WO2007002518A1 - Delayed release or extended-delayed release dosage forms of pramipexole - Google Patents
Delayed release or extended-delayed release dosage forms of pramipexole Download PDFInfo
- Publication number
- WO2007002518A1 WO2007002518A1 PCT/US2006/024665 US2006024665W WO2007002518A1 WO 2007002518 A1 WO2007002518 A1 WO 2007002518A1 US 2006024665 W US2006024665 W US 2006024665W WO 2007002518 A1 WO2007002518 A1 WO 2007002518A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pramipexole
- release
- delayed
- pharmaceutical composition
- polymer
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
- A61K9/1623—Sugars or sugar alcohols, e.g. lactose; Derivatives thereof; Homeopathic globules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid, pantothenic acid
- A61K31/198—Alpha-aminoacids, e.g. alanine, edetic acids [EDTA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/428—Thiazoles condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/006—Oral mucosa, e.g. mucoadhesive forms, sublingual droplets; Buccal patches or films; Buccal sprays
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0065—Forms with gastric retention, e.g. floating on gastric juice, adhering to gastric mucosa, expanding to prevent passage through the pylorus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2077—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets
- A61K9/2081—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets with microcapsules or coated microparticles according to A61K9/50
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
- A61K9/209—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat containing drug in at least two layers or in the core and in at least one outer layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/284—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone
- A61K9/2846—Poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/2853—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, polyethylene oxide, poloxamers, poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/286—Polysaccharides, e.g. gums; Cyclodextrin
- A61K9/2866—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2886—Dragees; Coated pills or tablets, e.g. with film or compression coating having two or more different drug-free coatings; Tablets of the type inert core-drug layer-inactive layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4808—Preparations in capsules, e.g. of gelatin, of chocolate characterised by the form of the capsule or the structure of the filling; Capsules containing small tablets; Capsules with outer layer for immediate drug release
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4891—Coated capsules; Multilayered drug free capsule shells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5026—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5031—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
- A61K9/5047—Cellulose ethers containing no ester groups, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
- A61K9/5078—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings with drug-free core
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0004—Osmotic delivery systems; Sustained release driven by osmosis, thermal energy or gas
Definitions
- a movement disorder is a neurological disturbance that involves one or more muscles or muscle groups. Movement disorders affect a significant portion of the population, causing disability as well as distress. Movement disorders include Parkinson's disease, Huntington's chorea, progressive supranuclear palsy, Wilson's disease, Tourette's syndrome, epilepsy, tardive dyskinesia, and various chronic tremors, tics and dystonias. Different clinically observed movement disorders can be traced to the same or similar areas of the brain. For example, abnormalities of basal ganglia (a large cluster of cells deep in the hemispheres of the brain) are postulated as a causative factor in diverse movement disorders.
- basal ganglia a large cluster of cells deep in the hemispheres of the brain
- Parkinson's disease is a common disabling disease of old age affecting about one percent of the population over the age of 60 in the United States. The incidence of Parkinson's disease increases with age and the cumulative lifetime risk of an individual developing the disease is about 1 in 40. It is a progressive neurodegenerative disorder of the extra pyramidal nervous system, and is associated with the depletion of dopamine from cells in the corpus striatum. The disease affects the mobility and control of the skeletal muscular system. Its characteristic features include resting tremor, bradykinetic movements, rigidity and postural change.
- Parkinson's disease A perceived pathophysiological cause of Parkinson's disease is progressive destruction of dopamine-producing cells in the basal ganglia which comprise the pars compartum of the substantia nigra, basal nuclei located in the brain stem. Loss of dopamineric neurons results in a relative excess of acetylcholine. See Jellinger, Post Mortem Studies in Parkinson 's Disease - Is It Possible to Detect Brain Areas For Specific Symptoms? J. Neural. Transm. 56(Supp): 1-29:1999. Parkinson's disease often begins with mild limb stiffness and infrequent tremors and progresses over a period often or more years to frequent tremors and memory impairment, to uncontrollable tremors and dementia.
- Pramipexole is a non-ergot potent dopamine receptor agonist and has been shown to be clinically effective in treating Parkinson's patients in early PD. It has preferential affinity for D3 receptor within the D2 subfamily of dopamine receptors and inhibits dopamine synthesis and release. It has been found effective as a monotherapy as well as an adjunct to levodopa therapy in patients with advance disabilities. Pramipexole is marketed as immediate release tablets by Pfizer under the trade name MIRAPEX ® .
- Tardive Dyskinesia is a chronic disorder of the nervous system, characterized by involuntary, irregular rhythmic movements of the mouth, tongue, and facial muscles. The upper extremities also may be involved. These movements may be accompanied, to a variable extent, by other involuntary movements and movement disorders. These include rocking, writhing, or twisting movements of the trunk (tardive dystonia), forcible eye closure (tardive blepharospasm), an irresistible impulse to move continually (tardive akathisia), jerking movements of the neck (tardive spasmodic torticollis), and disrupted respiratory movements (respiratory dyskinesia).
- TD TD cases are caused by the prolonged use of antipsychotic drugs (neuroleptics).
- a relatively small number are caused by the use of other medications, such as metoclopramide, that, like neuroleptics, block dopamine receptors.
- TD often manifests or worsens in severity after neuroleptic drug therapy is discontinued. Resumption of neuroleptic therapy will temporarily suppress the involuntary movements, but may aggravate them in the long run.
- TD affects approximately 15-20% of patients treated with neuroleptic drugs (Khot et al., Neuroleptics and Classic Tardive Dyskinesia, in Lang AE, Werner WJ (eds.): Drug Induced Movement Disorders, Futura Publishing Co., 1992, pp 121-166). Therefore, the condition affects hundreds of thousands of people in the United States alone. The cumulative incidence of TD is substantially higher in women, in older people, and in those being treated with neuroleptics for conditions other than schizophrenia, such as bipolar disorder (manic- depressive illness) (see, e.g., Hayashi et al, Clin. Neuropharmacol. 19: 390, 1996; Jeste et al., Arck Gen. Psychiatry 52: 756, 1995).
- TD does not respond in general to antiparkinson drugs (Decker et al, New Eng. J Med. Oct. 7, p. 861, 1971).
- Focal Dystonias (FD) are a class of related movement disorders involving the intermittent sustained contraction of a group of muscles. The prevalence of focal dystonias in one US county was estimated as 287 per million (Monroe County Study); this suggests that at least 70,000 people are affected in the US alone. The spasms of focal dystonia can last many seconds at a time, causing major disruption of the function of the affected area. Some of the focal dystonias are precipitated by repetitive movements; writer's cramp is the best known example.
- Focal dystonia can involve the face (e.g., blepharospasm, mandibular dystonia), the neck (torticollis), the limbs (e.g., writer's cramp), or the trunk.
- Dystonia can occur spontaneously or can be precipitated by exposure to neuroleptic drugs and other dopamine receptor blockers (tardive dystonia).
- neuroleptic drugs and other dopamine receptor blockers e.g., writer's cramp
- No systemic drug therapy is generally effective, but some drugs give partial relief to some patients. Those most often prescribed are anticholinergics, baclofen, benzodiazepines, and dopamine agonists and antagonists. The most consistently effective treatment is the injection of botulinum toxin into affected muscles.
- a tic is an abrupt repetitive movement, gesture, or utterance that often mimics a normal type of behavior.
- Motor tics include movements such as eye blinking, head jerks or shoulder shrugs, but can vary to more complex purposive-appearing behaviors such as facial expressions of emotion or meaningful gestures of the arms and head.
- the movement can be obscene (copropraxia) or self-injurious.
- Phonic or vocal tics range from throat clearing sounds to complex vocalizations and speech, sometimes with coprolalia (obscene speech) (Leckman et al, supra).
- Tics are irregular in time, though consistent regarding the muscle groups involved. Characteristically, they can be suppressed for a short time by voluntary effort.
- Tics are estimated to affect 1% to 13% of boys and 1% to 11% of girls, the male- female ratio being less than 2 to 1. Approximately 5% of children between the ages of 7 and 11 years are affected with tic behavior (Leckman et al, Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997). The estimated prevalence of multiple tics with vocalization, e.g., Tourette's syndrome, varies among different reports, ranging from 5 per 10,000 to 5 per 1,000.
- Gilles de Ia Tourette syndrome is the most severe tic disorder. Tourette's syndrome is 3-4 times more common in boys than girls and 10 times more common in children and adolescents than in adults (Leckman et al., Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997; Esper et al, Tenn. Med. 90: 18-20, 1997). Patients with TS have multiple tics, including at least one vocal (phonic) tic. TS becomes apparent in early childhood with the presentation of simple motor tics, for example, eye blinking or head jerks. Initially, tics may come and go, but in time tics become persistent and severe, and begin to have adverse effects on the child and the child's family.
- Phonic tics manifest, on average, 1 to 2 years after the onset of motor tics. By the age of 10, most children have developed an awareness of the premonitory urges that frequently precede a tic. Such premonitions may enable the individual to voluntary suppress the tic, yet premonition unfortunately adds to the discomfort associated with having the disorder. By late adolescence/early adulthood, tic disorders can improve significantly in certain individuals. However, adults who continue to suffer from tics often have particularly severe and debilitating symptoms. (Leckman et al., Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997).
- pramipexole therapy is usually associated with a number of undesirable side effects, and patient compliance is a significant obstacle for effective treatment.
- MIRAPEX ® tablets often have to be given three times a day in equally divided doses. Therefore, compliance is a major problem with patients.
- a second problem for the multiple dose regimen is that the daily "peak and trough" blood levels produced by multiple daily doses result in fluctuating stimulation of the dopaminergic neurons. These fluctuations may contribute to the pathogenesis of the motor complications in Parkinson disease.
- Commonly occurring adverse effects associated with MIRAPEX ® include nausea, vomiting / emesis, weakness, dizziness, fainting, agitation, confusion, hallucinations, muscle twitching, uncontrollable movements, a tingling sensation, chest pain, insomnia, somnolence, decreased appetite, dry mouth, sweating, headache, constipation and gastric intestinal complications.
- Episodes of sudden uncontrollable somnolence have been reported in 22% of PD patients receiving pramipexole in a dose related manner.
- pramipexole has been reported to include orthostatic hypotension, the incidence of which is dose- and peak-related.
- compulsive gambling behaviors see Dodd et al, Pathological Gambling Caused by Drugs Used to Treat Parkinson Disease, Archives of Neurology vol. 62, Sept. 2005; Driver- Dunckley et al., Pathological Gambling Associated With Dopamine Agonist Therapy in Parkinson's Disease, Neurology, Vol. 61, August 2003
- excessive shopping, overeating, and hypersexuality have also been linked to MIRAPEX ® treatment.
- MIRAPEX ® Although the main indication of MIRAPEX ® is PD treatment, MIRAPEX ® is also used in lower doses to treat other movement disorders such as restless legs syndrome ⁇ e.g. , 0.125 mg daily versus 4.5 mg daily for Parkinson's). It is also being used off-label to treat depression as well as some sleep disorders.
- the currently available immediate release pramipexole formulation is not ideal as it is associated with poor patient compliance as well as treatment-emergent side effects that lead to poor patient tolerance. Therefore, there remains a clear-cut need for new treatments and improved dosage forms for various movement disorders, such as using pramipexole in alleviating at least one adverse effect associated with the treatment of Parkinson's disease.
- pramipexole The pharmacokinetics of pramipexole is linear, with plasma concentrations increasing proportionate with increase in dosage. Pramipexole is rapidly absorbed, reaching peak concentration in approximately 2 hours. The absolute bioavailability of pramipexole is greater than 90%. It undergoes little presystemic metabolism and is excreted virtually unchanged in the urine. However, it is difficult to reach the upper limit of the dose range using the currently available immediate release formulation. This is partly because both gastrointestinal and CNS side effects are more frequent during the initial ascending phase of the plasma profile. Nausea was primarily reported at the moment of peak pramipexole plasma levels, and increased with increase in dose. Data from other studies suggest that pramipexole may induce a locally mediated nausea via gastric irritation as rapid onset of the nausea was observed prior to achieving peak plasma levels.
- the present invention is directed to pramipexole pharmaceutical compositions that allow for highly controlled, delayed administration, preferably once-daily administration that release pramipexole over an extended period of time.
- the delayed / extended release dosage form is preferably at least equivalent in effectiveness to the conventional immediate release, three-time daily regimen, and provides average steady-state blood levels of pramipexole over a course of treatment.
- a delayed and/or once-a-day administration of pramipexole is advantageous over thrice-a-day administration in terms of patient compliance and reduced adverse events, thus providing better treatment of the conditions for which the pramipexole is indicated.
- the invention provides an oral delayed immediate release (DIR or DR) or delayed extended release (DXR) dosage form that provides continuous and stable delivery of pramipexole over extended duration and maintains the desired therapeutic effects, while minimizing, if not eliminating, the undesired side effects and with improved patient compliance.
- DIR or DR delayed immediate release
- DXR delayed extended release
- pramipexole and/or its prodrug(s) and/or stereoisomers are released at a rate that results in reduction in the frequency or severity of at least one adverse effect associated with pramipexole therapy.
- the dosage form releases pramipexole and/or its prodrug and/or stereoisomer at a rate that results in reduction in the frequency or severity of at least one adverse event associated with current pramipexole therapies, or allows for a more convenient dosing regimen than current therapies.
- a delayed-release (DR) pramipexole pharmaceutical composition in an orally deliverable form, comprising an enteric coating, a pramipexole core, and one or more pharmaceutically acceptable carriers and excipients, wherein the enteric coating reduces or substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
- DR delayed-release
- pramipexole is first released and/or absorbed in intestine.
- the enteric coating delays the release of pramipexole by at least about 1.5 - 2 hours, or 2-3 hours after ingestion.
- the enteric coating is selected from: cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate, hydroxypropyl methylcellulose succinate, cellulose acetate succinate, cellulose acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of methylmethacrylic acid and methyl methacrylate, copolymer of methyl acrylate, methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and maleic anhydride (Gantrez ES series), ethyl methyacrylate-methylmethacrylate- chlorotrimethylammonium ethyl acrylate copolymer, natural resins such as zein, shellac and copal collophorium, carboxymethyl
- the enteric coating becomes soluble at above pH 4.5, such as around pH 5.5-6.8.
- the pramipexole pharmaceutical composition comprises one or more pramipexole salts, derivatives and/or stereoisomers.
- the pramipexole salt is pramipexole dihydrochloride monohydrate.
- the pramipexole core is formulated as an immediate release (IR) composition.
- the pramipexole core is formulated as an extended release (XR) composition.
- XR extended release
- the XR composition is prepared by coating pramipexole- layered inert pellets with a release-controlling polymer.
- the release-controlling polymer is ethylcellulose-based.
- the release-controlling polymer is selected from: EUDRAGIT ® RL; EUDRAGIT ® RS; cellulose derivatives selected from: ethylcellulose aqueous dispersions (AQUACOAT ® , SURELEASE ® ), hydroxyethyl cellulose, hydroxypropyl cellulose, or hydroxypropyl methylcellulose; polyvinylpyrrolidone; polyvinylpyrrolidone / vinyl acetate copolymer; OPADRY ® , or equivalents thereof.
