/44—Oxygen atoms attached in position 4
- C07D211/52—Oxygen atoms attached in position 4 having an aryl radical as the second substituent in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/56—Nitrogen atoms
- C07D211/58—Nitrogen atoms attached in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/06—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
Definitions
- Chemokines are chemotactic cytokines that are released by a wide variety of cells to attract macrophages, T cells, eosinophils, basophils and neutrophils to sites of inflammation (reviewed in Schall, Cvtokine. 3, 165-183 (1991) and Murphy, Rev. Immun.. 12, 593-633 (1994)).
- the -chemokines such as interleukin-8 (IL-8), neutrophil-activating protein-2 (NAP-2) and melanoma growth stimulatory activity protein (MGSA) are chemotactic primarily for neutrophils, whereas ⁇ -chemokines, such as RANTES, MlP-l ⁇ , MlP-l ⁇ , monocyte chemotactic protein-1 (MCP-1), MCP-2, MCP-3 and eotaxin are chemotactic for macrophages, T-cells, eosinophils and basophils (Deng, et al., Nature. 381. 661-666 (1996)).
- IL-8 interleukin-8
- NAP-2 neutrophil-activating protein-2
- MGSA melanoma growth stimulatory activity protein
- ⁇ -chemokines such as RANTES, MlP-l ⁇ , MlP-l ⁇ , monocyte chemotactic protein-1 (MCP-1), MCP-2, MCP-3 and eo
- chemokines bind specific cell-surface receptors belonging to the family of G-protein-coupled seven-transmembrane- domain proteins (reviewed in Horuk, Trends Pharm. Sci., 15. 159-165 (1994)) which are termed "chemokine receptors.” On binding their cognate ligands, chemokine receptors transduce an intracellular signal though the associated trimeric G protein, resulting in a rapid increase in intracellular calcium concentration.
- CCR-1 or "CKR-1" or "CC-CKR-1”
- MlP-l ⁇ , MlP-l ⁇ , MCP-3, RANTES a human chemokine receptor that bind or respond to ⁇ -chemokines with the following characteristic pattern: CCR-1 (or "CKR-1" or "CC-CKR-1") [MlP-l ⁇ , MlP-l ⁇ , MCP-3, RANTES] (Ben-Barruch, et al., J. Biol. Chem.. 270. 22123-22128 (1995); Beote, et al, Cell.
- CCR- 2A and CCR-2B (or "CKR-2A7"CKR-2A” or “CC-CKR-2A”/"CC-CKR- 2A") [MCP-1, MCP-3, MCP-4]; CCR-3 (or “CKR-3” or "CC-CKR-3") [eotaxin, RANTES, MCP-3] (Combadiere, et al., J. Biol. Chem.. 270. 16491-16494 (1995); CCR-4 (or "CKR-4" or "CC-CKR-4") [MlP-l ⁇ , RANTES, MCP-1] (Power, et al., J. Biol. Chem.. 270.
- ⁇ -chemokines include eotaxin, MIP ("macrophage inflammatory protein”), MCP ("monocyte chemoattractant protein”) and RANTES ("regulation-upon-activation, normal T expressed and secreted").
- Chemokine receptors such as CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, CXCR-4, have been implicated as being important mediators of inflammatory and immunoregulatory disorders and diseases, including asthma and allergic diseases, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis. Accordingly, agents which modulate chemokine receptors would be useful in such disorders and diseases.
- HIV-1 human immunodeficiency virus
- LAV human immunodeficiency virus
- HTLV-III human immunodeficiency virus
- Certain compounds have been demonstrated to inhibit the replication of HIV, including soluble CD4 protein and synthetic derivatives (Smith, et al., Science, 238, 1704-1707 (1987)), dextran sulfate, the dyes Direct Yellow 50, Evans Blue, and certain azo dyes (U.S. Patent No. 5,468,469). Some of these antiviral agents have been shown to act by blocking the binding of gpl20, the coat protein of HIV, to its target, the CD4 gyycoprotein of the cell.
- the principal cofactor for entry mediated by the envelope glycoproteins of primary macrophage-trophic strains of HIV-1 is CCR5, a receptor for the ⁇ - chemokines RANTES, MlP-l ⁇ and MlP-l ⁇ (Deng, et al., Nature. 381. 661-666 (1996)). HIV attaches to the CD4 molecule on cells through a region of its envelope protein, gpl20. It is believed that the CD-4 binding site on the gpl20 of Hr interacts with the CD4 molecule on the cell surface, and undergoes conformational changes which allow it to bind to another cell-surface receptor, such as CCR5 and/or CXCR-4.
- peptides eotaxin, RANTES, MlP-l ⁇ , MlP-l ⁇ , MCP-1, and MCP-3 are known to bind to chemokine receptors.
- the inhibitors of HIV-1 replication present in supernatants of CD8+ T cells have been characterized as the ⁇ -chemokines RANTES, MlP-l ⁇ and MlP-l ⁇ .
- PCT Patent Publication WO 97/10211 and EPO Patent Publication EP 0,673,928 disclose certain piperidines as tachykinin antagonists.
- the present invention is directed to compounds which are modulators of chemokine receptor activity and are useful in the prevention or treatment of certain inflammatory and immunoregulatory disorders and diseases, including asthma and allergic diseases, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis.
- the invention is also directed to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which chemokine receptors are involved.
- the present invention is further concerned with compounds which inhibit the entry of human immunodeficiency virus (HIV) into target cells and are of value in the prevention of infection by HPv 7 , the treatment of infection by HIV and the prevention and/or treatment of the resulting acquired immune deficiency syndrome (AIDS).
- HIV human immunodeficiency virus
- the present invention also relates to pharmaceutical compositions containing the compounds and to a method of use of the present compounds and other agents for the prevention and treatment of AIDS and viral infection by HIV.
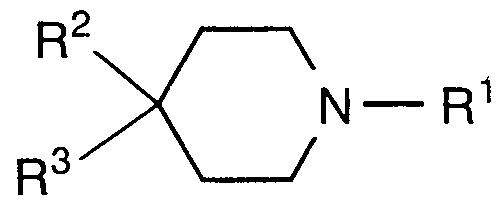
- the present invention is directed to compounds of formula I:
- R1 is selected from a group consisting of: linear or branched Cl-8 alkyl, linear or branched C2-8 alkenyl, wherein the Cl-8 alkyl or C2-8 alkenyl is optionally mono, di, tri or tetra substituted, where the substituents are independently selected from: (a) hydroxy,
- heteroaryl wherein heteroaryl is selected from the group consisting of:
- R4 and R ⁇ are independently selected from hydrogen, Cl-10 linear or branched alkyl, and C ⁇ -6 alkyl substituted with C3-8 cycloalkyl
- heteroaryl is selected from the group consisting of: (1') benzimidazolyl,
- 35 B ⁇ is selected from the group consisting of: (1) hydrogen,
- R 6 and R 7 may be joined together to form a 5-, 6-, or 7- membered monocyclic saturated ring containing 1 or 2 heteroatoms independently selected from nitrogen, oxygen, and sulfur, and in which the ring is unsubstituted or mono or di-substituted, the substituents independently selected from: (1) hydroxy,
- n is an integer selected from 0, 1 and 2 and pharmaceutically acceptable salts thereof.
- Preferred compounds of the present invention include those of formula la:
- R1 is selected from a group consisting of:
- R 7 is Cl-6 alkyl, benzyl or phenyl which is unsubsituted or substituted with halo, CF3, Ci-3alkyl, or Ci-3alkoxy,
- heteroaryl is selected from the group consisting of:
- R2 is selected from the group consisting of:
- R3 is selected from the group consisting of: (1) Ar,
- R4 is selected from hydrogen, Cl-10 linear or branched alkyl, and C ⁇ -6 alkyl substituted with C3-8 cycloalkyl,
- (o) CH 2 -heteroaryl, with the heteroaryl is selected from the group consisting of: (1') imidazolyl, (2') oxazolyl,
- n is an integer selected from 0, 1 and 2, with the proviso that the sum of m + n is 2; and pharmaceutically acceptable salts thereof.
- More preferred compounds of the present invention include those of formula lb:
- Rl, R2 and R ⁇ are as defined herein; and pharmaceutically acceptable salts thereof.
- R ⁇ is Ar, m is 1, n is 1, and R ⁇ is C5 alkyl which bears a group selected from: -NR 6 R 7 , -NR 6 COR 7 , -NR 6 CO2R 7 , or -NR ⁇ CONHR 7 , then R 1 does not bear a substituent which is 2,3-dichlorophenyl.
- pi bears at least one substituent which is selected from:
- R1 is selected from the group consisting of:
- R1 is selected from the group consisting of: C4, C5, or C6 linear alkyl, which is substituted, where the substituents are independently selected from:
- ! is C4 linear alkyl, which is substituted, where the substituents are independently selected from: (a) phenyl,
- B is selected from the group consisting of: (a) phenyl, and (b) di or tri-substituted phenyl, wherein the substituents on phenyl are independently selected from: chloro, methyl, phenyl, Ci-3alkoxy, and CF3;
- R" is Cl-3 alkyl, unsubstituted or substituted with cyclohexyl
- RlO is selected from the group consisting of:
- RU and Rl2 are independently selected from the group consisting of:
- R1 is selected from the group consisting of:
- R1 is selected from the group consisting of:
- R2 is selected from the group consisting of:
- R2 is selected from the group consisting of:
- R2 is hydrogen
- R3 is selected from the group consisting of: (1) Ar,
- R3 is selected from the group consisting of:
- R 4 is selected from hydrogen, Cl-10 linear or branched alkyl, and C ⁇ -6 alkyl substituted with C3-8 cycloalkyl, and
- Ar is selected from the group consisting of: (1) phenyl,
- CH -heteroaryl with the heteroaryl is selected from the group consisting of: (10 imidazolyl,
- Ar is selected from: phenyl, mono substituted phenyl or di-substituted phenyl, wherein the substituents are selected from the group consisting of:
- heteroaryl selected from the group consisting of:
- R3 is selected from:
- R is selected from hydrogen, Ci-6 linear or branched alkyl, and CH 2 substituted with
- R3 is: -N(R4)-CO-O-(CH 2 )-phenyl, wherein R is selected from hydrogen and Ci-6 alkyl.
- R 3 is:
- n is an integer selected from 0, 1 and 2 with the proviso that the sum of m + n is 2.
- n is 1.
- halo as used herein are intended to include chloro, fluoro, bromo and iodo.
- Cl-6, as in Ci-6alkyl is defined to identify the group as having 1, 2, 3, 4, 5, or 6 carbons, such that Cl-6alkyl specifically includes methyl, ethyl, propyl, butyl, pentyl, hexyl, and cyclohexyl.
