WO2004009058A1 - Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate - Google Patents
Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate Download PDFInfo
- Publication number
- WO2004009058A1 WO2004009058A1 PCT/EP2002/007961 EP0207961W WO2004009058A1 WO 2004009058 A1 WO2004009058 A1 WO 2004009058A1 EP 0207961 W EP0207961 W EP 0207961W WO 2004009058 A1 WO2004009058 A1 WO 2004009058A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- drug
- microcapsules
- coating
- process according
- acrylic polymer
- Prior art date
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J13/00—Colloid chemistry, e.g. the production of colloidal materials or their solutions, not otherwise provided for; Making microcapsules or microballoons
- B01J13/02—Making microcapsules or microballoons
- B01J13/06—Making microcapsules or microballoons by phase separation
- B01J13/08—Simple coacervation, i.e. addition of highly hydrophilic material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J13/00—Colloid chemistry, e.g. the production of colloidal materials or their solutions, not otherwise provided for; Making microcapsules or microballoons
- B01J13/02—Making microcapsules or microballoons
- B01J13/06—Making microcapsules or microballoons by phase separation
Definitions
- the present invention relates to the field of microencapsulation of active principles. A new process is described allowing to obtain pharmaceutical microcapsules with enhanced taste masking and an optimal dissolution profile. State of the art Achieving an effective encapsulation of active principles is important for the preparation of a variety of compositions; when microparticles of an active principle must be singly provided with an external coating, microencapsulation techniques are employed.
- microencapsulation process consists in coating small drug cores (microparticles) with a layer of polymer.
- the polymer layering may be achieved by different techniques; in particular the microencapsulation by phase separation (or coacervation), proved very reliable in obtaining coated microparticles (M.Calanchi, "Taste Masking of oral formulations", Pharmaceutical Manufacturing International, pp.139-141 , 1996; L. Dobetti, S. De Luigi, "Developments in Microencapsulation", Pharmaceutical Manufacturing and Packaging Sourcer, p. 39-40, Dec.1988).
- the production of microcapsules differs from normal drug coating techniques in that singly coated, discrete microparticles must be obtained, e.g.
- microencapsulation of active principles is applied in particular to prepare pharmaceutical multiparticulate compositions such as syrups, permanent or temporary suspensions, chewable or fast melting tablets, etc..
- the microencapsulation is used in particular to mask the taste of those drugs characterised by bitterness, throat-burning, saltiness and localised numbing of the tongue, etc.
- Microencapsulation is also used to modulate the drug release profile after administration. In principle, both taste masking and release-controlling properties are obtained by increasing the thickness of the microcapsule wall.
- taste-masked, slow-release microcapsules As a consequence, it is easy to prepare taste-masked, slow-release microcapsules, whereas it is more difficult to obtain taste-masked quick-release ones: the latter form is nevertheless very desired, in particular for those drugs with unpleasant taste which, for pharmacokinetic and pharmacodynamic reasons, must be delivered quickly in the stomach: one typical example is that of antibiotic drugs (for example Penicillins, Cephalosporins, Carbapenem, Penems, Penams, Aminoglycosides, Macrolides, Ketolides, Tetracyclines, Quinolones, etc.) which are often endowed with an unacceptable taste: they require a strong taste- masking, but at the same time they must be delivered and absorbed quickly in the stomach, so to ensure a quick onset of action and avoid disturbing the intestinal bacterial flora.
- antibiotic drugs for example Penicillins, Cephalosporins, Carbapenem, Penems, Penams, Aminoglycosides
- a second example is that of antinflammatory drugs or drugs for pain relief. Often this kind of drugs needs to be taste masked to avoid bitterness or throat burning, but at the same time a fast absorption is mandatory to assure a fast pain relief.
- Third example is that of drugs characterised by a narrow absorption window. These drugs require a fast release in the first part of the gastrointestinal tract to guarantee the proper bioavailability.
- the preferred and most widely used sealing polymer is ethylcellulose.
- This polymer is characterised by an efficient sealing capacity and is easily layered onto the drug microparticles; in addition it is an absolutely safe excipient, free from toxicity problems.