- the delayed-release pramipexole pharmaceutical composition is formulated to provide an effective dose over at least 4 - 24 hours after administration to the patient. In certain embodiments, the delayed-release pramipexole pharmaceutical composition is formulated to provide an effective plasma level over at least 8 - 16 hours after administration to the patient.
- the effective plasma level at a 0.375 mg dose is about 50 - 400 ⁇ g/mL for Parkinson's Disease treatment.
- the effective plasma levels may increase with an increase in dose levels, such as to 800-1800 pg/mL or even 400-4000 pg/mL.
- the pramipexole core comprises an XR portion and an IR portion.
- the XR portion and the IR portion are both present as multiparticulate beads / pellets embedded within an inactive dissolvable / disintegratable matrix.
- the XR portion and the IR portion are each present as a section of the pramipexole core.
- the XR portion is partially or completely covered by a rate- controlling coating that controls the release rate of the XR portion.
- the delayed-release pramipexole pharmaceutical composition is formulated as a once-a-day composition.
- the once-a-day composition contains about 0.375 mg, 0.5 mg, 1.0 mg, 1.5 mg, 3.0 mg, or 4.5 mg of pramipexole dihydrochloride monohydrate, or equivalent thereof.
- the delayed-release pramipexole pharmaceutical composition further comprises a bioadhesive layer that adheres to the lower GI tract.
- the bioadhesive layer comprises polymeric materials selected from polyamides, polyalkylene glycols, polyalkylene oxides, polyvinyl alcohols, polyvinylpyrrolidone, polyglycolides, polyurethanes, polymers of acrylic and methacrylic esters, polylactides, poly(butyric acid), polyanhydrides, polyorthoesters, poly(fumaric acid), poly(maleic acid), polycarbonates, polyalkylenes, polyalkylene terephthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polysiloxanes, polystyrene, poly(lactide-co-glycolide), chitosan, chitin, hyaluronic acid, hyaluronan, Carbopols, Corplex polymers, Polycarbophils-Cysteine (Thiomers), Chitosan-Thi
- the delayed-release pramipexole pharmaceutical composition upon administration to an individual, reduces or eliminates at least one undesirable side-effect selected from: nausea, emesis, insomnia, hallucination, somnolence, constipation, and gastric and/or intestinal complication as compared to treatment with a thrice-daily immediate release composition of the same overall dosage.
- the nausea or emesis results from a locally mediated gastric i ⁇ itation.
- the delayed-release pramipexole pharmaceutical composition provides substantially the same bioavailability and/or maximum blood concentration (C ma ⁇ ) compared to an immediate-release pramipexole pharmaceutical composition of the same dosage without the enteric coating.
- the delayed and/or extended release pharmaceutical composition provides a substantially reduced degree of fluctuation in plasma levels compared to an immediate release pharmaceutical composition of the pramipexole of the same dose administered three times daily.
- the delayed and/or extended release pharmaceutical composition is associated with reduced side effects (e.g., nausea, vomiting) compared to an immediate release pharmaceutical composition of the pramipexole of the same dose administered three times daily.
- the delayed-release pramipexole pharmaceutical composition is suitable for human treatment, or for veterinary treatment of a non-human mammal.
- Another aspect of the invention provides a method of preparing a pramipexole pharmaceutical composition, comprising coating a formulation comprising pramipexole with an enteric coating that reduces or substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
- GI gastrointestinal
- Another aspect of the invention provides a method of treating Parkinson's Disease in an individual, comprising administering to the individual a delayed-release pramipexole pharmaceutical composition as set forth above.
- Embodiments described herein are contemplated to be combined with each other embodiments as appropriate. Embodiments described in detail under one aspect of the invention may be equally applicable for the other aspects of the invention.
- Figures IA - IJ are schematic drawings (not to scale) illustrating cross-sectional views of exemplary designs for the subject delivery device.
- Figure 2 shows the results of an exemplary experiment comparing concentration of MIRAPEX ® 0.125 mg tablets over time in fed and fasted Beagle dogs.
- Figure 3 shows the results of an exemplary experiment comparing enteric-coated MIRAPEX ® 0.125 mg tablets and plain MIRAPEX ® 0.125 mg tablets in fasted Beagle dogs.
- Figure 4 shows the dissolution profile of pramipexole 0.375 mg Extended-Release (ER) and delayed extended release (DER) formulations.
- ER Extended-Release
- DER delayed extended release
- Figure 5 shows the results of comparing pramipexole 0.375 mg Extended Release (ER) and Delayed Extended Release (DER) formulations in fasted Beagles.
- Figure 6 shows the results of comparing pramipexole 0.375 mg Extended Release multiparticulate-based capsules and matrix-based tablet formulations, with 0.375 mg MIRAPAX ® Tablets Given in a Three-In-a-Day (TID) Dosing Regimen (0.125 mg x 3) in fed Beagles.
- Figure 8 shows a comparison between pramipexole 0.375 mg Extended Release Multiparticulate-based capsules formulations vs. 0.375mg Mirapex tablets given in a TID dosing regimen (0.125 mg x 3) in beagles.
- Figures 9A-C show the mean human pramipexole plasma concentration comparing pramipexole 0.375 mg extended release multiparticulate formulations with Mirapex® tablets, 0.375 mg (0.125 mg x 3).
- Figure 1OA is the in vitro dissolution profile of pramipexole extended release tablet formulation, 0.375 mg [5% coating (80 parts Surelease and 20 parts OPADRY), obtained using a USP II apparatus.
- Figure 1OB shows the in vivo PK performance of a pramipexole extended release tablet, 0.375 mg [5% coating (80 parts Surelease + 20 parts OPADRY)] and pramipexole extended release capsules, 0.375mg [8.3 % Ethocel and 5% Spheromer III coated) in fasted beagle dogs.
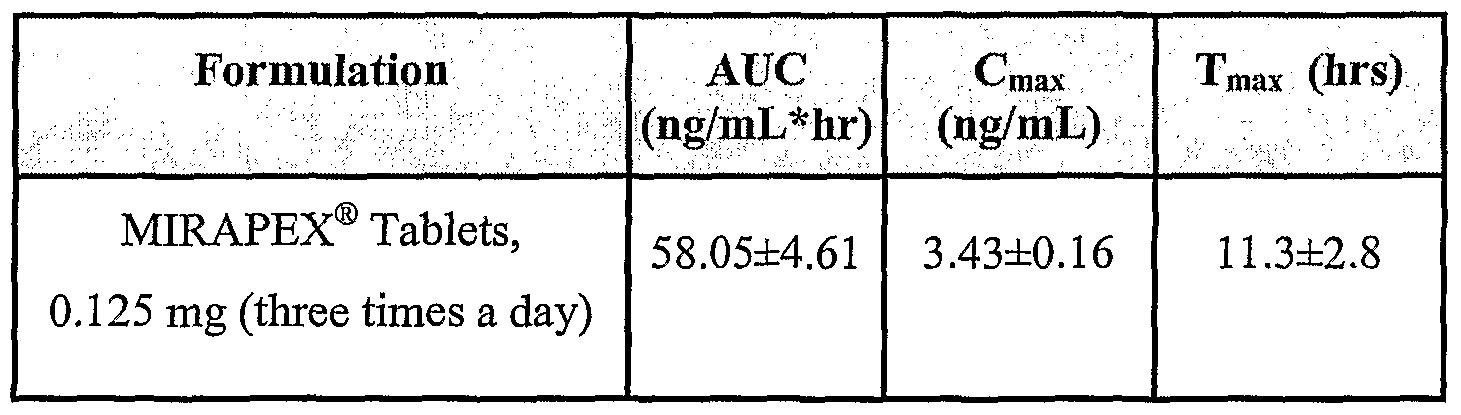
- Figure 11 is a comparison of pramipexole ER Formulations with Mirapex (0.125) mg tablets administered in three times a day dosing regimen.
- Figure 12 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
- Figure 13 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
- Figure 14 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
- Figure 15 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
- the present invention relates to pharmaceutical compositions and methods for the treatment of disorders for which pramipexole is administered. Such disorders include some sleep disorders and certain movement disorders (such as Parkinson's disease and restless legs syndrome, etc.).
- the present invention also relates to the methods for protecting neural cells.
- the pharmaceutical compositions and methods of the invention relate to the use of pramipexole either alone or in combination with other active agents or pharmaceutical compositions suitable for the treatment of such diseases or the prevention or inhibition of diseases using pramipexole as a neuroprotectant.
- the invention relates to particular pramipexole dosage forms (e.g., a delayed release, preferably once-a-day dosage form) that provide release profiles that are effective for the intended therapeutic use (e.g., ameliorating or overcoming symptoms of a movement disorder, such as Parkinson's disease), while reducing or avoiding at least one undesirable side-effect associated with conventional pramipexole treatment.
- a delayed release preferably once-a-day dosage form
- release profiles that are effective for the intended therapeutic use (e.g., ameliorating or overcoming symptoms of a movement disorder, such as Parkinson's disease), while reducing or avoiding at least one undesirable side-effect associated with conventional pramipexole treatment.
- pramipexole in the upper GI tract may cause local irritation and lead to at least one undesirable side effect of pramipexole treatment, including emesis and nausea.
- one or more undesirable side effects traditionally associated with administering pramipexole to an individual can be alleviated or even eliminated by delaying the release of pramipexole until the drug is in the lower GI tract, such as in the intestine (e.g., the small intestine, the colon, and/or the rectum).
- one way to reduce or eliminate the release of pramipexole in the stomach is to utilize an enteric coating, such that pramipexole is not substantially released in the acidic environment of the stomach.
- an enteric coating such that pramipexole is not substantially released in the acidic environment of the stomach.
- pramipexole is released either as an immediate release (IR) dosage form or as an extended release (XR) dosage form, or a mixture thereof. Since such dosage forms are also delayed-release dosage forms, they are referred to as delayed immediate release (DIR or DR) or delayed extended release (DXR) dosage forms, respectively.
- the DXR dosage form achieves the therapeutic benefit of the conventional thrice-a-day pramipexole regimen and yet is administered as a single daily administration, e.g., which contains substantially the same total dosage of pramipexole, yet releases the drug in a controlled manner and over an extended period of time, thus improving patient compliance, and alleviating and/or eliminating the undesirable daily "peak and trough" blood levels produced by multiple daily doses, and the associated fluctuating stimulation of the dopaminergic neurons.
- the dosage form may include bioadhesive layers that adhere to the lower GI tract, such as intestinal walls, to prolong the release of pramipexole in the lower GI tract.
- the bioadhesive layer may be inside or outside the enteric coating.
- the presence of the bioadhesive layer e.g., as a partial coating that is continuous or discontinuous
- the presence of the bioadhesive layer preferably does not substantially impede the release of pramipexole.
- the presence of the bioadhesive layer ⁇ e.g., as a partial coating that is continuous or discontinuous
- Pramipexole is not acid-sensitive, and thus need not be protected from the relative acidic environment of the upper GI tract per se. Indeed, in certain embodiments, pramipexole needs to be delivered to the lower GI tract, for example, for targeted treatment of certain colon diseases.
- the present invention is based in part on the unexpected discovery that by by-passing the release of pramipexole in the upper GI tract (e.g., the stomach) it is possible to avoid one or more undesirable side effects typically associated with pramipexole treatments, such as nausea and/or emesis.
- compositions of the present invention maybe in the form of, among others, a granule, tablet (including matrix or osmotic), pellet, powder, sachet, capsule, gel, dispersion, solution or suspension.
- a granule, tablet including matrix or osmotic
- pellet powder
- sachet capsule
- gel dispersion
- solution or suspension a granule, tablet or osmotic
- the dosage forms be composed in such a manner as to achieve the profiles set forth herein.
- Another aspect of the invention provides a method for making the pharmaceutical compositions with one or more features as described above.
- Another aspect of the invention provides a method for using the pharmaceutical compositions with one or more features as described above, in treating a movement disorder, such as Parkinson's disease.
- Another aspect of the invention provides the use of a pharmaceutical composition with one or more features as described above in manufacturing medicaments for the treatment of a movement disorder, such as Parkinson's disease.
- the subject preparations and methods can be used as part of treatments for human and/or other animal subjects.
- other animal subjects to which the invention is applicable extend to both domestic animals and livestock, raised either as laboratory animals, pets or zoo animals, or for commercial purposes. Examples are rodents such as mice, rats, hamsters, or rabbits; dogs; cats; cattle; horses; sheep; hogs; and goats.
- water-soluble herein means having solubility of at least about 10 mg/ml.
- solubility herein means solubility in water at 20-25 0 C at any physiologically acceptable pH, for example at any pH in the range of about 4 to about 8.
- reference herein to solubility in water pertains to the salt, not to the free base form of pramipexole.
- Solid fraction is the ratio of absolute to apparent density of a compact of the starch.
- a “compact” herein is a compressed tablet, prepared for example on a tablet press, consisting only of a sample of starch for which it is desired to measure tensile strength.
- a “solid fraction representative of the tablet” is a solid fraction selected to be similar to the solid fraction of tablets prepared according to the invention. Typically a solid fraction of about 0.75 to about 0.85, illustratively 0.8, will be selected.
- oral means suitable for oral, including peroral and intra-oral (e.g., sublingual or buccal) administration, but tablets of the present invention are adapted primarily for peroral administration, i.e., for swallowing, typically whole (or, in certain embodiments, broken), with the aid of water or other drinkable fluid.
- a “subject” herein is an animal of any species, preferably mammalian, most preferably human.
- Conditions and disorders in a subject for which a particular agent is said herein to be “indicated” are not restricted to conditions and disorders for which the agent has been expressly approved by a regulatory authority, but also include other conditions and disorders known or believed by a physician to be amenable to treatment with the agent.
- Treatment herein embraces prophylactic treatment unless the context requires otherwise.
- adrenergic refers to neurotransmitters or neuromodulators chemically related to adrenaline (epinephrine) or to neurons which release such adrenergic mediators. Examples are dopamine, norepinephrine, and epinephrine. Such agents are also referred to as catecholamines, which are derived from the amino acid tyrosine.
- catechol moiety refers to a moiety with the following generic structure:
- a polymer may be functionalized by covalently attaching catechol moieties or compounds comprising catechol moieties.
- a compound comprising a catechol moiety may be blended with a polymer to form a simple mixture with no covalent association between the catechol moieties and the polymer.
- catecholamines refers to neurotransmitters that have a catechol ring (e.g., a 3, 4-dihydroxylated benzene ring). Examples are dopamine, norepinephrine, and epinephrine.
- cholinergic refers to neurotransmitters or neuromodulators chemically related to choline or to neurons which release such cholinergic mediators.
- dopaminergic refers to neurotransmitters or neuromodulators chemically related to dopamine or to neurons which release such dopaminergic mediators.
- dopamine refers to an adrenergic neurotransmitter, as is known in the art.
- ED50 means the dose of a drug which produces 50% of its maximum response or effect.
- an "effective amount" of, e.g., a movement disorder pharmaceutical composition, with respect to the subject method of treatment refers to an amount of the pharmaceutical composition in a preparation which, when applied as part of the subject dosage regimen brings about the desired correction / suppression of the movement disorder (e.g., dyskinesis and/or bradykinesis) according to clinically acceptable standards.
- the desired correction / suppression of the movement disorder e.g., dyskinesis and/or bradykinesis
- LD 5 0 means the dose of a drug which is lethal in 50% of test subjects.