- Preferred compounds of the present invention include the compounds of the formula:
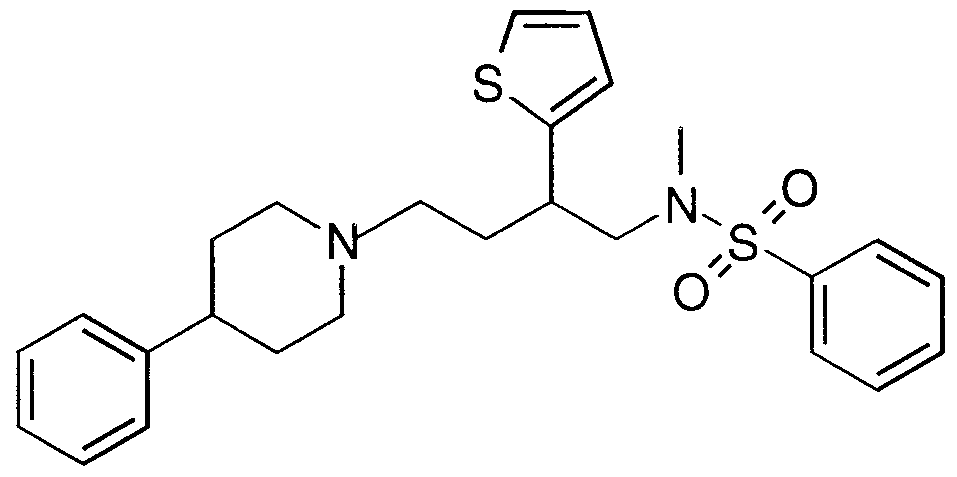
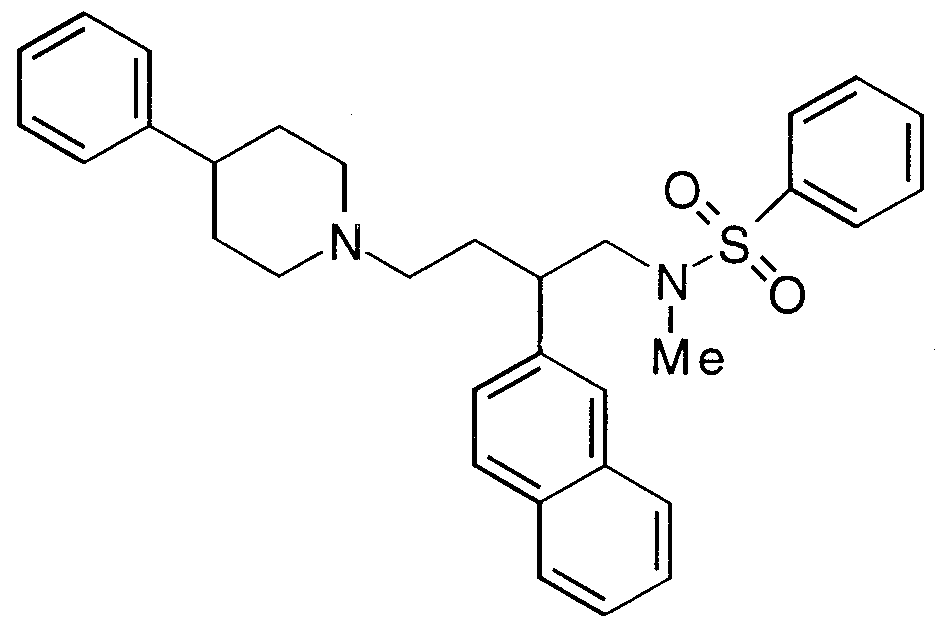
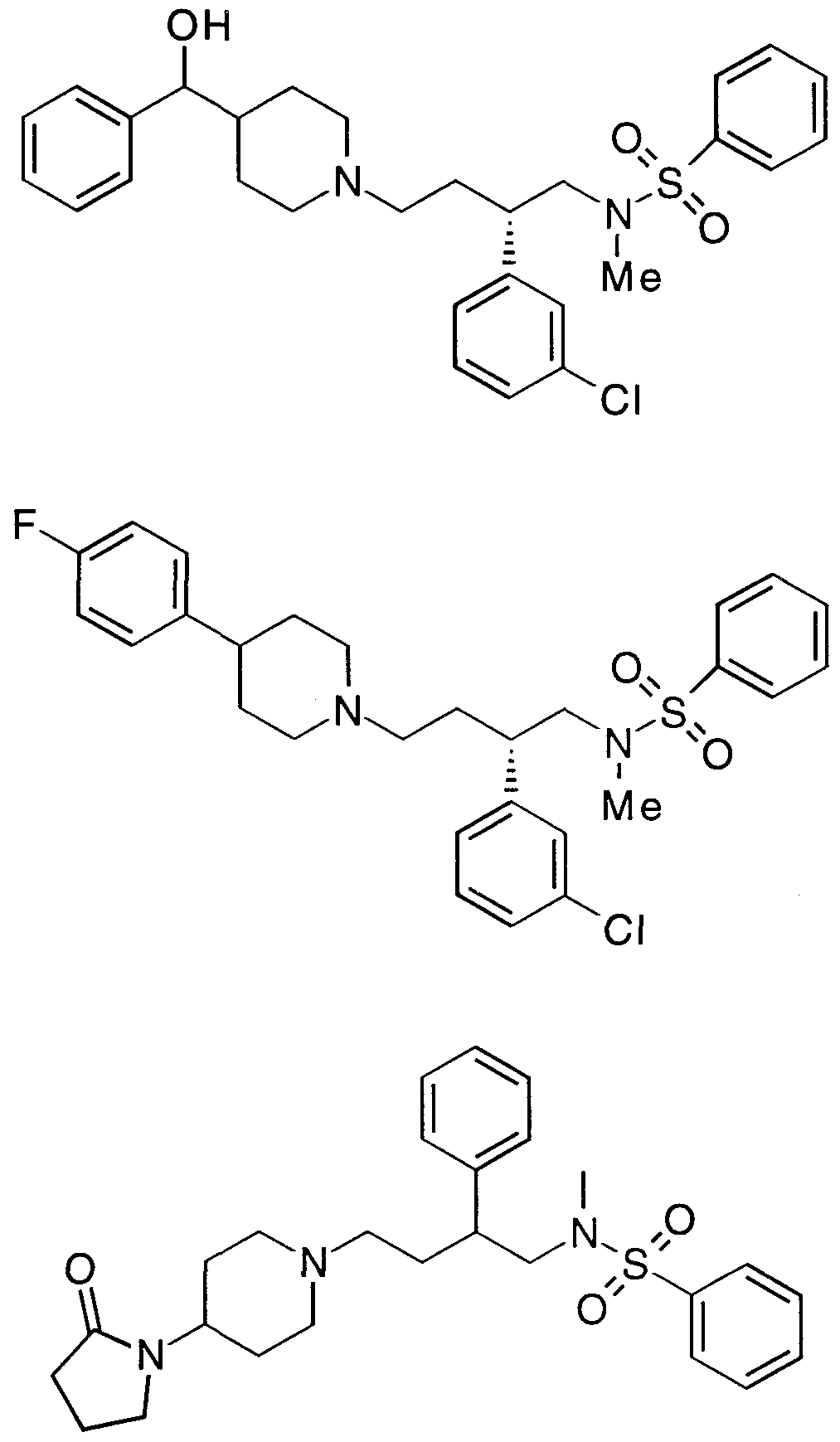
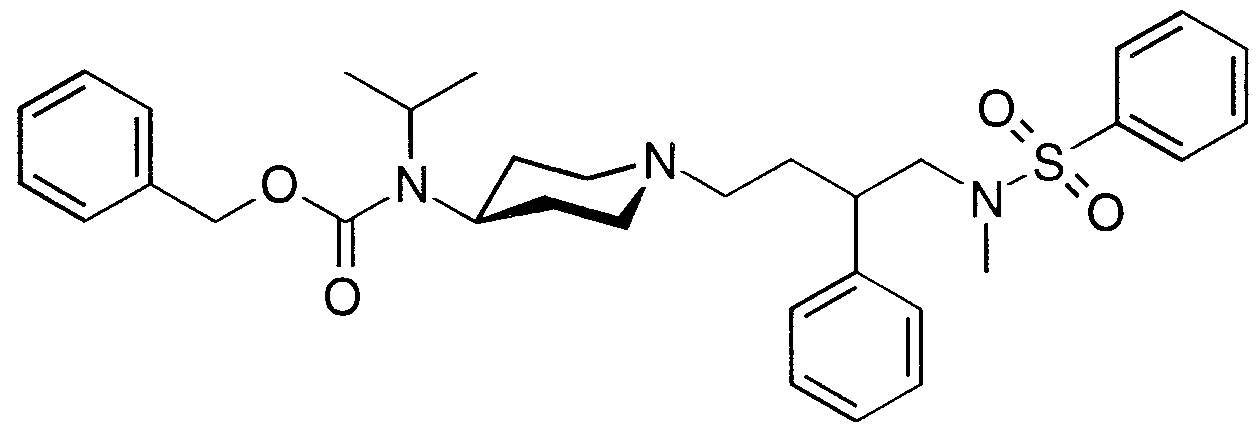
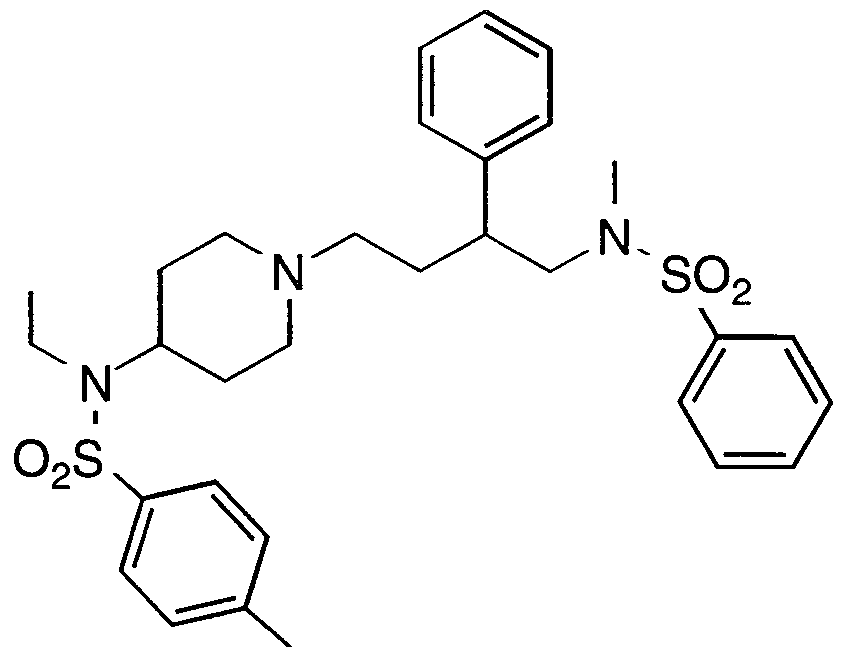
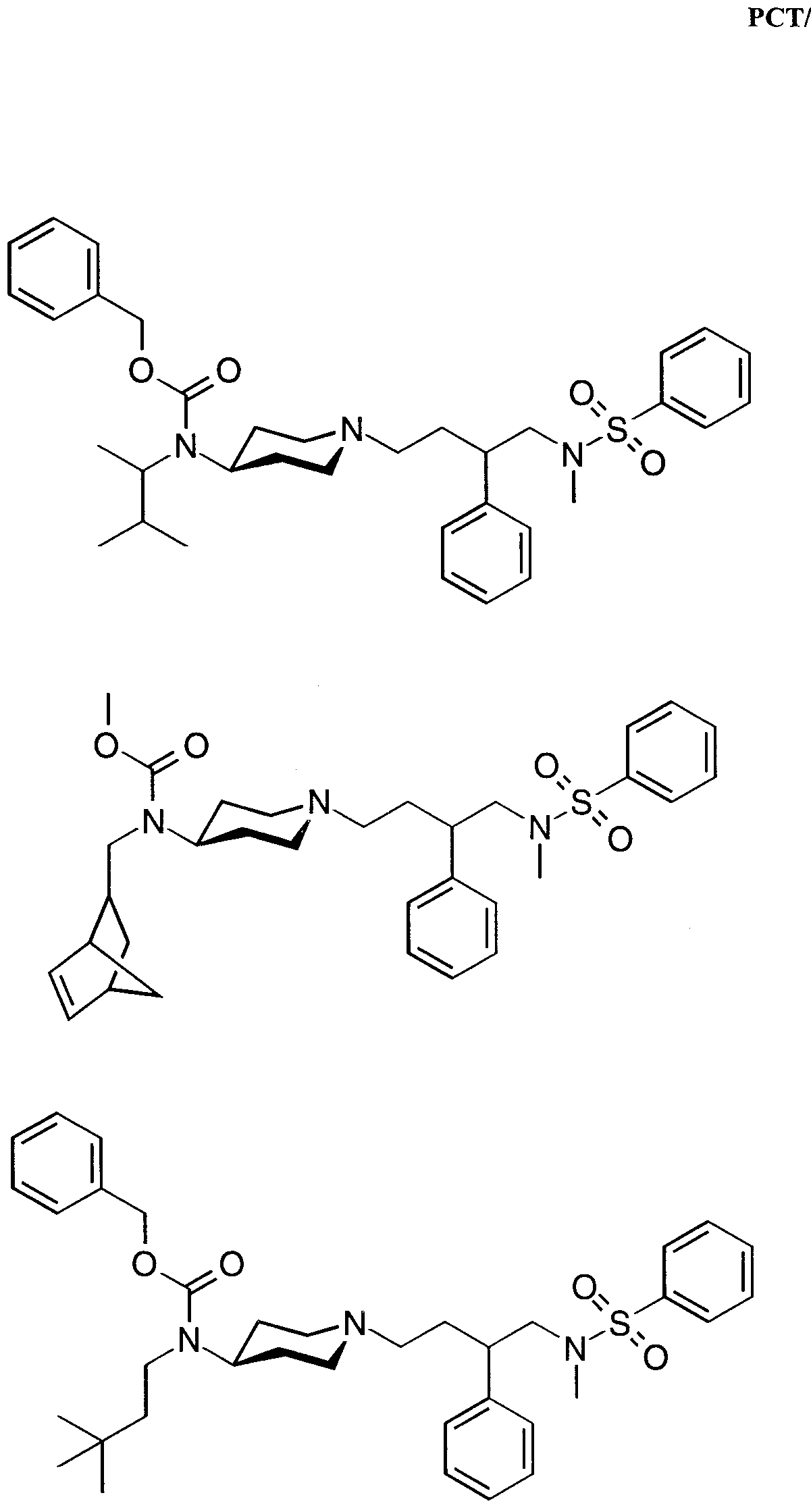
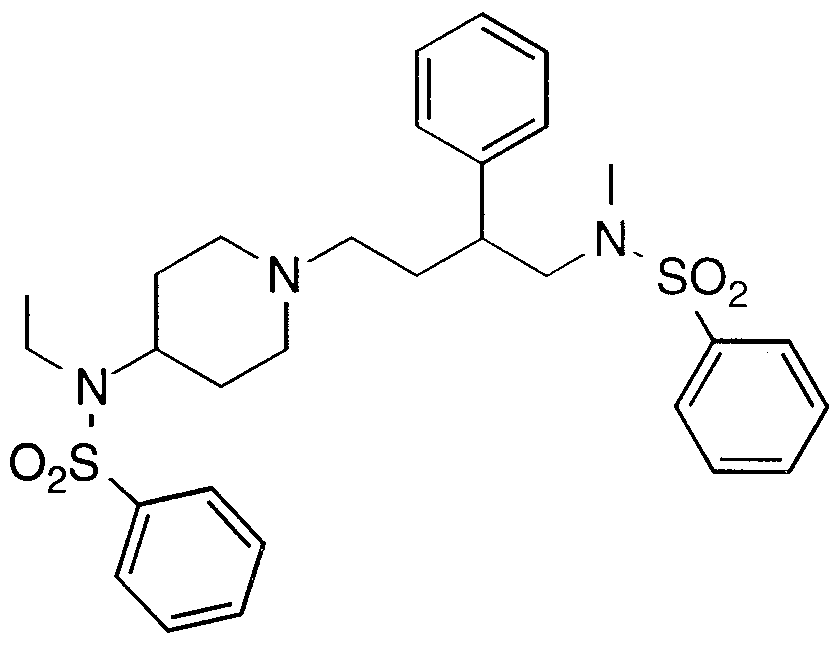
- Specific compounds within the present invention include a compound which selected from the group consisting of:
- the subject compounds are useful in a method of modulating chemokine receptor activity in a patient in need of such modulation comprising the administration of an effective amount of the compound.
- the present invention is directed to the use of the foregoing spiro-substituted azacycles as modulators of chemokine receptor activity.
