WO2006098852A2 - Hydroxyalkyl substituted imidazoquinolines - Google Patents
Hydroxyalkyl substituted imidazoquinolines Download PDFInfo
- Publication number
- WO2006098852A2 WO2006098852A2 PCT/US2006/006223 US2006006223W WO2006098852A2 WO 2006098852 A2 WO2006098852 A2 WO 2006098852A2 US 2006006223 W US2006006223 W US 2006006223W WO 2006098852 A2 WO2006098852 A2 WO 2006098852A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- amino
- alkyl
- compound
- salt
- Prior art date
Links
- 0 CC(CCCCNC)*C* Chemical compound CC(CCCCNC)*C* 0.000 description 4
- SEMRGRKFXAHOAV-UHFFFAOYSA-N CC(C)(C[n]1c(c(cccc2)c2nc2N)c2nc1CO)N Chemical compound CC(C)(C[n]1c(c(cccc2)c2nc2N)c2nc1CO)N SEMRGRKFXAHOAV-UHFFFAOYSA-N 0.000 description 1
- CCXRKOPFTDEGTC-UHFFFAOYSA-N Cc(cc1)ccc1S(NCCC[n]1c2c(cccc3)c3nc(N)c2nc1CCO)(=O)=O Chemical compound Cc(cc1)ccc1S(NCCC[n]1c2c(cccc3)c3nc(N)c2nc1CCO)(=O)=O CCXRKOPFTDEGTC-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Definitions
- IRMs immune response modifiers
- the present invention provides a new class of compounds which preferentially induce the biosynthesis of interferon ( ⁇ ) (IFN- ⁇ ) in animals.
- IFN- ⁇ interferon
- Such compounds are of the following Formulas I, II, and III:
- R, R 1 , G 1 , G 2 , m, and n are as defined below.
- the amount of TNF- ⁇ induced by the 2-(hydroxyalkyl) substituted compounds of the invention is substantially less than the amount of TNF- ⁇ induced by closely related analogs having an alkyl or alkyl ether substituent at the 2-position and that the compounds of the invention still retain the ability to induce the biosynthesis of IFN- ⁇ . See, for example, Figures 1-4 below.
- the reduction in the amount of TNF- ⁇ induced is seen over a broad range of test concentrations.
- the amount of TNF- ⁇ induced by the compounds of the invention is at least two-fold less than the amount of TNF- ⁇ induced by analogs having an alkyl or alkyl ether substituent at the 2-position.
- the amount of TNF- ⁇ induced by the compounds of the invention is at least three-fold less than the amount of TNF- ⁇ induced by analogs having an alkyl or alkyl ether substituent at the 2-position. In still other embodiments the amount of TNF- ⁇ induced by the compounds of the invention is at least four-fold less than the amount of TNF- ⁇ induced by analogs having an alkyl or alkyl ether substituent at the 2-position.
- substantially less than the amount of TNF- ⁇ means that there is at least a two-fold reduction in the maximal TNF- ⁇ response as determined using the test methods described herein.
- the compounds or salts of Formulas I, II, and III are especially useful as immune response modifiers due to their ability to preferentially induce interferon- ⁇ , thus providing a benefit over compounds that also induce pro-inflammatory cytokines (e.g. TNF- ⁇ ) or that induce pro-inflammatory cytokines at higher levels.
- a compound is said to preferentially induce IFN- ⁇ if, when tested according to the test methods described herein, the effective minimum concentration for IFN- ⁇ induction is less than the effective minimum concentration for TNF- ⁇ induction. In some embodiments, the effective minimum concentration for IFN- ⁇ induction is at least 3 -fold less than the effective minimum concentration for TNF- ⁇ induction.
- the effective minimum concentration for IFN- ⁇ induction is at least 6-fold less than the effective minimum concentration for TNF- ⁇ induction. In other embodiments, the effective minimum concentration for IFN- ⁇ induction is at least 9-fold less than the effective minimum concentration for TNF- ⁇ induction. In some embodiments, when tested according to the test methods described herein, the amount TNF- ⁇ induced by compounds of the invention is at or below the background level of TNF- ⁇ in the test method.
- the invention further provides pharmaceutical compositions containing an effective amount of a compound or salt of Formulas I, II, and/or III and methods of preferentially inducing the biosynthesis of IFN- ⁇ in an animal, and treating a viral infection or disease and/or treating a neoplastic disease in an animal by administering an effective amount of a compound or salt of Formulas I, II, and/or III or a pharmaceutical composition containing an effective amount of a compound or salt of Formulas I, II, and/or III to the animal.
- methods of synthesizing compounds of Formulas I, II, and III and intermediates useful in the synthesis of these compounds are provided.
- Figure 1 shows the IFN- ⁇ dose response curves (corresponding to values shown in Table 5 below) for Example 6, Analog 2, Analog 3, and Analog 5.
- FIG 2 shows the TNF- ⁇ dose response curves (corresponding to values shown in Table 5 below) for Example 6, Analog 2, Analog 3, and Analog 5.
- Figure 3 shows the IFN- ⁇ dose response curves (corresponding to values shown in Table 5 below) for Example 7, Analog 1, Analog 2, and Analog 4.
- FIG. 4 shows the TNF- ⁇ dose response curves (corresponding to values shown in Table 5 below) for Example 7, Analog 1, Analog 2, and Analog 4.
- R, Rj, G 1 , G 2 , m, and n are as defined below; and pharmaceutically acceptable salts thereof.
- the present invention provides a compound of the following Formula I:
- R is selected from the group consisting of Cj.io alkyl, C 1-10 alkoxy, halogen, and Ci-io haloalkyl;
- R 1 is selected from the group consisting of:
- X is straight chain or branched chain alkylene optionally interrupted by one -O- group
- Y is selected from the group consisting of -S(O) 0-2 -and -N(R 8 )-Q-;
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, wherein the alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, mercapto, cyano, aryl, aryloxy, heteroaryl, heteroaryloxy, heterocyclyl, amino, alkylamino, dialkylamino, and in the case of alkyl and alkenyl, o
- Het is selected from the group consisting of tetrahydropyranyl and tetrahydrofuranyl ;
- R 7 is C 2-7 alkylene;
- R 8 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, hydroxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl;
- R 10 is C 3-8 alkylene;
- A is selected from the group consisting of -O-, -C(O)-, -CH 2 -, -S(O) 0-2 -, and -N(Q-R 4 )-;
- Q is selected from the group consisting of a bond, -C(R 6 )-, -S(O) 2 , -C(R 6 )-N(R 8 )-,
- the present invention provides a compound of the following Formula II, which is a prodrug:
- G 1 is selected from the group consisting of:
- -CC NV)-R 1 , -CH(OH)-C(O)-OY 1 , -CH(OC 1-4 alkyl) Y 0 , -CH 2 Yi 5 and -CH(CH 3 )Y 1 ;
- R' and R" are independently selected from the group consisting of Ci -I0 alkyl, C 3-7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, C 1-6 alkyl, C 1-4 alkoxy, aryl, heteroaryl, aryl- C 1-4 alkylenyl, heteroaryl- C 1-4 alkylenyl, halo- C 1-4 alkylenyl, halo- C 1-4 alkoxy, -O-C(O)-CH 3 , -C(O)-O-CH 3 , -C(O)-NH 2 , -O-CH 2 -C(O)-NH 2 , -NH 2 , and -S(O) 2 -NH 2 , with the proviso that R
- Y' is selected from the group consisting of hydrogen, C 1-6 alkyl, and benzyl
- Y 0 is selected from the group consisting of C 1-6 alkyl, carboxy- C 1-6 alkylenyl, amino- C 1-4 alkylenyl, mono- N- C 1-6 alkylaniino- C 1-4 alkylenyl, and di- N,N-C 1-6 alkylamino- C 1-4 alkylenyl;
- Y 1 is selected from the group consisting of mono-N- C 1-6 alkylamino, di-N, N-C 1-6 alky larnino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4- C 1-4 alkylpiperazin- 1 -yl; m is 0 or 1 ; n is 1 or 2;
- R is selected from the group consisting of C 1-10 alkyl, C 1-10 alkoxy, halogen, and C 1-10 haloalkyl;
- Ri is selected from the group consisting of:
- X is straight chain or branched chain alkylene optionally interrupted by one -O- group
- Y is selected from the group consisting of -S(O) 0-2 -and -N(R 8 )-Q-;
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, wherein the alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, mercapto, cyano, aryl, aryloxy, heteroaryl, heteroaryloxy, heterocyclyl, amino, alkylamino, dialkylamino, and in the case of alkyl and alkenyl, o
- R 7 is C 2-7 alkylene
- R 8 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, hydroxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl;
- R 10 is C 3-8 alkylene; A is selected from the group consisting of -O-, -C(O)-, -CH 2 -, -S(O) 0-2 -, and
- Q is selected from the group consisting of a bond, -C(R 6 )-, -S(O) 2 , -C(Rg)-N(R 8 )-, -S(O) 2 -N(R 8 )-, -C(R 6 )-O-, and -C(R 6 )-S-; and a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7; with the proviso that when Y is -S(O )0-2 - then X can not contain an -O- group; or a pharmaceutically acceptable salt thereof.
- the present invention provides a compound of the following Formula III, which is a prodrug:
- G 2 is selected from the group consisting of: -X 2 -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ -aminoacyl,
- X 2 is selected from the group consisting of a bond; -CH 2 -O-; -CH(CHs)-O-; -C(CH 3 ) 2 -O-; and, in the case of -X 2 -C(O)-O-R', -CH 2 -NH-; R' and R" are independently selected from the group consisting ofC 1-10 alkyl,
- R is selected from the group consisting of C 1-10 alkyl, C 1-10 aikoxy, halogen, and C 1-10 haloalkyl;
- Ri is selected from the group consisting of:
- X is straight chain or branched chain alkylene optionally interrupted by one -O- group
- Y is selected from the group consisting of -S(O) 0-2 -and -N(R 8 )-Q-;
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, wherein the alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, mercapto, cyano, aryl, aryloxy, heteroaryl, heteroaryloxy, heterocyclyl, amino, alkylamino, dialkylamino, and in the case of alkyl and alkenyl, o
- R 7 is C 2-7 alkylene
- R 8 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, hydroxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl;
- R 10 is C 3-8 alkylene; A is selected from the group consisting of -O-, -C(O)-, -CH 2 -, -S(O) 0-2 -, and
- Q is selected from the group consisting of a bond, -C(R 6 )-, -S(O) 2 , -C(R 6 )-N(R 8 )-, -S(O) 2 -N(R 8 )-, -C(R 6 )-O-, and -C(R 6 )-S-; and a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7; with the proviso that when Y is -S(O) 0-2 - then X can not contain an -O- group; or a pharmaceutically acceptable salt thereof.
- alkyl alkenyl
- alkynyl alkynyl
- alk- cyclic groups
- these groups contain from 1 to 20 carbon atoms, with alkenyl groups containing from 2 to 20 carbon atoms, and alkynyl groups containing from 2 to 20 carbon atoms. In some embodiments, these groups have a total of up to 10 carbon atoms, up to 8 carbon atoms, up to 6 carbon atoms, or up to 4 carbon atoms.
- Cyclic groups can be monocyclic or polycyclic and preferably have from 3 to 10 ring carbon atoms.
- Exemplary cyclic groups include cyclopropyl, cyclobutyl, cyclopropylmethyl, cyclopentyl, cyclopentylmethyl, cyclohexyl, cyclohexylmethyl, adamantyl, and substituted and unsubstituted bornyl, norbornyl, and norbornenyl.
- alkylene alkenylene
- alkynylene are the divalent forms of the "alkyl”, “alkenyl”, and “alkynyl” groups defined above.
- alkylenyl alkenylenyl
- alkynylenyl use when “alkylene”, “alkenylene”, and “alkynylene,” respectively, are substituted.
- an arylalkylenyl group comprises an alkylene moiety to which an aryl group is attached.
- haloalkyl is inclusive of groups that are substituted by one or more halogen atoms, including perfluorinated groups. This is also true of other groups that include the prefix “halo-.” Examples of suitable haloalkyl groups are chloromethyl, chlorobutyl, trifluoromethyl, 2,2,2-trifluoroethyl, and the like.
- aryl as used herein includes carbocyclic aromatic rings or ring systems.
- aryl groups include phenyl, naphthyl, biphenyl, fluorenyl and indenyl.
- heteroatom refers to the atoms O, S, or N.
- heteroaryl includes aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N).
- heteroaryl includes a ring or ring system that contains 2-12 carbon atoms, 1-3 rings, 1-4 heteroatoms, and O, S, and N as the heteroatoms.
- heteroaryl groups include furyl, thienyl, pyridyl, quinolinyl, isoquinolinyl, indolyl, isoindolyl, triazolyl, pyrrolyl, tetrazolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, benzofuranyl, benzothiophenyl, carbazolyl, benzoxazolyl, pyrimidinyl, benzimidazolyl, quinoxalinyl, benzothiazolyl, naphthyridinyl, isoxazolyl, isothiazolyl, purinyl, quinazolinyl, pyrazinyl, 1-oxidopyridyl, pyridazinyl, triazinyl, tetrazinyl, oxadiazolyl, thiadiazolyl, and so on.
- heterocyclyl includes non-aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N) and includes all of the fully saturated and partially unsaturated derivatives of the above mentioned heteroaryl groups.
- heterocyclyl includes a ring or ring system that contains 2-12 carbon atoms, 1-3 rings, 1-4 heteroatoms, and O, S, and N as the heteroatoms.
- heterocyclyl groups include pyrrolidinyl, tetrahydrofuranyl, morpholinyl, thiomorpholinyl, 1,1- dioxothiomorpholinyl, piperidinyl, piperazinyl, thiazolidinyl, imidazolidinyl, isothiazolidinyl, tetrahydropyranyl, quinuclidinyl, homopiperidinyl (azepanyl), 1,4- oxazepanyl, homopiperazinyl (diazepanyl), 1,3-dioxolanyl, aziridinyl, azetidinyl, dihydroisoquinolin-( 1 H)-y ⁇ , octahydroisoquinolin-( 1 H)-yl, dihydroquinolin-(2/i)-yl, octahydroquinolin-(2H )-yl, dihydro-1H
- heterocyclyl includes bicylic and tricyclic heterocyclic ring systems. Such ring systems include fused and/or bridged rings and spiro rings. Fused rings can include, in addition to a saturated or partially saturated ring, an aromatic ring, for example, a benzene ring. Spiro rings include two rings joined by one spiro atom and three rings joined by two spiro atoms.