- lethal therapeutic index refers to the therapeutic index of a drug defined as
- metabolites refers to active derivatives produced upon introduction of a compound into a biological milieu, such as a patient.
- a "patient,” “individual,” or “subject” to be treated by the subject method can mean either a human or non-human animal.
- prevent means reducing the probability / risk of developing a condition in a subject (e.g., a human), or delaying the onset of a condition in the subject, or lessening the severity of one or more symptoms of a condition (e.g., a movement disorder) that may develop in the subject, or any combination thereof.
- a condition e.g., a movement disorder
- prodrug is intended to encompass compounds which, under physiologic conditions, are converted into the therapeutically active agents of the present invention.
- a common method for making a prodrug is to include one or more selected moieties which are hydrolyzed under physiologic conditions to reveal the desired molecule.
- the prodrug is converted by an enzymatic activity of the host animal.
- protecting group as used herein means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations. Examples of such protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively.
- the field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991).
- SeU5o means the dose of a drug which is produces a particular side-effect in 50% of test subjects.
- side-effect therapeutic index refers to the therapeutic index of a drug defined as SeDso/EDso.
- statically significant means that the obtained results are not likely to be due to chance fluctuations at the specified level of probability.
- the level of significance equal to 0.05 and 0.01 means that the probability of error is 5 out of 100 and 1 out of 100, respectively.
- treat means to counteract a medical condition (e.g., a movement disorder) to the extent that the medical condition is improved according to clinically acceptable standard(s).
- a medical condition e.g., a movement disorder
- to treat a movement disorder means to improve the movement disorder or relieve symptoms of the particular movement disorder in a patient, wherein the improvement and relief are evaluated with a clinically acceptable standardized test (e.g., a patient self-assessment scale) and/or an empirical test (e.g., PET scan).
- a clinically acceptable standardized test e.g., a patient self-assessment scale
- an empirical test e.g., PET scan
- Cmax means maximum plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
- Cmin means minimum plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
- Cavg means average plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets). Cavg is calculated by AUC over a 24 hours intervals divided by 24.
- Tmax means the time to achieve maximum plasma concentrations produced by ingestion of of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
- AUC as used herein means the area under the plasma concentration-time curve, as calculated by the trapezoidal rule over the 24 hour interval for all the formulations.
- DFL Degree of Fluctuation
- Cmin and “trough levels” should be considered synonyms.
- Cmax and “peak levels” should be considered synonyms.
- Pramipexole (formula I below) is a dopamine D2 receptor agonist useful in treatment of Parkinson's disease and complications associated therewith.
- Pramipexole as its dihydrochloride salt is commercially available in the United States as MIRAPEX ® tablets of Pharmacia & Upjohn / Pfizer. These are marketed as immediate-release tablets in 0.125 mg, 0.25 mg, 0.5 mg, 1.0 mg and 1.5 mg strengths, designed for thrice-a-day oral administration of a single tablet each to provide a daily dose of 0.375 to 4.5 mg. See Physicians' Desk Reference 57th edition (2003), 2768-2772.
- pramipexole dihydrochloride monohydrate unless otherwise specified; e.g., 1.0 mg pramipexole dihydrochloride monohydrate is equivalent to about 0.7 mg pramipexole base.
- Other salt forms can be readily converted from the amount of pramipexole base contained therein.
- pramipexole or a salt thereof herein embraces racemates, enantiomers, polymorphs, hydrates and solvates thereof.
- Pramipexole (I) is used preferably in the form of its S-enantiomer, (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)- benzothiazole.
- a preferred salt of pramipexole is the dihydrochloride salt, most preferably in the form of the monohydrate.
- Pramipexole compositions of the invention are preferably suitable for administration no more than once daily. Such compositions are useful in treatment of any CNS condition or disorder for which pramipexole has therapeutic utility, but especially Parkinson's disease and complications associated therewith.
- Pramipexole and its salts useful herein can be prepared by processes known per se, including processes disclosed in patents and other literature pertaining to pramipexole.
- the amount of the pramipexole salt present in a composition of the invention is sufficient to provide a daily dose in one to a small plurality, for example one to about 4, of tablets to be administered at one time.
- the full daily dose is delivered in a single tablet.
- An amount of pramipexole salt, expressed as pramipexole dihydrochloride monohydrate equivalent, of about 0.1 to about 10 mg per tablet, or about 0.05% to about 5% by weight of the composition will generally be suitable.
- an amount of about 0.2 to about 6 mg, more preferably an amount of about 0.3 to about 5 mg, per tablet is present.
- immediate release composition a dosage form that is formulated to release substantially all the active ingredient on administration with no enhanced, delayed or extended release effect.
- a composition may be in the form of a pellet (a term used interchangeably with “bead” or “beadlet” herein).
- the immediate release pellet can serve as a precursor to an extended or delayed release pellet, or be used with an extended or delayed release pellet.
- an immediate release pellet can be prepared by mixing pramipexole with a bulking agent. Additionally, one can add disintegrating agents, antiadherents and glidants to the formulation.
- Bulking agents employable in these compositions may be chosen from, among others: microcrystalline cellulose, for example, AVICEL® (FMC Corp.) or EMCOCEL ® (Mendell Inc.), which also has binder properties; dicalcium phosphate, for example, EMCOMPRESS ® (Mendell Inc.); calcium sulfate, for example, COMPACTROL ® (Mendell Inc.); and starches, for example, Starch 1500; and polyethylene glycols (CARBOWAX ® ).
- Such bulking agents are typically present in the range of about 5% to about 75% (w/w), with a preferred range of about 25% to about 50% (w/w).
- Suitable disintegrants include, but are not limited to: crosslinked sodium carboxymethyl cellulose (AC-DI-SOL ® ), sodium starch glycolate (EXPLOTAB ® , PRIMO JEL ® ) and crosslinked polyvinylpolypyrrolidone (PLASONE-XL ® ). Disintegrants are used to facilitate disintegration of the pellet upon administration and are typically present in an amount of about 3% to about 15% (w/w), with a preferred range of about 5% to about 10% (w/w).
- Antiadherents and glidants employable in such formulations can include talc, cornstarch, silicon dioxide, sodium lauryl sulfate, colloidal silica dioxide, and metallic stearates, among others.
- the immediate release composition may contain one or more binders to give the pellets cohesiveness.
- binders are well known in the art, and include such substances as microcrystalline cellulose, polyvinyl pyrrolidone, starch, maltrin, methylcellulose, hydroxypropyl methylcellulose, carboxymethyl cellulose, sucrose solution, dextrose solution, acacia, tragacanth and locust bean gum, which may be applied wet.
- the binding agent may be present in the composition in an amount of from about 0.2 wt % to about 40 wt %, preferably from about 5 wt % to about 30 wt %, or from about 10 wt % to about 15 wt %.
- the pellets can be made by, for example, simple granulation such as wet granulation or dry granulation, followed by sieving; extrusion and marumerization (spheronization); rotogranulation; or any agglomeration process that results in a pellet of reasonable size and robustness.
- simple granulation such as wet granulation or dry granulation, followed by sieving
- extrusion and marumerization spheronization
- rotogranulation or any agglomeration process that results in a pellet of reasonable size and robustness.
- the drug and other additives are granulated by addition of a binder solution.
- the wet mass is passed through an extruder equipped with a certain size screen, and the extrudates are spheronized in a marumerizer.
- the resulting pellets are dried and sieved for further applications.
- the granules are kneaded after wetting by the combined actions of mixing and milling.
- the resulting granules or pellets are dried and sieved for further applications.
- the immediate release beadlets or pellets are prepared by solution or suspension layering, whereby a drug solution or dispersion, with or without a binder and optionally an anti-tacking agent such as talc, is sprayed onto a core or starting seed (either prepared or a commercially available product) in a fluid bed processor or other suitable equipment.
- the cores or starting seeds can be, for example, sugar spheres or spheres made from microcrystalline cellulose.
- the binder in the formula can be present in amounts ranging from about 0% to about 5% by weight, and preferably about 0.5% to about 2% by weight.
- the amount of anti-tacking agent used can be from about 0% to about 5%, preferably about 0.5% to about 2% by weight.
- the drug thus is coated on the surface of the starting seeds.
- the drug may also be layered onto the drug-containing pellets described above, if desired. Following drug layering, the resulting drug-loaded pellets are dried for further applications.
- a protective layer, or overcoating, may be desired to ensure that the drug-loaded pellets do not aggregate during processing or upon storage.
- the protective coating layer may be applied immediately outside the core, either a drug-containing core or a drug-layered core, by conventional coating techniques such as pan coating or fluid bed coating using solutions of polymers in water or suitable organic solvents or by using aqueous polymer dispersions.
- OPADRY ® , OPADRY II ® (Colorcon) and corresponding color and colorless grades from Colorcon can be used to protect the pellets from being tacky and provide colors to the product.
- Different anhydride-based polymers e.g., sebacic/fumaric copolymers such as Spheromer I or Spheromer II from Spherics, Inc.
- the suggested levels of protective or color coating are from about 1% to about 6%, preferably about 2% to about 3% (w/w).
- ingredients can be incorporated into the overcoating formula, for example to provide a quicker immediate release, such as plasticizers: acetyltriethyl citrate, triethyl citrate, acetyltributyl citrate; dibutylsebacate, triacetin, polyethylene glycols, propylene glycol and the others; lubricants: talc, colloidal silica dioxide, magnesium stearate, calcium stearate, titanium dioxide, magnesium silicate, and the like.
- the immediate release composition may be prepared as an uncoated tablet, or a tablet core prior to coating, comprising starch and a hydrophilic polymer acting as a matrix for a water-soluble drug or prodrug requires to have a certain minimum hardness in order to be able to resist breakage and/or attrition due to mechanical stresses imposed during a high-speed tableting operation (including all steps up to and including filling of the tablets into containers).
- the minimum acceptable hardness will depend on a number of factors, including the severity of the mechanical stresses, but is typically at least about 20 SCU, preferably at least about 22 SCU, more preferably at least about 24 SCU (about 17 kp).
- Hardness can be increased by increasing the compression force applied by the tablet press, but only up to a certain level. At least in the case of tablets as described herein, above a certain compression force, further increases in compression force give little or no further increase in tablet hardness. There is, in other words, a maximum hardness achievable by compression of a particular starch / hydrophilic polymer / active agent composition. A starch providing a maximum hardness inadequate to withstand the mechanical stresses of a highspeed tableting operation is unsuitable for the present purpose. Certain pregelatinized starches provide a maximum hardness of 20 SCU or less; these are starches having low tensile strength (0.1 kN cm "2 or less).
- immediate release pellets are contemplated as being used in combination with extended release pellets and/or delayed release pellets in a single dosage form, and/or being modified to generate extended release (XR) pellets, delayed release (DR) pellets, and/or delayed and extended release (DXR) pellets in a single dosage form.
- XR extended release
- DR delayed release
- DXR delayed and extended release
- the delayed-release component has a coat applied to the surface of the active pellet that delays the release of the drug from the pellet after administration for a certain period of time. This delayed release can be accomplished by applying a coating of enteric materials.
- enteric materials are polymers that are substantially insoluble in the acidic environment of the stomach, but are predominantly soluble in intestinal fluids at various specific pH's, such as pH 4.5 or higher.
- the enteric materials are non-toxic, pharmaceutically acceptable polymers, and include, for example, cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate, hydroxypropyl methylcellulose succinate, cellulose acetate succinate, cellulose acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of methylmethacrylic acid and methyl methacrylate, copolymer of methyl acrylate, methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and maleic anhydride (Gantrez ES series), ethyl me
- coating materials can be employed in coating the surfaces in a range of from about 1.0% (w/w) to about 50% (w/w) of the pellet composition. Preferably, these coating materials are in the range of from about 10-20% (w/w).
- the pellets may be coated in a fluidized bed apparatus or pan coating, for example, in a conventional manner.
- the pramipexole With the enteric-coated pellets, there is no substantial release of pramipexole in the acidic stomach environment of below about pH 4.5.
- the pramipexole becomes available when the pH-sensitive enteric layer dissolves at a higher pH in the GI tract, after a certain delayed time, or after the unit passes through the stomach.
- the preferred delay time is in the range of about 0.5 to about 6 hours, but more preferable is about 0.5 to about 4 hours.
- certain DR pellets may be coated with EUDRAGIT ® L30D-55, which dissolves at about pH 5.5-6.0, i.e., in the upper intestines.
- the DR pellets may be coated with EUDRAGIT ® FS30D, which dissolves at about pH 7.0, i.e., in the lower intestine and colon.
- the XR pellet described above may be additionally coated with the enteric material to generate delayed and extended release (DXR) pellets.
- DXR delayed and extended release
- Such a dosage form is delayed release until the drug reaches non-acidic environment, such as the upper and/or lower intestine, 1 and thereupon releasing drugs over an extended period of time.
- Extended Release Composition (XR) Pramipexole extended release pellets can be prepared in many different ways to achieve an extended release profile.
- the subject pramipexole XR pellets can be prepared by coating drug layered inert pellets with release-controlling polymers.
- the inert pellet is coated with the drug layer, or a drug loaded granule is prepared, as described above.
- the active (drug loaded) pellet is coated with a release-controlling polymeric membrane.
- the release-controlling coating layer may be applied immediately outside the core (such as a drug-containing core or a drug-layered core), by conventional coating techniques, such as pan coating or fluid bed coating, using solutions of polymers in water or suitable organic solvents, or by using aqueous polymer dispersions.
- the release controlling membrane can separate additional drug layers on the core; for instance, after coating with the release controlling substance, another drug layer can be applied, which is followed by another release controlling layer, etc.
- Suitable materials for the release-controlling layer include EUDRAGIT ® RL, EUDRAGIT ® RS, cellulose derivatives such as ethylcellulose aqueous dispersions (AQUACOAT ® , SURELEASE ® ), hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, polyvinylpyrrolidone, polyvinylpyrrolidone/vinyl acetate copolymer, OPADRY ® ), and the like.
- the thickness of the coating affects the release profile, and so this parameter can be used to customize the profile.
- the suggested coating levels are from about 1% to about 40%, about 5% to about 30% (w/w), or about 20% or about 25% in other embodiments.
- a hydrophilic polymer matrix core can be inadequate to provide sustained release of sufficiently long duration to permit once daily administration. It is believed that such salts are readily leached out of the hydrophilic matrix when contacted by an aqueous medium such as gastrointestinal fluid.
- a release-controlling coating around the tablet to produce an extended-release (XR) tablet.
- XR extended-release
- Such a coating may comprise a hydrophobic or water-insoluble polymer component such as ethylcellulose together with a hydrophilic or water-soluble pore- forming component such as HPMC.
- a coating step exposure to mechanical stresses is also greatly increased.
- the composition is found to be especially suited to a high-speed tableting operation that includes a step of coating the tablet with a release-controlling layer.
- ethylcellulose and HPMC as components of a release coating layer
- cellulosic polymers ⁇ e.g., methylcellulose, hydroxypropylcellulose, hydroxyethylcellulose, carboxymethylcellulose sodium, cellulose esters such as cellulose acetate, etc.
- polyvinyl acetate polyvinyl pyrrolidone
- polymers and copolymers of acrylic acid and methacrylic acid and esters thereof polyethylene glycol, carrageenan and other gums, etc.
- a release-controlling layer typically constitutes about 1% to about 15%, preferably about 2.5% to about 10%, by weight of the tablet as a whole.