- these compounds are useful as modulators of the chemokine receptors, including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR- 3, CCR-4, CCR-5, CXCR-3, and/or CXCR-4.
- the utility of the compounds in accordance with the present invention as modulators of chemokine receptor activity may be demonstrated by methodology known in the art, such as the assay for CCR-1 and/or CCR-5 binding as disclosed by Van Riper, et al., J. Exp. Med.. 177. 851-856 (1993), and the assay for CCR-2 and/or CCR-3 binding as disclosed by Daugherty, et al., J. Exp. Med.. 183. 2349-2354 (1996).
- Cell lines for expressing the receptor of interest include those naturally expressing the receptor, such as EOL-3 or THP-1, or a cell engineered to express a recombinant receptor, such as CHO, RBL-2H3, HEK-293.
- a CCR3 transfected AML14.3D10 cell line has been placed on restricted deposit with American Type Culture Collection in Rockville, Maryland as ATCC No. CRL-12079, on April 5, 1996.
- the utility of the compounds in accordance with the present invention as inhibitors of the spread of HIV infection in cells may be demonstrated by methodology known in the art, such as the HIV quantitation assay disclosed by Nunberg, et al., J. Virology. 65 (9), 4887-4892 (1991).
- the compounds of the following examples had activity in binding to either the CCR-5 receptor or the CCR-3 receptor in the aforementioned assays, generally with an IC50 of less than about 10 ⁇ M. Such a result is indicative of the intrinsic activity of the compounds in use as modulators of chemokine receptor activity.
- Mammalian chemokine receptors provide a target for interfering with or promoting eosinophil and/or lymphocyte function in a mammal, such as a human.
- Compounds which inhibit or promote chemokine receptor function are particularly useful for modulating eosinophil and/or lymphocyte function for therapeutic purposes. Accordingly, the present invention is directed to compounds which are useful in the prevention and/or treatment of a wide variety of inflammatory and immunoregulatory disorders and diseases, including asthma and allergic diseases, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis.
- an instant compound which inhibits one or more functions of a mammalian chemokine receptor may be administered to inhibit (i.e., reduce or prevent) inflammation.
- a mammalian chemokine receptor e.g., a human chemokine receptor
- one or more inflammatory processes such as leukocyte emigration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, is inhibited.
- eosinophilic infiltration to inflammatory sites e.g., in asthma
- inflammatory sites e.g., in asthma
- an instant compound which promotes one or more functions of a mammalian chemokine receptor is administered to stimulate (induce or enhance) an inflammatory response, such as leukocyte emigration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, resulting in the beneficial stimulation of inflammatory processes.
- an inflammatory response such as leukocyte emigration, chemotaxis, exocytosis (e.g., of enzymes, histamine) or inflammatory mediator release, resulting in the beneficial stimulation of inflammatory processes.
- eosinophils can be recruited to combat parasitic infections.
- primates such as humans, a variety of other mammals can be treated according to the method of the present invention.
- mammals including, but not limited to, cows, sheep, goats, horses, dogs, cats, guinea pigs, rats or other bovine, ovine, equine, canine, feline, rodent or murine species can be treated.
- the method can also be practiced in other species, such as avian species (e.g., chickens).
- the disease or condition is one in which the actions of eosinophils and/or lymphocytes are to be inhibited or promoted, in order to modulate the inflammatory response.
- Diseases or conditions of humans or other species which can be treated with inhibitors of chemokine receptor function include, but are not limited to: inflammatory or allergic diseases and conditions, including respiratory allergic diseases such as asthma, allergic rhinitis, hypersensitivity lung diseases, hypersensitivity pneumonitis, eosinophilic pneumonias (e.g., Loeffler's syndrome, chronic eosinophilic pneumonia), delayed-type hypersentitivity, interstitial lung diseases (ILD) (e.g., idiopathic pulmonary fibrosis, or ILD associated with rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, systemic sclerosis, Sjogren's syndrome, polymyositis or dermatomyositis); systemic anaphylaxis or hypersensitivity responses, drug allergies (e.g., to penicillin, cephalosporins), insect sting allergies; autoimmune diseases, such as rheumatoid arthritis
- Other diseases or conditions in which undesirable inflammatory responses are to be inhibited can be treated, including, but not limited to, reperfusion injury, atherosclerosis, certain hematologic malignancies, cytokine-induced toxicity (e.g., septic shock, endotoxic shock), polymyositis, dermatomyositis.
- Immunosuppression such as that in individuals with immunodeficiency syndromes such as AIDS, individuals undergoing radiation therapy, chemotherapy, therapy for autoimmune disease or other drug therapy (e.g., corticosteroid therapy), which causes immunosuppression; immunosuppression due congenital deficiency in receptor function or other causes; and infectious diseases, such as parasitic diseases, including, but not limited to helminth infections, such as nematodes (round worms); (Trichuriasis, Enterobiasis, Ascariasis, Hookworm, Strongyloidiasis, Trichinosis, f ⁇ lariasis); trematodes (flukes) (Schistosomiasis, Clonorchiasis), cestodes (tape worms) (Echinococcosis, Taeniasis saginata, Cysticercosis);
- helminth infections such as nematodes (round worms); (Trichuriasis, Enterobias
- the compounds of the present invention are accordingly useful in the prevention and treatment of a wide variety of inflammatory and immunoregulatory disorders and diseases.
- the instant invention may be used to evaluate putative specific agonists or antagonists of chemokine receptors, including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, and CXCR-4.
- the present invention is directed to the use of these compounds in the preparation and execution of screening assays for compounds which modulate the activity of chemokine receptors.
- the compounds of this invention are useful for isolating receptor mutants, which are excellent screening tools for more potent compounds.
- the compounds of this invention are useful in establishing or determining the binding site of other compounds to chemokine receptors, e.g., by competitive inhibition.
- the compounds of the instant invention are also useful for the evaluation of putative specific modulators of the chemokine receptors, including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, and CXCR-4.
- putative specific modulators of the chemokine receptors including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, and CXCR-4.
- thorough evaluation of specific agonists and antagonists of the above chemokine receptors has been hampered by the lack of availability of non-peptidyl (metabolically resistant) compounds with high binding affinity for these receptors.
- the present invention is further directed to a method for the manufacture of a medicament for modulating chemokine receptor activity in humans and animals comprising combining
- the present invention is further directed to the use of these compounds in the prevention or treatment of infection by a retrovirus, in particular, the human immunodeficiency virus (HIV) and the treatment of, and delaying of the onset of consequent pathological conditions such as AIDS.
- Treating AIDS or preventing or treating infection by HIV is defined as including, but not limited to, treating a wide range of states of HIV infection: AIDS, ARC (AIDS related complex), both symptomatic and asymptomatic, and actual or potential exposure to HIV.
- the compounds of this invention are useful in treating infection by HIV after suspected past exposure to HIV by, e.g., blood transfusion, organ transplant, exchange of body fluids, bites, accidental needle stick, or exposure to patient blood during surgery.
- a subject compound may be used in a method of inhibiting the binding of a human immunodeficiency virus to a chemokine receptor, such as CCR-5 and/or CXCR-4, of a target cell, which comprises contacting the target cell with an amount of the compound which is effective at inhibiting the binding of the virus to the chemokine receptor.
- a chemokine receptor such as CCR-5 and/or CXCR-4
- the subject treated in the methods above is a mammal, preferably a human being, male or female, in whom modulation of chemokine receptor activity is desired.
- Modulation as used herein is intended to encompass antagonism, agonism, partial antagonism and/or partial agonism.
- therapeutically effective amount means the amount of the subject compound that will elicit the biological or medical response of a tissue, system, animal or human that is being sought by the researcher, veterinarian, medical doctor or other clinician.
- composition as used herein is intended to encompass a product comprising the specified ingredients in the specified amounts, as well as any product which results, directly or indirectly, from combination of the specified ingredients in the specified amounts.
- pharmaceutically acceptable it is meant the carrier, diluent or excipient must be compatible with the other ingredients of the formulation and not deleterious to the recipient thereof.
- administering a should be understood to mean providing a compound of the invention or a prodrug of a compound of the invention to the individual in need of treatment.
- Combined therapy to modulate chemokine receptor activity and thereby prevent and treat inflammatory and immunoregulatory disorders and diseases, including asthma and allergic diseases, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis, and those pathologies noted above is illustrated by the combination of the compounds of this invention and other compounds which are known for such utilities.
- the present compounds may be used in conjunction with an antiinflammatory or analgesic agent such as an opiate agonist, a lipoxygenase inhibitor, such as an inhibitor of 5-lipoxygenase, a cyclooxygenase inhibitor, such as a cyclooxygenase-2 inhibitor, an interleukin inhibitor, such as an interleukin-1 inhibitor, an NMDA antagonist, an inhibitor of nitric oxide or an inhibitor of the synthesis of nitric oxide, a non-steroidal antiinflammatory agent, or a cytokine- suppressing antiinflammatory agent, for example with a compound such as acetaminophen, asprin, codiene, fentanyl, ibuprofen, indomethacin, ketorolac, morphine, naproxen, phenacetin, piroxicam, a steroidal analgesic, sufentanyl, sunlindac, tenidap, and the like.
- the instant compounds may be administered with a pain reliever; a potentiator such as caffeine, an H2-antagonist, simethicone, aluminum or magnesium hydroxide; a decongestant such as phenyl ephrine, phenylpropanolamine, pseudophedrine, oxymetazoline, ephinephrine, naphazoline, xylometazoline, propylhexedrine, or levo- desoxy-ephedrine; an antiitussive such as codeine, hydrocodone, caramiphen, carbetapentane, or dextramethorphan; a diuretic; and a sedating or non-sedating antihistamine.
- a pain reliever such as caffeine, an H2-antagonist, simethicone, aluminum or magnesium hydroxide
- a decongestant such as phenyl ephrine, phenylpropanolamine, pseudophedrine, oxymetazoline,
- the present invention is further directed to combinations of the present compounds with one or more agents useful in the prevention or treatment of AIDS.
- the compounds of this invention may be effectively administered, whether at periods of pre-exposure and/or post-exposure, in combination with effective amounts of the AIDS antivirals, immunomodulators, anti-infectives, or vaccines known to those of ordinary skill in the art.
- Drug Name Manufacturer Indication 097 Hoechst/Bayer HIV infection, AIDS, ARC (non-nucleoside reverse transcriptase inhibitor)
- Cidofovir Gilead Science CMV retinitis, herpes, papillomavirus
- Virazole Viratek/ICN asymptomatic HIV Ribavirin (Costa Mesa, CA) positive, LAS, ARC
- Isethionate (IM & IV) (Rosemont, IL)
- combinations of the compounds of this invention with AIDS antivirals, immunomodulators, anti-infectives or vaccines is not limited to the list in the above Table, but includes in principle any combination with any pharmaceutical composition useful for the treatment of AIDS.
- Preferred combinations are simultaneous or alternating treatments of with a compound of the present invention and an inhibitor of HrV protease and/or a non-nucleoside inhibitor of HIV reverse transcriptase.
- An optional fourth component in the combination is a nucleoside inhibitor of HIV reverse transcriptase, such as AZT, 3TC, ddC or ddl.
- a preferred inhibitor of HIV protease is indinavir, which is the sulfate salt of N-(2(R)-hydroxy-l(S)-indanyl)-2(R)-phenylmethyl-4-(S)- hydroxy-5-(l-(4-(3-pyridyl-methyl)-2(S)-N'-(t-butylcarbo-xamido)- piperazinyl))-pentaneamide ethanolate, and is synthesized according to U.S. 5,413,999.
- Indinavir is generally administered at a dosage of 800 mg three times a day.
- Other preferred inhibitors of HrV protease include nelfinavir and ritonavir.
- Preferred non-nucleoside inhibitors of HIV reverse transcriptase include (-) 6-chloro-4(S)-cyclopropylethynyl- 4(S)-trifluoromethyl-l,4-dihydro-2H-3,l-benzoxazin-2-one, which may be prepared by methods disclosed in EP 0,582,455.
- the preparation of ddC, ddl and AZT are also described in EPO 0,484,071. These combinations may have unexpected effects on limiting the spread and degree of infection of HrV.
- Preferred combinations with the compounds of the present invention include the following (1) indinavir, with efavirenz or (-) 6-chloro-4(S)-cyclopropylethynyl-4(S)-trifluoromethyl-l,4-dihydro-2H- 3,l-benzoxazin-2-one, and, optionally, AZT and/or 3TC and or ddl and/or ddC; (2) indinavir, and any of AZT and/or ddl and/or ddC.
- the compound of the present invention and other active agents may be administered separately or in conjunction.
- the administration of one element may be prior to, concurrent to, or subsequent to the administration of other agent(s).
- the compounds of the present invention may be administered by oral, parenteral (e.g., intramuscular, intraperitoneal, intravenous, ICV, intracisternal injection or infusion, subcutaneous injection, or implant), by inhalation spray, nasal, vaginal, rectal, sublingual, or topical routes of administration and may be formulated, alone or together, in suitable dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles appropriate for each route of administration.
- parenteral e.g., intramuscular, intraperitoneal, intravenous, ICV, intracisternal injection or infusion, subcutaneous injection, or implant
- inhalation spray nasal, vaginal, rectal, sublingual, or topical routes of administration
- nasal, vaginal, rectal, sublingual, or topical routes of administration may be formulated, alone or together, in suitable dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles appropriate for each route of administration.
- the compounds of the invention are effective for
- compositions for the administration of the compounds of this invention may conveniently be presented in dosage unit form and may be prepared by any of the methods well known in the art of pharmacy. All methods include the step of bringing the active ingredient into association with the carrier which constitutes one or more accessory ingredients.
- the pharmaceutical compositions are prepared by uniformly and intimately bringing the active ingredient into association with a liquid carrier or a finely divided solid carrier or both, and then, if necessary, shaping the product into the desired formulation.