- ethylcellulose-coated microparticles are not capable to associate, to the good taste masking, an elevated dissolution rate in the stomach.
- attempts have been made to reduce the thickness of the microcapsule wall (i.e. using less encapsulating polymer); however this is not a good solution because the taste-masking is no longer ensured by the thinner coating.
- the present application discloses a microencapsulation process characterised by coating drug cores with a first layer of ethylcellulose and further coating the obtained microcapsules with a layer of an acrylic polymer.
- the obtained microcapsules show a high potency, an optimal taste masking, and ensure a quick release in the stomach.
- the invention allows thus to produce superior pharmaceutical formulations, especially useful in the case of drugs with unpleasant taste in particular drugs, which require an immediate delivery in the stomach, even if the administration in form of reconstitutable suspensions is required.
- Figure 1 Caffeine, microscope image of lot. B1 , described in the experimental part, showing an evident aggregation phenomena.
- Figure 2 Teophylline, particle size distribution of microcapsules of invention (lot.
- Figure 3 Fluoxetine, microscope image of lot.
- C3 X representing the microcapsules of the invention.
- Figure 4 Caffeine, microscope image of lot. C1 , representing the microcapsules of the invention.
- a first objective of the present invention is a process for the production of microcapsules containing a drug, characterised by the following steps: a. - coating drug microparticles with a layer of ethylcellulose b. - further coating the product of a. with a layer of an acrylic polymer
- the present process is particularly suitable for those drugs which have an unpleasant taste and require quick delivery into the stomach; however, any drug available in microparticular form can be subjected to the present process; for the purpose of the invention, the term "drug" includes also mixtures of two or more of them.
- the step a. obtains singly coated microcapsules.
- the coating step a. can be performed by microencapsulation techniques which, as such, are well-known in the art. Among them, microencapsulation by phase separation (also known as microencapsulation by coacervation ) is preferred.
- phase separation can be summarised in the following, non limitative, step sequence: (i) dispersion: the creation of a two phase system in which a liquid phase (e.g. ethylcellulose solution in cyclohexane) and a solid phase (drug particles) are present simultaneously; (ii) phase separation: thanks to the action of the coacervation-inducing agent (e.g. an ethylene polymer like epolene) a third phase is formed.
- This phase called coacervate is a highly concentrated polymer solution in solvent which spreads onto the surface of the suspended drug cores.
- the deposition of the polymeric membrane is promoted by a reduction of the total free interfacial energy brought about by the decrease of the coating material surface area during the coalescence of the liquid droplets;
- hardening the fluid polymeric film is hardened by cooling down the suspension to room temperature;
- separation microcapsules are separated from the liquid medium by settling. The supernatant is then removed and the microcapsules can be washed with fresh solvent to remove the residues of phase separation agent. Finally the microcapsules are filtered, dried and sifted.
- Another known technique applicable to perform step a is
- step b. is also performed by fluidized bed coating, the overall process is particularly advantageous in that it can be performed in the same reactor by simply changing the coating solution when passing from step a. to b.
- the product of step a. is an ethylcellulose microcapsule containing the drug.
- the obtained microcapsule has a drug / ethylcellulose weight ratio comprised between 1 :1 and 30:1 , more preferably between 3:1 and 15:1.
- the drug / ethylcellulose weight ratio is herein referred as PR (phase ratio).
- PR phase ratio
- the microcapsules obtained in step a. are suspended in a fluidised bed and sprayed with a solution or suspension of the acrylic polymer.
- the solvent used to form this solution or suspension is an acidic aqueous solvent, a hydroalcoholic solvent, an organic solvent, or mixtures thereof.
- a hydroalcoholic solution it preferably comprises the following weight percentages of components, calculated with respect to the total weight of the solution: acrylic polymer: 4-20%, preferably 7-20% alcohol (e.g. ethanol): 30-94%, preferably 40-75 water: 0-40%, preferably 10-35% micronised inorganic material (e.g. talc): 2-20%, preferably 5-9%.