- the hydrophobic or water-insoluble component preferably comprising ethylcellulose, typically constitutes about 1% to about 10%, preferably about 2% to about 7%, by weight of the tablet as a whole.
- the pore-forming component preferably comprising HPMC, is typically present in an amount of about 5% to about 50%, preferably about 10% to about 40%, by weight of the water- insoluble or hydrophobic component.
- a release-controlling layer in an amount of about 2.5% to about 5% by weight of the tablet core comprises an ethylcellulose-based material ⁇ e.g., SURELEASE ® of Colorcon) and an HPMC-based pore-forming material (e.g., OPADRY ® of Colorcon) in a weight ratio of about 3: 1 to about 4: 1.
- a release-controlling layer or coating is preferably applied at a relatively uniform thickness to provide even control of release rate of the pramipexole.
- the sustained-release tablet of the invention comprises a nonfunctional coating.
- a nonfunctional coating can comprise a polymer component, for example HPMC, optionally with other ingredients, for example one or more plasticizers, colorants, etc.
- HPMC polymer component
- other ingredients for example one or more plasticizers, colorants, etc.
- nonfunctional in the present context means having no substantial effect on release properties of the tablet, and does not imply that the coating serves no useful purpose.
- such a coating can impart a distinctive appearance to the tablet, provide protection against attrition during packaging and transportation, improve ease of swallowing, and/or have other benefits.
- a nonfunctional coating should be applied in an amount sufficient to provide complete coverage of the tablet.
- Uncoated tablets and cores of coated tablets of the invention can optionally contain one or more pharmaceutically acceptable excipients in addition to the starch and hydrophilic polymer components described above.
- excipients include without limitation glidants and lubricants.
- Other conventional excipients known in the art can also be included.
- a glidant can be used to improve powder flow properties prior to and during tableting and to reduce caking. Suitable glidants include colloidal silicon dioxide, magnesium trisilicate, powdered cellulose, starch, talc, tribasic calcium phosphate and the like.
- colloidal silicon dioxide is included as a glidant in an amount up to about 2%, preferably about 0.2% to about 0.6%, by weight of the tablet.
- a lubricant can be used to enhance release of a tablet from apparatus on which it is formed, for example by preventing adherence to the face of an upper punch ("picking") or lower punch ("sticking").
- Suitable lubricants include magnesium stearate, calcium stearate, canola oil, glyceryl palmitostearate, hydrogenated vegetable oil, magnesium oxide, mineral oil, poloxamer, polyethylene glycol, polyvinyl alcohol sodium benzoate, sodium lauryl sulfate, sodium stearyl fumarate, stearic acid, talc, hydrogenated vegetable oil, zinc stearate and the like.
- magnesium stearate is included as a lubricant in an amount of about 0.1 % to about 1.5%, preferably about 0.3% to about 1%, by weight of the tablet.
- coated extended-release (XR) tablets of pramipexole dihydrochloride of the invention are prepared using the composition shown in the table below:
- Tablet cores were prepared as following: all ingredients except the lubricant (magnesium stearate) were screened to remove lumps and were blended thoroughly in a low- shear mixer operating at 24 rpm for 10-30 minutes. The lubricant was then screened into the mixer and the materials were blended for a further 2-5 minutes. The resulting lubricated mixture was compressed into 350 mg tablets using a GlobePharma Manual Tablet Compaction Machine.
- lubricant magnesium stearate
- pramipexole was layered onto the lactose particles to achieve its uniform dispersion.
- a coating solution was prepared as follows: OPADRY ® HPMC-based material in an amount of 8.002 g was added to 171.735 g water and mixed for 45 minutes to provide an HPMC mixture. Next, 128.032 g SURELEASE ® ethylcellulose-based material was added to the HPMC mixture and mixed for an additional 30 minutes to provide a coating solution.
- Coating to a 5% total weight gain and curing of the coated tablets were performed as following: the coating solution was applied to the tablet cores in an amount providing a 5% weight gain.
- the resulting coated tablets were cured using a 12 inch (about 30 cm) Vector LCDS or 24 inch (about 60 cm) Thomas Accela-Coata coating pan for about 15 minutes at a bed temperature of at least about 70 0 C. After curing, temperature was ramped down over a period of about 8 minutes to an exhaust temperature of about 45°C.
- the extended release pellets typically contain the same amount of total pramipexole used for thrice-a-day conventional treatment, e.g., about 0.375 mg for the 0.125 mg regimen.
- compositions of the present invention can be in a number of different forms, such as tablets, powders, suspensions, solutions, etc.
- the composition is preferably in pellet/beadlet form, which can be incorporated into hard gelatin or other kinds of capsules, either with additional excipients, or alone.
- the dosage formulations described herein may contain one or more excipients, carriers or diluents. These excipients, carriers or diluents can be selected, for example, to control the disintegration rate of a tablet or drug eluting device to fit the desired release profile according to the instant invention.
- the one or more carriers (additives) and/or diluents may be pharmaceutically acceptable.
- pharmaceutically acceptable carrier means a pharmaceutically acceptable material, composition or vehicle, such as a liquid or solid filter, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting the subject regulators from one organ, or portion of the body, to another organ, or portion of the body.
- a pharmaceutically acceptable material, composition or vehicle such as a liquid or solid filter, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting the subject regulators from one organ, or portion of the body, to another organ, or portion of the body.
- Each carrier must be “acceptable” in the sense of being compatible with the other ingredients of the formulation and not injurious to the patient.
- materials which can serve as pharmaceutically acceptable carriers include (1) sugars, such as lactose, glucose and sucrose; (2) starches, such as corn starch and potato starch; (3) cellulose, and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; (4) powdered tragacanth; (5) malt; (6) gelatin; (7) talc; (8) excipients, such as cocoa butter and suppository waxes; (9) oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10) glycols, such as propylene glycol; (11) polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; (12) esters such as ethyl oleate and ethyl laurate; (13) agar; (14) buffering agents, such as magnesium hydroxide and aluminum hydroxide; (15)
- Typical excipients to be added to a capsule formulation include, but are not limited to: fillers such as microcrystalline cellulose, soy polysaccharides, calcium phosphate dihydrate, calcium sulfate, lactose, sucrose, sorbitol, or any other inert filler.
- fillers such as microcrystalline cellulose, soy polysaccharides, calcium phosphate dihydrate, calcium sulfate, lactose, sucrose, sorbitol, or any other inert filler.
- there can be flow aids such as fumed silicon dioxide, silica gel, magnesium stearate, calcium stearate or any other materials that impart good flow properties.
- a lubricant can also be added if desired, such as polyethylene glycol, leucine, glyceryl behenate, magnesium stearate or calcium stearate.
- the formulations can conveniently be presented in unit dosage form and can be prepared by any of the methods well known in the art of pharmacy. All methods include bringing into association the drug with the carrier or diluent which constitutes one or more accessory ingredients. In general, the formulations are prepared by uniformly and intimately bringing into association the agent with the carriers and then, if necessary, dividing the product into unit dosages thereof. It will be understood by those skilled in the art that any vehicle or carrier conventionally employed and which is inert with respect to the active agent, and preferably does not interfere with bioadhesion in embodiments employing a bioadhesive coating, may be utilized for preparing and administering the pharmaceutical compositions of the present invention. Illustrative of such vehicles and carriers are those described, for example, in Remington's Pharmaceutical Sciences, 18th ed. (1990), the disclosure of which is incorporated herein by reference.
- Examples of carriers and diluents include pharmaceutically accepted hydrogels such as alginate, chitosan, methylmethacrylates, cellulose and derivatives thereof (microcrystalline cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, carboxymethylcellulose, ethylcellulose), agarose and POVIDONETM, kaolin, magnesium stearate, starch, lactose, sucrose, density-controlling agents such as barium sulfate and oils, dissolution enhancers such as aspartic acid, citric acid, glutamic acid, tartartic acid, sodium bicarbonate, sodium carbonate, sodium phosphate, glycine, tricine, Tromethamine, and TRIS.
- pharmaceutically accepted hydrogels such as alginate, chitosan, methylmethacrylates, cellulose and derivatives thereof (microcrystalline cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, carboxymethylcellulose, ethylcellulose),
- excipients, carriers or diluents can also be selected to control the time until a dosage form detaches from a mucosal membrane.
- the addition of one or more disintegrating agents will reduce the time until a tablet or drug eluting device detaches.
- an agent that interferes with the mucosa-tablet / device adhesion can be used to control the time until detachment occurs.
- certain components, such as pramipexole, of the present pharmaceutical compositions may contain a basic functional group, such as amino or alkylamino, and are thus capable of forming pharmaceutically acceptable salts with pharmaceutically acceptable acids.
- pharmaceutically acceptable salts refers to the relatively non-toxic, inorganic and organic acid addition salts of compounds of the present invention. These salts can be prepared in situ during the final isolation and purification of the compounds of the invention, or by separately reacting a purified compound of the invention in its free base form with a suitable organic or inorganic acid, and isolating the salt thus formed.
- Representative salts include but are not limited to following: 2-hydroxyethanesulfonate, 2-naphthalenesulfonate, 3-hydroxy-2-naphthoate, 3- phenylpropionate, acetate, adipate, alginate, amsonate, aspartate, benzenesulfonate, benzoate, besylate, bicarbonate, bisulfate, bitartrate, borate, butyrate, calcium edetate, camphorate, camphorsulfonate, camsylate, carbonate, citrate, clavulariate, cyclopentanepropionate, digluconate, dodecylsulfate, edetate, edisylate, estolate, esylate, ethanesulfonate, fumarate, gluceptate, glucoheptanoate, gluconate, glutamate, glycerophosphate, glycollylarsanilate, hemisul
- the pharmaceutically acceptable salts of compounds include the conventional non-toxic salts of the compounds, e.g., from non-toxic organic or inorganic acids. Particularly suitable are salts of weak acids.
- such conventional non-toxic salts include those derived from inorganic acids such as hydrochloric, hydrobromic, hydriodic, cinnamic, gluconic, sulfuric, sulfamic, phosphoric, nitric, and the like; and the salts prepared from organic acids such as acetic, propionic, succinic, glycolic, stearic, lactic, maleic, tartaric, citric, ascorbic, palmitic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicyclic, sulfanilic, 2-acetoxybenzoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulf
- the components of formulations of the present invention may contain one or more acidic functional groups and, thus, are capable of forming pharmaceutically acceptable salts with pharmaceutically acceptable bases.
- pharmaceutically acceptable salts refers to the relatively non-toxic, inorganic and organic base addition salts of compounds of the present invention. These salts can likewise be prepared in situ during the final isolation and purification of the compounds, or by separately reacting the purified compound in its free acid form with a suitable base, such as the hydroxide, carbonate or bicarbonate of a pharmaceutically acceptable metal cation, with ammonia, or with a pharmaceutically acceptable organic primary, secondary or tertiary amine.
- Representative alkali or alkaline earth salts include the lithium, sodium, potassium, calcium, and magnesium salts and the like.
- Representative organic amines useful for the formation of base addition salts include ethylamine, diethylamine, ethylenediamine, tromethamin, ethanolamine, diethanolamine, piperazine and the like. (See, for example, Berge et al, supra).
- wetting agents such as sodium lauryl sulfate and magnesium stearate, as well as coloring agents, release agents, coating agents, sweetening, flavoring and perfuming agents, preservatives and antioxidants can also be present in the compositions.
- antioxidants may also be included.
- examples of pharmaceutically acceptable antioxidants include: (1) water soluble antioxidants, such as ascorbic acid, cysteine ny ⁇ rocnionde, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like; (2) oil-soluble antioxidants, such as ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin, propyl gallate, alpha- tocopherol, and the like; and (3) metal chelating agents, such as citric acid, ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid, phosphoric acid, and the like.
- water soluble antioxidants such as ascorbic acid, cysteine ny ⁇ rocnionde, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like
- oil-soluble antioxidants such as ascorbyl palmitate, butylated
- the disintegration time of a composition may be formulated to effect a substantially zero-order release, over a period of 2, 4, 6, 8, 12, or 24 hours, for instance.
- multiparticulate capsules are preferred because they provide an increased surface area as opposed to a tablet or matrix, and thus allow for better release profiles and bioavailability.
- the pellets described above can be incorporated into a tablet, in particular by incorporation into a tablet matrix, which rapidly disperses the particles after ingestion.
- a filler/binder must be used in the tableting process that will not allow the destruction of the pellets during the tableting process.
- Materials that are suitable for this purpose include, but are not limited to, microcrystalline cellulose (AVICEL ® ), soy polysaccharide (EMCOSOY ® ), pre-gelatinized starches (STARCH ® 1500, NATIONAL ® 1551), and polyethylene glycols (CARBOWAX ® ). These materials should be present in the range of about 5%-75% (w/w), and preferably between about 25%-50% (w/w).
- disintegrants may be added to the tablets in order to disperse the beads once the tablet is ingested.
- Suitable disintegrants include, but are not limited to: crosslmked sodium carboxymethyl cellulose (AC-DI-SOL ® ), sodium starch glycolate (EXPLOTAB ® , PRIMOJEL ® ), and crosslinked polyvinylpolypyrrolidone (Plasone-XL). These materials should be present in the range of about 3%-15% (w/w), with a preferred range of about 5%- 10% (w/w).
- Lubricants may also be added to assure proper tableting, and these can include, but are not limited to: magnesium stearate, calcium stearate, stearic acid, polyethylene glycol, leucine, glyceryl behenate, and hydrogenated vegetable oil. These lubricants should be present in amounts from about 0.1%- 10% (w/w), with a preferred range of about 0.3%-3.0% (w/w).
- Tablets are formed, for example, as follows.
- the pellets are introduced into a blender along with AVICEL ® , disintegrants and lubricant, mixed for a set number of minutes to provide a homogeneous blend which is then put in the hopper of a tablet press with which tablets are compressed.
- the compression force used is adequate to form a tablet; however, it is not sufficient to fracture the beadlets or coatings.
- the subject once-a-day dosage forms of pramipexole typically contain the same total amount of therapeutically effective amount of pramipexole that is administered to a patient during a conventional pramipexole treatment.
- one conventional thrice-a-day regimen comprises 3 times a day of 0.125 mg pramipexole each (other dosages are available as 0.5, 0.75, 1.0, 1.5, 3.0, and 4.5 mg, etc.).
- the total amount of pramipexole for PD treatment is about 0.375 mg (3 x 0.125 mg) in the subject once-a-day dosage forms.
- the total amount of pramipexole used may be adjusted upward or downward by, for example, 5-30%, or 10-20%, etc., depending on specific patient's age, weight, gender, race, health condition, and other considerations.
- the subject pharmaceutical composition is formulated for variable dosing, such as customized dosing for individual patients.
- more than one type of drugs can be present in a tablet or a drug eluting device of the invention, e.g., for combination therapy with other pharmaceutical compositions effective for treating PD or other movement disorders (see below).
- the drugs can be evenly distributed throughout a medicament or can be heterogeneously distributed in a medicament, such that one drug is fully or partially released before a second drug. See different embodiments of the drug devices and/or layering in other parts of this specification.
- Dosage forms of the invention typically weigh at least about 50 mg. Dosage forms (such as the various shell designs of the invention) can also weigh at least 100 mg, at least 150 mg, at least 250 mg, at least 500 mg, or at least 1000 mg, etc.