- the active object compound is included in an amount sufficient to produce the desired effect upon the process or condition of diseases.
- composition is intended to encompass a product comprising the specified ingredients in the specified amounts, as well as any product which results, directly or indirectly, from combination of the specified ingredients in the specified amounts.
- compositions containing the active ingredient may be in a form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions, hard or soft capsules, or syrups or elixirs.
- Compositions intended for oral use may be prepared according to any method known to the art for the manufacture of pharmaceutical compositions and such compositions may contain one or more agents selected from the group consisting of sweetening agents, flavoring agents, coloring agents and preserving agents in order to provide pharmaceutically elegant and palatable preparations. Tablets contain the active ingredient in admixture with non-toxic pharmaceutically acceptable excipients which are suitable for the manufacture of tablets.
- excipients may be for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, for example, corn starch, or alginic acid; binding agents, for example starch, gelatin or acacia, and lubricating agents, for example magnesium stearate, stearic acid or talc.
- the tablets may be uncoated or they may be coated by known techniques to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monostearate or glyceryl distearate may be employed. They may also be coated by the techniques described in the U.S. Patents 4,256,108; 4,166,452; and 4,265,874 to form osmotic therapeutic tablets for control release.
- Formulations for oral use may also be presented as hard gelatin capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient is mixed with water or an oil medium, for example peanut oil, liquid paraffin, or olive oil.
- an inert solid diluent for example, calcium carbonate, calcium phosphate or kaolin
- water or an oil medium for example peanut oil, liquid paraffin, or olive oil.
- Aqueous suspensions contain the active materials in admixture with excipients suitable for the manufacture of aqueous suspensions.
- excipients are suspending agents, for example sodium carboxymethylcellulose, methylcellulose, hydroxy- propylmethylcellulose, sodium alginate, polyvinyl- pyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for example lecithin, or condensation products of an alkylene oxide with fatty acids, for example polyoxyethylene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for example polyethylene sorbitan mono
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents, such as sucrose or saccharin.
- preservatives for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- coloring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- flavoring agents for example ethyl, or n-propyl, p-hydroxybenzoate
- sweetening agents such as sucrose or saccharin.
- Oily suspensions may be formulated by suspending the active ingredient in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example beeswax, hard paraffin or cetyl alcohol.
- Sweetening agents such as those set forth above, and flavoring agents may be added to provide a palatable oral preparation.
- These compositions may be preserved by the addition of an anti-oxidant such as ascorbic acid.
- Dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide the active ingredient in admixture with a dispersing or wetting agent, suspending agent and one or more preservatives. Suitable dispersing or wetting agents and suspending agents are exemplified by those already mentioned above. Additional excipients, for example sweetening, flavoring and coloring agents, may also be present.
- the pharmaceutical compositions of the invention may also be in the form of oil-in-water emulsions.
- the oily phase may be a vegetable oil, for example olive oil or arachis oil, or a mineral oil, for example liquid paraffin or mixtures of these.
- Suitable emulsifying agents may be naturally- occurring gums, for example gum acacia or gum tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin, and esters or partial esters derived from fatty acids and hexitol anhydrides, for example sorbitan monooleate, and condensation products of the said partial esters with ethylene oxide, for example polyoxyethylene sorbitan monooleate.
- the emulsions may also contain sweetening and flavoring agents.
- Syrups and elixirs may be formulated with sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents.
- sweetening agents for example glycerol, propylene glycol, sorbitol or sucrose.
- Such formulations may also contain a demulcent, a preservative and flavoring and coloring agents.
- the pharmaceutical compositions may be in the form of a sterile injectable aqueous or oleagenous suspension.
- This suspension may be formulated according to the known art using those suitable dispersing or wetting agents and suspending agents which have been mentioned above.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example as a solution in 1,3-butane diol.
- the acceptable vehicles and solvents that may be employed are water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the compounds of the present invention may also be administered in the form of suppositories for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- a suitable non-irritating excipient which is solid at ordinary temperatures but liquid at the rectal temperature and will therefore melt in the rectum to release the drug.
- Such materials are cocoa butter and polyethylene glycols.
- topical application For topical use, creams, ointments, jellies, solutions or suspensions, etc., containing the compounds of The present invention are employed. (For purposes of this application, topical application shall include mouth washes and gargles.)
- compositions and method of the present invention may further comprise other therapeutically active compounds as noted herein which are usually applied in the treatment of the above mentioned pathological conditions.
- an appropriate dosage level will generally be about 0.01 to 500 mg per kg patient body weight per day which can be administered in single or multiple doses.
- the dosage level will be about 0.1 to about 250 mg/kg per day; more preferably about 0.5 to about 100 mg/kg per day.
- a suitable dosage level may be about 0.01 to 250 mg/kg per day, about 0.05 to 100 mg/kg per day, or about 0.1 to 50 mg/kg per day. Within this range the dosage may be 0.05 to 0.5, 0.5 to 5 or 5 to 50 mg/kg per day.
- compositions are preferably provided in the form of tablets containing 1.0 to 1000 milligrams of the active ingredient, particularly 1.0, 5.0, 10.0, 15.0. 20.0, 25.0, 50.0, 75.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, 500.0, 600.0, 750.0, 800.0, 900.0, and 1000.0 milligrams of the active ingredient for the symptomatic adjustment of the dosage to the patient to be treated.
- the compounds may be administered on a regimen of 1 to 4 times per day, preferably once or twice per day.
- the compounds of the present invention are prepared by alkylating heterocycle I under appropriate conditions to provide compound II (Scheme 1).
- the required starting materials for preparing heterocycle I are available commercially or can be prepared using the methods given below.
- heterocycle I is combined with the appropriate aldehyde and the intermediate imine or iminium species is reduced to the tertiary amine chemically (e.g. using sodium cyanoborohydride, sodium borohydride, or sodium triacetoxyborohydride) or catalytically (e.g. using hydrogen and palladium on carbon or Raney nickel catalyst) (Scheme 1).
- the aldehyde needed for this reaction can be prepared by methods generally known in the chemical literature; for the purposes of the present invention one preparation of a representative aldehyde is described in Hale, J.J.; Finke, P.E.; MacCoss, M. Bioorganic & Medicinal Chemistry Letters 1993,3, 319-322.
- heterocycle I can be alkylated with an alkyl halide or alkyl sulfonate ester (with or without an added base to neutralize the mineral acid or sulfonic acid by-product) to give the desired compound (Scheme 1).
- the alkyl halide or alkyl sulfonate needed for this reaction can be prepared by methods generally known in the chemical literature; for the purposes of the present invention an aldehyde, prepared as described above, can be reduced to an alcohol with sodium borohydride, diisobutylaluminum hydride or lithium aluminum hydride, and the product alcohol converted to either the alkyl halide using methods described in March J. "Advanced Organic Chemistry", 4th ed., John
- I can be acylated to give a tertiary amide; subsequent reduction with a strong reducing agent (e.g. diborane; borane in THF; borane dimethylsulfide, or lithium aluminum hydride) will give the desired compound (Scheme 1).
- a strong reducing agent e.g. diborane; borane in THF; borane dimethylsulfide, or lithium aluminum hydride
- the acylating agent needed for this reaction can be prepared by methods generally known in the chemical literature; for the purposes of the present invention an aldehyde, prepared as described above, can be oxidized using such commonly used reagents as permanganate in acid or silver oxide, and the resulting acid activated as an acid chloride or mixed anhydride which can be used to acylate I.
- the product amide can in and of itself be a chemokine receptor modulator or can be reduced as noted above to give the tertiary amine.
- compound II may be further modified in subsequent reactions, as illustrated below.
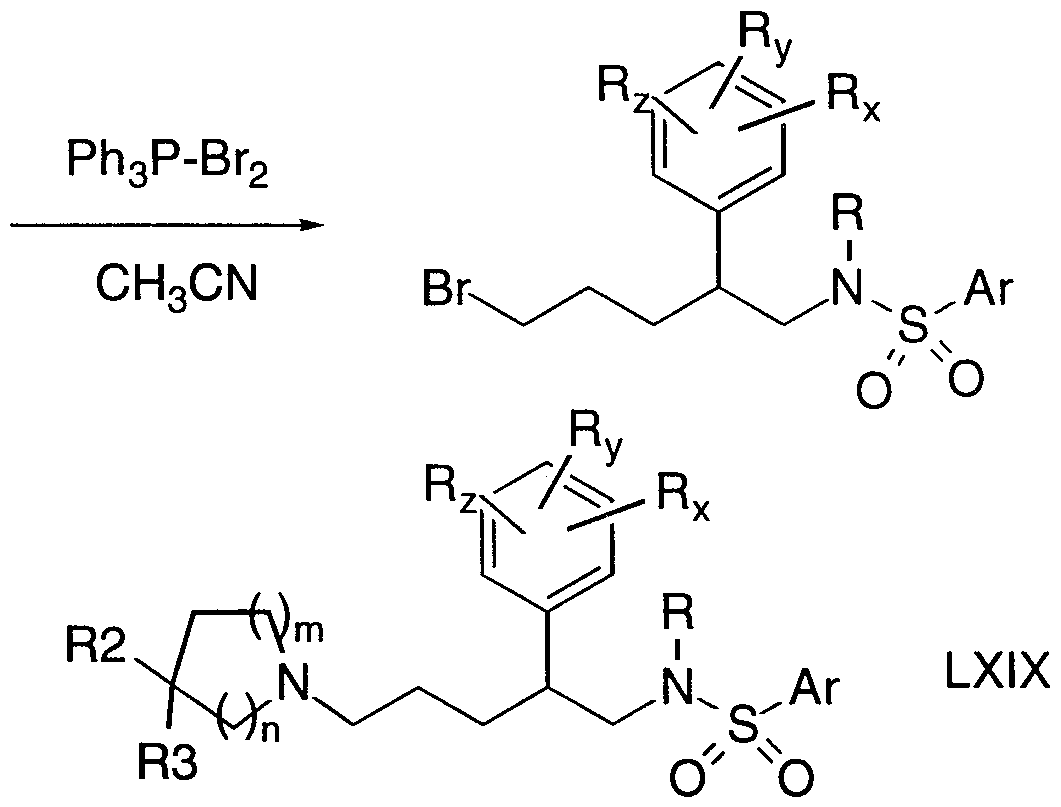
- compounds of interest can be prepared by activating the hydroxyl groups of l,4-dihydroxy-2-butyne, for example by treatment with triphenyl- phosphine dibromide in acetonitrile, to give l,4-dibromo-2-butyne (Scheme 2).
- Palladium-catalysed hydrostannylation preferentially forms the 3- tributylstannyl olefin IV.
- the minor product from this reaction can also isolated and carried through the sequence described below.
- Compound IV can be converted to the corresponding 3-aryl derivative V by treatment with an aryl bromide (wherein Rx, Ry and Rz are substituents on the phenyl or heteroaryl as defined herein) in the presence of a suitable palladium catalyst at or above room temperature.
- Suitable catalysts include palladium acetate and triphenylphosphine, bis(triphenylphosphine) palladium (II) chloride, or palladium (0) bis(dibenzylidineacetone) in the presence of triphenylphosphine or tri-2- furylphosphine.
- Suitable solvents include 1,4-dioxane, DMF, and N- methyl pyrrolidinone.
- a base such as potassium carbonate or potassium phosphate may also be employed.
- Compound V may be employed as a chemokine receptor modulator itself or it can be reduced to saturated derivative VI by standard conditions, for example catalytic hydrogenation with palladium on carbon or with palladium hydroxide in the presence of a mild acid such as acetic acid.
- the allyl acid VII (prepared, for example, as described in Hale et al; see above) can be converted into the N-methyl-N-methoxy amide VIII, which is then treated with an alkyl or aryl metal reagent, for example methylhthium or butyllithium, to provide the ketone IX (Scheme 3).
- the ketone can be converted into an imine which can then be reduced to secondary amine X chemically, (e.g using sodium cyanoborohydride or sodium borohydride), or catalytically (e.g. using hydrogen and palladium on carbon or Raney nickel catalyst). Acylation under standard conditions, for example with an acid chloride, provides the corresponding amide.
- amine X can be sulfonylated, for example with a alkyl or aryl sulfonyl chloride or an alkyl or aryl sulfonic anhydride, to give (for aryl substituted sulfonylating reagents) sulfonamide XL
- the allyl group in XI can be oxidatively cleaved to aldehyde XII with osmium tetroxide followed by sodium periodate or with ozone at low temperature. Reductive amination of aldehyde XII with azacycle I can then be carried out under the conditions described above to give the desired product XIII.
- oxazolidinone imide XV is prepared from acid XIV, by formation of the corresponding acid chloride (by treatment with oxalyl chloride or thionyl chloride) and addition of N- lithio 2(S)-benzyl oxazolidinone.
- the enolate azidation can be accomplished by a variety of methods, such as the procedure of Evans, D. A.; et. al. J. Am. Chem. Soc. 1990, 112, 4011-4030.
- Reduction of the oxazolidinone moiety of XVI can be carried out by a variety of metal hydride reagents (e.g.
- Acid VII can be homologated under Arndt-Eistert conditions to give the chain-extended acid XrV, which can be derivatized under standard acylating conditions with, for example, an aniline derivative, to give the amide XXI.
- Oxidative cleavage of the olefin with osmium tetroxide or ozone then provides aldehyde XXII as an intermediate suitable for coupling as described earlier.
- ketone derivatives are prepared by an extension of the chemistry given above, as shown in Scheme 6.
- An Arndt-Eistert chain extension of acid XIV provides heptenoic acid XXIII, which after conversion into N-methoxy-N-methyl amide XXIV, can be reacted with an aryl organometallic reagent, such as an aryl magnesium bromide, to provide ketone XXV. Routine oxidative cleavage then gives the desired aldehyde XXVI, which can be coupled with an appropriate amine as described above.