- the acrylic polymer can be layered indifferently during one or more layering steps: in the latter case a multilayered acrylic coating is obtained.
- the product of step b. has an acrylic polymer content comprised between 5% and 40% by weight ; an optimal range of this polymer is 10-25%
- the acrylic polymer used in step b. is chosen among acrylic polymers for pharmaceutical use: they are well-known in pharmaceutical technology, and can be indifferently linear, branched and/or cross-linked polymers of acrylic and/or methacrylic acid.; the chosen polymer must be soluble at acidic pH, (e.g. 1 g dissolves in 1N HCI); Representative, but not limitative examples of these polymers are the products of the class comprising Eudragit E (cationic copolymer based on dimethylaminoethyl methacrylate and neutral methacrylic esters).
- a further object of the present invention are the microcapsules obtained by the process above described.
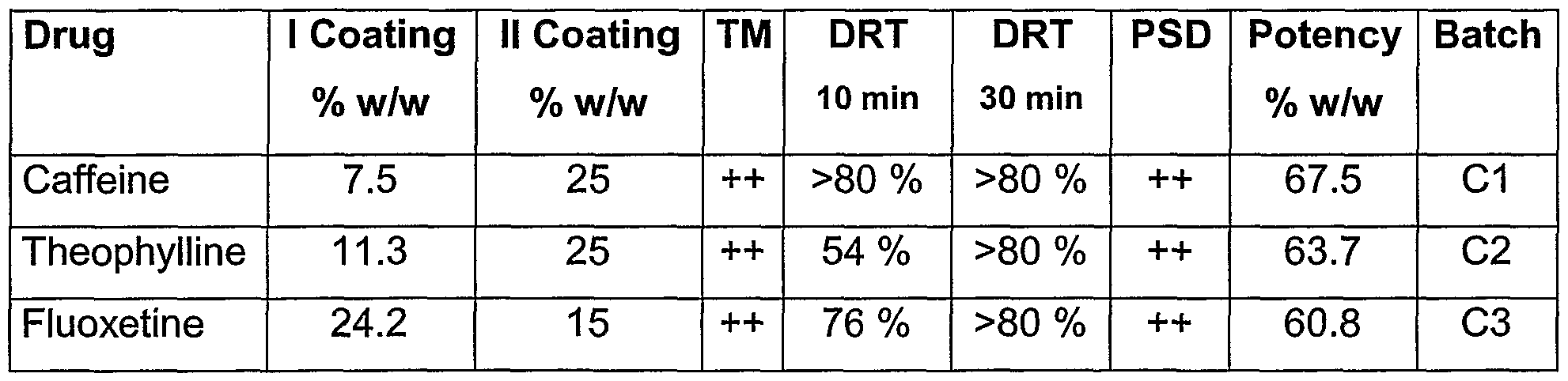
- the process according to the present invention allows to obtain small taste-masked microcapsules (i.e. having a weight median diameter comprised between 20 - 800 ⁇ m, preferably 100 - 400 ⁇ m, with potency (i.e. mg drug/g of the end product of step b.) comprised between 400 and 950 mg/g, and capable to release at least 80% of the drug contained therein within 30 minutes, preferably in 10 minutes in a simulated gastric fluid test or in acidic media.
- the high level of potency is a pharmaceutically advantageous feature which allows to obtain, at constancy of drug content, smaller tablets or capsules, (i.e.
- the reduction in the amounts of coating polymers involves the further advantage that the present compositions can dissolve in water without forming thickened viscous solutions around the drug cores: this further eases the drug diffusion and the establishing of a fast onset of action.
- the obtained microcapsules further show the advantage of an improved suspendability in water, i.e. they do not form aggregates, do not float on the surface of a suspending medium, nor they adhere to side walls of a glass: therefore they do not require a separated wetting treatment with surfactants, such as instead required in case of ethylcellulose microcapsules.
- microcapsules show the capability of maintaining the taste masking properties when suspended in neutral or basic aqueous media.
- the use of resuspended dosage form is often required for easiness and effectiveness of administration (e.g. dosage form as monodose sachet and dry powders for extemporaneous suspension).