- Dosage forms of the invention typically measure at least 2 mm in one direction.
- dosage forms can measure at least 5 mm, at least 10 mm, at least 15 mm or at least 20 mm in one direction.
- the diameter of the dosage forms is 2 to 40 mm, preferably 10 to 30 mm such as 20 to 26 mm.
- Mini-tablets have a diameter of 2 mm to about 5 mm.
- Such dosage forms can measure at least 2 mm, at least 5 mm, at least 10 mm, at least 15 mm or least 20 mm in a second direction and, optionally, a third direction.
- the dosage form is of a size that facilitates swallowing by a subject.
- the volume of a typical dosage form of the invention is at least 0.008 mL, at least 0.01 mL, at least 0.05 mL, at least 0.1 mL, at least 0.125 mL, at least 0.2 mL, at least 0.3 mL, at least 0.4 mL or at least 0.5 mL.
- Dosage forms of the invention may be a tablet that can be of any suitable size and shape, for example, round, oval polygonal or pillow-shaped, and optionally bear nonfunctional surface markings. Especially in the case of coated tablets, they are preferably designed to be swallowed whole and are therefore typically not provided with a breaking score. Tablets of the invention can be packaged in a container, e.g., accompanied by a package insert providing pertinent information such as, for example, dosage and administration information, contraindications, precautions, drug interactions and adverse reactions.
- the drugs may be formulated as bilayer (or other multilayer) tablets or shells (e.g., stacked layer of cakes, each may represent an independent formulation).
- the drugs may be formulated as a tablet within a tablet or bead (not limited to two nested layers).
- a bioadhesive layer may be coated over part or all of a gel capsule (or other forms of delivery device) to enhance the stay of the device within a certain area of the GI tract, such as the intestine.
- the drugs may be formulated into a core tablet held in a recessed fashion within an annular ring of drug material.
- a dosage form is described in U.S. patent application Ser. No. 10/419,536 entitled “Dosage Form with a Core Tablet of Active Ingredient Sheathed in a Compressed Angular Body of Powder or Granular Material, and Process and Tooling for Producing It," filed on Apr. 21, 2003 and Ser. No. 10/379,338 entitled “Controlled Release Dosage Forms,” filed on Mar. 3, 2003 and are incorporated herein by reference. This design may be used for many embodiments of the subject dosage forms.
- the outer annular ring is formulated for either immediate release (IR) or extended release (XR) delivery for a desired amount of time.
- the inner core(s) of the dosage form may be released after a delay which may be formulated for the desired release profile.
- the enteric coating covers the tablet to delay drug delivery until the tablet enters a non-acidic environment.
- Another embodiment of this invention may be achieved by formulating each of the drugs as pellets / beads, each with its own release profile and delay where applicable, and delivering the mixture of the pellets (e.g., IR, DR, DXR, etc.) in a shell using methods commonly known in the art.
- the proportions of the different types of pellets / beads may be altered or customized by a skilled artisan ⁇ e.g., qualified physician or pharmacologist), based on an individual patient's characteristics, such as weight, age, gender, ethnicity, and/or specific genetic backgrounds. Such customization may be effected with the aid of, or automatically executed by a computer program based on relevant parameters such as those described above.
- the drug-releasing beads are characterized by a dissolution profile wherein 0 to 20% (e.g., 1-20%) of the beads undergo dissolution and release the drug in 0 to 2 hours, 20 to 40% undergo dissolution and release the drug in 2 to 4 hours, 40 to 60% exhibit dissolution and release in 4 to 6 hours, 60 to 80% in 6 to 8 hours, and 80 to 100% in 8 to 10 hours or longer.
- the drug-releasing beads can include a central composition or core comprising a drug and pharmaceutically acceptable composition forming ingredients including a lubricant, antioxidant, and buffer.
- the beads comprise increasing doses of drug, for example, 0.1 mg, 0.2 mg, 0.5 mg, and so forth to a high dose.
- the beads may be coated with a release rate-controlling polymer that can be selected utilizing the dissolution profile disclosed above.
- the manufacture of the beads can be adapted from, for example, Liu et ah, Inter. J. ofPharm. 112: 105-116, 1994; Liu et at, Inter. J. ofPharm. 112: 117-124, 1994; Pharm. ScL, by Remington, 14th Ed. pp. 1626-1628 (1970); Fincher et al., J. Pharm. Set 57: 1825-1835, 1968; and U.S. Pat. No. 4,083,949.
- the tablet is a longitudinally compressed tablet.
- the core of the tablet is a slow- eroding active core 1 with pramipexole and other pharmaceutical excipients.
- the side of the core is coated with a bioadhesive polymer layer 4, while the two ends of the core are coated with an insoluble plug 2 and an enteric polymer plug 3, respectively.
- the enteric polymer plug 3 will only dissolve in a relatively higher pH environment (e.g., about pH 4.5 and higher), such as those found in intestine or colon. Once the enteric polymer layer 3 is dissolved, the active core 1 starts to release its contents.
- the bioadhesive layer 4 is selectively adhesive to intestine or colon, such that the content of the active core 1 may be released over a prolonged period of time.
- Figure IB shows a slight variation of the device depicted in Figure IA, in that the insoluble plug 2 in replaced by a second enteric polymer plug 2.
- both enteric polymer plugs 2 will dissolve in relatively higher pH environments, either substantially simultaneously, or at different time, such that the rate of release from the slow-eroding active core 1 may be regulated.
- Figure 1C shows yet another alternative embodiment, in that the slow eroding active core 1 in Figure IA becomes two consecutive layers - an immediate release active core layer
- the immediate release layer 2 provides a rapid drug release, which is maintained by more sustained drug release from the slow-eroding active core 1.
- Figure ID shows a schematic (not necessarily to scale) drawing of another embodiment of the delivery device containing multiparticulate beads / pellets.
- the multiparticulate dosage form combines two types of pellets - the immediate release pellets 1 and the controlled release active pellets (DR or XR) 2 — both embedded in an appropriate matrix of excipients ⁇ e.g., HPMC, MCC, lactose).
- the matrix is inside a hard gelatin capsule
- enteric material 4 which in turn is coated by enteric material 4.
- This type of dosage form will provide multiple pulses of drug release, with the effect being a more or less sustained blood level of drug within the acceptable range.
- the release is delayed by the enteric coating 4 in order to by-pass the upper GI tract.
- the IR pellets are designed to provide an effective blood level soon after the start of the drug release, which is subsequently maintained by the DR and/or XR combinations.
- the DR portion provides an immediate release after a delay. IfXR pellets are also used, the XR portion provides an extended release profile that maintains the effective blood level of pramipexole throughout the remaining course of the day.
- the total dose of pramipexole in this composition is usually no greater than 0.375 mg.
- the IR pellets may comprise 1/3 (or 0.125 mg) of the total, while the remaining 2/3 is provided by the DR and/or XR.
- an enteric coating 3 covers an inner core with two (asymmetric) portions - the immediate release active layer (IR) 1 and the controlled release active layer (DR or XR) 2.
- the ratio of IR to DR / XR may be anywhere between 1 : 10 to 2: 1. In a preferred embodiment, the ratio may be 1 :2.
- Figure IF the complex core of Figure IE is replaced with a uniform slow-eroding or non-eroding active matrix core 1, from which pramipexole is released after the enteric coating 2 is dissolved.
- Figure IG presents yet another embodiment, wherein an enteric coating 4 delays the release its drug contents.
- the immediate release active core 1 is quickly dissolved, effectively splitting the core into two halves of controlled- release active core 2, each coated by a layer of rate-controlling coating 3 at surfaces not in contact with the immediate release active core 1.
- the rate gradually increases as the immediate release active core 1 dissolves, exposing more surface area of the two controlled-release active cores 2 not coated by the rate-controlling coating 3.
- Release profile may be controlled by, for example, the amount of the immediate release active core 1, the thickness and material of the rate-controlling coating 3, the geometric shape / surface area of the controlled-release active core 2 directly in contact with the immediate release active core 1, etc.
- the active core 1 is substantially covered by a layer of semi-permeable coating 3, which contains one or more small openings / orifices 2.
- the outermost portion of the whole device is further coated with a layer of delayed-release coating / enteric coating 4. Once coating 4 is dissolved, the orifice(s) is exposed, allowing direct release of the active core 1 through the orifice(s) 2.
- Different release profiles may be obtained, for example, by controlling the number and/or size of the orifice(s) 2, the thickness and/or material of the semi-permeable coating 3.
- the core comprises three layers, with the middle layer being the active core 1. Underneath the active core 1 is a push layer 2 that will swell after the tablet comes into contact with body fluid and when the fluid enter the tablet through the semi-permeable coating 5. Above the active core is a delayed- release layer 3 having access to one or more orifice(s) for drug release. The swelling push layer 2 will cause first the delayed-release layer 3, and then the active core 1 to be released through the orifice 4.
- the delayed release layer may also have the IR component or an immediate release component in between the delayed release and slow release core layer.
- the immediate release beads / pellets 1 and the controlled-release (XR and/or DR and/or DXR) beads / pellets 2 are embedded within the enteric polymer material 3 as multiparticulate beads / pellets.
- the enteric polymer material 3 may additionally comprise compression enhancers or fillers, or any other materials described herein that are customarily used in tablet production.
- the IR portion of the dosage form is formulated as a matrix for embedding one or more other portions of the same dosage form (DR, XR, DXR, etc.).
- the IR may be coated by enteric layer to avoid release in upper GI tract.
- Each controlled release portion (DR, XR, DXR, etc.) is optionally coated by a bioadhesive coat and/or a delayed release coat.
- Each CR portion may be formed as microparticles ⁇ e.g. , beads) suspended in the first portion ⁇ e.g., IR portion) matrix. The disintegration of the matrix leads to the release of the embedded microparticles, which may re-adhere to the gut or other tissues (if coated by bioadhesive layer), and provided for sustained release.
- Figure 12 features yet another configuration of the delivery device, in which a drug portion 1201 is sandwiched between two adhesive layers 1202 ⁇ e.g., a layered cross section) or inside one continuous adhesive layer 1202 ⁇ e.g., configured as a filled tube).
- SPHEROMERTM I Ip(FASA)] and SPHEROMERTM III are exemplary such bioadhesive layers.
- the portion / layer can (but need not) be substantially flat.
- an immediate release portion IR 1203 may be present, and is coated over all or a part of the adhesive layer 1202.
- the rapid dissolution of the IR portion exposes a drug surface not in contact with the adhesive material.
- the dissolution of the IR portion does not substantially change the release rate of the drug portion. This multilayer configuration is finally applied with an enteric or delayed release coating.
- Figure 13 features yet another configuration of the subject delivery device, which may be used in general to deliver any kind of drugs (or prodrugs/metabolic precursors thereof, etc). It should be understood that the subject delivery devices (such as the one described in Figure 13), dosage form, and methods of making and using are not limited to these specific exemplary drug compositions described herein.
- any drug to be delivered e.g., pramipexole
- a bioadhesive polymer composition e.g., poly(ethylene glycol)
- pharmaceutically acceptable excipients may be formulated using the subject granulation- extrusion-spheronization process into multiparticulate pellets, which in turn may be dispersed in certain matrix materials, or simply encapsulated in capsules, e.g., according to the various embodiments disclosed above.
- Suitable excipients for use in the subject granulation-extrusion-spheronization process include: Starcap-1500, starch-1500, and glycerine monostearate.
- the mixture is substantially free of microcrystalline cellulose.
- about 30-90%, about 40-85%, or about 50-80% (v/v) of the mixture (and the pellets formed therefrom) is effective ingredient (e.g., drug composition), rather than excipients or polymers.
- effective ingredient e.g., drug composition
- Such loadings can be achieved using any drug or combination of drugs that are suitably cohesive, plastic, and engage in hydrogen bonding. Pramipexole is an example of such drugs, though others will be known to or can be easily identified by those of skill in the art.
- a planetary type mixer e.g., Hobart Mixer with a 5-qt mixing bowl, operating at the speed setting #1, for about 5-15 min.
- the blending process is done in small volume to reduce any possible loss of the ingredients due to their non-specific adherence to the blending device.
- the blending step is typically done to ensure the formation of a uniform dry mix of the ingredients, typically over a period of, e.g., 5-15 min.
- Granulation fluids may be purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro-alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated " solvent or in a ketone, etc. or any suitable mixture thereof
- the granulation process is conducted in a small volume, such as in a 500-mL cylindrical vessel.
- the granulation process is conducted with manual mixing, or conducted mechanically, e.g., in a planetary type mixer (such as a Hobart Mixer with a 5-qt mixing bowl). If the Hobart Mixer is used, it can be operated at its speed setting #1, depending on the batch size. Other types of mechanical mixers may also be used, with their respective appropriate settings, to achieve substantially the same result.
- the wet granulation is extruded through the screen of a screen-type extruder.
- a Caleva Model 20 (or Model 25) Extruder may be used, operating at 10-20 rpm, and forming breakable wet strands ("the extrudate").
- the screen aperture may be set at 0.8, 1 , or 1.5 mm.
- Other types of extruders may be used to achieve substantially the same result.
- the extrudate is then spheronized in a spheronizer.
- a spheronizer For example, a Caleva Model 250 spheronizer equipped with a 2.5-mm spheronization plate may be used, which may be operated at a speed of about 1000-2000 rpm, typically for 5-10 min., in order to form spheronized pellets.
- Other types of spheronizer may be used to achieve substantially the same result.
- the spheronized pellets are then dried.
- the drying may be conducted in a fluidized bed drier, such as a Vector MFL.01 Micro Batch Fluid Bed System.
- a fluidized bed drier such as a Vector MFL.01 Micro Batch Fluid Bed System.
- the Vector drier may be operated at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of about 50 0 C.
- the pellets may be dried in an ACT (Applied Chemical Technology) fluidized bed drier, operating at an inlet air flow rate of 140- 150 fpm (foot per minute) and an inlet air temperature of 104 0 F.
- ACT Applied Chemical Technology
- Other types of driers may also be used to achieve substantially the same result.
- the drying temperature for a drier similar to the Vector drier may be between 35-70 0 C, or 40-65 0 C, or 45-6O 0 C, or 45-55 0 C, etc.
- the drying temperature for a drier similar to the ACT drier may be between 70-140 0 F, or 80-130 0 F, or 90-120 0 F, or 100-110 0 F, etc.
- the spheronized pellets may be dried in an oven, such as a Precision gravity oven, operating at about 50 0 C, for 4-48 hrs, or 8-24 hrs.
- the oven drying temperature for a drier similar to the Precision gravity oven may be between 35-70 0 C, or 40-65 0 C, or 45-6O 0 C, or 45-55 0 C, etc.
- the dried pellets are then screened and/or classified. This can be done by using a stack of sieves, such as stainless steel sieves U.S. standard mesh sizes 8, 10, 12, 14, 16, 18, 20, 25, 30, 40, 45, or 60, etc., and using a mechanical sieve shaker (e.g., W.S. Tyler Sieve Shaker Ro-Tap Rx-29, operated for 5 min.). Particle size and distribution of pellet formulations can then be analyzed, and the classified pellets ranging from 0.25 mm (mesh # 60) to 2 mm (mesh # 10) may be selected for use or future formulation, such as additional film coating or other experimentation.