- Alcohol containing compounds are prepared according to procedures given in Scheme 7. Formation of the N-methyl-N-methoxy amide of acid VII followed by oxidative cleavage of the olefin provides intermediate aldehyde XXVII. Coupling with an appropriate amine provides amide XXVIII. Addition of an organometallic reagent to compound XXVIII provides illustrated ketone XXLX. Treatment with a hydride reducing agent, such as sodium borohydride, then yields the desired alcohol XXX.
- a hydride reducing agent such as sodium borohydride
- allyl acid VII can be reduced to alcohol XXXI with, for example, lithium aluminum hydride.
- This alcohol can be alkylated by a Williamson ether synthesis, by deprotonation with a strong base such as sodium hydride or sodium hexamethyldisilazide followed by reaction with a benzyl halide such as benzyl bromide.
- the resulting ether XXXrV can be processed through the oxidative cleavage steps described earlier to provide aldehyde XXXV.
- This aldehyde can then be coupled with an appropriate amine under reductive amination conditions to give XXXVI.
- reduction of XXXV to the corresponding alcohol followed by conversion to the bromide allows for alkylation with an amine to provide XXXVI.
- CBZ-protected piperidine XLV can be allowed to react with oxalyl chloride and then sodium azide, to provide the corresponding acyl azide, which can then be thermally rearranged to isocyanate XLVI (Scheme 11).
- Compound XLVI can be treated with an alcohol ROH or an amine RR'NH to form carbamate XLVII or urea XLVIII, respectively, each of which can be deprotected with hydrogen in the presence of palladium on carbon to secondary amines XLLX or L.
- a suitable base such as sodium hydride, lithium hexamethyldisilazide or potassium t-butoxide
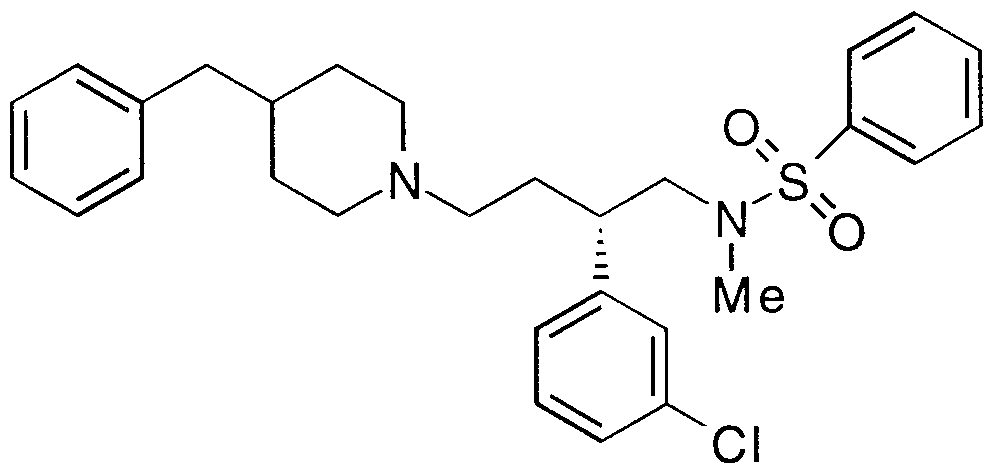
- Step A (R,S)-N-(2-Phenylpent-4-en-l-yl)-N-methylbenzene- sulfonamide
- reaction mixture was diluted with methylene chloride and washed with water containing 20 mL of 2 N HC1. The aqueous layer was reextracted with methylene chloride and the organic layers were washed with brine, combined, dried over sodium sulfate and concentrated in vacuo. The residue was purified by flash column chromatography (FCC) eluting with 5% ethyl acetate/hexanes to afford 3.2 g of the title compound.
- FCC flash column chromatography
- Step B (R,S)-N-(2-Phenyl-4-oxobut-l-yl)-N-methylbenzene- sulfonamide
- a solution of 1.0 g (3.2 mmol) of (R,S)-N-(2-phenylpent-4- en-l-yl)-N-methylbenzenesulfonamide from Step A in 7 mL of acetone, 3.5 mL of t-butanol and 3.5 mL of water was added 413 mg (3.5 mmol) of N-methylmorpholine-N-oxide followed by 0.14 mL of 4% osmium tetroxide in water.
- the reaction was stirred at rt for 16 h and was then quenched with aqueous sodium bisulfite and concentrated in vacuo.
- the residue was diluted with water and extracted twice with ether.
- the ether layers were each washed with brine, combined, dried over sodium sulfate and concentrated in vacuo.
- the residue was purified by FCC eluting with 5% methanol in methylene chloride to afford the diol intermediate.
- the above product was taken up in 10 mL of THF and 755 mg (3.5 mmole) of sodium periodate in 3 mL of water was added. The mixture was stirred at rt for 3 h, poured into water and extracted twice with ether.
- Step C (R,S)-N-[2-Phenyl-4-(4-phenylpiperidin-l-yl)but-l-yl]-N- methylbenzenesulfonamide hydrochioride salt
- Step A N-[4-(4-Phenylpiperidin-l-yl)but-2-yn-l-yl]-N- methylbenzenesulfonamide To a suspension of triphenylphosphine dibromide at 0 C
- a solution of the sodium salt of N-methylbenzenesulfonamide in 50 mL of DMF was prepared at 0 C under nitrogen by portionwise addition of 3.2 g (80 mmol) of 60% sodium hydride over 0.5 h and then stirred with cooling for 0.5 h.
- This salt solution was added via canula over 15 min with cooling to the above dibromide solution.
- 18.0 g (110 mmol) of 4-phenylpiperidine was added and the reaction was stirred a further 2 h at rt.
- the reaction was diluted with water and extracted three times with ether. The ether layers were each washed with a portion of brine, dried over sodium sulfate, combined and concentrated in vacuo.
- Step B N-[4-(4-Phenylpiperidin-l-yl)-2-tributylstannylbut-2-en-l-yl]-
- N-[4-(4-phenyl-piperidin- l-yl)but-2-yn- 1-yl] -N-methylbenzenesulfonamide from Step A in 75 mL of
- the mixture was heated at 70 C for 24 h, cooled, treated with aqueous sodium fluoride for 10 min, and partitioned between water and ether. The water layer was reextracted with ether and each organic layer was washed with brine, dried over sodium sulfate, combined and concentrated in vacuo. The residue was purified by FCC eluting with 25- 50% ethyl acetate/hexanes to give 57 mg of product contaminated with stannane biproduct. The product was further purified by prep TLC (50% ethyl acetate/hexanes) to afford 53 mg of title compound.
- Step D (R,S)-N-[4-(4-Phenylpiperidin-l-yl)-2-(3-methylphenyl)-but-l- yl] -N-methylbenzenesulfonamide
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA002296314A CA2296314A1 (en) | 1997-07-25 | 1998-07-21 | Cyclic amine modulators of chemokine receptor activity |
| EP98936920A EP1003514A4 (en) | 1997-07-25 | 1998-07-21 | Cyclic amine modulators of chemokine receptor activity |
| JP50994999A JP2002510327A (en) | 1997-07-25 | 1998-07-21 | Cyclic amine chemokine receptor activity modulator |
| AU85760/98A AU8576098A (en) | 1997-07-25 | 1998-07-21 | Cyclic amine modulators of chemokine receptor activity |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US5375497P | 1997-07-25 | 1997-07-25 | |
| US60/053,754 | 1997-07-25 | ||
| GBGB9800958.2A GB9800958D0 (en) | 1998-01-16 | 1998-01-16 | Cyclic amine modulators of chemokine activity |
| GB9800958.2 | 1998-01-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1999004794A1 true WO1999004794A1 (en) | 1999-02-04 |
Family
ID=26312958
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1998/014990 WO1999004794A1 (en) | 1997-07-25 | 1998-07-21 | Cyclic amine modulators of chemokine receptor activity |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1003514A4 (en) |
| JP (1) | JP2002510327A (en) |
| AU (1) | AU8576098A (en) |
| CA (1) | CA2296314A1 (en) |
| WO (1) | WO1999004794A1 (en) |
Cited By (98)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000029377A1 (en) * | 1998-11-17 | 2000-05-25 | F. Hoffmann-La Roche Ag | 4-aroyl-piperidin-ccr-3 receptor antagonists iii |
| EP1013276A1 (en) * | 1998-12-23 | 2000-06-28 | Pfizer Inc. | Aminoazacycloalkanes as CCR5 modulators |
| WO2000038680A1 (en) * | 1998-12-23 | 2000-07-06 | Pfizer Limited | Azabicycloalkanes as ccr5 modulators |
| WO2000039125A1 (en) * | 1998-12-23 | 2000-07-06 | Pfizer Limited | Piperidines as ccr5 modulators |
| WO2000056729A1 (en) * | 1999-03-24 | 2000-09-28 | Anormed Inc. | Chemokine recpetor binding heterocyclic compounds |
| EP1050307A1 (en) * | 1999-05-06 | 2000-11-08 | Applied Research Systems ARS Holding N.V. | CCR4 antagonists in sepsis |
| WO2000066558A1 (en) * | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperazine derivatives useful as ccr5 antagonists |
| WO2000066559A1 (en) * | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| WO2000076972A1 (en) * | 1999-06-11 | 2000-12-21 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| WO2001014333A1 (en) * | 1999-08-24 | 2001-03-01 | Astrazeneca Uk Limited | Substituted piperidine compounds useful as modulators of chemokine receptor activity |
| WO2001066525A1 (en) * | 2000-03-10 | 2001-09-13 | Astrazeneca Ab | New ccr5 modulators: benzimidazoles or benzotriazoles |
| WO2001087839A1 (en) * | 2000-05-17 | 2001-11-22 | Astrazeneca Ab | Pharmaceutically active piperidine derivatives, in particular as modulators of chemokine receptor activity |
| WO2001090106A2 (en) * | 2000-05-26 | 2001-11-29 | Pfizer Limited | Tryasolyl tropane derivatives as ccr5 modulators |
| WO2001092227A1 (en) * | 2000-05-31 | 2001-12-06 | Astrazeneca Ab | Chemical compounds |
| US6331545B1 (en) | 1998-12-18 | 2001-12-18 | Soo S. Ko | Heterocycyclic piperidines as modulators of chemokine receptor activity |
| WO2002004420A1 (en) * | 2000-07-12 | 2002-01-17 | Novartis Ag | Piperidine coumpounds for use as ccr-3 inhibitors |
| EP1180513A1 (en) * | 1999-04-28 | 2002-02-20 | Takeda Chemical Industries, Ltd. | Cyclic amide compounds, process for the preparation of the same and uses thereof |
| US6358979B1 (en) | 1999-06-11 | 2002-03-19 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| US6387930B1 (en) | 1999-05-04 | 2002-05-14 | Schering Corporation | Piperidine derivatives useful as CCR5 antagonists |
| US6391865B1 (en) | 1999-05-04 | 2002-05-21 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| US6432981B1 (en) | 1999-06-11 | 2002-08-13 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| WO2002079186A2 (en) * | 2001-03-30 | 2002-10-10 | F. Hoffmann-La Roche Ag | Aminopiperidine derivatives as modulators of chemokine receptor activity |
| US6472410B1 (en) | 1999-06-11 | 2002-10-29 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| US6486180B1 (en) | 1998-12-18 | 2002-11-26 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US6492400B1 (en) | 1998-12-18 | 2002-12-10 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US6500844B1 (en) | 1999-06-11 | 2002-12-31 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| US6506777B1 (en) | 1999-06-11 | 2003-01-14 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| US6511826B2 (en) | 1995-06-06 | 2003-01-28 | Human Genome Sciences, Inc. | Polynucleotides encoding human G-protein chemokine receptor (CCR5) HDGNR10 |
| US6525070B2 (en) | 2000-04-08 | 2003-02-25 | Astrazeneca Ab | Bipiperidine derivatives as modulators of CCR3 activity and as H1 antagonists |
| US6538002B1 (en) | 1999-06-11 | 2003-03-25 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| US6562978B1 (en) | 1999-10-01 | 2003-05-13 | Takeda Chemical Industries, Ltd. | Cyclic amine compounds as CCR5 antagonists |
| WO2003077907A1 (en) * | 2002-03-15 | 2003-09-25 | Novartis Ag | Azetidine derivatives as ccr-3 receptor antagonists |
| US6627629B2 (en) | 2000-06-30 | 2003-09-30 | Bristol-Myers Squibb Pharma | N-ureidoheterocycloalkyl-piperidines as modulators of chemokine receptor activity |
| US6656953B2 (en) | 2000-12-06 | 2003-12-02 | Sepracor Inc. | 4,4-Disubstituted piperidines, and methods of use thereof |
| US6667314B2 (en) | 2000-05-26 | 2003-12-23 | Pfizer, Inc. | Tropane derivatives useful in therapy |
| US6689765B2 (en) | 1999-05-04 | 2004-02-10 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| US6720325B2 (en) | 2001-03-29 | 2004-04-13 | Schering Corporation | CCR5 antagonists useful for treating aids |
| US6743594B1 (en) | 1995-06-06 | 2004-06-01 | Human Genome Sciences, Inc. | Methods of screening using human G-protein chemokine receptor HDGNR10 (CCR5) |
| US6750348B1 (en) | 1999-03-24 | 2004-06-15 | Anormed, Inc. | Chemokine receptor binding heterocyclic compounds |
| WO2004054974A2 (en) * | 2002-12-13 | 2004-07-01 | Smithkline Beecham Corporation | Piperidine derivatives as ccr5 antagonists |
| US6787650B1 (en) | 1999-10-05 | 2004-09-07 | Takeda Chemical Industries, Ltd. | Urea compounds, process for producing the same and use thereof |
| US6919356B2 (en) | 2002-09-26 | 2005-07-19 | Bristol Myers Squibb Company | N-substituted heterocyclic amines as modulators of chemokine receptor activity |
| WO2005073192A1 (en) * | 2004-02-02 | 2005-08-11 | Astrazeneca Ab | Novel piperidines as chemokine modulators (ccr) |