- the above described microcapsules simultaneously ensuring elevated taste masking / elevated potency / elevated dissolution rate, are new and represent a further object of the present invention.
- These microcapsules can be further processed, optionally in presence of suitable pharmaceutical excipients, into suitable pharmaceutical formulations, e.g. dry powders for extemporaneous suspensions, tablets, minitablets, microcapsule-containing capsules, monodose sachets, fast disintegrating tablets, syrups, etc.
- Active ingredients useful with this invention include antibiotic and antibacterial agents such as ketolides; antiviral agents, analgesics, anesthetics, anorexics, antiarthritics, antiasthmatic agents, anticonvulsants, antidepressants, antidiabetic agents, antidiarrheals, antihistamines, anti- inflammatory agents, antiemetics, antineoplastics, antiparkinsonism drugs, antipruritics, antipsychotics, antipyretics, antispasmodics, H2 antagonists, cardiovascular drugs, antiarrhythmics, antihypertensives, ACE inhibitors, diuretics, vasodilators, hormones, hypnotics, immunosuppressives, muscle relaxants, parasympatholytics, parasympathomimetics, psychostimul
- Phase separation 3000 g of cyclohexane were poured into a 5L jacketed stainless steel reactor. Then, under a gentle stirring ensured by a helix, a fixed amount of drug, ethylcellulose and polyethylene were added.
- the stirring rate was then increased to 500 rpm.
- the system was then heated to
- microcapsules were dried in an oven overnight at 40°C and sifted by 500 ⁇ m screen.
- a fixed amount microcapsules obtained as described in the previous paragraph were loaded in a Glatt GPCG 1 fluid-bed equipped with 4" Wurster insert, plate type B, spraying nozzle 1.0 mm, and sprayed with a coating suspension having the following qualitative composition:
- the second layer of coating suspension were subsequently applied.
- the final product was sifted by 500 ⁇ m screen.
- the coating level obtained was calculated as microcapsules theoretical weight gain.
- Residual cyclohexane, residual ethanol and residual polyethylene were well within the acceptance limits for pharmaceuticals.
- Particle Size Distribution Particle Size Distribution
- the coating i.e. ethylcellulose
- the drug microparticles were first coated with a layer of ethylcellulose and further with a layer of an acrylic polymer, according to what described in the present invention.
- the overall coating amount is relatively low, so ensuring the possibility to obtain suitable potency.
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP02807606A EP1534251A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
| JP2004522144A JP4357422B2 (en) | 2002-07-17 | 2002-07-17 | Method for producing microcapsule preparation having enhanced taste masking ability and high dissolution rate |
| US10/521,598 US20050269722A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
| PCT/EP2002/007961 WO2004009058A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
| AU2002368090A AU2002368090A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced tastemasking and high dissolution rate |
| CA2492789A CA2492789C (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/EP2002/007961 WO2004009058A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2004009058A1 true WO2004009058A1 (en) | 2004-01-29 |
Family
ID=30470213
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2002/007961 WO2004009058A1 (en) | 2002-07-17 | 2002-07-17 | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20050269722A1 (en) |
| EP (1) | EP1534251A1 (en) |
| JP (1) | JP4357422B2 (en) |
| AU (1) | AU2002368090A1 (en) |
| CA (1) | CA2492789C (en) |
| WO (1) | WO2004009058A1 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007052121A3 (en) * | 2005-11-02 | 2007-10-18 | Warner Lambert Co | Multi-layered coating technology for taste masking |