- heterocyclyl contains a nitrogen atom
- the point of attachment of the heterocyclyl group may be the nitrogen atom
- arylene is the divalent forms of the "aryl”, “heteroaryl”, and “heterocyclyl” groups defined above.
- arylenyl is used when “arylene”, “heteroarylene”, and “heterocyclylene”, respectively, are substituted.
- an alkylarylenyl group comprises an arylene moiety to which an alkyl group is attached.
- each group is independently selected, whether explicitly stated or not.
- each R 8 group is independently selected for the formula -N(R 8 )-C(O)-N(R 8 )- each R 8 group is independently selected.
- the invention is inclusive of the compounds described herein in any of their pharmaceutically acceptable forms, including isomers (e.g., diastereomers and enantiomers), salts, solvates, polymorphs, and the like.
- isomers e.g., diastereomers and enantiomers
- the invention specifically includes each of the compound's enantiomers as well as racemic mixtures of the enantiomers.
- compound includes any or all of such forms, whether explicitly stated or not (although at times, “salts” are explicitly stated).
- prodrug means a compound that can be transformed in vivo to yield an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above.
- the prodrug itself, may be an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above.
- the transformation may occur by various mechanisms, such as through a chemical (e.g., solvolysis or hydrolysis, for example, in the blood) or enzymatic biotransformation.
- a chemical e.g., solvolysis or hydrolysis, for example, in the blood
- enzymatic biotransformation e.g., a chemical (e.g., solvolysis or hydrolysis, for example, in the blood) or enzymatic biotransformation.
- T. Higuchi and W. Stella "Pro-drugs as Novel Delivery Systems," Vol. 14 of the A. C. S. Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987.
- each one of the following variables e.g., Y, X, R 1 , Q, G 1 , G 2 , n, and so on
- each one of the following variables in any of its embodiments can be combined with any one or more of the other variables in any of their embodiments and associated with any one of the formulas described herein, as would be understood by one of skill in the art.
- Each of the resulting combinations of variables is an embodiment of the present invention.
- n is 1.
- n is 2.
- m is 0.
- Ri is -X-Y-R 4 wherein X is straight chain or branched chain C 1-6 alkylene which may be interrupted by one -O- group; Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-C(O)-N(R 8 )-, and -S(O) 2 - wherein R 8 is selected from hydrogen and methyl; and R 4 is selected from the group consisting of C 1-6 alkyl, isoquinolinyl, N-methylimidazolyl, pyridinyl, quinolinyl, phenyl, and phenyl substituted by a substituent selected from the group consisting of chloro, cyano, fluoro, hydroxy, and methyl; with the proviso that when Y is -S(O) 2
- Ri is selected from the group consisting of 2-[(cyclo ⁇ ropylcarbonyl)amino]ethyl, 4- [(cyclo ⁇ ropylcarbonyl)amino]butyl, 2-[(cyclohexylcarbonyl)amino]-2-methylpropyl, 2- ⁇ [(1 -methylethyl)carbonyl] amino ⁇ ethyl, 4- ⁇ [(l -methylethyl)carbonyl]amino ⁇ butyl, 2- methyl-2- ⁇ [(I -methylethyl)carbonyl]amino ⁇ propyl, 2-[(methylsulfonyl)amino]ethyl, 4- [(methylsulfonyl)amino]butyl, 2-methyl-2-[(methylsulfonyl)amino]propyl, 2-methyl-2- W
- R 1 is -X-Y-R 4 wherein X is straight chain or branched chain C 1-8 alkylene which may be interrupted by one -O- group; Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-S(O) 2 -N(R 8a )-, -N(R 8 )-C(O)-N(R 8a )-, and -S(O) 2 - wherein R 8 is hydrogen, methyl, benzyl, or pyridin-3-ylmethyl; R 8a is hydrogen, methyl, or ethyl, and R 4 is selected from the group consisting of C 1-7 alkyl, haloC 1-4 alkyl, hydroxyC 1-4 alkyl, phenyl, benzyl, 1-pheny
- Y is selected from the group consisting of -N(R 8 )-C(0)-, -N(Rs)-S(O) 2 -, and -N(R 8 )-C(O)-N(R 8a )-.
- R 8a is hydrogen.
- R 8a is methyl.
- R 8 is hydrogen.
- R 8 is benzyl.
- R 8 is pyridin-3-ylmethyl.
- Y is -S(O) 2 -.
- X is C 1-6 alkylene.
- R 5 is ( CH 2 ) b
- R 8 is hydrogen, methyl, or pyridin-3-ylmethyl
- A is -0-, -CH 2 -, or -N(CH 3 )-
- a is 1 or 2
- b is 2.
- R 1 is selected from the group consisting of 4-(l,l-dioxidoisothiazolidin-2-yl)butyl, 4-[(4- morpholinecarbonyl)amino]butyl, and 2-[(4-morpholinecarbonyl)amino]ethyl.
- Ri is -Ci -4 alkylenyl-Het.
- Het is selected from the group consisting of tetrahydropyranyl and tetrahydrofuranyl.
- Ri is tetrahydro-2H-pyran-4-ylmethyl.
- the present invention provides a compound selected from the group consisting of iV-[4-(4-amino-2- hydroxymethyl-lH-imidazo[4,5-c]quinolin-l-yl)butyl]methanesulfonamide and7V- ⁇ 4-[4- amino-2-(2-hydroxyethyl)-l/i-imidazo[4,5-c]quinolin-l-yl]butyl] ⁇ methanesulfonamide, or a pharmaceutically acceptable salt thereof.
- the present invention provides N- ⁇ 2-[4-amino-2-(hydroxymethyl)- lH " -imidazo[4,5-c]quinolin- 1 -yl]- l,l-dimethylethyl ⁇ methanesulfonamide or a pharmaceutically acceptable salt thereof.
- the present invention provides a pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of Formula I, II, III, or of any one of the above embodiments and a pharmaceutically acceptable carrier.
- the present invention provides a method of preferentially inducing the biosynthesis of IFN- ⁇ in an animal comprising administering an effective amount of a compound or salt of Formula I, II, III, or of any one of the above embodiments or the above pharmaceutical composition to the animal.
- the present invention provides a method of treating a viral disease in an animal in need thereof comprising administering a therapeutically effective amount of a compound or salt of Formula I, II, III, or of any one of the above embodiments or the above pharmaceutical composition to the animal.
- the present invention provides a method of treating a neoplastic disease in an animal in need thereof comprising administering a therapeutically effective amount of a compound or salt of Formula I, II, III, or of any one of the above embodiments or the above pharmaceutical composition to the animal.
- the compound or salt or pharmaceutical composition is administered systemically.
- R is selected from the group consisting of Ci -I0 alkyl, C 1-10 alkoxy, halogen, and C 1-10 haloalkyl.
- R 1 is selected from the group consisting Of-X-Y-R 4 , -X-R 5 , and -X-Het.
- Ri is -X-Y-R 4 .
- R 1 is -X-Y-R 4 wherein X is straight chain or branched chain Ci -6 alkylene which may be interrupted by one -O- group; Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-C(O)-N(R 8 )-, and -S(O) 2 - wherein R 8 is selected from hydrogen and methyl; and R 4 is selected from the group consisting of C 1-6 alkyl, isoquinolinyl, N-methylimidazolyl, pyridinyl, quinolinyl, phenyl, and phenyl substituted by a substituent selected from the group consisting of chloro, cyano, fluoro, hydroxy, and methyl.
- R 1 is selected from the group consisting of 2- [(cyclopropylcarbonyl)amino]ethyl, 4-[(cyclopropylcarbonyl)amino]butyl, 2- [(cyclohexylcarbonyl)amino]-2-methylpropyl, 2- ⁇ [(I -methylethyl)carbonyl]amino ⁇ ethyl, 4- ⁇ [(I -methylethyl)carbonyl]amino ⁇ butyl, 2-methyl-2- ⁇ [( 1 - methylethyl)carbonyl]amino ⁇ propyl, 2-[(methylsulfonyl)amino]ethyl, 4- [(methylsulfonyl)amino]butyl, 2-methyl-2- [(methylsulfonyl)amino]propyl, 2-methyl-2- ( ⁇ [(l-methylethyl)amino]carbonyl ⁇ amino)propyl, 2-methyl
- R 1 is -X-R 5 .
- R 1 is -X-R 5 wherein X is straight chain or branched
- R 1 is selected from the group consisting of 4-(I 9 I- dioxidoisothiazolidin-2-yl)butyl, 4-[(4-morpholinecarbonyl)amino]butyl, and 2-[(4- morpholinecarbonyl)amino]ethyl.
- R 1 is -X-Het.
- R 1 is - C 1-4 alkylenyl-Het.
- Ri is tetrahydro-2H -pyran-4-ylmethyl.
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, wherein the alkyl, alkenyl, aryl, arylalkylenyl, heteroaryl, and heteroarylalkylenyl, groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, mercapto, cyano, aryl, aryloxy, heteroaryl, heteroaryloxy, heterocyclyl, amino, alkylaniino, dialkylamino, and in the case of alkyl and alkenyl, oxo.
- R 4 is selected from the group consisting of C 1-6 alkyl, isoquinolinyl, iV-methylimidazolyl, pyridinyl, quinolinyl, phenyl, and phenyl substituted by a substituent selected from the group consisting of chloro, cyano, fluoro, hydroxy, and methyl.
- R 4 is selected from the group consisting of C 1-7 alkyl, haloC 1-4 alkyl, hydroxy C 1-4 alkyl, phenyl, benzyl, 1-phenylethyl, 2-phenylethyl, 2- phenylethenyl, phenylcyclopropyl, pyridinyl, thienyl, JV-methylimidazolyl, 3,5- dimethylisoxazolyl, wherein benzyl is unsubstituted or substituted by a methyl group, and phenyl is unsubstituted or substituted by one or two substituents independently selected from the group consisting of methyl, fluoro, chloro, cyano, hydroxy, and dimethylamino.
- R 4 is C 1-7 alkyl.
- R 4 is C 1-4 alkyl.
- R 4 is phenyl which is unsubstituted or substituted by one or two substituents independently selected from the group consisting of methyl, fluoro, chloro, cyano, hydroxy, and dimethylamino.
- R 5 is selected from the group consisting of
- R 5 i is
- R 6 O.
- R 7 is C 2-7 alkylene.
- R 7 is C 2-4 alkylene.
- R 8 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, hydroxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl.
- R 8 is selected from the group consisting of hydrogen, C 1-4 alkyl, and C 1-4 alkoxy C 1-4 alkylenyl .
- R 8 is arylalkylenyl.
- R 8 is benzyl
- R 8 is heteroarylalkylenyl.
- R 8 is pyridin-3-ylmethyl.
- R 8 is hydrogen or C 1-4 alkyl.
- R 8 is selected from hydrogen and methyl
- R 8 is hydrogen
- R 10 is C 3-8 alkylene.
- R 10 is C 4-6 alkylene.
- A is selected from the group consisting of -O-, -C(O)-,
- A is -O-, -CH 2 -, or -N(Q-R 4 )-.
- A is -O-, -CH 2 -, -S-, or -S(O) 2 -.
- A is -O- or -S(O) 2 -.
- A is -O-.
- A is -CH 2 -.
- A is -N(Q-R 4 )-.
- A is -N(CH 3 )-.
- R' and R" are independently selected from the group consisting of C 1-10 alkyl, C 3-7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen,
- G 1 is selected from the group consisting of -C(O)-R', ⁇ -aminoacyl, and -C(O)-O-R'.
- R' contains one to ten carbon atoms.
- ⁇ -aminoacyl is an ⁇ -C 2-11 aminoacyl group derived from an ⁇ -amino acid selected from the group consisting of racemic, D-, and L-amino acids containing a total of at least 2 carbon atoms and a total of up to 11 carbon atoms, and may also include one or more heteroatoms selected from the group consisting of O, S, and N.
- G 2 is selected from the group consisting of -X 2 -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ - aminoacyl, -X 2 -C(O)-O-R', and -C(O)-N(R")R'.
- X 2 is selected from the group consisting of a bond; -CH 2 -O-; -CH(CH 3 )-O-; -C(CH 3 ) 2 -O-; and, in the case Of -X 2 -C(O)-O-R, -CH 2 -NH-; R and R" are independently selected from the group consisting of C 1-10 alkyl, C 3-7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, C 1-6 alkyl, C 1-4 alkoxy, aryl, heteroaryl, aryl- C 1-4 alkylenyl, heteroaryl- C 1-4 alkylenyl, halo- C 1-4 alkylenyl, halo- C 1-4 alk
- G 2 is selected from the group consisting of -C(O)-R' and ⁇ -aminoacyl, wherein R' is Ci -6 alkyl or phenyl which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, C 1-6 alkyl, C 1-4 alkoxy, aryl, heteroaryl, aryl- C 1-4 alkylenyl, heteroaryl- C 1-4 alkylenyl, halo-C 1-4 alkylenyl, halo-C 1-4 alkoxy, -O-C(O)-CH 3 , -C(O)-O-CH 3 , -C(O)-NH 2 , -O-CH 2 -C(O)-NH 2 , -NH 2 , and -S(O) 2 -NH 2 .
- G 2 is selected from the group consisting of ⁇ -amino-C 2-5 alkanoyl, C 2-6 alkanoyl, d.galkoxycarbonyl, and C 1-6 alkylcarbamoyl.
- ⁇ -aminoacyl is an ⁇ -aminoacyl group derived from a naturally occuring ⁇ -amino acid selected from the group consisting of racemic, D-, and L- amino acids.
- ⁇ -aminoacyl is an ⁇ -aminoacyl group derived from an ⁇ - amino acid found in proteins, wherein the the amino acid is selected from the group consisting of racemic, D-, and L-amino acids.
- the hydrogen atom of the hydroxy group of Formula II is an ⁇ -aminoacyl group derived from an ⁇ - amino acid found in proteins, wherein the the amino acid is selected from the group consisting of racemic, D-, and L-amino acids.