| US6949643B2 (en) | 2001-04-12 | 2005-09-27 | Astrazeneca Ab | Thiazolopytimidines and their use as modulators of chemokine receptor activity |
| US6958343B2 (en) | 2000-02-11 | 2005-10-25 | Astrazeneca, Ab | Thiazolopyrimidines and their use as modulators of chemokine receptor activity |
| US6958350B2 (en) | 2001-02-19 | 2005-10-25 | Astrazeneca Ab | Chemical compounds |
| US6958344B2 (en) | 2000-02-11 | 2005-10-25 | Astrazeneca Ab | Pyrimidine compounds and their use as modulators of chemokine receptor activity |
| US6960602B2 (en) | 2001-03-22 | 2005-11-01 | Astrazeneca Ab | Piperidine derivatives as modulators of chemokine receptors |
| US6974869B2 (en) | 2001-09-18 | 2005-12-13 | Bristol-Myers Squibb Pharma Company | Piperizinones as modulators of chemokine receptor activity |
| US6992091B2 (en) | 2002-09-12 | 2006-01-31 | Bristol-Myers Squibb Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US7071193B2 (en) | 2000-10-20 | 2006-07-04 | Astrazeneca Ab | 7-amino-2-alkylthiopteridin-4-yl-amines for the treatment of chemokine-related diseases |
| US7144903B2 (en) | 2001-05-23 | 2006-12-05 | Amgen Inc. | CCR4 antagonists |
| WO2006129679A1 (en) | 2005-05-31 | 2006-12-07 | Ono Pharmaceutical Co., Ltd. | Spiropiperidine compound and medicinal use thereof |
| US7186718B2 (en) | 2001-08-22 | 2007-03-06 | Astrazeneca Ab | Piperidinyl-morpholinyl derivatives as modulators of chemokine receptor activity |
| US7192973B2 (en) | 2001-11-15 | 2007-03-20 | Astrazeneca Ab | Piperidine derivatives and their use as modulators of chemokine receptor activity (especially CCR5) |
| WO2007049771A1 (en) | 2005-10-28 | 2007-05-03 | Ono Pharmaceutical Co., Ltd. | Compound containing basic group and use thereof |
| WO2007058322A1 (en) | 2005-11-18 | 2007-05-24 | Ono Pharmaceutical Co., Ltd. | Basic group-containing compound and use thereof |
| US7238691B2 (en) | 2001-09-18 | 2007-07-03 | Astrazeneca Ab | Piperidine derivatives and their use as modulators of chemokine (especially CCR3) activity |
| US7262204B2 (en) | 2000-10-11 | 2007-08-28 | Amgen Inc. | Modulation of CCR4 function |
| US7265227B2 (en) | 2001-07-23 | 2007-09-04 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| WO2007105637A1 (en) | 2006-03-10 | 2007-09-20 | Ono Pharmaceutical Co., Ltd. | Nitrogenated heterocyclic derivative, and pharmaceutical agent comprising the derivative as active ingredient |
| CN100343240C (en) * | 2002-03-29 | 2007-10-17 | 先灵公司 | Stereoselective alkylation of chiral 2-methyl-4-protected piperazines |
| US7294636B2 (en) | 2003-05-09 | 2007-11-13 | Astrazeneca Ab | Chemical compounds |
| WO2007132846A1 (en) | 2006-05-16 | 2007-11-22 | Ono Pharmaceutical Co., Ltd. | Compound having acidic group which may be protected, and use thereof |
| US7304077B2 (en) | 2000-09-04 | 2007-12-04 | Astrazeneca Ab | Chemical compounds |
| US7307090B2 (en) | 2001-07-02 | 2007-12-11 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| WO2008016006A1 (en) | 2006-07-31 | 2008-02-07 | Ono Pharmaceutical Co., Ltd. | Compound having cyclic group bound thereto through spiro binding and use thereof |
| US7482363B2 (en) | 2002-03-19 | 2009-01-27 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| US7485732B2 (en) | 2003-06-11 | 2009-02-03 | Merck & Co., Inc. | Substituted 3-alkyl and 3-alkenyl azetidine derivatives |
| US7495013B2 (en) | 2003-04-01 | 2009-02-24 | Astrazeneca Ab | Chemical compounds |
| US7517989B2 (en) | 2002-03-19 | 2009-04-14 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| US7579342B2 (en) | 2000-02-23 | 2009-08-25 | Astrazeneca | Pteridine compounds for the treatment of psoriasis |
| US7585867B2 (en) | 2002-09-20 | 2009-09-08 | Astrazeneca Ab | Substituted thiazolo[4,5-d]pyrimidin-2(3H)-one |
| US7615555B2 (en) | 2004-04-23 | 2009-11-10 | Astrazeneca Ab | Piperidine derivatives as modulators of chemokine receptor CCR5 |
| US7709500B2 (en) | 2002-02-18 | 2010-05-04 | Astrazeneca Ab | Chemical compounds |
| US7790883B2 (en) | 2003-12-05 | 2010-09-07 | Astrazeneca Ab | Process for the preparation of thiazolopyrimidines |
| WO2011011652A1 (en) | 2009-07-24 | 2011-01-27 | Glaxosmithkline Llc | Therapeutic compounds |
| US7906652B2 (en) | 2005-11-28 | 2011-03-15 | Merck Sharp & Dohme Corp. | Heterocycle-substituted 3-alkyl azetidine derivatives |
| EP2364982A1 (en) | 2003-04-18 | 2011-09-14 | ONO Pharmaceutical Co., Ltd. | Spiro-piperidine compounds as chemokine receptor antagonists and medicinal use thereof |
| EP2385040A1 (en) | 2003-03-14 | 2011-11-09 | ONO Pharmaceutical Co., Ltd. | Nitrogen-containing heterocyclic derivatives and drugs containing the same as the active ingredient |
| US8143261B2 (en) | 1999-10-01 | 2012-03-27 | Astrazeneca Ab | Thiazolo (4,5-D) pyrimidine compounds |
| US8314127B2 (en) | 2005-07-21 | 2012-11-20 | Astrazeneca Ab | Piperidine derivatives |
| EP2546234A1 (en) | 2004-09-13 | 2013-01-16 | Ono Pharmaceutical Co., Ltd. | Nitrogeneous heterocyclic derivative and medicine containing the same as an active ingredient |
| WO2013024022A1 (en) | 2011-08-12 | 2013-02-21 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical compositions for treatment of pulmonary hypertension |
| US8778967B2 (en) | 2001-12-21 | 2014-07-15 | Genzyme Corporation | Chemokine receptor binding heterocyclic compounds with enhanced efficacy |
| US9206186B2 (en) | 2003-03-24 | 2015-12-08 | Axikin Pharmaceuticals, Inc. | 2-phenoxy- and 2-phenylsulfonamide derivatives with CCR3 antagonistic activity for the treatment of inflammatory or immunological disorders |
| US10117931B2 (en) | 2009-04-28 | 2018-11-06 | Kameran Lashkari | Methods for treatment of age-related macular degeneration |
| CN110483490A (en) * | 2019-08-29 | 2019-11-22 | 苏州汉德创宏生化科技有限公司 | A kind of synthetic method of 3- (piperidin-4-yl) oxazolidine -2- ketone and its salt |
| US10548889B1 (en) | 2018-08-31 | 2020-02-04 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| US10610527B2 (en) | 2015-12-22 | 2020-04-07 | X4 Pharmaceuticals, Inc. | Methods for treating immunodeficiency disease |
| US10633336B2 (en) | 2014-12-19 | 2020-04-28 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| US10752588B2 (en) | 2014-12-19 | 2020-08-25 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| US10759796B2 (en) | 2016-06-21 | 2020-09-01 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US10953003B2 (en) | 2015-12-14 | 2021-03-23 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US10988465B2 (en) | 2016-06-21 | 2021-04-27 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11332470B2 (en) | 2016-06-21 | 2022-05-17 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11337969B2 (en) | 2016-04-08 | 2022-05-24 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US11357742B2 (en) | 2015-12-14 | 2022-06-14 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1558250A4 (en) * | 2002-10-30 | 2006-11-02 | Merck & Co Inc | Gamma-aminoamide modulators of chemokine receptor activity |
| CA2534550A1 (en) * | 2003-09-15 | 2005-03-24 | Novartis Ag | 1, 3-disubstituted azetidine derivatives for use as ccr-3 receptor antagonists in the treatment of inflammatory and allergic diseases |
| WO2006138350A2 (en) * | 2005-06-15 | 2006-12-28 | Anormed Inc. | Chemokine receptor binding compounds |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5434158A (en) * | 1994-04-26 | 1995-07-18 | Merck & Co., Inc. | Spiro-substituted azacycles as neurokinin-3 antagonists |
| WO1996024582A1 (en) * | 1995-02-10 | 1996-08-15 | Zeneca Limited | 5-(4-subst.-piperidinyl-1)-3-aryl-pentanoic acid derivatives as tachykinin receptor antagonist |
| US5576333A (en) * | 1992-11-03 | 1996-11-19 | Zeneca Limited | Carboxamide derivatives |
| US5789422A (en) * | 1996-10-28 | 1998-08-04 | Schering Corporation | Substituted arylalkylamines as neurokinin antagonists |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9100505D0 (en) * | 1991-01-10 | 1991-02-20 | Shell Int Research | Piperidine derivatives |
| GB9310713D0 (en) * | 1993-05-24 | 1993-07-07 | Zeneca Ltd | Aryl substituted heterocycles |
| FR2719311B1 (en) * | 1994-03-18 | 1998-06-26 | Sanofi Sa | Compounds that are selective antagonists of the human NK3 receptor and their use as drugs and diagnostic tools. |
| EP0883613A1 (en) * | 1996-02-09 | 1998-12-16 | Smithkline Beecham Plc | Sulfonamide derivatives as 5ht7 receptor antagonists |
| IL125658A0 (en) * | 1997-08-18 | 1999-04-11 | Hoffmann La Roche | Ccr-3 receptor antagonists |
-
1998
- 1998-07-21 EP EP98936920A patent/EP1003514A4/en not_active Withdrawn
- 1998-07-21 JP JP50994999A patent/JP2002510327A/en active Pending
- 1998-07-21 AU AU85760/98A patent/AU8576098A/en not_active Abandoned
- 1998-07-21 CA CA002296314A patent/CA2296314A1/en not_active Abandoned
- 1998-07-21 WO PCT/US1998/014990 patent/WO1999004794A1/en not_active Application Discontinuation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5576333A (en) * | 1992-11-03 | 1996-11-19 | Zeneca Limited | Carboxamide derivatives |
| US5434158A (en) * | 1994-04-26 | 1995-07-18 | Merck & Co., Inc. | Spiro-substituted azacycles as neurokinin-3 antagonists |
| WO1996024582A1 (en) * | 1995-02-10 | 1996-08-15 | Zeneca Limited | 5-(4-subst.-piperidinyl-1)-3-aryl-pentanoic acid derivatives as tachykinin receptor antagonist |
| US5789422A (en) * | 1996-10-28 | 1998-08-04 | Schering Corporation | Substituted arylalkylamines as neurokinin antagonists |
Non-Patent Citations (8)
| Title |
|---|
| Chemical Abstracts Service (C A S); 1 January 1900 (1900-01-01), XP002912835, Database accession no. 127-5325 * |
| Chemical Abstracts Service (C A S); 1 January 1900 (1900-01-01), XP002912836, Database accession no. 111-146934 * |
| CHEMICAL ABSTRACTS, 1 January 1900, Columbus, Ohio, US; abstract no. 108-68302, XP002912830 * |
| CHEMICAL ABSTRACTS, 1 January 1900, Columbus, Ohio, US; abstract no. 115-182801, XP002912833 * |
| CHEMICAL ABSTRACTS, 1 January 1900, Columbus, Ohio, US; abstract no. 117-191696, XP002912831 * |
| CHEMICAL ABSTRACTS, 1 January 1900, Columbus, Ohio, US; abstract no. 122-205210, XP002912832 * |
| HIRSCHMANN R: "PEPTIDE RELATED RESEARCH AS A VEHICLE TOWARDS CHEMICAL AND BIOLOGICAL UNDERSTANDING", BOOKS OF ABSTRACTS. DIVISION OF MEDICINAL CHEMISTRY, XX, XX, 13 April 1997 (1997-04-13), XX, pages COMPLETE, XP002912834 * |
| See also references of EP1003514A4 * |
Cited By (169)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6743594B1 (en) | 1995-06-06 | 2004-06-01 | Human Genome Sciences, Inc. | Methods of screening using human G-protein chemokine receptor HDGNR10 (CCR5) |
| US6511826B2 (en) | 1995-06-06 | 2003-01-28 | Human Genome Sciences, Inc. | Polynucleotides encoding human G-protein chemokine receptor (CCR5) HDGNR10 |
| US6759519B2 (en) | 1995-06-06 | 2004-07-06 | Human Genome Sciences, Inc. | Antibodies to human G-protein chemokine receptor HDGNR10 (CCR5receptor) |
| US6800729B2 (en) | 1995-06-06 | 2004-10-05 | Human Genome Sciences, Inc. | Human G-Protein chemokine receptor HDGNR10 (CCR5 receptor) |
| ES2160525A1 (en) * | 1998-11-17 | 2001-11-01 | Hoffmann La Roche | 4-aroyl-piperidin-ccr-3 receptor antagonists iii |
| WO2000029377A1 (en) * | 1998-11-17 | 2000-05-25 | F. Hoffmann-La Roche Ag | 4-aroyl-piperidin-ccr-3 receptor antagonists iii |
| US6759411B2 (en) | 1998-12-18 | 2004-07-06 | Bristol-Myers Squibb Pharma Company | Heterocyclic piperidines as modulators of chemokine receptor activity |