| US8071128B2 (en) | 1996-06-14 | 2011-12-06 | Kyowa Hakko Kirin Co., Ltd. | Intrabuccally rapidly disintegrating tablet and a production method of the tablets |
| US8367111B2 (en) | 2002-12-31 | 2013-02-05 | Aptalis Pharmatech, Inc. | Extended release dosage forms of propranolol hydrochloride |
| US8580313B2 (en) | 2009-12-02 | 2013-11-12 | Aptalis Pharma Limited | Fexofenadine microcapsules and compositions containing them |
| CN103655483A (en) * | 2012-09-26 | 2014-03-26 | 扬州市星斗药业有限公司 | Grain with tebipenem and preparation method thereof |
| US8747895B2 (en) | 2004-09-13 | 2014-06-10 | Aptalis Pharmatech, Inc. | Orally disintegrating tablets of atomoxetine |
| CN103893150A (en) * | 2014-03-28 | 2014-07-02 | 北京联合大学 | Penicillin V potassium micro-capsule and preparation method thereof |
| EP2749273A1 (en) * | 2012-12-28 | 2014-07-02 | I.P.S. International Products & Services S.r.l. | Solid oral preparation with modified release |
| US9040086B2 (en) | 2001-10-04 | 2015-05-26 | Aptalis Pharmatech, Inc. | Timed, sustained release systems for propranolol |
| US9161919B2 (en) | 2005-05-02 | 2015-10-20 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US9884014B2 (en) | 2004-10-12 | 2018-02-06 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions |
| US10471017B2 (en) | 2004-10-21 | 2019-11-12 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions with gastrosoluble pore-formers |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6572244B2 (en) | 2014-02-25 | 2019-09-04 | オービス バイオサイエンシズ, インク.Orbis Biosciences, Inc. | Taste masking drug formulation |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0378137A2 (en) * | 1989-01-13 | 1990-07-18 | Kali-Chemie Pharma GmbH | Galenical form |
| WO2000030617A1 (en) * | 1998-11-25 | 2000-06-02 | Cima Labs Inc. | Taste masking rapid release coating system |
| US6136347A (en) * | 1992-01-15 | 2000-10-24 | Bayer Aktiengesellschaft | Flavor-masked pharmaceutical compositions |
| FR2795962A1 (en) * | 1999-07-08 | 2001-01-12 | Prographarm Laboratoires | PROCESS FOR MANUFACTURING MASK TASTE PELLETS AND IMMEDIATE RELEASE OF THE ACTIVE INGREDIENT |
| WO2001049270A2 (en) * | 1999-12-30 | 2001-07-12 | Ancile Pharmaceuticals, Inc. | Odor-masking coating for a pharmaceutical preparation |
| WO2001052848A2 (en) * | 2000-01-20 | 2001-07-26 | Eurand Pharmaceuticals Ltd. | Functional coating of linezolid microcapsules for oral administration |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6197347B1 (en) * | 1998-06-29 | 2001-03-06 | Andrx Pharmaceuticals, Inc. | Oral dosage for the controlled release of analgesic |
-
2002
- 2002-07-17 EP EP02807606A patent/EP1534251A1/en not_active Withdrawn
- 2002-07-17 US US10/521,598 patent/US20050269722A1/en not_active Abandoned
- 2002-07-17 WO PCT/EP2002/007961 patent/WO2004009058A1/en not_active Application Discontinuation
- 2002-07-17 JP JP2004522144A patent/JP4357422B2/en not_active Expired - Fee Related
- 2002-07-17 AU AU2002368090A patent/AU2002368090A1/en not_active Abandoned
- 2002-07-17 CA CA2492789A patent/CA2492789C/en not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0378137A2 (en) * | 1989-01-13 | 1990-07-18 | Kali-Chemie Pharma GmbH | Galenical form |
| US6136347A (en) * | 1992-01-15 | 2000-10-24 | Bayer Aktiengesellschaft | Flavor-masked pharmaceutical compositions |
| WO2000030617A1 (en) * | 1998-11-25 | 2000-06-02 | Cima Labs Inc. | Taste masking rapid release coating system |
| FR2795962A1 (en) * | 1999-07-08 | 2001-01-12 | Prographarm Laboratoires | PROCESS FOR MANUFACTURING MASK TASTE PELLETS AND IMMEDIATE RELEASE OF THE ACTIVE INGREDIENT |
| WO2001049270A2 (en) * | 1999-12-30 | 2001-07-12 | Ancile Pharmaceuticals, Inc. | Odor-masking coating for a pharmaceutical preparation |
| WO2001052848A2 (en) * | 2000-01-20 | 2001-07-26 | Eurand Pharmaceuticals Ltd. | Functional coating of linezolid microcapsules for oral administration |