- G 2 (including any one of its embodiments) is replaced by G 2 , wherein G 2 is defined as in any one of the above embodiments of G 2 .
- Het is selected from the group consisting of tetrahydropyranyl and tetrahydrofuranyl.
- Het is tetrahydro-2H-pyran-4-yl.
- Q is selected from the group consisting of a bond, -C(R 6 )-, -S(O) 2 , -C(R 6 )-N(R 8 )-, -S(O) 2 -N(R 8 )-, -C(R 6 )-O-, and -C(R 6 )-S-.
- Q is selected from the group consisting of a bond, -C(R 6 )-, -S(O) 2 -, and -C(R 6 )-N(R 8 )-.
- Q is selected from the group consisting of -C(O)-, -S(O) 2 -, and -C(O)-N(R 8 )-.
- R 8 is hydrogen or methyl.
- Q is -C(O)-.
- Q is -S(O) 2 -.
- Q is -C(R 6 )-N(R 8 )-.
- Q is -C(O)-N(R 8 )- wherein R 8 is hydrogen or methyl.
- X is straight chain or branched chain alkylene optionally interrupted by one -O- group.
- X is straight chain or branched chain C 1-6 alkylene which may be interrupted by one -O- group.
- X is straight chain or branched chain C 1-8 alkylene.
- X is straight chain or branched chain C 1-6 alkylene.
- X is straight chain or branched chain C 1-4 alkylene.
- X is ethylene
- X is propylene
- X is butylene
- X is -CH 2 -C(CH 3 ) 2 -.
- Y is selected from the group consisting of -S(O) 0-2 -and
- Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-C(O)-N(R 8 )-, and -S(O) 2 -, with the proviso that when Y is -S(O) 2 - then X does not contain an -O- group.
- R 8 is selected from hydrogen and methyl.
- Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-S(O) 2 -N(R 8a )-, -N(R 8 )-C(O)-N(R 8a )-, and -S(O) 2 -.
- Y is selected from the group consisting of -N(R 8 )-C(O)-, -N(R 8 )-S(O) 2 -, -N(R 8 )-C(O)-N(R 8a )-.
- Y is -S(O) 2 -.
- a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7.
- a and b are each independently 1 to 3.
- a and b are each 2.
- a is 1, 2, or 3, and b is 2.
- a is 1 or 2, and b is 2.
- n is 1 or 2.
- n is 1.
- n is 2.
- ni is 0 or 1.
- m is 0.
- m is 1.
- Compounds of the invention may be synthesized by synthetic routes that include processes analogous to those well known in the chemical arts, particularly in light of the description contained herein.
- the starting materials are generally available from commercial sources such as Aldrich Chemicals (Milwaukee, Wisconsin, USA) or are readily prepared using methods well known to those skilled in the art (e.g., prepared by methods generally described in Louis F. Fieser and Mary Fieser, Reagents for Organic Synthesis, v. 1-19, Wiley, New York, (1967-1999 ed.); Alan R. Katritsky, Otto Meth- Cohn, Charles W. Rees, Comprehensive Organic Functional Group Transformations, v. 1- 6, Pergamon Press, Oxford, England, (1995); Barry M.
- Suitable amino protecting groups include acetyl, trifluoroacetyl, fert-butoxycarbonyl (Boc), benzyloxycarbonyl, and 9- fluorenylmethoxycarbonyl (Fmoc).
- Suitable hydroxy protecting groups include acetyl and silyl groups such as the tert-butyl dimethylsilyl group.
- Such techniques may include, for example, all types of chromatography (high performance liquid chromatography (HPLC), column chromatography using common absorbents such as silica gel, and thin layer chromatography), recrystallization, and differential (i.e., liquid- liquid) extraction techniques.
- HPLC high performance liquid chromatography
- column chromatography using common absorbents such as silica gel
- thin layer chromatography such as silica gel
- recrystallization i.e., differential (i.e., liquid- liquid) extraction techniques.
- compounds of the invention can be prepared according to Reaction Scheme I, wherein R 1 , R, m, and n are as defined above and alkyl is methyl or ethyl.
- an ether substituted 1H -imidazo[4,5-c]quinolin-4-amine of Formula X is cleaved to provide a hydroxyalkyl substituted 1H-imidazo[4,5-c]quinolin-4- amine of Formula I.
- the reaction is conveniently carried out by adding a solution of boron tribromide in a suitable solvent such as dichloromethane to a solution or suspension of a compound of Formula X in a suitable solvent such as dichloromethane at ambient or at a sub-ambient temperature, for example, at 0°C.
- the product or pharmaceutically acceptable salt thereof can be isolated using conventional methods.
- compounds of the invention can be prepared according to Reaction Scheme II, wherein R 1 , G 1 , and n are as defined above.
- the amino group of a compound of Formula I can be converted by conventional methods to a functional group such as an amide, carbamate, urea, amidine, or another hydrolyzable group.
- a compound of this type can be made by the replacement of a hydrogen atom in an amino group with a group such as -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ -aminoacyl, -C(O)-O-R',
- R' and R" are independently selected from the group consisting of Ci -10 alkyl, C 3-7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, C 1-6 alkyl, C 1-4 alkoxy, aryl, heteroaryl, aryl-C 1-4 alkylenyl, heteroaryl-C 1-4 alkylenyl, halo-C 1-4 alkylenyl, halo ⁇ C 1-4 alk
- amides derived from carboxylic acids containing one to ten carbon atoms are amides derived from carboxylic acids containing one to ten carbon atoms, amides derived from amino acids, and carbamates containing one to ten carbon atoms.
- the reaction can be carried out, for example, by combining a compound of Formula I with a chloroformate or acid chloride, such as ethyl chloroformate or acetyl chloride, in the presence of a base such as triethylamine in a suitable solvent such as dichloromethane at ambient temperature.
- a chloroformate or acid chloride such as ethyl chloroformate or acetyl chloride
- the hydroxy group on a compound of Formula I can be protected using a suitable silyl group such as tert-butyl dimethylsilyl using conventional methods.
- the G 1 group may then be installed using conventional methods followed by the removal of the hydroxy protecting group under acidic conditions to provide a compound of Formula II.
- compounds of the invention can be prepared according to Reaction Scheme III, wherein Ri, G 2 , and n are as defined above.
- Compounds of Formula I can be prepared according to the method described above.
- the hydrogen atom of the alcohol group of a compound of Formula I can be replaced using conventional methods with a group such as X 2 -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ -aminoacyl, -X 2 -C(O)-O-R', and -C(O)-N(R")R'; wherein X 2 is selected from the group consisting of a bond; -CH 2 -O-; -CH(CHs)-O-; -C(CH 3 ) 2 -O-; and, in the case of -X 2 -C(O)-O-R', -CH 2 -NH-; R' and R" are independently selected from the group consisting of C 1-10 alkyl, C 3-7
- Particularly useful compounds of Formula III are esters made from carboxylic acids containing one to six carbon atoms, unsubstituted or substituted benzoic acid esters, or esters made from naturally occurring amino acids.
- the reaction can be carried out by treating a compound of Formula I with a carboxylic acid or amino acid under Mitsunobu reaction conditions by adding triphenylphosphine and a carboxylic acid to a solution or suspension of a compound of Formula I in a suitable solvent such as tetrahydrofuran and then slowly adding diisopropyl azodicarboxylate.
- the reaction can be run at a sub-ambient temperature such as 0 0 C.
- compounds of the invention can also be prepared using the synthetic methods described in the EXAMPLES below.
- compositions of the invention contain a therapeutically effective amount of a compound or salt described above in combination with a pharmaceutically acceptable carrier.
- a therapeutically effective amount and “effective amount” mean an amount of the compound or salt sufficient to induce a therapeutic or prophylactic effect, such as cytokine induction, immunomodulation, antitumor activity, and/or antiviral activity. Cytokine induction can include preferentially inducing the biosynthesis of IFN- ⁇ .
- amount of compound or salt used in a pharmaceutical composition of the invention will vary according to factors known to those of skill in the art, such as the physical and chemical nature of the compound or salt, the nature of the carrier, and the intended dosing regimen.
- compositions of the invention will contain sufficient active ingredient or prodrug to provide a dose of about 100 nanograms per kilogram (ng/kg) to about 50 milligrams per kilogram (mg/kg), preferably about 10 micrograms per kilogram ( ⁇ g/kg) to about 5 mg/kg, of the compound or salt to the subject.
- the method includes administering sufficient compound to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- dosage forms may be used, such as tablets, lozenges, capsules, parenteral formulations (e.g., intravenous formulations), syrups, creams, ointments, aerosol formulations, transdermal patches, transmucosal patches and the like.
- These dosage forms can be prepared with conventional pharmaceutically acceptable carriers and additives using conventional methods, which generally include the step of bringing the active ingredient into association with the carrier.
- the compounds or salts of the invention can be administered as the single therapeutic agent in the treatment regimen, or the compounds or salts described herein may be administered in combination with one another or with other active agents, including additional immune response modifiers, antivirals, antibiotics, antibodies, proteins, peptides, oligonucleotides, etc.
- Compounds or salts of the invention have been shown to induce the production of certain cytokines in experiments performed according to the tests set forth below. These results indicate that the compounds or salts are useful for modulating the immune response in a number of different ways, rendering them useful in the treatment of a variety of disorders.
- the compounds or salts of the invention are especially useful as immune response modifiers due to their ability to preferentially induce interferon- ⁇ , thus providing a benefit over compounds that also induce pro-inflammatory cytokines (e.g. TNF- ⁇ ) or that induce pro-inflammatory cytokines at higher levels.
- pro-inflammatory cytokines e.g. TNF- ⁇
- interferon- ⁇ and pro-inflammatory cytokines are beneficial in treating certain conditions, interferon- ⁇ preferentially induced is believed to be better tolerated by patients, because the significantly lower levels of pro-inflammatory cytokines can result in fewer or less severe adverse side effects experienced by patients. For example, if a subject is treated for a 006/006223
- hepatitis C metastatic cancer
- the compound may also cause side effects, such as severe and/or widespread inflammation, tissue destruction, or emesis, that render the subject unable or unwilling to receive the treatment.
- side effects such as severe and/or widespread inflammation, tissue destruction, or emesis
- the compound may treat the disease with less risk of adverse side effects from proinflammatory cytokines such as TNF- ⁇ . Therefore, by maintaining the ability to treat a condition and reducing adverse side effects, compounds that preferentially induce IFN- ⁇ provide an advantage over compounds that would also induce pro-inflammatory cytokines, such as TNF- ⁇ , at higher levels.
- the ability of the compounds or salts of the invention to preferentially induce the biosynthesis of IFN- ⁇ may be particularly advantageous when administered systemically, since adverse side effects, including for example widespread inflammation, may be reduced or even eliminated.
- Compounds of the invention may be administered systemically in a number of ways, including but not limited to oral and intravenous administration.
- Cytokines whose biosynthesis may be induced by compounds or salts of the invention include IFN- ⁇ , IP-IO, MCP-I , and a variety of other cytokines.
- cytokines such as TNF- ⁇ , IL- 12 may be induced, albeit at significantly reduced levels.
- these and other cytokines can inhibit virus production and tumor cell growth, making the compounds or salts useful in the treatment of viral diseases and neoplastic diseases.
- the invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of the invention to the animal.
- the animal to which the compound or salt is administered for induction of cytokine biosynthesis may have a disease as described infra, for example a viral disease or a neoplastic disease, and administration of the compound or salt may provide therapeutic treatment.
- the compound or salt may be administered to the animal prior to the animal acquiring the disease so that administration of the compound or salt may provide a prophylactic treatment.
- compounds or salts of the invention can affect other aspects of the innate immune response. For example, the compounds or salts may cause maturation of dendritic cells or proliferation and differentiation of B-lymphocytes.
- the compound or salt or composition may be administered alone or in combination with one or more active components as in, for example, a vaccine adjuvant.
- the compound or salt or composition and other component or components may be administered separately; together but independently such as in a solution; or together and associated with one another such as (a) covalently linked or (b) non-covalently associated, e.g., in a colloidal suspension.
- Conditions for which compounds or salts or compositions identified herein may be used as treatments include, but are not limited to:
- viral diseases such as, for example, diseases resulting from infection by an adenovirus, a herpesvirus (e.g., HSV-I, HSV-II, CMV, or VZV), a poxvirus (e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum), a picornavirus
- a herpesvirus e.g., HSV-I, HSV-II, CMV, or VZV
- a poxvirus e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum
- a picornavirus e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum
- a coronavirus e.g., SARS
- a papovavirus e.g., papillomaviruses, such as those that cause genital warts, common warts, or plantar warts
- a hepadnavirus e.g., hepatitis B virus
- a flavivirus e.g., hepatitis C virus or Dengue virus
- a retrovirus e.g., a lentivirus such as HIV
- bacterial diseases such as, for example, diseases resulting from infection by bacteria of, for example, the genus Escherichia, Enterobacter, Salmonella, Staphylococcus, Shigella, Listeria, Aerobacter, Helicobacter, Klebsiella, Proteus, Pseudomonas, Streptococcus, Chlamydia, Mycoplasma, Pneumococcus, Neisseria, Clostridium, Bacillus, Corynebacterium, Mycobacterium, Campylobacter, Vibrio, Serratia, Providencia, Chromobacterium, Brucella, Yersinia, Haemophilus, or Bordetella;
- neoplastic diseases such as intraepithelial neoplasias, cervical dysplasia, actinic keratosis, basal cell carcinoma, squamous cell carcinoma, renal cell carcinoma, Kaposi's sarcoma, melanoma, leukemias including but not limited to acute myeloid leukemia, acute lymphocytic leukemia, chronic myeloid leukemia, chronic lymphocytic leukemia, multiple myeloma, Hodgkin's lymphoma, non-Hodgkin's lympho
- atopic diseases such as atopic dermatitis or eczema, eosinophilia, asthma, allergy, allergic rhinitis, and Ommen's syndrome;
- diseases associated with wound repair such as, for example, inhibition of keloid formation and other types of scarring (e.g., enhancing wound healing, including chronic wounds).