- a stack of sieves such as stainless steel sieves U.S. standard mesh sizes 8, 10, 12, 14, 16, 18, 20, 25, 30, 40, 45, or 60, etc.
- a mechanical sieve shaker e.g., W.S. Tyler Sieve Shaker Ro-Tap Rx-29, operated for 5 min.
- the selected pellets may be film-coated, e.g., with a delayed- release coating (such as an entaric coating), a controlled-release (CR) coating, a bioadhesive polymeric composition, and/or a dispersion-promoting coating, etc.
- a delayed- release coating such as an entaric coating
- CR controlled-release
- the pellet core may be optionally surrounded by a CR coating, such as polymeric substance based on acrylates and/or methacrylates, e.g., a EUDRAGITTM polymer (sold by Rohm America, Inc.).
- EUDRAGITTM polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent.
- EUDRAGITTM RL, EUDRAGITTM NE, and EUDRAGITTM RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films.
- EUDRAGITTM RL is freely permeable and EUDRAGITTM RS is slightly permeable, independent of pH. In contrast, the permeability of EUDRAGITTM L is pH dependent. EUDRAGITTM L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester. It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable. If desired, two or more types of polymeric substances may be mixed for use as the CR coating. Other polymers suitable for CR coatings, such as ethyl cellulose and cellulose acetate, can also be used in the CR coating. In certain embodiments, the CR coating may comprise one or more suitable polymers, such as a combination of two or more of the polymers discussed above.
- the pellets may also be coated by a bioadhesive polymeric composition.
- the adhesive material may facilitate the adhesion of the pellets to a desired surface, such as a preferred GI tract surface.
- the pellets / beads may be coated by a top-layer of a bioadhesive polymer such as SPHEROMERTM I [p(FASA)], SPHEROMERTM II, SPHEROMERTM III, SPHEROMERTM IV, or mixtures thereof.
- the functions of a CR coating and bioadhesive coating can be combined in a single layer by using a mixture of polymers including a bioadhesive polymer and a polymer suitable for controlled release, i.e., a single layer may be both the CR layer and the bioadhesive layer of a particle.
- the pellets can also be film-coated with an additional layer of a so-called "non-functional polymer,” such as OPADRYTM II, EUDRAGITTM E, AcrylEZETM, hydroxypropylmethyl cellulose, hydroxypropyl cellulose, polyvinyl alcohol, polyvinylacetate, polyanhydride, etc.
- This layer may serve as a dispersion-promoting coating that inhibits clumping and aggregation of the particles during dispersion.
- this layer is preferably sufficiently strong or resilient to remain substantially intact during the compression process. This layer may also be protected by including a cushioning material among the excipients of the tablet matrix.
- the coating material (such as bioadhesive polymers and/or functional / nonfunctonal polymers) maybe dissolved in an appropriate solvent, such as methylene chloride ⁇ e.g., for SPHEROMERTM I), methanol (e.g., for SPHEROMERTM III), a binary mixture of methanol and methylene chloride (e.g., for SPHEROMERTM I and SPHEROMERTM III), methanol or a binary mixture of ethanol and water (3:1 v/v) (e.g., for SPHEROMERTM IV), or methanol, ethanol, or isopropanol, or their binary mixture with acetone (e.g., functional or nonfunctional polymer).
- an appropriate solvent such as methylene chloride ⁇ e.g., for SPHEROMERTM I), methanol (e.g., for SPHEROMERTM III), a binary mixture of methanol and methylene chloride (e.g., for SPHEROMERTM I and SP
- the film coating may be performed in a fluidized bed coater, such as a Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert, operating at an inlet air flow rate of 100-300 lpm (liters per minute), and an inlet air temperature of about 25-45 0 C, or about 30-40 0 C, depending on the specific drugs and coatings (e.g., 25-30 0 C for SPHEROMERTM I-coated pramipexole; about 35 0 C for SPHEROMERTM Ill-coated pramipexole, etc.).
- the pellets may be pre- warmed at 35 0 C for 2-5 min., and after film-coating, post-dried at about 30 0 C for about 15-30 min.
- pellets may be coated in a fluid bed processor, such as a Fluid Air Model 5 fluid bed processor equipped with a Wurster insert, operating at an inlet air flow rate of about 70 cfm (cubic foot per minute) and an inlet air temperature of about 35 0 C.
- a fluid bed processor such as a Fluid Air Model 5 fluid bed processor equipped with a Wurster insert, operating at an inlet air flow rate of about 70 cfm (cubic foot per minute) and an inlet air temperature of about 35 0 C.
- the pellets may be pre-warmed at 40 0 C for 5-7 min., and after film-coating, post-dried at about 35 0 C for about 30 min.
- Different lots of the same pellets produced using the subject method may optionally be mixed, e.g., by using a blender (such as a GlobePharma Maxiblend Blender equipped with an 8-qt stainless steel V-shell).
- a blender such as a GlobePharma Maxiblend Blender equipped with an 8-qt stainless steel V-shell.
- pellets may be mixed. For example, some pellets may have no coating other than a core comprising the effective ingredients. Other pellets, such as those identically made, may have additionally been coated by one or more types of coatings, e.g., bioadhesive coating, delayed-release coating, controlled-release coating, and/or dispersion-promoting coating, etc.
- coatings e.g., bioadhesive coating, delayed-release coating, controlled-release coating, and/or dispersion-promoting coating, etc.
- pellets produced using the methods of the invention may be encapsulated in capsules, such as hard gelatin capsules or pullulan capsules (NPcapsTM), each with a predetermined amount of effective ingredients.
- pellets produced using the methods of the invention may be dispersed in a matrix material to assist the delivery of the effective ingredients of the pellets.
- a matrix material to assist the delivery of the effective ingredients of the pellets.
- Figure 13 shows a schematic drawing (not to scale) of one such configuration.
- the active components 1301 such as the pellets produced using the subject method, which are not necessarily round in shape
- the carrier matrix 1302 can rapidly disintegrate, e.g., dissolve substantially completely (superdisintegrant) within about 15 minutes, 10 minutes, 8 minutes, 7 minutes, 6 minutes, 5 minutes, 3 minutes, 2 minutes, or about 1 minute or less.
- the inactive material 1302 may additionally comprise one or more cushioning material(s) dispersed throughout, e.g., sufficient to protect the active components 1301 when preparing the delivery device, by substantially absorbing the impact of compacting, and/or reducing friction on the surface of the particles 1301 (to prevent damaging the substructure of the particles, see below).
- the particles 1301 may be in any suitable size and shape (rods, beads, or other regular or irregular shapes).
- the particles are beads with a diameter of less than about 2 mm, about 1.5 mm, about 1 mm, about 0.8 mm, about 0.5 mm, about 0.3 mm, or about 0.1 mm.
- the pellet size is about 0.8 - 1 mm. Particles are formulated to these sizes in order to enable high drug loading when needed.
- particles 1301 may have substructures, such as various coating layers surrounding a drug / prodrug core.
- substructures such as various coating layers surrounding a drug / prodrug core.
- the core by itself may be an immediate release portion, or may have release- controlling components (e.g., CR portion), and preferably, the core is made by extrusion, such as the granulation-extrusion-spheronization process.
- the core is optionally surrounded by a CR coating, such as polymeric substance based on acrylates and/or methacrylates, e.g., a EUDRAGITTM polymer (sold by Rohm America, Inc.).
- a EUDRAGITTM polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent.
- EUDRAGITTM RL, EUDRAGITTM NE, and EUDRAGITTM RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films.
- EUDRAGITTM RL is freely permeable and EUDRAGITTM RS is slightly permeable, independent of pH.
- the permeability of EUDRAGITTM L is pH dependent.
- EUDRAGITTM L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester.
- CR coating It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable.
- two or more types of polymeric substances may be mixed for use as the CR coating.
- Other polymers suitable for CR coatings such as ethyl cellulose and cellulose acetate, can be used in the CR coating.
- the CR coating may comprise one or more suitable polymers, such as a combination of two or more of the polymers discussed above.
- the CR coating is itself coated by a layer of adhesive material that facilitates the adhesion of the particles / beads to a desired surface, such as a preferred GI tract surface.
- a desired surface such as a preferred GI tract surface.
- suitable adhesive materials are described herein above.
- the pellets / beads may be coated by a top-layer of a bioadhesive polymer such as SPHEROMERTM I [p(FASA)], SPHEROMERTM III, SPHEROMERTM IV, or mixtures thereof.
- the functions of a CR coating and bioadhesive coating can be combined in a single layer by using a mixture of polymers including a bioadhesive polymer and a polymer suitable for controlled release, i.e., a single layer may be both the CR layer and the bioadhesive layer of a particle.
- pellets can be further film-coated with an additional layer of a so-called "non-functional polymer” such as OPADRYTM II, EUDRAGITTM E, AcryloezeTM, hydroxypropylmethyl cellulose, hydroxypropyl cellulose, polyvinyl alcohol, polyvinylacetate, polyanhydride, etc.
- This layer may serve as a dispersion-promoting coating that inhibits clumping and aggregation of the particles during dispersion.
- this layer is preferably sufficiently strong or resilient to remain substantially intact during the compression process. This layer may also be protected by including a cushioning material among the excipients of the tablet matrix.
- an IR portion is included in the particle, such as over the dispersion- promoting coating, or between the dispersion-promoting coating and the adhesive layer, etc.
- particles 1301 are not embedded within the inactive material 1302, but are instead disposed loose in a capsule that dissolves and releases the particles in the GI tract.
- Figure 14 features yet another embodiment of the delivery device, in which particles described herein above (e.g., with respect to Figure 19) are embedded within a slow eroding material 1401 (e.g., that gradually erodes over 30 minutes, 45 minutes, 1 hr, 2 hrs, 4 hrs, 6 hrs, or longer). At least a portion of the eroding material 1401 is covered by an IR portion 1402, which disintegrates relatively rapidly to expose a surface of eroding material 1401. A portion of the slow eroding material 1401 is also optionally covered by a passive polymer support layer and/or an adhesive material 1403 as described herein above. In certain embodiments, the IR portion 1402 may be disposed on the adhesive layer 1403 instead of the eroding material 1401 as depicted.
- a slow eroding material 1401 e.g., that gradually erodes over 30 minutes, 45 minutes, 1 hr, 2 hrs, 4 hrs, 6 hrs, or longer.
- any drug to be delivered e.g., pramipexole
- a bioadhesive polymer composition e.g., a bioadhesive polymer composition, and/or pharmaceutically acceptable excipients
- ingredients such as those described above are weighed and mixed. These ingredients, possibly with the exception of any lubricants, can then be blended together in any suitable device, such as an end-over-end ATR rotator (e.g., model RKVS), or a planetary type mixer (e.g., Hobart Mixer).
- the blending process is done in small volume to reduce any possible loss of the ingredients due to their non-specific adherence to the blending device.
- the blending step is typically done to ensure the formation of a uniform dry mix of the ingredients, typically over a period of, e.g., 5-15 min.
- Granulation fluids may be purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro-alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated solvent or in a ketone, etc.
- the granulation process is conducted in a small volume, such as in a 500-mL cylindrical vessel.
- the granulation process is conducted with manual mixing, or conducted mechanically, e.g., in a planetary type mixer (such as a Hobart Mixer with a 5-qt mixing bowl). If the Hobart Mixer is used, it can be operated at its speed setting #1, depending on the batch size. Other types of mechanical mixers may also be used, with their respective appropriate settings, to achieve substantially the same result.
- a planetary type mixer such as a Hobart Mixer with a 5-qt mixing bowl.
- the Hobart Mixer can be operated at its speed setting #1, depending on the batch size.
- Other types of mechanical mixers may also be used, with their respective appropriate settings, to achieve substantially the same result.
- the wet granulation is dried.
- the wet granulation is dried in an oven (e.g., a Precision gravity oven, operating at about 50 0 C, for 8- 24 hrs; or similar appropriate conditions for other types of ovens).
- the granulation may be dried in a fluidized bed drier, such as a Vector MFL.01 Micro Batch Fluid Bed System, operating at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of about 50 0 C.
- the drying temperature is generally around 50 0 C. However, depending on different types of drugs / compositions, the temperature may be 35- 70 0 C, or 40-65 0 C, or 45-60 0 C, or 45-55 0 C, etc.
- the dried granulation is then grinded, e.g., by using a pestle in a mortar, optionally followed by sieving the ground material, e.g., through an appropriate-sized screen (such as a U.S. Std. mesh # 60 screen), depending on the desired size of the granules.
- a pestle in a mortar
- sieving e.g., through an appropriate-sized screen (such as a U.S. Std. mesh # 60 screen), depending on the desired size of the granules.
- the sieved granulation may be blended with a lubricant.
- the blending is conducted using an end-over-end ATR rotator (e.g., model RKVS).
- the blending is conducted using a planetary type mixer (e.g., Hobart Mixer, operating at the speed setting #1, for 5-15 min.). As a result, a uniformly lubricated dry mix is formed, which is then ready for compression.
- the lubricated dry mix may be passed through a sieve or screen, e.g., a U.S. Std. mesh # 60 screen.
- Different components of the pharmaceutical composition may be prepared as a mixture or separately using the subject methods.
- the dry mixes can be compressed into single layer or multilayer tablets.
- the lubricated dry mix may be pressed into tablets, such as by using a single-station manual tablet press (e.g., GlobePharma Manual Tablet Compaction Machine MTCM-I, equipped with adequate die and punch set).
- a single-station manual tablet press e.g., GlobePharma Manual Tablet Compaction Machine MTCM-I, equipped with adequate die and punch set.
- tablets may be prepared, e.g., at a pressure ranging from 250 to 4000 pounds per square inch (psi), and a compression time of, e.g., 1 to 4 seconds.
- Other machines may also be used to achieve substantially the same result.
- tablets may be produced with wet granulation of active ingredients followed by direct compression.
- multilayer tablets may be produced, with each layer comprising a different ingredient.
- a single-station manual tablet press e.g., GlobePharma Manual Tablet Compaction Machine MTCM-I, equipped with adequate die and punch set
- the compression process may include:
- pre-compressing the two layers together e.g., at a pressure ranging from 250 to 500 pounds per square inch (psi) and a compression time of, e.g., 1 to 5 seconds.
- the process can be repeated or modified if more than two layers of ingredients are to be used.
- the tablet can be made with a pre-compressed insert with effective ingredients.
- Such pre-compressed inserts may be produced with direct compression.
- the same press machine may be used for this process.
- tablet inserts may be prepared, e.g., at a pressure ranging from 500 to 1000 pounds per square inch (psi), and a compression time of, e.g., 1 to 2 seconds.
- psi pounds per square inch
- Other machines may also be used to achieve substantially the same result.
- the pre-compressed insert may be used as one of the layers ⁇ e.g., the second layer) in the tablet, or embedded in the middle of another layer ⁇ e.g., the second layer).
- the tablets may be coated with one or more coating compositions, such as in the form of successive layers.
- the coating compositions may include bioadhesive layers, delayed release layers, controlled-release layers, and/or other functional / non-functional polymers etc. (supra).
- tablets may be film-coated for this purpose, using a pan coater (e.g., O'Hara Labcoat, operating at an inlet air flow rate of about 60 cfrn (cubic foot per minute) and an inlet air temperature of about 35 0 C).