Non-Patent Citations (1)
| Title |
|---|
| MARTIN F: "Oral 5-aminosalicylic acid preparations in treatment of inflammatory bowel disease. An update.", DIGESTIVE DISEASES AND SCIENCES. UNITED STATES DEC 1987, vol. 32, no. 12 Suppl, December 1987 (1987-12-01), pages 57S - 63S, XP009002753, ISSN: 0163-2116 * |
Cited By (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8945618B2 (en) | 1996-06-14 | 2015-02-03 | Kyowa Hakko Kirin Co., Ltd. | Intrabuccally rapidly disintegrating tablet and a production method of the tablets |
| US8071128B2 (en) | 1996-06-14 | 2011-12-06 | Kyowa Hakko Kirin Co., Ltd. | Intrabuccally rapidly disintegrating tablet and a production method of the tablets |
| US8357396B2 (en) | 1996-06-14 | 2013-01-22 | Kyowa Hakko Kirin Co., Ltd. | Intrabuccally rapidly disintegrating tablet and a production method of the tablets |
| US8956650B2 (en) | 1996-06-14 | 2015-02-17 | Kyowa Hakko Kirin Co., Ltd. | Intrabuccally rapidly disintegrating tablet and a production method of the tablets |
| US9358214B2 (en) | 2001-10-04 | 2016-06-07 | Adare Pharmaceuticals, Inc. | Timed, sustained release systems for propranolol |
| US9040086B2 (en) | 2001-10-04 | 2015-05-26 | Aptalis Pharmatech, Inc. | Timed, sustained release systems for propranolol |
| US8367111B2 (en) | 2002-12-31 | 2013-02-05 | Aptalis Pharmatech, Inc. | Extended release dosage forms of propranolol hydrochloride |
| US8747895B2 (en) | 2004-09-13 | 2014-06-10 | Aptalis Pharmatech, Inc. | Orally disintegrating tablets of atomoxetine |
| US10568832B2 (en) | 2004-10-12 | 2020-02-25 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions |
| US11452689B2 (en) | 2004-10-12 | 2022-09-27 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions |
| US10130580B2 (en) | 2004-10-12 | 2018-11-20 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions |
| US9884014B2 (en) | 2004-10-12 | 2018-02-06 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions |
| US10471017B2 (en) | 2004-10-21 | 2019-11-12 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions with gastrosoluble pore-formers |
| US10952971B2 (en) | 2004-10-21 | 2021-03-23 | Adare Pharmaceuticals, Inc. | Taste-masked pharmaceutical compositions with gastrosoluble pore-formers |
| US9161918B2 (en) | 2005-05-02 | 2015-10-20 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US10500161B2 (en) | 2005-05-02 | 2019-12-10 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US9566249B2 (en) | 2005-05-02 | 2017-02-14 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US9579293B2 (en) | 2005-05-02 | 2017-02-28 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US11147772B2 (en) | 2005-05-02 | 2021-10-19 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US10045946B2 (en) | 2005-05-02 | 2018-08-14 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| US9161919B2 (en) | 2005-05-02 | 2015-10-20 | Adare Pharmaceuticals, Inc. | Timed, pulsatile release systems |
| WO2007052121A3 (en) * | 2005-11-02 | 2007-10-18 | Warner Lambert Co | Multi-layered coating technology for taste masking |
| US10166220B2 (en) | 2009-12-02 | 2019-01-01 | Adare Pharmaceuticals S.R.L. | Fexofenadine microcapsules and compositions containing them |
| US9233105B2 (en) | 2009-12-02 | 2016-01-12 | Adare Pharmaceuticals S.R.L. | Fexofenadine microcapsules and compositions containing them |
| US10729682B2 (en) | 2009-12-02 | 2020-08-04 | Adare Pharmaceuticals S.R.L. | Fexofenadine microcapsules and compositions containing them |
| US8580313B2 (en) | 2009-12-02 | 2013-11-12 | Aptalis Pharma Limited | Fexofenadine microcapsules and compositions containing them |
| CN103655483A (en) * | 2012-09-26 | 2014-03-26 | 扬州市星斗药业有限公司 | Grain with tebipenem and preparation method thereof |
| EP2749273A1 (en) * | 2012-12-28 | 2014-07-02 | I.P.S. International Products & Services S.r.l. | Solid oral preparation with modified release |