- a compound or salt identified herein may be useful as a vaccine adjuvant for use in conjunction with any material that raises either humoral and/or cell mediated immune response, such as, for example, live viral, bacterial, or parasitic immunogens; inactivated viral, tumor-derived, protozoal, organism-derived, fungal, or bacterial immunogens; toxoids; toxins; self-antigens; polysaccharides; proteins; glycoproteins; peptides; cellular vaccines; DNA vaccines; autologous vaccines; recombinant proteins; and the like, for use in connection with, for example, BCG, cholera, plague, typhoid, hepatitis A, hepatitis B, hepatitis C, influenza A, influenza B, parainfluenza, polio, rabies, measles, mumps, rubella, yellow fever, tetanus, diphtheria, hemophilus influenza b, tuberculosis, meningo
- Compounds or salts identified herein may be particularly helpful in individuals having compromised immune function.
- compounds or salts may be used for treating the opportunistic infections and tumors that occur after suppression of cell mediated immunity in, for example, transplant patients, cancer patients and HIV patients.
- one or more of the above diseases or types of diseases for example, a viral disease or a neoplastic disease may be treated in an animal in need thereof (having the disease) by administering a therapeutically effective amount of a compound or salt of the invention to the animal.
- An animal may also be vaccinated by administering an effective amount of a compound or salt described herein, as a vaccine adjuvant.
- a method of vaccinating an animal comprising administering an effective amount of a compound or salt described herein to the animal as a vaccine adjuvant.
- An amount of a compound or salt effective to induce cytokine biosynthesis is an amount sufficient to cause one or more cell types, such as dendritic cells and B-cells to produce an amount of one or more cytokines such as, for example, IFN- ⁇ , IP-IO 5 and MCP-I that is increased (induced) over a background level of such cytokines.
- the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- the amount is expected to be a dose of, for example, from about 0.01 mg/m to about 5.0 mg/m 2 , (computed according to the Dubois method as described above) although in some embodiments the induction of cytokine biosynthesis may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt or composition to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m to about 1.2 mg/m".
- the invention provides a method of treating a disease which is responsive to the induction of cytokine biosynthesis, particularly the preferential induction of IFN- ⁇ , including a method of treating a viral infection in an animal and a method of treating a neoplastic disease in an animal, comprising administering an effective amount of a compound or salt or composition of the invention to the animal.
- An amount effective to treat or inhibit a viral infection is an amount that will cause a reduction in one or more of the manifestations of viral infection, such as viral lesions, viral load, rate of virus production, and mortality as compared to untreated control animals.
- the precise amount that is effective for such treatment will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- An amount of a compound or salt effective to treat a neoplastic condition is an amount that will cause a reduction in tumor size or in the number of tumor foci.
- the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- the amount is expected to be a dose of, for example, from about 0.01 mg/m to about 5.0 mg/m , (computed according to the Dubois method as described above) although in some embodiments either of these methods may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt to provide a dose of from about 0.1 mg/m to about 2.0 mg/ m to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- pre HPLC normal high performance flash chromatography
- COMBIFLASH an automated high-performance flash purification product available from Teledyne Isco, Inc., Lincoln, California, USA
- HORIZON HPFC an automated high-performance flash purification product available from Biotage, Inc, Charlottesville, Virginia, USA.
- the eluent used for each purification is given in the example.
- the solvent mixture 80/18/2 v/v/v chloroform/methanol/concentrated ammonium hydroxide (CMA) was used as the polar component of the eluent. In these separations, CMA was mixed with chloroform in the indicated ratio.
- CMA was mixed with chloroform in the indicated ratio.
- This material was purified by prep HPLC (COMB IFLASH system eluting first with a gradient of 0 to 5% methanol in dichloromethane containing 1% ammonium hydroxide and then with a gradient of 5 to 10% methanol in dichloromethane containing 1% ammonium hydroxide) to provide a white solid.
- This material was suspended in hot acetonitrile, allowed to cool, and then the solvent was decanted.
- This material was purified by prep HPLC (COMBIFLASH system eluting first with a gradient of 0 to 5% methanol in dichloromethane containing 1% ammonium hydroxide and then with a gradient of 5 to 10% methanol in dichloromethane containing 1% ammonium hydroxide) to provide a white solid.
- This material was suspended in hot acetonitrile, allowed to cool, and then the solvent was decanted.
- 3-Methoxypropionyl chloride (15.4 g, 126 mmol) was added dropwise over a period of 20 minutes to a chilled (ice bath) solution of tert-butyl iV- ⁇ 4-[(3-aminoquinolin- 4-yl)amino]butyl ⁇ carbamate (38 g, 115 mmol, U.S. Patent No. 6,541,485, Example 2, Part B) in pyridine.
- the reaction mixture was stirred for 4 hours and then allowed to stand at ambient temperature over the weekend.
- Pyridine hydrochloride (3.9 g, 34 mmol) was added and the reaction mixture was heated at reflux overnight.
- the reaction mixture was concentrated under reduced pressure and the residue was diluted with dichloromethane (250 mL) and aqueous sodium bicarbonate (250 mL). The layers were separated. The separatory funnel was rinsed with a small amount of methanol to remove a residue coating the walls. The combined organics were concentrated under reduced pressure.
- 3-Chloroperoxybenzoic acid (20 g of 77%) was added in a single portion to a solution of the material from Part A (18 g, 45.2 mmol) in dichloroethane (170 mL). After 2 hours concentrated ammonium hydroxide (150 mL) was added and the reaction mixture was stirred until the phases were mixed well.
- Para-Toluenesulfonyl chloride (10.6 g, 54 mmol) was added in a single portion along with a small amount of dichloroethane. The reaction mixture was stirred overnight at ambient temperature and then diluted with water and dichloromethane. The layers were separated and the aqueous layer was extracted with dichloromethane (x2).
- the material from Part B was combined with a solution of hydrochloric acid in dioxane (325 mL of 4 M) and stirred at ambient temperature for 3 hours.
- the reaction mixture was concentrated under reduced pressure.
- the residue was dissolved in methanol (30 mL) and 6 M sodium hydroxide was added with stirring to about pH 9. Attempts to extract with dichloromethane and ethyl acetate were not successful.
- the organic and aqueous layers were concentrated under reduced pressure and combined to provide a dark orange solid.
- Triethylamine (10.5 mL, 75.0 mmol) was added to a mixture of a portion (4.7 g, 15 mmol) of the material from Part C in pyridine (50 mL). The reaction mixture was stirred for several minutes and then methanesulfonyl chloride (1.27 mL, 16.5 mmol) was added dropwise. The reaction mixture was stirred at ambient temperature for 2 hours and then at 50 °C for 2 hours. More methanesulfonyl chloride (0.5 eq) was added and the reaction mixture was stirred at 50 0 C for 2 hours. Another portion of methanesulfonyl chloride (0.25 eq) was added and the reaction mixture was stirred at ambient temperature overnight.
- a solution of sodium hydroxide (1.8 g of solid sodium hydroxide dissolved in 45 mL of water) was added slowly to a solution of the material from Part A (41.1 mmol) in tetrahydrofuran (96 mL).
- the layers were separated and the tetrahydrofuran was removed under reduced pressure to provide a mixture.
- the mixture was diluted with water (200 mL) and then extracted with dichloromethane (2 x 100 mL). The organics were combined, washed sequentially with aqueous sodium carbonate (2 x 150 mL) and brine (100 mL), dried over sodium sulfate and magnesium sulfate, filtered, and then concentrated under reduced pressure. The residue was triturated with heptane (75 mL) for 15 minutes at 65 °C and then filtered while hot.
- a Parr vessel was charged with 5% Pt/C (0.5 g) and acetonitrile (10 mL). A solution of the material from Part B in acetonitrile (450 mL) was added. The vessel was placed on a Parr shaker under hydrogen pressure (40 psi, 2.8 x 10 5 Pa) for 5 hours. The reaction mixture was filtered through a layer of CELITE filter aid to remove the catalyst. The filtrate was carried on to the next step.
- the powder was dissolved in ethanol (25 mL), combined with a solution of sodium hydroxide (0.21 g) in water (25 mL), and then heated at 50 °C for 3 hours. The ethanol was removed under reduced pressure and the solids were isolated by filtration to provide 1.2 g of a light tan powder.
- the powder was dissolved in a mixture of acetonitrile (100 mL), water (2 mL) and ethanol (25 mL). The solution was allowed to stand overnight and was then concentrated to a volume of 5 mL to provide a white paste. The paste was triturated with warm (70 °C) acetonitrile (50 mL) for 30 minutes, heated to reflux, and then allowed to cool to ambient temperature.
- Triethylamine (39.3 mL, 0.282 mol) was added to a chilled (ice bath) solution of N 1 -(2-chloro-3 -nitroquinolin-4-yl)-2-methylpropane-l,2-diamine (41.42 g, 0.141 mol) in dichloromethane (about 500 mL). Under a nitrogen atmosphere a solution of methanesulfonic anhydride in (29.47 g, 0.169 mol) in dichloromethane (100 mL) was added via a cannula to the reaction mixture over a period of 45 minutes. After the addition was complete the ice bath was removed and the reaction mixture was allowed to stir at ambient temperature overnight.
- reaction mixture was washed sequentially with saturated aqueous sodium bicarbonate (x2) and brine, dried over a mixture of sodium sulfate and magnesium sulfate, filtered, and then concentrated under reduced pressure to provide 46.22 g of an orange solid.
- This material was recrystallized from toluene (about 1 L), isolated by filtration, rinsed with cold toluene, and dried under high vacuum at 60 °C to provide 33.09 g of N - ⁇ 2-[(2-chloro-3-nitroquinolin-4-yl)amino]-l,l- dimethylethyl ⁇ methanesulfonamide.
- Part B A hydrogenation vessel was charged with 5% Pt/C (4.14 g) and a solution of N- ⁇ 2-
- boron tribromide (3.5 mL of 1 M in dichloromethane) was added dropwise to a chilled (0 °C) solution of iV- ⁇ 2-[4-amino-2-(2- methoxyethyl)- 1H-imidazo[4,5-c]quinolin- 1 -yl]- 1 , 1 -dimethylethyl ⁇ methanesulfonamide (0.55 g, 1.40 mmol) in dichloromethane (20 mL). The reaction was allowed to warm to ambient temperature overnight. The reaction was quenched with methanol (10 mL) and the solvent was removed under reduced pressure.
- This material was purified by prep ⁇ PLC (HORIZON HPFC system, eluting with a gradient of 30-50% CMA in chloroform for 15 column volumes followed by 50% CMA in chloroform for 5 column volumes) and then dried under high vacuum to provide 250 mg of N- ⁇ 2-[4-amino-2-(2-hydroxyethyl)- 1 H -imidazo [4, 5 -c] quinolin- 1 -yl] -1,1 -dimethylethyl ⁇ methanesulfonamide as white solid, m.p. 209 - 212°C.
- a pressure vessel was charged with a solution of of N- ⁇ 2-[(2-chloro-3- nitroquinolin-4-yl)amino]-1,1-dimethylethyl ⁇ methanesulfonamide (5 g, 13 mmol) in acetonitrile (150 mL ). Catalyst was added (0.5 g of 5% Pt/C) and the vessel was placed under hydrogen pressure (50 psi, 3.4 X 10 5 Pa) for 2 hours. The reaction mixture was filtered through a layer of CELITE filter aid. Part B
- the material from Part C was combined with a solution of ammonia in methanol (50 mL of 7 N) and heated at 150 °C for 10 hours. The reaction mixture was allowed to cool to ambient temperature. A precipitate was isolated by filtration, rinsed with methanol (20 mL), slurried with water (50 mL), isolated by filtration, washed with water (20 mL), and dried to provide 2.7 g of a brown crystalline solid.
- a reagent (1.1 eq) from Table 1 below was added to a test tube containing a solution of l-(4-aminobutyl)-2-(2-methoxyethyl)-1H-imidazo[4,5-c]quinolin-4-amine (73 mg) in N,N-dimethylacetamide (1 mL) containing N, N-diisopropylethylamine (2 eq).
- the test tube was placed on a shaker overnight. The solvent was removed by vacuum centrifugation.
- the reaction mixtures were separated by solid-supported liquid-liquid extraction according to the following procedure.
- a reagent (1.1 eq) from Table 2 below was added to a test tube containing a solution of 1 -(3 -aminopropyl)-2-(2-methoxyethyl)- 1 H-imidazo [4,5 -c]quinolin-4-amine (30 mg) in chloroform (1 mL) containing N,N-diisopropylethylamine (1.5 eq).
- the test tube was placed on a shaker overnight.
- the reaction mixtures were separated by solid- supported liquid-liquid extraction according to the following procedure. Each reaction mixture was loaded onto diatomaceous earth that had been equilibrated with de-ionized water (600 ⁇ L) for about 20 minutes.
- the material from Part A was combined with dichloromethane (40 mL), stirred until homogeneous, and then chilled in an ice bath. Boron tribromide (40 mL of 1 M in dichloromethane) was slowly added. The ice bath was removed and the reaction mixture was stirred overnight at ambient temperature. The reaction mixture was concentrated under reduced pressure. The residue was combined with methanol (50 mL) and hydrochloric acid (50 mL of 6 N) and heated at 50 °C for 2 hours. The solution was adjusted to pH 9 with sodium hydroxide (6 M) and then extracted first with ethyl acetate (3 x 100 mL) and then with dichloromethane.
- a pressure vessel was charged with the material from Part B, 5% Pt/C (4.24 g), and acetonitrile (1.5 L). The mixture was placed under hydrogen pressure for 20 hours and then filtered through a layer of CELITE filter aid. The filter cake was rinsed with additional acetonitrile. The filtrate was concentrated under reduced pressure. The residue was dissolved in toluene (750 mL) and then concentrated under reduced pressure to remove residual water. The toluene concentration was repeated.
- Part E 3-Chloroperoxybenzoic acid (26.33 g of 60%, 91.56 mmol) was added in portions over a period of 5 minutes to a chilled solution of the material from Part D in chloroform (350 mL).
- the reaction mixture was allowed to slowly warm to ambient temperature. After 2 hours the reaction mixture was chilled in an ice bath and ammonium hydroxide (100 mL) was added with vigorous stirring to homogenize.
- Para-toluenesulfonyl chloride (15.27 g, 80.12 mmol) was added in portions over a period of 10 minutes. The ice bath was removed and the reaction mixture was stirred for 30 minutes.