- the tablets may be pre-warmed at 35 0 C for 5-10 min., and after film coating, may be post-dried at about 30 0 C for about 15-30 min.
- Other coaters may also be used to achieve substantially the same result.
- Figure 15 features yet another embodiment of the delivery device, in which particles 1500 described herein above (e.g., with respect to Figure 13) are disposed on the surface of a bioadhesive film 1501.
- the film may optionally be dried or cured, e.g., without disrupting the particle adhesion.
- the film may then be folded and placed in a capsule 1502 for administration to a patient. If needed the capsule containing the active containing bioadhesive film is coated with delayed release coating to allow the film to adhere to the proximal part of the GI tract. If needed, the film may first be folded or cut to a suitable shape or size. Once administered to a patient, the capsule releases the film, which then rehydrates (if necessary) and adheres to a mucosal surface, allowing the particles spreaded and adhered thereto to release the active components.
- biocompatible polymers including hydrogels
- biodegradable polymers undergo chemical decomposition to form soluble monomers or soluble polymer units.
- the biodegradation of polymers usually involves chemically or enzymatically catalyzed hydrolysis.
- biodegradable polymers comprise a member selected from biodegradable poly(amides), poly(amino acids), poly(esters), poly(lactic acid), poly(glycolic acid), poly(orthoesters), poly(anhydrides), biodegradable poly(dehydropyrans), and poly(dioxinones).
- the polymers are known to the art in Controlled Release of Drugs, by Rosoff, Ch. 2, pp. 53-95 (1989); and in U.S. Pat. Nos. 3,811,444; 3,962,414; 4,066,747; 4,070,347; 4,079,038; and 4,093,709.
- representative dosage forms include hydrogel matrix containing a plurality of tiny pills or other particles.
- the hydrogel matrix comprises a hydrophilic polymer, such as selected from a polysaccharide, agar, agarose, natural gum, alkali alginate including sodium alginate, carrageenan, fucoidan, furcellaran, laminaran, hypnea, gum arabic, gum ghatti, gum karaya, gum tragacanth, locust bean gum, pectin, aniylopectin, gelatin and a hydrophilic colloid.
- a hydrophilic polymer such as selected from a polysaccharide, agar, agarose, natural gum, alkali alginate including sodium alginate, carrageenan, fucoidan, furcellaran, laminaran, hypnea, gum arabic, gum ghatti, gum karaya, gum tragacanth, locust bean gum, pectin,
- the hydrogel matrix comprises a plurality of tiny pills or particles (such as 4 to 50), each tiny pill or particle may comprise a different portion of the subject pramipexole compositions (e.g., IR, XR, DR, DXR, etc.).
- wall-forming materials include a triglyceryl ester selected from glyceryl tristearate, glyceryl monostearate, glyceryl dipalmitate, glyceryl laureate, glyceryl didecenoate and glyceryl tridecenoate.
- Other wall forming materials comprise polyvinyl acetate phthalate, methylcellulose phthalate, and microporous vinyl olefins. Procedures for manufacturing tiny pills are disclosed in U.S. Pat. Nos. 4,434,153; 4,721,613; 4,853,229; 2,996,431; 3,139,383 and 4,752,470, which are incorporated by reference herein.
- the invention employs a dosage form comprising a polymer that releases a drug by diffusion, flux through pores, or by rupture of a polymer matrix.
- the dosage form matrix can be made by procedures known to the polymer art.
- An example of providing a dosage form comprises blending a pharmaceutically acceptable carrier, like polyethylene glycol, with a known dose of the subject pharmaceutical composition, and adding it to a silastic medical grade elastomer with a cross-linking agent, like stannous octanoate, followed by casting in a mold. The step is repeated for each successive layer. The system is allowed to set, e.g., for 1 hour, to provide the dosage form.
- Representative polymers suitable for manufacturing the dosage form include olefin and vinyl polymers, condensation polymers, carbohydrate polymers, and silicon polymers as represented by poly(ethylene), poly(propylene), polyvinyl acetate), poly(methyl acrylate), poly(isobutyl methacrylate), poly(alginate), poly(amide), and poly(silicone).
- the polymers and manufacturing procedures are known in Polymers, by Coleman et al, Vol. 31, pp. 1187- 1230 (1990); Drug Carrier Systems, by Roerdink et ah, Vol. 9, pp. 57-109 (1989); Adv. Drug Delivery Rev., by Leong et al, Vol. 1, pp. 199-233 (1987); Handbook of Common Polymers, Compiled by Roff et at, (1971) published by CRC Press; and U.S. Pat. No. 3,992,518.
- a composition of the invention is administered in combination therapy with one or more additional drugs or prodrugs.
- the term "combination therapy” herein means a treatment regimen wherein the agent provided by the composition of the invention and a second agent are administered individually or together, sequentially or simultaneously, in such a way as to provide a beneficial effect from co-action of these therapeutic agents.
- beneficial effect can include, but is not limited to, pharmacokinetic or pharmacodynamic co-action of the therapeutic agents.
- Combination therapy can, for example, enable administration of a lower dose of one or both agents than would normally be administered during monotherapy, thus decreasing risk or incidence of adverse effects associated with higher doses.
- combination therapy can result in increased therapeutic effect at the normal dose of each agent in monotherapy.
- compositions of the invention can be especially suited to combination therapies, particularly where the second agent is one that is, or can be, administered once daily.
- the second agent is one that is, or can be, administered once daily.
- the two components of the combination therapy can be administered in separate dosage forms or in coformulation, i.e., in a single dosage form
- the second agent can be administered by any suitable route and in any pharmaceutically acceptable dosage form, for example by a route and/or in a dosage form other than the present composition.
- both components of the combination therapy are formulated together in a single dosage form.
- the second components of the subject combination therapy include L-dopa, selegiline, apomorphine and anticholinergics.
- L-dopa (levo-dihydroxy-phenylalanine) is a dopamine precursor which can cross the blood-brain barrier and be converted to dopamine in the brain.
- L-dopa has a short half life in the body and it is typical after long use (z. e. , after about 4-5 years) for the' effect of L-dopa to become sporadic and unpredictable, resulting in fluctuations in motor function, dyskinesias and psychiatric side effects.
- L-dopa can cause B vitamin deficiencies to arise.
- the gastrointestinal absorption of orally administered levodopa depends on the gastrointestinal transit rates as absorption occurs primarily in the proximal third of the intestine (duodenum/jejunum) and not in the stomach (Rivera-Calimlim et al. Europ. J. Clin. Invest. 1, 1313-1320, 1971).
- a delayed release dosage form containing levodopa/carbidopa or levodopa/carbidopa/entacapone with pramipexole will allow the levodopa to be released in the target proximal intestine region and release levodopa is a sustained manner similar to enteral infusion of levodopa.
- Selegiline (Deprenyl, Eldepryl) has been used as an alternative to L-dopa, and acts by reducing the breakdown of dopamine in the brain. Unfortunately, selegiline becomes ineffective after about nine months of use.
- Apomorphine a dopamine receptor agonist, has been used to treat Parkinson's disease, although is causes severe vomiting when used on its own, as well as skin reactions, infection, drowsiness and some psychiatric side effects.
- Systemically administered anticholinergic drugs have also been used to treat Parkinson's disease and act by reducing the amount of acetylcholine produced in the brain and thereby redress the dopamine/acetylcholine imbalance present in Parkinson's disease.
- systemically administered anticholinergics develop serious neuropsychiatric side effects, including hallucinations, as well as dyskinetic movements, and other effects resulting from wide anticholinergic distribution, including vision effects, difficulty swallowing, dry mouth, and urine retention. See e.g. Playfer, Parkinson's Disease, Postgrad Med J 13: 257-264, 1997 and Nadeau, Parkinson's Disease, J Am GerSoc 45: 233- 240, 1997.
- Newer drug refinements and developments include direct-acting dopamine agonists, slow-release L-dopa formulations, inhibitors of the dopamine degrading enzymes catechol-O- methyltransferase (COMT) and monoamine oxidase B (MAO-B), and dopamine transport blockers.
- These treatments enhance central dopaminergic neurotransmission during the early stages of Parkinson's disease, ameliorate symptoms associated with Parkinson's disease, and temporarily improve the quality of life.
- L-dopa for treating Parkinson's disease, the benefits accorded by these dopaminergic therapies are temporary, and their efficacy declines with disease progression.
- thalamotomy a malignant neoplasm originating from thalamic cells.
- thalamotomy a malignant neoplasm originating from thalamic cells.
- Thalamotomy destroys part of the thalamus, a brain region involved in movement control.
- Unilateral stereotactic thalamotomy has proven to be effective for controlling contralateral tremor and rigidity, but carries a risk of hemiparesis.
- Bilateral thalamotomy carries an increased risk of speech and swallowing disorders resulting.
- Pallidotomy surgical ablation of part of the globus pallidus (a basal ganglia), has also be used with some success.
- Pallidotomy is performed by inserting a wire probe into the globus pallidus and heating the probe to destroy nearby tissue.
- Pallidotomy is most useful for the treatment of peak-dose diskinesias and for dystonia that occurs at the end of a dose.
- Therapeutic methods aimed at controlling suspected causative factors associated with Parkinson's disease ⁇ e.g., therapies which control oxidative stress and excitotoxicity) have also been developed.
- Clinical trials have shown that administration of antioxid ⁇ tive agents vitamin E and deprenyl provided little or no neuroprotective function (Shoulson et at, Ann. Neurol. 43: 318-325, 1998).
- Glutamate-receptor blockers and neuronal nitric oxide synthase (NOS) inhibitors have been proposed as therapies for Parkinson's disease; however, no experimental results from human studies have yet been published (Rodriguez, Ann. Neurol. 44: S175-S188, 1998).
- GDNF glial cell line-derived neurotrophic factor
- neurotrophic factors which may have therapeutic value have been proposed based on in vitro and animal model systems, including neurturin, basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), neurotrophins 3 and 4/5, ciliary neurotrophic factor and transforming growth factor ⁇ (TGF- ⁇ ).
- bFGF basic fibroblast growth factor
- BDNF brain-derived neurotrophic factor
- TGF- ⁇ transforming growth factor ⁇
- dopaminergic neurons for use in the transplantation process have been tried in animal experiments, including the use of mesencephalic dopamine neurons obtained from human embryo cadavers, immature neuronal precursor cells (i.e., neuronal stem cells), dopamine secreting non-neuronal cells, terminally differentiated teratocarcinoma- derived neuronal cell lines (Dunnett and Bjorkland, supra), genetically modified cells (Raymon et al., Exp. Neurol. 144: 82-91, 1997; and Kang, Mov. Dis.
- Additional therapies are also available, such as physical therapy, occupational therapy, or speech / language therapy.
- Exercise, diet, nutrition, patient/caregiver education, and psychosocial interventions have also been shown to have a positive effect on the mental and/or physical state of a person suffering from Parkinson's disease.
- Various methods of evaluating Parkinson's disease in a patient include Hoehn and Yahr Staging of Parkinson's Disease, Unified Parkinson Disease Rating Scale (UPDRS), and Schwab and England Activities of Daily Living Scale.
- UPDS Unified Parkinson Disease Rating Scale
- a person suffering from Parkinson's disease should avoid contraindicated and potentially contraindicated drugs such as antipsychotic drugs, Haloperidol (Haldol), Perphenazine (Trilafon), Chlorpromazine (Thorazine), Trifluoperazine (Stelazine), Flufenazine (Prolixin, Permitil) Thiothixene (Navane), Thioridazine (Mellaril); antidepressant drug, combination of Perphenazine and Amitriptyline (Triavil); anti-vomiting drugs, Prochlorperazine (Compazine), Metoclopramide (Reglan, Maxeran), Thiethylperazine (Torecan), Reserpine (Serpasil), Tetrabenazine (Nitoman); blood pressure drug, Alpha- methyldopa (Aldomet); anti-seizure drug, Phenytoin (Dilantin); mood stabilizing drug, lithium; and anti
- the method includes administering, conjointly with the subject pharmaceutical composition, one or more of other therapeutic compositions useful for the treatment of diseases, for which pramipexole is indicated for.
- pramipexole may be coadministered with a dopamine precursor, a dopaminergic agent, a dopaminergic and anticholinergic agent, an anti-ch ⁇ linergic agent, a dopamine agonist, a MAO-B (monoamine oxidase B) inhibitor, a COMT (catechol 0-methyltransferase) inhibitor, a muscle relaxant, a sedative, an anticonvulsant agent, a dopamine reuptake inhibitor, a dopamine blocker, a ⁇ - blocker, a carbonic anhydrase inhibitor, a narcotic agent, a GABAergic agent, or an alpha antagonist.
- a dopamine precursor e.g., a dopaminergic agent, a dopaminergic and anticholinergic agent
- the subject packages, preparations, pharmaceutical compositions, and methods for the treatment of movement disorders further comprise one or more therapeutic agents for treating Parkinson's disease selected from a dopamine precursor, such as L-dopa; a dopaminergic agent, such as Levodopa-carbidopa (SINEMET ® , SINEMET CR ® ) or Levodopa-benserazide (PROLOPA ® , MADOPAR ® , MADOPAR HBS ® ); a dopaminergic and anti-cholinergic agent, such as amantadine (SYMMETRYL ® , SYMADINE ® ); an anti-cholinergic agent, such as trihexyphenidyl (ARTANE ® ), benztropine (COGENTIN ® ), ethoproprazine (PARSITAN ® ), or procyclidine (KEMADRIN ® ); a dopamine agonist, such as apomorphine
- US20030045539 discloses a combination treatment of cabergoline and pramipexole provided concurrently to a patient suffering from various central nervous system diseases, and in particular for the treatment of Parkinson's Disease (PD).
- the initial dose of cabergoline is administered to the patient at a dose of 0.5 to 1 mg/patient/day and is adjusted upward at weekly intervals to a therapeutic dosage of 2, 4, 6, 8 or 10 mg/patient/day and where the initial dose of pramipexole is started at 0.375 mg/patient/day and is adjusted upward every 5 to 7 days to a therapeutic dosage of 3, 4, 5, 6, or 7 mg/patient/day.
- At least one portion of the subject pharmaceutical composition may additional comprises cabergoline and pramipexole for treating Parkinson's disease.
- US20040166159 discloses a pharmaceutical dosage forms having immediate and controlled release properties that contain an aromatic amino acid decarboxylase (AAAD) inhibitor (such as carbidopa), levodopa, and optionally a catechol-0-methyltransferase (COMT) inhibitor, for the treatment of medical conditions associated with reduced dopamine levels in a patient's brain.
- the dosage form may comprise up to about 1000 mg, or about 20-500 mg, about 50-500 mg, or about 100-200 mg of COMT inhibitor.
- the COMT inhibitor may be contained only within the immediate release component, or only within the sustained release component, or both.
- the COMT inhibitor may be CGP-28014, entacapone, or tolcapone.
- the dosage form may further comprise one or more drugs such as anticholinergics, beta 2-agonists, cyclooxygenase-2 (COX-2) inhibitors, dopamine receptor agonists, monoamine oxidase (MAO) inhibitors, opiate delta receptor agonists, opiate delta receptor antagonists, and N-methyl-D-aspartate (NMDA) antagonists.
- drugs such as anticholinergics, beta 2-agonists, cyclooxygenase-2 (COX-2) inhibitors, dopamine receptor agonists, monoamine oxidase (MAO) inhibitors, opiate delta receptor agonists, opiate delta receptor antagonists, and N-methyl-D-aspartate (NMDA) antagonists.