| US6331545B1 (en) | 1998-12-18 | 2001-12-18 | Soo S. Ko | Heterocycyclic piperidines as modulators of chemokine receptor activity |
| US7312222B2 (en) | 1998-12-18 | 2007-12-25 | Bristol-Myers Squibb Pharma Company | Heterocyclic piperidines as modulators of chemokine receptor activity |
| US6486180B1 (en) | 1998-12-18 | 2002-11-26 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US6875776B2 (en) | 1998-12-18 | 2005-04-05 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US6492400B1 (en) | 1998-12-18 | 2002-12-10 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US6780857B2 (en) | 1998-12-18 | 2004-08-24 | Bristol-Myers Squibb Pharma Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| WO2000039125A1 (en) * | 1998-12-23 | 2000-07-06 | Pfizer Limited | Piperidines as ccr5 modulators |
| US7041667B1 (en) | 1998-12-23 | 2006-05-09 | Pfizer, Inc. | CCR5 modulators |
| US7217714B1 (en) | 1998-12-23 | 2007-05-15 | Agouron Pharmaceuticals, Inc. | CCR5 modulators |
| WO2000038680A1 (en) * | 1998-12-23 | 2000-07-06 | Pfizer Limited | Azabicycloalkanes as ccr5 modulators |
| HRP20010468B1 (en) * | 1998-12-23 | 2012-06-30 | Pfizer Inc. | Azabicycloalkanes as ccr5 modulators |
| US6586430B1 (en) | 1998-12-23 | 2003-07-01 | Pfizer Inc. | CCR5 modulators |
| AP1697A (en) * | 1998-12-23 | 2006-12-22 | Pfizer | Azabicycloalkanes as CCR5 modulators. |
| EP1013276A1 (en) * | 1998-12-23 | 2000-06-28 | Pfizer Inc. | Aminoazacycloalkanes as CCR5 modulators |
| WO2000056729A1 (en) * | 1999-03-24 | 2000-09-28 | Anormed Inc. | Chemokine recpetor binding heterocyclic compounds |
| US6750348B1 (en) | 1999-03-24 | 2004-06-15 | Anormed, Inc. | Chemokine receptor binding heterocyclic compounds |
| US7629337B2 (en) | 1999-03-24 | 2009-12-08 | Genzyme Corporation | Chemokine receptor binding heterocyclic compounds |
| US7183273B2 (en) | 1999-03-24 | 2007-02-27 | Anormed, Inc. | Chemokine receptor binding heterocyclic compounds |
| EP1180513A1 (en) * | 1999-04-28 | 2002-02-20 | Takeda Chemical Industries, Ltd. | Cyclic amide compounds, process for the preparation of the same and uses thereof |
| EP1180513A4 (en) * | 1999-04-28 | 2002-07-10 | Takeda Chemical Industries Ltd | Cyclic amide compounds, process for the preparation of the same and uses thereof |
| US6689765B2 (en) | 1999-05-04 | 2004-02-10 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| US8114879B2 (en) | 1999-05-04 | 2012-02-14 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| WO2000066558A1 (en) * | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperazine derivatives useful as ccr5 antagonists |
| JP2011219493A (en) * | 1999-05-04 | 2011-11-04 | Schering Corp | Piperazine derivative useful as ccr5 antagonist |
| WO2000066559A1 (en) * | 1999-05-04 | 2000-11-09 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| EP1659111A3 (en) * | 1999-05-04 | 2007-05-09 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| EP1659111A2 (en) * | 1999-05-04 | 2006-05-24 | Schering Corporation | Piperidine derivatives useful as ccr5 antagonists |
| US6387930B1 (en) | 1999-05-04 | 2002-05-14 | Schering Corporation | Piperidine derivatives useful as CCR5 antagonists |
| CZ301161B6 (en) * | 1999-05-04 | 2009-11-18 | Schering Corporation | Piperidinopiperidyl derivative usable as CCR5 antagonist and pharmaceutical composition in which the derivative is comprised |
| EP1632479A3 (en) * | 1999-05-04 | 2007-05-09 | Schering Corporation | Piperazine derivatives useful as ccr5 antagonists |
| US7384944B2 (en) | 1999-05-04 | 2008-06-10 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| US6391865B1 (en) | 1999-05-04 | 2002-05-21 | Schering Corporation | Piperazine derivatives useful as CCR5 antagonists |
| US6602885B2 (en) | 1999-05-04 | 2003-08-05 | Schering Corporation | Piperidine derivatives useful as CCR5 antagonists |
| EP1050307A1 (en) * | 1999-05-06 | 2000-11-08 | Applied Research Systems ARS Holding N.V. | CCR4 antagonists in sepsis |
| WO2000067791A1 (en) * | 1999-05-06 | 2000-11-16 | Glaxo Group Limited | Ccr4 antagonists in sepsis |
| US6358979B1 (en) | 1999-06-11 | 2002-03-19 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| US6432981B1 (en) | 1999-06-11 | 2002-08-13 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| WO2000076972A1 (en) * | 1999-06-11 | 2000-12-21 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| JP4688381B2 (en) * | 1999-06-11 | 2011-05-25 | メルク・シャープ・エンド・ドーム・コーポレイション | N-cyclopentyl modulator of chemokine receptor activity |
| US6472410B1 (en) | 1999-06-11 | 2002-10-29 | Merck & Co., Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| US6593346B2 (en) | 1999-06-11 | 2003-07-15 | Merck & Co. Inc. | N-cyclopentyl modulators of chemokine receptor activity |
| AU772321B2 (en) * | 1999-06-11 | 2004-04-22 | Merck Sharp & Dohme Corp. | N-cyclopentyl modulators of chemokine receptor activity |
| US6500844B1 (en) | 1999-06-11 | 2002-12-31 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| US6538002B1 (en) | 1999-06-11 | 2003-03-25 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| US6506777B1 (en) | 1999-06-11 | 2003-01-14 | Merck & Co., Inc. | Cyclopentyl modulators of chemokine receptor activity |
| JP2003502315A (en) * | 1999-06-11 | 2003-01-21 | メルク エンド カムパニー インコーポレーテッド | N-cyclopentyl modulator of chemokine receptor activity |
| US6903085B1 (en) | 1999-08-24 | 2005-06-07 | Astrazeneca, Ab | Substituted piperidine compounds useful as modulators of chemokine receptor activity |
| WO2001014333A1 (en) * | 1999-08-24 | 2001-03-01 | Astrazeneca Uk Limited | Substituted piperidine compounds useful as modulators of chemokine receptor activity |
| US6562978B1 (en) | 1999-10-01 | 2003-05-13 | Takeda Chemical Industries, Ltd. | Cyclic amine compounds as CCR5 antagonists |
| US8143261B2 (en) | 1999-10-01 | 2012-03-27 | Astrazeneca Ab | Thiazolo (4,5-D) pyrimidine compounds |
| US7348324B2 (en) | 1999-10-01 | 2008-03-25 | Takeda Pharmaceutical Company Limited | Cyclic amine compounds as CCR5 antagonists |
| US6787650B1 (en) | 1999-10-05 | 2004-09-07 | Takeda Chemical Industries, Ltd. | Urea compounds, process for producing the same and use thereof |
| US6958344B2 (en) | 2000-02-11 | 2005-10-25 | Astrazeneca Ab | Pyrimidine compounds and their use as modulators of chemokine receptor activity |
| US6958343B2 (en) | 2000-02-11 | 2005-10-25 | Astrazeneca, Ab | Thiazolopyrimidines and their use as modulators of chemokine receptor activity |
| US7579342B2 (en) | 2000-02-23 | 2009-08-25 | Astrazeneca | Pteridine compounds for the treatment of psoriasis |
| US6989393B2 (en) | 2000-03-10 | 2006-01-24 | Astrazeneca Ab | Ccr5 modulators benzimidazoles or benzotriazoles |
| WO2001066525A1 (en) * | 2000-03-10 | 2001-09-13 | Astrazeneca Ab | New ccr5 modulators: benzimidazoles or benzotriazoles |
| US6525070B2 (en) | 2000-04-08 | 2003-02-25 | Astrazeneca Ab | Bipiperidine derivatives as modulators of CCR3 activity and as H1 antagonists |
| US7179922B2 (en) | 2000-04-08 | 2007-02-20 | Astrazeneca Ab | Chemical compounds |
| US6903115B2 (en) | 2000-04-08 | 2005-06-07 | Astrazeneca Ab | Bipiperidine compounds |
| US7238811B2 (en) | 2000-04-08 | 2007-07-03 | Astrazeneca Ab | Chemical compounds |
| JP2003533510A (en) * | 2000-05-17 | 2003-11-11 | アストラゼネカ・アクチエボラーグ | Pharmaceutically active piperidine derivatives, especially as modulators of chemokine receptor activity |
| WO2001087839A1 (en) * | 2000-05-17 | 2001-11-22 | Astrazeneca Ab | Pharmaceutically active piperidine derivatives, in particular as modulators of chemokine receptor activity |
| EA005382B1 (en) * | 2000-05-26 | 2005-02-24 | Пфайзер Инк. | Tropane derivatives useful in therapy |
| WO2001090106A3 (en) * | 2000-05-26 | 2002-03-28 | Pfizer Ltd | Tryasolyl tropane derivatives as ccr5 modulators |
| CN100355753C (en) * | 2000-05-26 | 2007-12-19 | 辉瑞大药厂 | Tropan derivatives useful in therapy, method for obtaining thereof and intermediate compounds |
| HRP20020938B1 (en) * | 2000-05-26 | 2011-05-31 | Pfizer Inc. | Tropane derivatives useful in therapy |
| WO2001090106A2 (en) * | 2000-05-26 | 2001-11-29 | Pfizer Limited | Tryasolyl tropane derivatives as ccr5 modulators |
| EP1526134A2 (en) * | 2000-05-26 | 2005-04-27 | Pfizer Limited | Triazolyl tropane derivatives as ccr5 modulators |
| US7368460B2 (en) | 2000-05-26 | 2008-05-06 | Pfizer, Inc. | Tropane derivatives useful in therapy |
| US7576097B2 (en) | 2000-05-26 | 2009-08-18 | Pfizer, Inc. | Tropane derivatives useful in therapy |
| US6667314B2 (en) | 2000-05-26 | 2003-12-23 | Pfizer, Inc. | Tropane derivatives useful in therapy |
| EP1990341A1 (en) * | 2000-05-26 | 2008-11-12 | Pfizer Inc. | Triazolyl tropane derivatives as CCR5 modulators |
| EP1526134A3 (en) * | 2000-05-26 | 2006-11-29 | Pfizer Limited | Triazolyl tropane derivatives as ccr5 modulators |
| US7348341B2 (en) | 2000-05-31 | 2008-03-25 | Astrazeneca Ab | Chemical compounds |
| WO2001092227A1 (en) * | 2000-05-31 | 2001-12-06 | Astrazeneca Ab | Chemical compounds |
| US6627629B2 (en) | 2000-06-30 | 2003-09-30 | Bristol-Myers Squibb Pharma | N-ureidoheterocycloalkyl-piperidines as modulators of chemokine receptor activity |
| US6949546B2 (en) | 2000-06-30 | 2005-09-27 | Bristol-Myers Squibb Pharma Company | N-ureidoheterocycloalkyl-piperidines as modulators of chemokine receptor activity |
| WO2002004420A1 (en) * | 2000-07-12 | 2002-01-17 | Novartis Ag | Piperidine coumpounds for use as ccr-3 inhibitors |
| US6670379B2 (en) | 2000-07-12 | 2003-12-30 | Novartis Ag | Piperidine compounds for use as ccr-3 inhibitors |
| US7304077B2 (en) | 2000-09-04 | 2007-12-04 | Astrazeneca Ab | Chemical compounds |
| US7262204B2 (en) | 2000-10-11 | 2007-08-28 | Amgen Inc. | Modulation of CCR4 function |
| US7071193B2 (en) | 2000-10-20 | 2006-07-04 | Astrazeneca Ab | 7-amino-2-alkylthiopteridin-4-yl-amines for the treatment of chemokine-related diseases |
| US7217823B2 (en) | 2000-12-06 | 2007-05-15 | Sepracor Inc. | 4,4-Disubstituted piperidines, and methods of use thereof |
| US6656953B2 (en) | 2000-12-06 | 2003-12-02 | Sepracor Inc. | 4,4-Disubstituted piperidines, and methods of use thereof |
| US6958350B2 (en) | 2001-02-19 | 2005-10-25 | Astrazeneca Ab | Chemical compounds |
| US6960602B2 (en) | 2001-03-22 | 2005-11-01 | Astrazeneca Ab | Piperidine derivatives as modulators of chemokine receptors |
| US6900211B2 (en) | 2001-03-29 | 2005-05-31 | Schering Corporation | CCR5 antagonists useful for treating aids |
| US7008946B2 (en) | 2001-03-29 | 2006-03-07 | Schering Corporation | CCR5 antagonists useful for treating AIDS |
| US6720325B2 (en) | 2001-03-29 | 2004-04-13 | Schering Corporation | CCR5 antagonists useful for treating aids |
| US7098213B2 (en) | 2001-03-29 | 2006-08-29 | Schering Corporation | CCR5 antagonists useful for treating AIDS |
| US7060701B2 (en) | 2001-03-29 | 2006-06-13 | Schering Corporation | CCR5 antagonists useful for treating AIDS |
| WO2002079186A2 (en) * | 2001-03-30 | 2002-10-10 | F. Hoffmann-La Roche Ag | Aminopiperidine derivatives as modulators of chemokine receptor activity |
| WO2002079186A3 (en) * | 2001-03-30 | 2003-05-01 | Hoffmann La Roche | Aminopiperidine derivatives as modulators of chemokine receptor activity |
| US6949643B2 (en) | 2001-04-12 | 2005-09-27 | Astrazeneca Ab | Thiazolopytimidines and their use as modulators of chemokine receptor activity |
| US7144903B2 (en) | 2001-05-23 | 2006-12-05 | Amgen Inc. | CCR4 antagonists |