- the reaction mixture was diluted with water (100 mL) and chloroform (250 mL).
- the layers were separated.
- the organic layer was washed with 10% sodium carbonate (200 mL) and water (200 mL).
- the combined aqueous was back extracted with chloroform (100 mL).
- the combined organics were washed with brine (200 mL), dried over magnesium sulfate, filtered, and then concentrated under reduced pressure to provide a light brown foam.
- the foam was purified by column chromatography (silica gel, eluting with 95/5 chloroform/methanol) and then recrystallized from acetonitrile to provide 3.75 g of l-[2-(4-amino-2- ethoxymethyl-lH-imidazo[4,5-c]quinolin-l-yl)-l,l-dimethylethyl]-3-(l-methylethyl)urea as an off white solid.
- reaction mixture was diluted with acetonitrile (10 mL) and the reaction mixture was stirred overnight.
- the reaction mixture was diluted with dichloromethane (10 mL) and acetonitrile (10 mL), stirred for an additional 16 hours, quenched with methanol (25 mL), and then concentrated under reduced pressure to provide an orange foam.
- the foam was dissolved in hydrochloric acid (25 mL of 6 N) and heated at 50 0 C for 2 hours. The solution was neutralized with 50% sodium hydroxide. The resulting gummy precipitate was extracted with chloroform (3 x 15 mL).
- the reaction was quenched with aqueous hydrochloric acid (IN, 20 mL) to afford a homogeneous mixture.
- aqueous hydrochloric acid (IN, 20 mL)
- the layers were separated and the aqueous layer washed with dichloromethane (20 mL).
- the pH of the aqueous layer was adjusted to 12 by addition of aqueous sodium hydroxide (50%) at which time a solid precipitated out of solution.
- the solid was stirred for 18 hours, collected by filtration and washed with water.
- the crude product was purified by chromatography over silica gel (eluting with CMA) to afford a white powder.
- the powder was triturated with methanol (20 mL).
- the crude product absorbed on silica was purified by chromatography using a HORIZON HPFC system (silica cartridge, eluting with 0-35% CMA in chloroform over 2.6 L) followed by recrystallization from acetonitrile to provide 170 mg ofiV- ⁇ 2-[4-ammo-2-(hydroxymethyl)-1N-imidazo[4,5-c]quinolin-1-yl]ethyl ⁇ -N'- isopropylurea as an off-white solid, mp >240 °C.
- the reaction was quenched slowly with methanol (100 mL) and then concentrated under reduced pressure. The residue was combined with 6 M hydrochloric acid (100 mL), heated to 50°C, and stirred for 2 hours. The resulting solution was cooled (ice bath) and then free-based (p ⁇ 9) with the addition of 6 M aqueous sodium hydroxide. A brown gummy solid formed in the basic aqueous solution. The aqueous liquid was decanted from the solid and acetonitrile was added (30 mL). A white precipitate formed and was isolated by filtration.
- This material was purified by prep HPLC (Analogix Separation System, Biotage Si 40+M column, eluted with a gradient of 0-20% methanol in dichloromethane with 1 % ammonium hydroxide) to provide a light brown solid.
- the residue was combined with 6 M hydrochloric acid (5OmL), heated to 50°C, and stirred for 2 hours.
- the resulting solution was concentrated under reduced pressure to a slurry that cooled (ice bath) and then free-based with the addition of 7 M ammonia in methanol (40 mL).
- the mixture was concentrated under reduced pressure and the addition of 7 M ammonia in methanol (4OmL) was repeated 2 more times.
- the concentrated brown sludge like material was purified by prep HPLC (ISCO Combiflash Separation System, Biotage Si 40+M column, eluted with a gradient of methanol in dichloromethane with 1 % ammonium hydroxide) to provide a light brown solid.
- the residue was purified by solid-supported liquid-liquid extraction according to the following procedure.
- the sample was dissolved in chloroform (1 mL) then loaded onto diatomaceous earth that had been equilibrated with 1 M sodium hydroxide (600 ⁇ L) for about 20 minutes. After 10 minutes chloroform (500 ⁇ L) was added to elute the product from the diatomaceous earth into a well of a collection plate. After an additional 10 minutes the process was repeated with additional chloroform (500 ⁇ L). The solvent was then removed by vacuum centrifugation. Part B
- the solvents were removed by vacuum centrifugation.
- the compounds were purified according to the method described in Examples 8 - 72.
- the table below shows the reagent used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
- l-(4- Ben2ylaminobutyl)-2-ethoxymethy- lH-imidazo[4,5-c]quinolin-4-amine was prepared according to the general method of Part A of Examples 246 - 257 using benzaldehyde in lieu of pyridine 3-carboxaldehyde and l-(4-aminobutyl)-2-ethoxymethyl-lH-imidazo[4,5- ⁇ ?]quinolin-4-amine in lieu of l-(4-arninobutyl)-2-methoxymethyl-l/i-imidazo[4,5- c]quinolin-4-amine.
- the table below shows the reagent used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
- the compounds in the table below were prepared according to the general method of Examples 111 - 140.
- the table shows a reference for the ether starting material, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
- Certain exemplary compounds including some of those described above in the Examples, have the following Formula Ib and the following substituents n and R 1 wherein each line of the table is matched to Formula Ib to represent a specific embodiment of the invention.
- cytokine induction An in vitro human blood cell system is used to assess cytokine induction. Activity is based on the measurement of interferon ( ⁇ ) and tumor necrosis factor ( ⁇ ) (IFN- ⁇ and TNF- ⁇ , respectively) secreted into culture media as described by Testerman et. al. in “Cytokine Induction by the Immunomodulators Imiquimod and S-27609", Journal of Leukocyte Biology, 58, 365-372 (September, 1995).
- ⁇ interferon
- ⁇ tumor necrosis factor
- PBMC Peripheral blood mononuclear cells
- HISTOPAQUE- 1077 Sigma, St. Louis, MO
- Ficoll-Paque Plus Amersham Biosciences Piscataway, NJ
- Blood is diluted 1:1 with Dulbecco's Phosphate Buffered Saline (DPBS) or Hank's Balanced Salts Solution (HBSS).
- DPBS Dulbecco's Phosphate Buffered Saline
- HBSS Hank's Balanced Salts Solution
- PBMC whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium.
- the PBMC layer is collected and washed twice with DPBS or HBSS and re-suspended at 4 x 10 6 cells/mL in RPMI complete.
- the PBMC suspension is added to 96 well flat bottom sterile tissue culture plates containing an equal volume of RPMI complete media containing test compound.
- the compounds are solubilized in dimethyl sulfoxide (DMSO).
- DMSO concentration should not exceed a final concentration of 1% for addition to the culture wells.
- the compounds are generally tested at concentrations ranging from 30-0.014 ⁇ M.
- Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with reference compound. Incubation
- test compound is added at 60 ⁇ M to the first well containing RPMI complete and serial 3 fold dilutions are made in the wells.
- the PBMC suspension is then added to the wells in an equal volume, bringing the test compound concentrations to the desired range (usually 30-0.014 ⁇ M).
- the final concentration of PBMC suspension is 2 x 10 6 cells/mL.
- the plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere.
- IFN- ⁇ concentration is determined with a human multi-subtype colorimetric sandwich ELISA (Catalog Number 41105) from PBL Biomedical Laboratories,
- TNF- ⁇ concentration is determined by ORIGEN M-Series Immunoassay and read on an IGEN M-8 analyzer from BioVeris Corporation, formerly known as IGEN
- the immunoassay uses a human TNF- ⁇ capture and detection antibody pair (Catalog Numbers AHC3419 and AHC3712) from Biosource
- the data output of the assay consists of concentration values of TNF- ⁇ and
- IFN- ⁇ (y-axis) as a function of compound concentration (x-axis).
- the reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ - dimethyl-l//-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from the past 61 experiments.
- the minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound.
- the minimum effective concentration ( ⁇ molar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ).
- the maximal response (pg/mL) is the maximal response attained in the dose response curve.
- the compounds of Examples 6 and 7 and several closely related analogs were tested using the test method described above.
- the IFN- ⁇ dose response curves for Example 6, Analog 2, Analog 3 and Analog 5 are shown in Figure 1.
- the TNF- ⁇ dose response curves for Example 6, Analog 2, Analog 3 and Analog 5 are shown in Figure 2.
- the IFN- ⁇ dose response curves for Example 7, Analog 1, Analog 2 and Analog 4 are shown in Figure 3.
- the TNF- ⁇ dose response curves for Example 7, Analog 1, Analog 2 and Analog 4 are shown in Figure 4.
- the minimum effective concentration for the induction of IFN- ⁇ , minimum effective concentration for the induction of TNF- ⁇ , the maximal response for IFN- ⁇ , and the maximal response for TNF- ⁇ are shown in Table 5 below where # is the number of separate experiments in which the compound was tested. When a compound was tested in more than one experiment the values shown are the median values.
- Analogs 1-11, 17-33, 68, 72, and 77 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent Nos. 6,331,539 and 6,677,349.
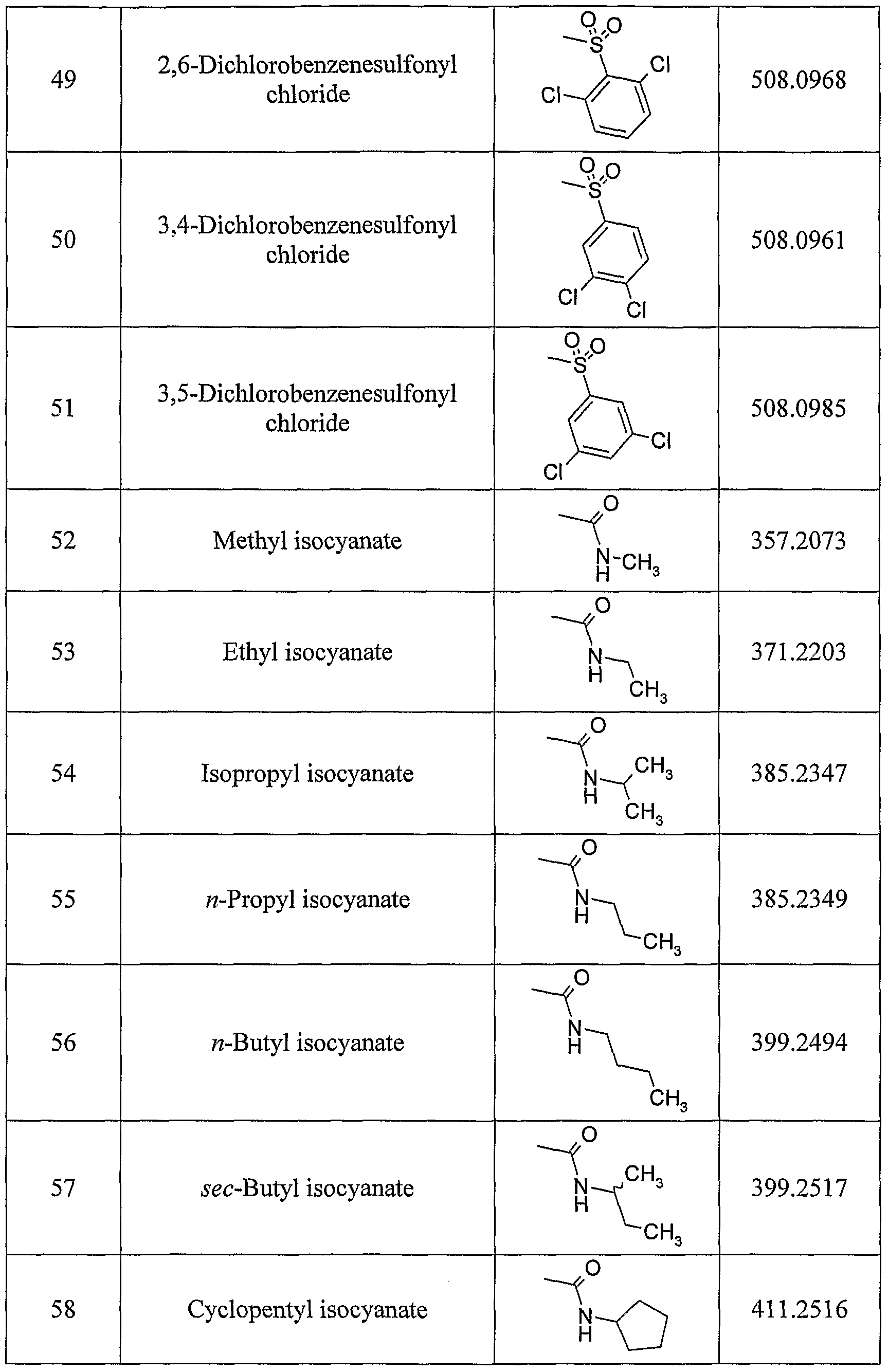
- Analogs 12-16, 40-42, 46-50, 56, 57, 62, 63, 66, and 67 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent Nos. 6,541,485 and 6,573,273.
- Analogs 32-35 and 83 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S.
- Analogs 36-39 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent
- Analogs 43-45, 58, 59, 70, and 73 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent Nos. 6,069,149 and 6,677,349.
- Analogs 52-55, 60, 61, 64, 65, 69, 71, 74, 75, 78, and 82 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent Nos. 6,451,810 and 6,756,382.