| EP3865124A1 (en) * | 2012-12-28 | 2021-08-18 | I.P.S. International Products & Services S.r.l. | Polyvalent polymeric matrix for modified release solid oral preparations and method of preparation thereof |
| CN103893150A (en) * | 2014-03-28 | 2014-07-02 | 北京联合大学 | Penicillin V potassium micro-capsule and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2492789A1 (en) | 2004-01-29 |
| EP1534251A1 (en) | 2005-06-01 |
| JP2005537270A (en) | 2005-12-08 |
| CA2492789C (en) | 2012-07-03 |
| AU2002368090A1 (en) | 2004-02-09 |
| US20050269722A1 (en) | 2005-12-08 |
| JP4357422B2 (en) | 2009-11-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6445063B2 (en) | Microencapsulation processes and products | |
| KR101571198B1 (en) | Controlled release pharmaceutical composition with resistance against the influence of ethanol employing a coating comprising neutral vinyl polymers and excipients | |
| AU763643B2 (en) | Pharmaceutical composition of topiramate | |
| JP4615124B2 (en) | Coated pharmaceutical form with controlled agent release | |
| JP4555980B2 (en) | Hygroscopic microcapsules having a core coated with a hydrophobic polymer | |
| US20030180352A1 (en) | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions | |
| CA2492789C (en) | Process for the preparation of pharmaceutical microcapsules with enhanced taste-masking and high dissolution rate | |
| SK1032000A3 (en) | Oral pharmaceutical preparation comprising an antiulcer activity compound, and process for its production | |
| JPH07103015B2 (en) | Sustained-release pharmaceutical formulation with pH-controlled membrane coating | |
| HRP20020119A2 (en) | Taste masked pharmaceutical liquid formulations | |
| DE10353196A1 (en) | Multilayer dosage form with a matrix influencing the delivery of a modulatory substance | |
| EP1781275B2 (en) | Sustained release pharmaceutical composition of tolterodine | |
| JP4234427B2 (en) | Microgranules based on active ingredients and process for their production | |
| AU2005215108A1 (en) | Pharmaceutical composition for oral application and method for preparing thereof | |
| MXPA05008193A (en) | Taste-masking coated particles, process for the preparation thereof and orodispersible tablets containing said coated particles. | |
| DE60114887T2 (en) | PARTICULAR COMPOSITION WITH SIGMOIDEM RELEASE PATTERN CONTAINING ELETRIPTAN HYDROBROMIDE | |
| US20020076444A1 (en) | Novel method for obtaining microspheres and resulting products | |
| US20070154550A1 (en) | Pharmaceutical composition comprising anticonvulsant with taste mask coating | |
| US10966928B2 (en) | Oral dosage form | |
| Sarkar et al. | Study of ethyl cellulose based sustained release microspheres of naproxen sodium | |
| EP2046303A1 (en) | Pharmaceutical compositions comprising a combination of piperidinoalkanol and decongestant | |
| EP3892263A1 (en) | Oral dosage form | |
| Huanbutta et al. | Factors affecting preparations of chitosan microcapsules for colonic drug delivery | |
| JP3343144B2 (en) | Micro capsule | |
| WO2001035930A1 (en) | Taste masked oral compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ OM PH PL PT RO RU SD SE SG SI SK SL TJ TM TN TR TT TZ UA UG US UZ VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LU MC NL PT SE SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2492789 Country of ref document: CA Ref document number: 2004522144 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 10521598 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2002368090 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2002807606 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 538314 Country of ref document: NZ |
|
| WWP | Wipo information: published in national office |
Ref document number: 2002807606 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 2002807606 Country of ref document: EP |