| US7307090B2 (en) | 2001-07-02 | 2007-12-11 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| US7265227B2 (en) | 2001-07-23 | 2007-09-04 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| US7186718B2 (en) | 2001-08-22 | 2007-03-06 | Astrazeneca Ab | Piperidinyl-morpholinyl derivatives as modulators of chemokine receptor activity |
| US7514430B2 (en) | 2001-09-18 | 2009-04-07 | Bristol-Myers Squibb Pharma Company | Piperizinones as modulators of chemokine receptor activity |
| US7238691B2 (en) | 2001-09-18 | 2007-07-03 | Astrazeneca Ab | Piperidine derivatives and their use as modulators of chemokine (especially CCR3) activity |
| US6974869B2 (en) | 2001-09-18 | 2005-12-13 | Bristol-Myers Squibb Pharma Company | Piperizinones as modulators of chemokine receptor activity |
| US7192973B2 (en) | 2001-11-15 | 2007-03-20 | Astrazeneca Ab | Piperidine derivatives and their use as modulators of chemokine receptor activity (especially CCR5) |
| US8778967B2 (en) | 2001-12-21 | 2014-07-15 | Genzyme Corporation | Chemokine receptor binding heterocyclic compounds with enhanced efficacy |
| US10322111B2 (en) | 2001-12-21 | 2019-06-18 | Genzyme Corporation | Chemokine receptor binding heterocyclic compounds with enhanced efficacy |
| US7709500B2 (en) | 2002-02-18 | 2010-05-04 | Astrazeneca Ab | Chemical compounds |
| US7288537B2 (en) | 2002-03-15 | 2007-10-30 | Novartis Ag | Azetidine derivatives as CCR-3 receptor antagonists |
| KR100706270B1 (en) * | 2002-03-15 | 2007-04-11 | 노파르티스 아게 | Azetidine derivatives as ???-3 receptor antagonists |
| JP2009102325A (en) * | 2002-03-15 | 2009-05-14 | Novartis Ag | Azetidine derivative as ccr-3 receptor antagonist |
| AU2003227072B2 (en) * | 2002-03-15 | 2006-08-10 | Novartis Ag | Azetidine derivatives as CCR-3 receptor antagonists |
| WO2003077907A1 (en) * | 2002-03-15 | 2003-09-25 | Novartis Ag | Azetidine derivatives as ccr-3 receptor antagonists |
| US7482363B2 (en) | 2002-03-19 | 2009-01-27 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| US7517989B2 (en) | 2002-03-19 | 2009-04-14 | Astrazeneca Ab | Piperidine derivatives useful as modulators of chemokine receptor activity |
| CN100343240C (en) * | 2002-03-29 | 2007-10-17 | 先灵公司 | Stereoselective alkylation of chiral 2-methyl-4-protected piperazines |
| US6992091B2 (en) | 2002-09-12 | 2006-01-31 | Bristol-Myers Squibb Company | N-ureidoalkyl-piperidines as modulators of chemokine receptor activity |
| US7585867B2 (en) | 2002-09-20 | 2009-09-08 | Astrazeneca Ab | Substituted thiazolo[4,5-d]pyrimidin-2(3H)-one |
| US6919356B2 (en) | 2002-09-26 | 2005-07-19 | Bristol Myers Squibb Company | N-substituted heterocyclic amines as modulators of chemokine receptor activity |
| WO2004054974A3 (en) * | 2002-12-13 | 2004-09-02 | Smithkline Beecham Corp | Piperidine derivatives as ccr5 antagonists |
| WO2004054974A2 (en) * | 2002-12-13 | 2004-07-01 | Smithkline Beecham Corporation | Piperidine derivatives as ccr5 antagonists |
| US7645771B2 (en) | 2002-12-13 | 2010-01-12 | Smithkline Beecham Corp. | CCR5 antagonists as therapeutic agents |
| EP2385040A1 (en) | 2003-03-14 | 2011-11-09 | ONO Pharmaceutical Co., Ltd. | Nitrogen-containing heterocyclic derivatives and drugs containing the same as the active ingredient |
| US9206186B2 (en) | 2003-03-24 | 2015-12-08 | Axikin Pharmaceuticals, Inc. | 2-phenoxy- and 2-phenylsulfonamide derivatives with CCR3 antagonistic activity for the treatment of inflammatory or immunological disorders |
| US7495013B2 (en) | 2003-04-01 | 2009-02-24 | Astrazeneca Ab | Chemical compounds |
| EP2364982A1 (en) | 2003-04-18 | 2011-09-14 | ONO Pharmaceutical Co., Ltd. | Spiro-piperidine compounds as chemokine receptor antagonists and medicinal use thereof |
| US7294636B2 (en) | 2003-05-09 | 2007-11-13 | Astrazeneca Ab | Chemical compounds |
| US7485732B2 (en) | 2003-06-11 | 2009-02-03 | Merck & Co., Inc. | Substituted 3-alkyl and 3-alkenyl azetidine derivatives |
| US7906503B2 (en) | 2003-06-11 | 2011-03-15 | Merck Sharp & Dohme Corp. | Substituted 3-alkyl and 3-alkenyl azetidine derivatives |
| US7790883B2 (en) | 2003-12-05 | 2010-09-07 | Astrazeneca Ab | Process for the preparation of thiazolopyrimidines |
| WO2005073192A1 (en) * | 2004-02-02 | 2005-08-11 | Astrazeneca Ab | Novel piperidines as chemokine modulators (ccr) |
| US7956070B2 (en) | 2004-02-02 | 2011-06-07 | Astrazeneca Ab | Piperidines as chemokine modulators (CCR) |
| US7615555B2 (en) | 2004-04-23 | 2009-11-10 | Astrazeneca Ab | Piperidine derivatives as modulators of chemokine receptor CCR5 |
| EP2546234A1 (en) | 2004-09-13 | 2013-01-16 | Ono Pharmaceutical Co., Ltd. | Nitrogeneous heterocyclic derivative and medicine containing the same as an active ingredient |
| WO2006129679A1 (en) | 2005-05-31 | 2006-12-07 | Ono Pharmaceutical Co., Ltd. | Spiropiperidine compound and medicinal use thereof |
| US8314127B2 (en) | 2005-07-21 | 2012-11-20 | Astrazeneca Ab | Piperidine derivatives |
| WO2007049771A1 (en) | 2005-10-28 | 2007-05-03 | Ono Pharmaceutical Co., Ltd. | Compound containing basic group and use thereof |
| EP2657235A1 (en) | 2005-10-28 | 2013-10-30 | Ono Pharmaceutical Co., Ltd. | Compound containing basic group and use thereof |
| WO2007058322A1 (en) | 2005-11-18 | 2007-05-24 | Ono Pharmaceutical Co., Ltd. | Basic group-containing compound and use thereof |
| US7906652B2 (en) | 2005-11-28 | 2011-03-15 | Merck Sharp & Dohme Corp. | Heterocycle-substituted 3-alkyl azetidine derivatives |
| WO2007105637A1 (en) | 2006-03-10 | 2007-09-20 | Ono Pharmaceutical Co., Ltd. | Nitrogenated heterocyclic derivative, and pharmaceutical agent comprising the derivative as active ingredient |
| WO2007132846A1 (en) | 2006-05-16 | 2007-11-22 | Ono Pharmaceutical Co., Ltd. | Compound having acidic group which may be protected, and use thereof |
| WO2008016006A1 (en) | 2006-07-31 | 2008-02-07 | Ono Pharmaceutical Co., Ltd. | Compound having cyclic group bound thereto through spiro binding and use thereof |
| US10117931B2 (en) | 2009-04-28 | 2018-11-06 | Kameran Lashkari | Methods for treatment of age-related macular degeneration |
| WO2011011652A1 (en) | 2009-07-24 | 2011-01-27 | Glaxosmithkline Llc | Therapeutic compounds |
| US8592395B2 (en) | 2009-07-24 | 2013-11-26 | Glaxosmithkline Llc | Therapeutic compounds |
| WO2013024022A1 (en) | 2011-08-12 | 2013-02-21 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical compositions for treatment of pulmonary hypertension |
| US11498896B2 (en) | 2014-12-19 | 2022-11-15 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| US10633336B2 (en) | 2014-12-19 | 2020-04-28 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| US10752588B2 (en) | 2014-12-19 | 2020-08-25 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| US11357742B2 (en) | 2015-12-14 | 2022-06-14 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US10953003B2 (en) | 2015-12-14 | 2021-03-23 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US11219621B2 (en) | 2015-12-22 | 2022-01-11 | X4 Pharmaceuticals, Inc. | Methods for treating immunodeficiency disease |
| US10610527B2 (en) | 2015-12-22 | 2020-04-07 | X4 Pharmaceuticals, Inc. | Methods for treating immunodeficiency disease |
| US11337969B2 (en) | 2016-04-08 | 2022-05-24 | X4 Pharmaceuticals, Inc. | Methods for treating cancer |
| US10988465B2 (en) | 2016-06-21 | 2021-04-27 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11306088B2 (en) | 2016-06-21 | 2022-04-19 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11332470B2 (en) | 2016-06-21 | 2022-05-17 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US10759796B2 (en) | 2016-06-21 | 2020-09-01 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11780837B2 (en) | 2016-06-21 | 2023-10-10 | X4 Pharmaceuticals, Inc. | CXCR4 inhibitors and uses thereof |
| US11045461B2 (en) | 2018-08-31 | 2021-06-29 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| US10548889B1 (en) | 2018-08-31 | 2020-02-04 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| US11672793B2 (en) | 2018-08-31 | 2023-06-13 | X4 Pharmaceuticals, Inc. | Compositions of CXCR4 inhibitors and methods of preparation and use |
| CN110483490A (en) * | 2019-08-29 | 2019-11-22 | 苏州汉德创宏生化科技有限公司 | A kind of synthetic method of 3- (piperidin-4-yl) oxazolidine -2- ketone and its salt |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2296314A1 (en) | 1999-02-04 |
| AU8576098A (en) | 1999-02-16 |
| EP1003514A1 (en) | 2000-05-31 |
| JP2002510327A (en) | 2002-04-02 |
| EP1003514A4 (en) | 2000-10-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6136827A (en) | Cyclic amine modulations of chemokine receptor activity | |
| EP1003514A1 (en) | Cyclic amine modulators of chemokine receptor activity | |
| US6166037A (en) | Pyrrolidine and piperidine modulators of chemokine receptor activity | |
| US6140349A (en) | Cyclic amine modulators of chemokine receptor activity | |
| EP1052992A1 (en) | Cyclic amine modulators of chemokine receptor activity | |
| US6013644A (en) | Spiro-substituted azacycles as modulators of chemokine receptor activity | |
| US6303593B1 (en) | 3-thienyl and 3-furanyl pyrrolidine modulators of chemokine receptor activity | |
| EP1009405A1 (en) | Pyrrolidine and piperidine modulators of chemokine receptor activity | |
| US5962462A (en) | Spiro-substituted azacycles as modulators of chemokine receptor activity | |
| US6362201B1 (en) | 3-cyclopropyl and 3-cyclobutyl pyrrolidine modulators of chemokine receptor activity | |
| US6124319A (en) | 3,3-disubstituted piperidines as modulators of chemokine receptor activity | |
| US6489354B1 (en) | 3-alkyl substituted pyrrolidine modulators of chemokine receptor activity | |
| WO1998025604A1 (en) | Spiro-substituted azacycles as modulators of chemokine receptor activity | |
| US6432981B1 (en) | Cyclopentyl modulators of chemokine receptor activity | |
| WO1998025617A1 (en) | Substituted aryl piperazines as modulators of chemokine receptor activity | |
| WO1998025605A1 (en) | Spiro-substituted azacycles as modulators of chemokine receptor activity | |
| CA2278309A1 (en) | 3,3-disubstituted piperidines as modulators of chemokine receptor activity | |
| US6372764B1 (en) | Pyrrolidine modulators of chemokine receptor activity | |
| US6511994B2 (en) | Modulators of CCR5 chemokine receptor activity | |
| US6506777B1 (en) | Cyclopentyl modulators of chemokine receptor activity | |
| US6500844B1 (en) | Cyclopentyl modulators of chemokine receptor activity | |
| US6472410B1 (en) | N-cyclopentyl modulators of chemokine receptor activity | |
| US6538002B1 (en) | Cyclopentyl modulators of chemokine receptor activity | |
| EP1192133B1 (en) | N-cyclopentyl modulators of chemokine receptor activity | |
| US6455548B2 (en) | 4-alkyl piperidinyl pyrrolidine modulators of chemokine receptor activity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AL AM AU AZ BA BB BG BR BY CA CN CU CZ EE GE HR HU ID IL IS JP KG KR KZ LC LK LR LT LV MD MG MK MN MX NO NZ PL RO RU SG SI SK SL TJ TM TR TT UA US UZ VN YU |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1998936920 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2296314 Country of ref document: CA Ref country code: CA Ref document number: 2296314 Kind code of ref document: A Format of ref document f/p: F |
|
| NENP | Non-entry into the national phase |
Ref country code: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 85760/98 Country of ref document: AU |
|
| WWP | Wipo information: published in national office |
Ref document number: 1998936920 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1998936920 Country of ref document: EP |