- Analogs 79-81 are either specifically exemplified in or are readily prepared using the synthetic methods disclosed in U.S. Patent
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06758163A EP1851224A2 (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
| JP2007557115A JP2008543725A (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
| US11/885,005 US8178677B2 (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
| CA002598695A CA2598695A1 (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
| AU2006223634A AU2006223634A1 (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US65538005P | 2005-02-23 | 2005-02-23 | |
| US60/655,380 | 2005-02-23 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006098852A2 true WO2006098852A2 (en) | 2006-09-21 |
| WO2006098852A3 WO2006098852A3 (en) | 2007-05-31 |
Family
ID=36992185
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/006223 WO2006098852A2 (en) | 2005-02-23 | 2006-02-22 | Hydroxyalkyl substituted imidazoquinolines |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8178677B2 (en) |
| EP (1) | EP1851224A2 (en) |
| JP (1) | JP2008543725A (en) |
| AU (1) | AU2006223634A1 (en) |
| CA (1) | CA2598695A1 (en) |
| WO (1) | WO2006098852A2 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| WO2012167081A1 (en) * | 2011-06-03 | 2012-12-06 | 3M Innovative Properties Company | Hydrazino 1h-imidazoquinolin-4-amines and conjugates made therefrom |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| JP2014043458A (en) * | 2008-03-24 | 2014-03-13 | 4Sc Discovery Gmbh | Novel substituted imidazoquinoline |
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| WO2014107663A2 (en) | 2013-01-07 | 2014-07-10 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating cutaneous t cell lymphoma |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| EP3085373A1 (en) | 2006-02-22 | 2016-10-26 | 3M Innovative Properties Company | Immune response modifier conjugates |
| WO2017184746A1 (en) * | 2016-04-19 | 2017-10-26 | Ifm Therapeutics, Inc | Nlrp3 modulators |
| US10005772B2 (en) | 2006-12-22 | 2018-06-26 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
| WO2019166946A1 (en) | 2018-02-28 | 2019-09-06 | Pfizer Inc. | Il-15 variants and uses thereof |
| WO2019224715A1 (en) | 2018-05-23 | 2019-11-28 | Pfizer Inc. | Antibodies specific for cd3 and uses thereof |
| WO2019224716A2 (en) | 2018-05-23 | 2019-11-28 | Pfizer Inc. | Antibodies specific for gucy2c and uses thereof |
| US10526309B2 (en) | 2015-10-02 | 2020-01-07 | The University Of North Carolina At Chapel Hill | Pan-TAM inhibitors and Mer/Axl dual inhibitors |
| US10533005B2 (en) | 2017-02-17 | 2020-01-14 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| US10533007B2 (en) | 2016-04-19 | 2020-01-14 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| WO2020128893A1 (en) | 2018-12-21 | 2020-06-25 | Pfizer Inc. | Combination treatments of cancer comprising a tlr agonist |
| CN112400801A (en) * | 2020-12-07 | 2021-02-26 | 天津医科大学第二医院 | Laryngeal precancerous lesion animal model and construction method and application thereof |
| WO2021124073A1 (en) | 2019-12-17 | 2021-06-24 | Pfizer Inc. | Antibodies specific for cd47, pd-l1, and uses thereof |
| WO2022013775A1 (en) | 2020-07-17 | 2022-01-20 | Pfizer Inc. | Therapeutic antibodies and their uses |
| EP4178574A4 (en) * | 2020-07-08 | 2024-04-10 | Purdue Research Foundation | Compounds, compositions, and methods for the treatment of fibrotic diseases and cancer |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6677347B2 (en) * | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| US20040265351A1 (en) | 2003-04-10 | 2004-12-30 | Miller Richard L. | Methods and compositions for enhancing immune response |
| US20040214851A1 (en) * | 2003-04-28 | 2004-10-28 | 3M Innovative Properties Company | Compositions and methods for induction of opioid receptors |
| AU2004315876B2 (en) | 2003-10-03 | 2011-05-26 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US20090075980A1 (en) * | 2003-10-03 | 2009-03-19 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and Analogs Thereof |
| EP1701955A1 (en) * | 2003-12-29 | 2006-09-20 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| EP1699788A2 (en) * | 2003-12-30 | 2006-09-13 | 3M Innovative Properties Company | Imidazoquinolinyl, imidazopyridinyl and imidazonaphthyridinyl sulfonamides |
| US8697873B2 (en) * | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8017779B2 (en) * | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US7897609B2 (en) * | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| US8541438B2 (en) | 2004-06-18 | 2013-09-24 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| WO2006009826A1 (en) * | 2004-06-18 | 2006-01-26 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US20090270443A1 (en) * | 2004-09-02 | 2009-10-29 | Doris Stoermer | 1-amino imidazo-containing compounds and methods |
| EP1819364A4 (en) * | 2004-12-08 | 2010-12-29 | 3M Innovative Properties Co | Immunomodulatory compositions, combinations and methods |
| AU2006338521A1 (en) | 2005-02-09 | 2007-10-11 | Coley Pharmaceutical Group, Inc. | Oxime and hydroxylamine substituted thiazolo(4,5-c) ring compounds and methods |
| WO2006086449A2 (en) * | 2005-02-09 | 2006-08-17 | Coley Pharmaceutical Group, Inc. | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines |
| WO2006091394A2 (en) * | 2005-02-11 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Substituted imidazoquinolines and imidazonaphthyridines |
| AU2006223634A1 (en) | 2005-02-23 | 2006-09-21 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinolines |
| US8846710B2 (en) * | 2005-02-23 | 2014-09-30 | 3M Innovative Properties Company | Method of preferentially inducing the biosynthesis of interferon |
| AU2006216798A1 (en) * | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinoline compounds and methods |
| AU2006287270A1 (en) * | 2005-09-09 | 2007-03-15 | Coley Pharmaceutical Group, Inc. | Amide and carbamate derivatives of N-{2-[4-amino-2- (ethoxymethyl)-1H-imidazo[4,5-c]quinolin-1-yl]-1,1-dimethylethyl}methanesulfonamide and methods |
| ZA200803029B (en) | 2005-09-09 | 2009-02-25 | Coley Pharm Group Inc | Amide and carbamate derivatives of alkyl substituted /V-[4-(4-amino-1H-imidazo[4,5-c] quinolin-1-yl)butyl] methane-sulfonamides and methods |
| KR20080083270A (en) | 2005-11-04 | 2008-09-17 | 콜레이 파마시티컬 그룹, 인코포레이티드 | Hydroxy and alkoxy substituted 1h-imidazoquinolines and methods |
| WO2007106854A2 (en) * | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| US8178539B2 (en) * | 2006-09-06 | 2012-05-15 | 3M Innovative Properties Company | Substituted 3,4,6,7-tetrahydro-5H-1,2a,4a,8-tetraazacyclopenta[cd]phenalenes and methods |
| WO2009111337A1 (en) * | 2008-03-03 | 2009-09-11 | Irm Llc | Compounds and compositions as tlr activity modulators |
| KR101856462B1 (en) | 2009-03-25 | 2018-05-10 | 보드 오브 리전츠, 더 유니버시티 오브 텍사스 시스템 | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| EP3222621B1 (en) | 2010-08-17 | 2023-03-08 | 3M Innovative Properties Company | Lipidated immune response modifier compound and its medical use |
| WO2012167088A1 (en) | 2011-06-03 | 2012-12-06 | 3M Innovative Properties Company | Heterobifunctional linkers with polyethylene glycol segments and immune response modifier conjugates made therefrom |
| WO2016044839A2 (en) | 2014-09-19 | 2016-03-24 | The Board Of Regents Of The University Of Texas System | Compositions and methods for treating viral infections through stimulated innate immunity in combination with antiviral compounds |
| CN108137586B (en) * | 2015-09-14 | 2021-04-13 | 辉瑞大药厂 | Novel imidazo [4,5-c ] quinoline and imidazo [4,5-c ] [1,5] naphthyridine derivatives as LRRK2 inhibitors |
| WO2019089521A1 (en) * | 2017-10-30 | 2019-05-09 | Theracaine Llc | Hydrophobic acid addition salts |
| EP3728255B1 (en) | 2017-12-20 | 2022-01-26 | 3M Innovative Properties Company | Amide substituted imidazo[4,5-c]quinoline compounds with a branched chain linking group for use as an immune response modifier |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992015582A1 (en) * | 1991-03-01 | 1992-09-17 | Minnesota Mining And Manufacturing Company | 1-SUBSTITUTED, 2-SUBSTITUTED 1H-IMIDAZO[4,5-c]QUINOLIN-4-AMINES |
| WO1992015581A1 (en) * | 1991-03-01 | 1992-09-17 | Minnesota Mining And Manufacturing Company | PROCESS FOR IMIDAZO[4,5-c]QUINOLIN-4-AMINES |
| US5389640A (en) * | 1991-03-01 | 1995-02-14 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
Family Cites Families (183)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3314941A (en) | 1964-06-23 | 1967-04-18 | American Cyanamid Co | Novel substituted pyridodiazepins |
| IL73534A (en) | 1983-11-18 | 1990-12-23 | Riker Laboratories Inc | 1h-imidazo(4,5-c)quinoline-4-amines,their preparation and pharmaceutical compositions containing certain such compounds |
| ZA848968B (en) | 1983-11-18 | 1986-06-25 | Riker Laboratories Inc | 1h-imidazo(4,5-c)quinolines and 1h-imidazo(4,5-c)quinolin-4-amines |
| US5238944A (en) | 1988-12-15 | 1993-08-24 | Riker Laboratories, Inc. | Topical formulations and transdermal delivery systems containing 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| US5756747A (en) | 1989-02-27 | 1998-05-26 | Riker Laboratories, Inc. | 1H-imidazo 4,5-c!quinolin-4-amines |
| US4929624A (en) | 1989-03-23 | 1990-05-29 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo(4,5-c)quinolin-4-amines |
| US5037986A (en) | 1989-03-23 | 1991-08-06 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo[4,5-c]quinolin-4-amines |
| NZ232740A (en) | 1989-04-20 | 1992-06-25 | Riker Laboratories Inc | Solution for parenteral administration comprising a 1h-imidazo(4,5-c) quinolin-4-amine derivative, an acid and a tonicity adjuster |
| US4988815A (en) | 1989-10-26 | 1991-01-29 | Riker Laboratories, Inc. | 3-Amino or 3-nitro quinoline compounds which are intermediates in preparing 1H-imidazo[4,5-c]quinolines |
| WO1992006093A1 (en) | 1990-10-05 | 1992-04-16 | Minnesota Mining And Manufacturing Company | Process for the preparation of imidazo[4,5-c]quinolin-4-amines |
| US5268376A (en) | 1991-09-04 | 1993-12-07 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5266575A (en) | 1991-11-06 | 1993-11-30 | Minnesota Mining And Manufacturing Company | 2-ethyl 1H-imidazo[4,5-ciquinolin-4-amines |
| IL105325A (en) | 1992-04-16 | 1996-11-14 | Minnesota Mining & Mfg | Immunogen/vaccine adjuvant composition |
| US6608201B2 (en) | 1992-08-28 | 2003-08-19 | 3M Innovative Properties Company | Process for preparing 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5395937A (en) | 1993-01-29 | 1995-03-07 | Minnesota Mining And Manufacturing Company | Process for preparing quinoline amines |
| US5648516A (en) | 1994-07-20 | 1997-07-15 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| US5352784A (en) | 1993-07-15 | 1994-10-04 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| KR100341341B1 (en) | 1993-07-15 | 2002-11-25 | 미네소타 마이닝 앤드 매뉴팩춰링 캄파니 | IMIDAZO[4,5-c]PYRIDIN-4-AMINES |
| US5644063A (en) | 1994-09-08 | 1997-07-01 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-c]pyridin-4-amine intermediates |
| US5482936A (en) | 1995-01-12 | 1996-01-09 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-C]quinoline amines |
| US5741908A (en) | 1996-06-21 | 1998-04-21 | Minnesota Mining And Manufacturing Company | Process for reparing imidazoquinolinamines |
| US5693811A (en) | 1996-06-21 | 1997-12-02 | Minnesota Mining And Manufacturing Company | Process for preparing tetrahdroimidazoquinolinamines |
| JP4391592B2 (en) | 1996-10-25 | 2009-12-24 | スリーエム カンパニー | Immune response modifying compounds for the treatment of TH2-mediated and related diseases |
| US5939090A (en) | 1996-12-03 | 1999-08-17 | 3M Innovative Properties Company | Gel formulations for topical drug delivery |
| US6069149A (en) | 1997-01-09 | 2000-05-30 | Terumo Kabushiki Kaisha | Amide derivatives and intermediates for the synthesis thereof |
| UA67760C2 (en) | 1997-12-11 | 2004-07-15 | Міннесота Майнінг Енд Мануфакчурінг Компані | Imidazonaphthyridines and use thereof to induce the biosynthesis of cytokines |
| US6110929A (en) | 1998-07-28 | 2000-08-29 | 3M Innovative Properties Company | Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof |
| JP2000119271A (en) | 1998-08-12 | 2000-04-25 | Hokuriku Seiyaku Co Ltd | 1h-imidazopyridine derivative |
| US20020058674A1 (en) | 1999-01-08 | 2002-05-16 | Hedenstrom John C. | Systems and methods for treating a mucosal surface |
| EP1140091B1 (en) | 1999-01-08 | 2005-09-21 | 3M Innovative Properties Company | Formulations comprising imiquimod or other immune response modifiers for treating cervical dysplasia |
| US6486168B1 (en) | 1999-01-08 | 2002-11-26 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| US6558951B1 (en) | 1999-02-11 | 2003-05-06 | 3M Innovative Properties Company | Maturation of dendritic cells with immune response modifying compounds |
| JP2000247884A (en) | 1999-03-01 | 2000-09-12 | Sumitomo Pharmaceut Co Ltd | Arachidonic acid-induced skin disease-treating agent |
| US6451810B1 (en) | 1999-06-10 | 2002-09-17 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6756382B2 (en) | 1999-06-10 | 2004-06-29 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6573273B1 (en) | 1999-06-10 | 2003-06-03 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6541485B1 (en) | 1999-06-10 | 2003-04-01 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6331539B1 (en) | 1999-06-10 | 2001-12-18 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US6916925B1 (en) | 1999-11-05 | 2005-07-12 | 3M Innovative Properties Co. | Dye labeled imidazoquinoline compounds |
| US6376669B1 (en) | 1999-11-05 | 2002-04-23 | 3M Innovative Properties Company | Dye labeled imidazoquinoline compounds |
| US6894060B2 (en) | 2000-03-30 | 2005-05-17 | 3M Innovative Properties Company | Method for the treatment of dermal lesions caused by envenomation |
| US20020055517A1 (en) | 2000-09-15 | 2002-05-09 | 3M Innovative Properties Company | Methods for delaying recurrence of herpes virus symptoms |
| US6525064B1 (en) | 2000-12-08 | 2003-02-25 | 3M Innovative Properties Company | Sulfonamido substituted imidazopyridines |
| US6660735B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6677348B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Aryl ether substituted imidazoquinolines |
| US6660747B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US6664260B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Heterocyclic ether substituted imidazoquinolines |
| UA75622C2 (en) | 2000-12-08 | 2006-05-15 | 3M Innovative Properties Co | Aryl ether substituted imidazoquinolines, pharmaceutical composition based thereon |
| US6545017B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Urea substituted imidazopyridines |
| US6664264B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US6677347B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| US6667312B2 (en) | 2000-12-08 | 2003-12-23 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US20020107262A1 (en) | 2000-12-08 | 2002-08-08 | 3M Innovative Properties Company | Substituted imidazopyridines |
| JP2008531580A (en) | 2000-12-08 | 2008-08-14 | スリーエム イノベイティブ プロパティズ カンパニー | Compositions and methods for targeted delivery of immune response modifiers |
| US6545016B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Amide substituted imidazopyridines |
| US6664265B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| KR100455713B1 (en) | 2001-01-29 | 2004-11-06 | 호남석유화학 주식회사 | Multinuclear metallocene catalysts for olefin polymerization and process for olefin polymerization using the same |
| US7226928B2 (en) | 2001-06-15 | 2007-06-05 | 3M Innovative Properties Company | Methods for the treatment of periodontal disease |
| US20040235867A1 (en) | 2001-07-24 | 2004-11-25 | Bilodeau Mark T. | Tyrosine kinase inhibitors |
| CA2458876A1 (en) | 2001-08-30 | 2003-03-13 | 3M Innovative Properties Company | Methods of maturing plasmacytoid dendritic cells using immune response modifier molecules |
| JP4445262B2 (en) | 2001-10-09 | 2010-04-07 | アムジェン インコーポレイテッド | Imidazole derivatives as anti-inflammatory agents |
| US20030139364A1 (en) | 2001-10-12 | 2003-07-24 | University Of Iowa Research Foundation | Methods and products for enhancing immune responses using imidazoquinoline compounds |
| ATE416771T1 (en) | 2001-11-16 | 2008-12-15 | 3M Innovative Properties Co | N-Ä4-(4-AMINO-2-ETHYL-1H-IMIDAZOÄ4,5-CUCHINOLINE 1-YL)BUTYLUMETHANESULFONAMIDE, PHARMACEUTICAL COMPOSITION CONTAINING SAME AND USE THEREOF |
| ES2312659T3 (en) | 2001-11-29 | 2009-03-01 | 3M Innovative Properties Company | PHARMACEUTICAL FORMULATIONS THAT INCLUDE A MODIFIER OF THE IMMUNE RESPONSE. |
| US6677349B1 (en) | 2001-12-21 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| JP2005518433A (en) | 2002-02-22 | 2005-06-23 | スリーエム イノベイティブ プロパティズ カンパニー | Methods for reducing and treating UVB-induced immunosuppression |
| CA2484049A1 (en) | 2002-03-19 | 2003-10-02 | Powdermed Limited | Imidazoquinoline adjuvants for vaccines |
| GB0211649D0 (en) | 2002-05-21 | 2002-07-03 | Novartis Ag | Organic compounds |
| AU2003233519A1 (en) | 2002-05-29 | 2003-12-19 | 3M Innovative Properties Company | Process for imidazo(4,5-c)pyridin-4-amines |
| JP2005538057A (en) | 2002-06-07 | 2005-12-15 | スリーエム イノベイティブ プロパティズ カンパニー | Ether-substituted imidazopyridine |
| DK1543002T3 (en) | 2002-07-23 | 2006-12-27 | Teva Gyogyszergyar Zartkoeruee | Preparation of 1H-imidazo (4,5-c) quinoline-4-amines via 1H-imidazo (4,5-c) quinoline-4-phthalimide intermediates |
| PL374866A1 (en) | 2002-07-26 | 2005-11-14 | Teva Gyogyszergyar Zartköruen Muködo Reszvenytarsasag | Preparation of 1h-imidazo [4,5-c] quinolin-4-amines via novel 1h-imidazo [4,5-c] quinolin-4-cyano and 1h-imidazo [4,5-c] quinolin-4-carboxamide intermediates |
| AU2003299863B2 (en) | 2002-08-15 | 2009-09-24 | 3M Innovative Properties Company | Immunostimulatory compositions and methods of stimulating an immune response |
| US6818650B2 (en) | 2002-09-26 | 2004-11-16 | 3M Innovative Properties Company | 1H-imidazo dimers |
| WO2004053057A2 (en) | 2002-12-11 | 2004-06-24 | 3M Innovative Properties Company | Gene expression systems and recombinant cell lines |
| AU2003287316A1 (en) | 2002-12-11 | 2004-06-30 | 3M Innovative Properties Company | Assays relating to toll-like receptor activity |
| CA2510375A1 (en) | 2002-12-20 | 2004-07-15 | 3M Innovative Properties Company | Aryl / hetaryl substituted imidazoquinolines |
| WO2004060319A2 (en) | 2002-12-30 | 2004-07-22 | 3M Innovative Properties Company | Immunostimulatory combinations |
| WO2004071459A2 (en) * | 2003-02-13 | 2004-08-26 | 3M Innovative Properties Company | Methods and compositions related to irm compounds and toll-like receptor 8 |
| JP2006519020A (en) | 2003-02-27 | 2006-08-24 | スリーエム イノベイティブ プロパティズ カンパニー | Selective regulation of TLR-mediated biological activity |
| WO2004078138A2 (en) | 2003-03-04 | 2004-09-16 | 3M Innovative Properties Company | Prophylactic treatment of uv-induced epidermal neoplasia |
| US7163947B2 (en) | 2003-03-07 | 2007-01-16 | 3M Innovative Properties Company | 1-Amino 1H-imidazoquinolines |
| JP4891066B2 (en) | 2003-03-13 | 2012-03-07 | スリーエム イノベイティブ プロパティズ カンパニー | How to improve skin quality |
| US7699057B2 (en) | 2003-03-13 | 2010-04-20 | 3M Innovative Properties Company | Methods for treating skin lesions |
| EP1603476A4 (en) | 2003-03-13 | 2010-01-13 | 3M Innovative Properties Co | Method of tattoo removal |
| EP1613956A2 (en) | 2003-03-25 | 2006-01-11 | 3M Innovative Properties Company | Selective activation of cellular activities mediated through a common toll-like receptor |
| US20040192585A1 (en) | 2003-03-25 | 2004-09-30 | 3M Innovative Properties Company | Treatment for basal cell carcinoma |
| US20040265351A1 (en) | 2003-04-10 | 2004-12-30 | Miller Richard L. | Methods and compositions for enhancing immune response |
| WO2004108072A2 (en) | 2003-04-10 | 2004-12-16 | 3M Innovative Properties Company | Delivery of immune response modifier compounds using metal-containing particulate support materials |
| US20040214851A1 (en) | 2003-04-28 | 2004-10-28 | 3M Innovative Properties Company | Compositions and methods for induction of opioid receptors |
| US7731967B2 (en) | 2003-04-30 | 2010-06-08 | Novartis Vaccines And Diagnostics, Inc. | Compositions for inducing immune responses |
| US20050032829A1 (en) | 2003-06-06 | 2005-02-10 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| US6943255B2 (en) | 2003-06-06 | 2005-09-13 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| US20050106300A1 (en) | 2003-06-30 | 2005-05-19 | Purdue Research Foundation | Method for producing a material having an increased solubility in alcohol |
| AU2004261987A1 (en) | 2003-07-31 | 2005-02-10 | 3M Innovative Properties Company | Compositions for encapsulation and controlled release |
| WO2005016273A2 (en) | 2003-08-05 | 2005-02-24 | 3M Innovative Properties Company | Infection prophylaxis using immune response modifier compounds |
| WO2005018556A2 (en) | 2003-08-12 | 2005-03-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazo-containing compounds |
| EP1653959B1 (en) | 2003-08-14 | 2015-05-27 | 3M Innovative Properties Company | Lipid-modified immune response modifiers |
| CA2551075A1 (en) | 2003-08-25 | 2005-03-03 | 3M Innovative Properties Company | Immunostimulatory combinations and treatments |
| WO2005020912A2 (en) | 2003-08-25 | 2005-03-10 | 3M Innovative Properties Company | Delivery of immune response modifier compounds |
| RU2006105101A (en) | 2003-08-27 | 2007-10-10 | 3М Инновейтив Пропертиз Компани (US) | Aryloxy and arylalkylene-substituted substituted imidazoquinolines |
| AU2004268665A1 (en) | 2003-09-02 | 2005-03-10 | 3M Innovative Properties Company | Methods related to the treatment of mucosal associated conditions |
| AU2004270201A1 (en) | 2003-09-05 | 2005-03-17 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| NZ545536A (en) | 2003-09-05 | 2010-04-30 | Anadys Pharmaceuticals Inc | TLR7 ligands for the treatment of hepatitis C |
| EP1664342A4 (en) | 2003-09-17 | 2007-12-26 | 3M Innovative Properties Co | Selective modulation of tlr gene expression |
| WO2005033049A2 (en) | 2003-10-01 | 2005-04-14 | Taro Pharmaceuticals U.S.A., Inc. | METHOD OF PREPARING 4-AMINO-1H-IMIDAZO(4,5-c)QUINOLINES AND ACID ADDITION SALTS THEREOF |
| KR101154101B1 (en) | 2003-10-03 | 2012-06-11 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | Alkoxy substituted imidazoquinolines |
| US7544697B2 (en) | 2003-10-03 | 2009-06-09 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and analogs thereof |
| US20090075980A1 (en) | 2003-10-03 | 2009-03-19 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and Analogs Thereof |
| US20050096259A1 (en) | 2003-10-31 | 2005-05-05 | 3M Innovative Properties Company | Neutrophil activation by immune response modifier compounds |
| ITMI20032121A1 (en) | 2003-11-04 | 2005-05-05 | Dinamite Dipharma Spa In Forma Abbr Eviata Dipharm | PROCEDURE FOR THE PREPARATION OF IMIQUIMOD AND ITS INTERMEDIATES |
| CN1906192A (en) | 2003-11-14 | 2007-01-31 | 3M创新有限公司 | Hydroxylamine substituted imidazo ring compounds |
| JP2007511527A (en) | 2003-11-14 | 2007-05-10 | スリーエム イノベイティブ プロパティズ カンパニー | Oxime-substituted imidazo ring compounds |
| BRPI0416801A (en) | 2003-11-21 | 2007-01-09 | Novartis Ag | 1h-imidazoquinoline derivatives as protein synase inhibitors |
| AR046845A1 (en) | 2003-11-21 | 2005-12-28 | Novartis Ag | DERIVATIVES OF 1H-IMIDAZO [4,5-C] QUINOLINE FOR THE TREATMENT OF PROTEIN-KINASE DEPENDENT DISEASES |
| NZ547467A (en) | 2003-11-25 | 2010-06-25 | 3M Innovative Properties Co | Substituted imidazo ring system and methods |
| AU2004293096A1 (en) | 2003-11-25 | 2005-06-09 | 3M Innovative Properties Company | Hydroxylamine and oxime substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| EP1689361A4 (en) | 2003-12-02 | 2009-06-17 | 3M Innovative Properties Co | Therapeutic combinations and methods including irm compounds |
| AR048289A1 (en) | 2003-12-04 | 2006-04-19 | 3M Innovative Properties Co | ETERES OF RING IMIDAZO SULFONA REPLACED. |
| EP1701955A1 (en) | 2003-12-29 | 2006-09-20 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| AU2004312510A1 (en) | 2003-12-29 | 2005-07-21 | 3M Innovative Properties Company | Piperazine, [1,4]diazepane, [1,4]diazocane, and [1,5]diazocane fused imidazo ring compounds |
| EP1699788A2 (en) | 2003-12-30 | 2006-09-13 | 3M Innovative Properties Company | Imidazoquinolinyl, imidazopyridinyl and imidazonaphthyridinyl sulfonamides |
| WO2005065678A1 (en) | 2003-12-30 | 2005-07-21 | 3M Innovative Properties Company | Immunomodulatory combinations |
| EP1699398A4 (en) | 2003-12-30 | 2007-10-17 | 3M Innovative Properties Co | Enhancement of immune responses |
| JP4991520B2 (en) | 2004-03-15 | 2012-08-01 | スリーエム イノベイティブ プロパティズ カンパニー | Immune response modulator formulation and method |
| US8697873B2 (en) | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| JP2007532572A (en) | 2004-04-09 | 2007-11-15 | スリーエム イノベイティブ プロパティズ カンパニー | Methods, compositions and preparations for delivering immune response modifiers |
| EP1755665A4 (en) | 2004-04-28 | 2010-03-03 | 3M Innovative Properties Co | Compositions and methods for mucosal vaccination |
| US20080015184A1 (en) | 2004-06-14 | 2008-01-17 | 3M Innovative Properties Company | Urea Substituted Imidazopyridines, Imidazoquinolines, and Imidazonaphthyridines |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| WO2006009826A1 (en) | 2004-06-18 | 2006-01-26 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7884207B2 (en) | 2004-06-18 | 2011-02-08 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| WO2006093514A2 (en) | 2004-06-18 | 2006-09-08 | 3M Innovative Properties Company | Aryl and arylalkylenyl substituted thiazoloquinolines and thiazolonaphthyridines |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| WO2006009832A1 (en) | 2004-06-18 | 2006-01-26 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US20060045885A1 (en) | 2004-08-27 | 2006-03-02 | Kedl Ross M | Method of eliciting an immune response against HIV |
| US20060045886A1 (en) | 2004-08-27 | 2006-03-02 | Kedl Ross M | HIV immunostimulatory compositions |
| US20090270443A1 (en) | 2004-09-02 | 2009-10-29 | Doris Stoermer | 1-amino imidazo-containing compounds and methods |
| WO2006029115A2 (en) | 2004-09-02 | 2006-03-16 | 3M Innovative Properties Company | 2-amino 1h imidazo ring systems and methods |
| WO2006028962A2 (en) | 2004-09-02 | 2006-03-16 | 3M Innovative Properties Company | 1-alkoxy 1h-imidazo ring systems and methods |
| US20080193468A1 (en) | 2004-09-08 | 2008-08-14 | Children's Medical Center Corporation | Method for Stimulating the Immune Response of Newborns |
| CN101824034A (en) | 2004-09-14 | 2010-09-08 | 诺华疫苗和诊断公司 | Imidazoquinolie compounds |
| WO2006042254A2 (en) | 2004-10-08 | 2006-04-20 | 3M Innovative Properties Company | Adjuvant for dna vaccines |
| WO2006063152A2 (en) | 2004-12-08 | 2006-06-15 | 3M Innovative Properties Company | Immunostimulatory combinations and methods |
| US8080560B2 (en) | 2004-12-17 | 2011-12-20 | 3M Innovative Properties Company | Immune response modifier formulations containing oleic acid and methods |
| JP5543068B2 (en) | 2004-12-30 | 2014-07-09 | スリーエム イノベイティブ プロパティズ カンパニー | Chiral fused [1,2] imidazo [4,5-c] cyclic compound |
| AU2005326708C1 (en) | 2004-12-30 | 2012-08-30 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| EP1830876B1 (en) | 2004-12-30 | 2015-04-08 | Meda AB | Use of imiquimod for the treatment of cutaneous metastases derived from a breast cancer tumor |
| AR052447A1 (en) | 2004-12-30 | 2007-03-21 | 3M Innovative Properties Co | 1- (2-METIPROPIL) ETANSULFONATE -1H-IMIDAZO [4,5-C] [1,5] NAFTIRIDIN-4- AMINA AND 1- (2- METIPROPIL) -1H-IMIDAZO [4,5-C ] [1,5] NAFTIRIDIN-4-AMINA |
| US20080119508A1 (en) | 2004-12-30 | 2008-05-22 | 3M Innovative Properties Company | Multi-Route Administration Of Immune Response Modifier Compounds |
| US8436176B2 (en) | 2004-12-30 | 2013-05-07 | Medicis Pharmaceutical Corporation | Process for preparing 2-methyl-1-(2-methylpropyl)-1H-imidazo[4,5-c][1,5]naphthyridin-4-amine |
| EP1848967B1 (en) | 2005-02-02 | 2012-02-08 | Mocon, Inc. | Instrument and method for detecting and reporting the size of leaks in hermetically sealed packaging |
| WO2006084251A2 (en) | 2005-02-04 | 2006-08-10 | Coley Pharmaceutical Group, Inc. | Aqueous gel formulations containing immune reponse modifiers |
| AU2006338521A1 (en) | 2005-02-09 | 2007-10-11 | Coley Pharmaceutical Group, Inc. | Oxime and hydroxylamine substituted thiazolo(4,5-c) ring compounds and methods |
| WO2006086449A2 (en) | 2005-02-09 | 2006-08-17 | Coley Pharmaceutical Group, Inc. | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| CA2597595A1 (en) | 2005-02-11 | 2006-08-17 | Coley Pharmaceutical Group, Inc. | Substituted fused [1,2]imidazo[4,5-c] ring compounds and methods |
| WO2006091394A2 (en) | 2005-02-11 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Substituted imidazoquinolines and imidazonaphthyridines |
| US8846710B2 (en) | 2005-02-23 | 2014-09-30 | 3M Innovative Properties Company | Method of preferentially inducing the biosynthesis of interferon |
| AU2006223634A1 (en) | 2005-02-23 | 2006-09-21 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinolines |
| CA2598639A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazonaphthyridines |
| AU2006216798A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinoline compounds and methods |
| WO2006099275A2 (en) | 2005-03-14 | 2006-09-21 | 3M Innovative Properties Company | Method of treating actinic keratosis |
| EP1863814A1 (en) | 2005-04-01 | 2007-12-12 | Coley Pharmaceutical Group, Inc. | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| JP2008538119A (en) | 2005-04-01 | 2008-10-09 | コーリー ファーマシューティカル グループ,インコーポレーテッド | Pyrazolo [3,4-c] quinolines, pyrazolo [3,4-c] naphthyridines, analogs thereof, and methods |
| WO2006107853A2 (en) | 2005-04-01 | 2006-10-12 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridine-1,4-diamines and analogs thereof |
| AU2006241166A1 (en) | 2005-04-25 | 2006-11-02 | 3M Innovative Properties Company | Immunostimulatory compositions |
| MX2008002414A (en) | 2005-09-02 | 2008-03-27 | Pfizer | Hydroxy substituted 1h-imidazopyridines and methods. |
| ZA200803029B (en) | 2005-09-09 | 2009-02-25 | Coley Pharm Group Inc | Amide and carbamate derivatives of alkyl substituted /V-[4-(4-amino-1H-imidazo[4,5-c] quinolin-1-yl)butyl] methane-sulfonamides and methods |
| US8889154B2 (en) | 2005-09-15 | 2014-11-18 | Medicis Pharmaceutical Corporation | Packaging for 1-(2-methylpropyl)-1H-imidazo[4,5-c] quinolin-4-amine-containing formulation |
| BRPI0616338A2 (en) | 2005-09-23 | 2011-06-14 | Coley Pharm Group Inc | Method for 1h-imidazo [4,5-c] pyridines and analogs thereof |
| KR20080083270A (en) | 2005-11-04 | 2008-09-17 | 콜레이 파마시티컬 그룹, 인코포레이티드 | Hydroxy and alkoxy substituted 1h-imidazoquinolines and methods |
| WO2007079203A2 (en) | 2005-12-28 | 2007-07-12 | 3M Innovative Properties Company | Treatment for cutaneous t cell lymphoma |
| WO2007092641A2 (en) | 2006-02-10 | 2007-08-16 | Coley Pharmaceutical Group, Inc. | Method for substituted 1h-imidazo[4,5-c]pyridines |
| EP3085373A1 (en) | 2006-02-22 | 2016-10-26 | 3M Innovative Properties Company | Immune response modifier conjugates |
| WO2007106852A2 (en) | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Substituted fused[1,2]imidazo[4,5-c] ring compounds and methods |
| WO2007106854A2 (en) | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| WO2007149802A2 (en) | 2006-06-19 | 2007-12-27 | 3M Innovative Properties Company | Formulation for delivery of immune response modifiers |
| WO2008008432A2 (en) | 2006-07-12 | 2008-01-17 | Coley Pharmaceutical Group, Inc. | Substituted chiral fused( 1,2) imidazo (4,5-c) ring compounds and methods |
| AU2007279376B2 (en) | 2006-07-31 | 2012-09-06 | Wirra Ip Pty Ltd | Immune response modifier compositions and methods |
| US8178539B2 (en) | 2006-09-06 | 2012-05-15 | 3M Innovative Properties Company | Substituted 3,4,6,7-tetrahydro-5H-1,2a,4a,8-tetraazacyclopenta[cd]phenalenes and methods |
| US20080149123A1 (en) | 2006-12-22 | 2008-06-26 | Mckay William D | Particulate material dispensing hairbrush with combination bristles |
-
2006
- 2006-02-22 AU AU2006223634A patent/AU2006223634A1/en not_active Abandoned
- 2006-02-22 US US11/885,005 patent/US8178677B2/en not_active Expired - Fee Related
- 2006-02-22 JP JP2007557115A patent/JP2008543725A/en not_active Withdrawn
- 2006-02-22 WO PCT/US2006/006223 patent/WO2006098852A2/en active Application Filing
- 2006-02-22 EP EP06758163A patent/EP1851224A2/en not_active Withdrawn
- 2006-02-22 CA CA002598695A patent/CA2598695A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992015582A1 (en) * | 1991-03-01 | 1992-09-17 | Minnesota Mining And Manufacturing Company | 1-SUBSTITUTED, 2-SUBSTITUTED 1H-IMIDAZO[4,5-c]QUINOLIN-4-AMINES |
| WO1992015581A1 (en) * | 1991-03-01 | 1992-09-17 | Minnesota Mining And Manufacturing Company | PROCESS FOR IMIDAZO[4,5-c]QUINOLIN-4-AMINES |
| US5389640A (en) * | 1991-03-01 | 1995-02-14 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5605899A (en) * | 1991-03-01 | 1997-02-25 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
Cited By (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US10071156B2 (en) | 2005-02-04 | 2018-09-11 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| EP3085373A1 (en) | 2006-02-22 | 2016-10-26 | 3M Innovative Properties Company | Immune response modifier conjugates |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US10144735B2 (en) | 2006-12-22 | 2018-12-04 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
| US10005772B2 (en) | 2006-12-22 | 2018-06-26 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
| JP2014043458A (en) * | 2008-03-24 | 2014-03-13 | 4Sc Discovery Gmbh | Novel substituted imidazoquinoline |
| EP3153180A1 (en) | 2011-06-03 | 2017-04-12 | 3M Innovative Properties Company | Heterobifunctional linkers with polyethylene glycol segments and immune response modifier conjugates made therefrom |
| WO2012167081A1 (en) * | 2011-06-03 | 2012-12-06 | 3M Innovative Properties Company | Hydrazino 1h-imidazoquinolin-4-amines and conjugates made therefrom |
| US9585968B2 (en) * | 2011-06-03 | 2017-03-07 | 3M Innovative Properties Company | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom |
| AU2012261959B2 (en) * | 2011-06-03 | 2015-12-03 | Solventum Intellectual Properties Company | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom |
| US20150352218A1 (en) * | 2011-06-03 | 2015-12-10 | 3M Innovative Properties Company | Hydrazino 1h-imidazoquinolin-4-amines and conjugates made therefrom |
| US10406142B2 (en) * | 2011-06-03 | 2019-09-10 | 3M Lnnovative Properties Company | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom |
| CN103582640A (en) * | 2011-06-03 | 2014-02-12 | 3M创新有限公司 | Hydrazino 1H-imidazoquinolin-4-amines and conjugates made therefrom |
| EP3756669A1 (en) | 2013-01-07 | 2020-12-30 | The Trustees of the University of Pennsylvania | Compositions for use for treating cutaneous t cell lymphoma |
| WO2014107663A2 (en) | 2013-01-07 | 2014-07-10 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating cutaneous t cell lymphoma |
| US10526309B2 (en) | 2015-10-02 | 2020-01-07 | The University Of North Carolina At Chapel Hill | Pan-TAM inhibitors and Mer/Axl dual inhibitors |
| US11072612B2 (en) | 2016-04-19 | 2021-07-27 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| JP2019513809A (en) * | 2016-04-19 | 2019-05-30 | イネイト・テューマー・イミュニティ・インコーポレイテッドInnate Tumor Immunity, Inc. | NLRP3 modifier |
| CN109071535A (en) * | 2016-04-19 | 2018-12-21 | 先天肿瘤免疫公司 | NLRP3 regulator |
| US10533007B2 (en) | 2016-04-19 | 2020-01-14 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| US10556903B2 (en) | 2016-04-19 | 2020-02-11 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| CN109071535B (en) * | 2016-04-19 | 2021-10-08 | 先天肿瘤免疫公司 | NLRP3 modulators |
| WO2017184746A1 (en) * | 2016-04-19 | 2017-10-26 | Ifm Therapeutics, Inc | Nlrp3 modulators |
| US11827632B2 (en) | 2017-02-17 | 2023-11-28 | Innate Tumor Immunity, Inc | NLRP3 modulators |
| US10533005B2 (en) | 2017-02-17 | 2020-01-14 | Innate Tumor Immunity, Inc. | NLRP3 modulators |
| WO2019166946A1 (en) | 2018-02-28 | 2019-09-06 | Pfizer Inc. | Il-15 variants and uses thereof |
| WO2019224716A2 (en) | 2018-05-23 | 2019-11-28 | Pfizer Inc. | Antibodies specific for gucy2c and uses thereof |
| US11434292B2 (en) | 2018-05-23 | 2022-09-06 | Pfizer Inc. | Antibodies specific for CD3 and uses thereof |
| WO2019224715A1 (en) | 2018-05-23 | 2019-11-28 | Pfizer Inc. | Antibodies specific for cd3 and uses thereof |
| WO2020128893A1 (en) | 2018-12-21 | 2020-06-25 | Pfizer Inc. | Combination treatments of cancer comprising a tlr agonist |
| WO2021124073A1 (en) | 2019-12-17 | 2021-06-24 | Pfizer Inc. | Antibodies specific for cd47, pd-l1, and uses thereof |
| EP4178574A4 (en) * | 2020-07-08 | 2024-04-10 | Purdue Research Foundation | Compounds, compositions, and methods for the treatment of fibrotic diseases and cancer |
| WO2022013775A1 (en) | 2020-07-17 | 2022-01-20 | Pfizer Inc. | Therapeutic antibodies and their uses |
| CN112400801A (en) * | 2020-12-07 | 2021-02-26 | 天津医科大学第二医院 | Laryngeal precancerous lesion animal model and construction method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1851224A2 (en) | 2007-11-07 |
| US20090029988A1 (en) | 2009-01-29 |
| CA2598695A1 (en) | 2006-09-21 |
| WO2006098852A3 (en) | 2007-05-31 |
| US8178677B2 (en) | 2012-05-15 |
| JP2008543725A (en) | 2008-12-04 |
| AU2006223634A1 (en) | 2006-09-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2006098852A2 (en) | Hydroxyalkyl substituted imidazoquinolines | |
| JP5247458B2 (en) | Hydroxy and alkoxy substituted 1H-imidazoquinolines and methods | |
| US8343993B2 (en) | Hydroxyalkyl substituted imidazonaphthyridines | |
| US8846710B2 (en) | Method of preferentially inducing the biosynthesis of interferon | |
| JP5209312B2 (en) | 1-alkoxy 1H-imidazo ring systems and methods | |
| US8143270B2 (en) | 2-amino 1H-in-imidazo ring systems and methods | |
| WO2006091567A2 (en) | Hydroxyalkyl substituted imidazoquinoline compounds and methods | |
| WO2006038923A2 (en) | Aryl substituted imidazonaphthyridines | |
| WO2006107853A2 (en) | Pyrazolopyridine-1,4-diamines and analogs thereof | |
| AU2006216997A1 (en) | Substituted imidazoquinolines and imidazonaphthyridines | |
| WO2007079086A1 (en) | Pyrazoloalkyl substituted imidazo ring compounds and methods | |
| WO2005123080A2 (en) | Nitrogen-containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006223634 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006758163 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2598695 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007557115 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2006223634 Country of ref document: AU Date of ref document: 20060222 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11885005 Country of ref document: US |