- drugs such as anticholinergics, beta 2-agonists, cyclooxygenase-2 (COX-2) inhibitors, dopamine receptor agonists, monoamine oxidase (MAO) inhibitors, opiate delta receptor agonists, opiate
- the dosage form may further comprise one or more drugs selected from albuterol, alpha- lipoic acid, amantadine, andropinirole, apomorphine, baclofen, biperiden, benztropine, bromocriptine, budipine, cabergoline, clozapine, deprenyl, dextromethorphan, dihydroergokryptine, dihydrolipoic acid, eliprodil, eptastigmine, ergoline, formoterol, galanthamine, lazabemide, lysuride, mazindol, memantine, mofegiline, orphenadrine, pergolide, pirbuterol, propentofylline, procyclidine, rasagiline, remacemide, riluzole, rimantadine, ropinirole, salmeterol, selegiline, spheramine, terguride, and trihexyphenidyl.
- the invention further comprises one or more therapeutic agents for treating dystonia selected from an anti-cholinergic agent, such as trihexyphenidyl (ARTANE ® ), benztropine (COGENTIN ® ), ethoproprazine (PARSITAN ® ), or procyclidine (KEMADRIN ® ); a dopaminergic agent, such as Levodopa-carbidopa (SINEMET ® , SINEMET CR ® ) or Levodopa-benserazide (PROLOPA ® , MADOPAR ® , MADOPAR HBS ® ); a muscle relaxant, such as baclofen (LIORESAL ® ); a sedative, such as Clonazepam (RIVOTRIL
- an anti-cholinergic agent such as trihexyphenidyl (ARTANE ® ), benztropine (COGENTIN ® ), ethoproprazine (PARSITAN
- the invention further comprises one or more therapeutic agents for treating tremor selected from a /3-blocker, such as propranolol (INDERAL ® , INDERAL-LA ® ); an anticonvulsant agent, such as primidone (MYSOLINE ® ); or a carbonic anhydrase inhibitor, such as acetalzolamide (DIAMOX ® ) or methazolamide (NEPTAZANE ® ).
- a /3-blocker such as propranolol (INDERAL ® , INDERAL-LA ®
- an anticonvulsant agent such as primidone (MYSOLINE ® )
- a carbonic anhydrase inhibitor such as acetalzolamide (DIAMOX ® ) or methazolamide (NEPTAZANE ® ).
- the invention further comprises one or more therapeutic agents for treating myoclonus selected from a sedative, such as clonazepam (RIVOTRIL ® ); or an anticonvulsant agent, such as valproic acid (EPIVAL ® ).
- a sedative such as clonazepam (RIVOTRIL ® )
- an anticonvulsant agent such as valproic acid (EPIVAL ® ).
- the invention further comprises one or more therapeutic agents for treating chorea selected from a dopamine blocker, such as haloperidol (HALDOL ® ); or a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN ® ).
- a dopamine blocker such as haloperidol (HALDOL ® )
- a dopamine reuptake inhibitor such as tetrabenazine (NITOMAN ® ).
- the invention further comprises one or more therapeutic agents for treating restless leg syndrome selected from a dopaminergic, such as Levodopa-carbidopa (SINEMET ® , SINEMET CR ® ) or Levodopa-benserazide (PROLOPA ® , MADOPAR ® , MADOPAR HBS ® ); a sedative, such as clonazepam (RIVOTRIL ® ); a dopamine agonists, such as bromocriptine (PARLODEL ® ), pergolide (PERMAX ® ), or ropinirole (REQUIP ® ); a narcotic agent, such as codeine (TYLENOL # 3 ® ); or a GABAergic agent, such as gabapentin (NEURONTIN ® ).
- a dopaminergic such as Levodopa-carbidopa (SINEMET ® , SINEMET CR ®
- the invention further comprises one or more therapeutic agents for treating tics selected from a sedative, such as clonazepam (RIVOTRIL ® ); an alpha antagonist, such as clonidine (CATAPRESS ® ); a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN ® ); or a dopamine blocker, such as haloperidol (HALDOL ® ) or perphenazine.
- a sedative such as clonazepam (RIVOTRIL ® ); an alpha antagonist, such as clonidine (CATAPRESS ® ); a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN ® ); or a dopamine blocker, such as haloperidol (HALDOL ® ) or perphenazine.
- a sedative such as clonazepam (RIVOTRIL ®
- the method includes administering, conjointly with the pharmaceutical composition, one or more of physical therapy, occupational therapy, or speech/language therapy.
- An agent to be administered conjointly with a subject compound may be formulated together with a subject compound as a single pharmaceutical preparation, e.g., as a pill or other medicament including both agents, or may be administered as a separate pharmaceutical preparation.
- Another aspect of the invention provides a packaged pharmaceutical composition, comprising the subject pharmaceutical composition in an amount sufficient to treat or prevent a movement disorder in a patient, which may additionally include a pharmaceutically acceptable carrier, and instructions (written and/or pictorial) describing the use of the formulation for treating the patient, wherein the patient suffers from ataxia, corticobasal ganglionic degeneration (CBGD), dyskinesia, dystonia, tremors, hereditary spastic paraplegia, Huntington's disease, multiple system atrophy, myoclonus, Parkinson's disease, progressive supranuclear palsy, restless legs syndrome, Rett syndrome, spasticity, Sydenham's chorea, other choreas, athetosis, ballism, stereotypy, tardive dyskinesia/dystonia, tics, Tourette's syndrome, olivopontocerebellar atrophy (OPCA), diffuse Lewy body disease, hemibalismus, hemi-facial spa
- the movement disorder is Parkinson's disease.
- the present invention contemplates modes of treatment and/or prophylaxis ⁇ e.g., treating or preventing the development of symptoms in high-risk populations), which utilize one or more of the subject dosage forms for decreasing or overcoming the defects in a movement disorder patient.
- the improvement and/or restoration of mental or physical state in an organism has positive behavioral, social, and psychological consequences.
- Parkinson's disease is the second most common neurodegenerative disorder, affecting nearly 1 million people in North America. The disease is characterized by symptoms such as muscle rigidity, tremor and bradykinesia.
- Parkinson's disease Early studies of Parkinson's disease showed unusual inclusions in the cytoplasm of neurons (i.e., Lewy bodies), occurring predominantly in the substantia nigra, which innervate the striatal region of the forebrain. Although Lewy bodies were also found in other neurodegenerative conditions, the presence of Lewy bodies in Parkinson's disease is accompanied by cell loss in the substantia nigra. This cell loss is considered to be the defining pathological feature of Parkinson's disease.
- Parkinson's disease incidence leading to the search for environmental factors
- MPTP l-methyl-4-phenyl-l,2,3,6- tetrahydropyridine
- Parkinson's disease has also identified genes associated with Parkinson's disease (Mizuno et al, Biomed. Pharmacother. 53(3): 109-116, 1999; Dunnett and Bjorklund, Nature 399 (6738 Suppl): A32-A39, 1999); namely, the ⁇ -synuclein gene (Polymeropouos et al, Science 276: 2045-2047, 1997), the parkin gene (Kitada et al, Nature 392: 605-608, 1998), and the UCH-Ll thiol protease gene (Leroy et al, Nature 395: 451-452, 1998).
- Parkinson's disease Mizuno et al, Biomed. Pharmacother. 53(3): 109-116, 1999; Dunnett and Bjorklund, Nature 399 (6738 Suppl): A32-A39, 1999
- ⁇ -synuclein gene Polymeropouos et al, Science 276: 20
- Parkinson's disease is associated with the progressive loss of dopamine neurons in the ventral mesencephalon of the substantia nigra (Shoulson, Science 282: 1072- 1074, 1998), which innervates the major motor-control center of the forebrain, the striatum.
- a gradual decline in the number of neurons and dopamine content of the basal ganglia is normally associated with increasing age, progressive dopamine loss is pronounced in people suffering from Parkinson's disease, resulting in the appearance of symptoms when about 70-80% of striatal dopamine and 50% of nigral dopamine neurons are lost (Dunnett and Bjorklund, supra).
- Parkinson's disease This loss of dopamine-producing neurons resulting in a dopamine deficiency is believed to be responsible for the motor symptoms of Parkinson's disease. Although the cause of dopaminergic cell death remains unknown, it is believed that dopaminergic cell death is affected by a combination of necrotic and apoptotic cell death. Mechanisms and signals responsible for the progressive degeneration of nigral dopamine neurons in Parkinson's disease have been proposed (Olanow et al, Ann. Neurol. 44: Sl -S 196, 1998), and include oxidative stress (from the generation of reactive oxygen species), mitochondrial dysfunction, excitotoxicity, calcium imbalance, inflammatory changes and apoptosis as contributory and interdependent factors in Parkinson's disease neuronal cell death.
- Apoptosis ⁇ i.e., programmed cell death
- accelerated apoptosis is believed to underlie many neurodegenerative diseases, including Parkinson's disease (Barinaga, Science 281: 1303-1304, 1998; Mochizuki et al, J. Neurol. Set 137: 120- 123, 1996; and Oo et al, Neuroscience 69: 893-901, 1995).
- apoptotic death can be initiated by a variety of external stimuli, and the biochemical nature of the intracellular apoptosis effectors is at least partially understood.
- the subject dosage form is administered orally to the lower gastrointestinal (GI) tract.
- GI lower gastrointestinal
- An orally ingested product can adhere to either the epithelial surface or the mucus lining of the GI tract.
- a polymeric drug delivery device adhere to the epithelium or to the mucous layer.
- Bioadhesion in the GI tract may proceed in two stages: (1) viscoelastic deformation at the point of contact of the synthetic material into the mucus substrate, and (2) formation of bonds between the adhesive synthetic material and the mucus or the epithelial cells.
- adhesion of polymers to tissues may be achieved by (i) physical or mechanical bonds, (ii) primary or covalent chemical bonds, and/or (iii) secondary chemical bonds ⁇ e.g., ionic).
- Physical or mechanical bonds can result from deposition and inclusion of the adhesive material in the crevices of the mucus or the folds of the mucosa.
- Secondary chemical bonds, contributing to bioadhesive properties, consist of dispersive interactions ⁇ e.g., van der Waals interactions) and stronger specific interactions, which include hydrogen bonds.
- the hydrophilic functional groups primarily responsible for forming hydrogen bonds are the hydroxyl and the carboxylic groups.
- Bioadhesion is defined as the ability of a material to adhere to a biological tissue for an extended period of time. Bioadhesion is one solution to the problem of inadequate residence time resulting from intestinal peristalsis, and from displacement by ciliary movement. For sufficient bioadhesion to occur, an intimate contact must exist between the bioadhesive and the receptor tissue, the bioadhesive must penetrate into the crevice of the tissue surface and/or mucus, and mechanical, electrostatic, or chemical bonds must form. Polycarbophils and acrylic acid polymers usually have the best adhesive properties. Duchene et al, in Drug Dev. bid.
- bioadhesive systems for drug delivery (incorporated herein by reference). These bioadhesive systems may be adapted for use in the instant invention. Other bioadhesive systems that may be adapted for use in the instant application are described in WO 93/21906; Smart et al, J. Pharm. Pharmacol. 36: 295-299, 1984; Gurney et al, Biomaterials 5: 336- 340, 1984; Park et al, "Alternative Approaches to Oral Controlled Drug Delivery: Bioadhesives and In-Situ Systems," in J. M. Anderson and S. W.
- the subject dosage forms having increased lower gastrointestinal retention time.
- intestinal residence time is the time required for a dosage form to transit through the intestine to the pyloric sphincter.
- a dosage form of the invention has an intestinal residence time of at least 3 hours, at least 4 hours, at least 6 hours, at least 8 hours or at least 12 hours.
- the dosage forms of the invention may have an increased retention time in the small and/or large intestine, or in the area of the gastrointestinal tract that absorbs the drug contained in the dosage form.
- dosage forms of the invention can be retained in the small intestine (or one or two portions thereof, selected from the duodenum, the jejunum and the ileum) for at least 6 hours, at least 8 hours or at least 12 hours, such as from 16 to 18 hours.
- Suitable bioadhesive polymeric coatings are disclosed in U.S. Patent Nos. 6,197,346, 6,217,908 and 6,365,187 (the contents of which are incorporated herein by reference), and include soluble and insoluble, biodegradable and nonbiodegradable polymers. These can be hydrogels or thermoplastics, homopolymers, copolymers or blends, and/or natural or synthetic polymers.
- the preferred polymers are synthetic polymers, with controlled synthesis and degradation characteristics.
- Particularly preferred polymers are anhydride copolymers of fumaric acid and sebacic acid (P(FA:SA)), which have exceptionally good bioadhesive properties when administered to the GI tract.
- P(FA: SA) copolymers examples include those having a 1:99 to 99:1 ratio of fumaric acid to sebacic acid, such as 5:95 to 75:25, for example, 10:90 to 60:40 or at least 15:85 to 25:75. Specific examples of such copolymers have a 20:80 or a 50:50 ratio of fumaric acid to sebacic acid.
- Polymers used in dosage forms of the invention produce a bioadhesive interaction (fracture strength) of at least 100 N/m 2 (10 mN/cm ) when applied to the mucosal surface of rat intestine.
- the fracture strength of the dosage forms is advantageously at least 250 N/m 2 , at least 500 N/m 2 or at least 1000 N/m 2 .
- the fracture strength of a polymer- containing dosage form can be from 100 to 500 N/m 2 .
- the forces described herein refer to measurements made upon rat intestinal mucosa, unless otherwise stated. The same adhesive measurements made on a different species of animal will differ from those obtained using rats.
- the fracture strength of dosage forms of the invention on rat intestine is generally at least 125 N/m 2 , such as at least 150 N/m 2 , at least 250 N/m 2 , at least 500 N/m 2 or at least 1000 N/m 2 .
- the fracture strength of a dosage form can be measured according to the methods disclosed by Duchene et at Briefly, the dosage form is attached on one side to a tensile tester and is contacted with a testing surface (e.g., a mucosal membrane) on the opposite surface.
- the tensile tester measures the force required to displace the dosage form from the testing surface.
- Common tensile testers include a Texture Analyzer and the Instron tensile tester.
- dosage forms are pressed using flat-faced tooling, 0.3750" (9.525 mm) in diameter. Dosage form weight will depend on composition; in most cases, the dosage forms have a final weight of 200 mg. These dosage forms are then glued to a plastic 10 mm diameter probe using a common, fast-drying cyanoacrylate adhesive. Once the dosage forms are firmly adhered to the probe, the probe is attached to the Texture Analyzer. The Texture Analyzer is fitted with a 1 kg load cell for maximum sensitivity. The following settings are used:
- Test and Post-Test Speeds are as low as the instrument will allow, to ensure a maximum number of data points captured.
- the Pre-Test speed is used only until the probe encounters the Trigger Force; i.e., prior to contacting the tissue.
- the Proportional, Integral, and Differential Gain are set to 0. These settings, when optimized, maintain the system at the Applied Force for the duration of the Contact Time. With soft tissue as a substrate, however, the probe and dosage form are constantly driven into the deformable surface. This results in visible damage to the tissue. Thus, the probe and dosage form are allowed to relax gradually from the Applied Force by setting these parameters to 0. The tracking speed, which is a measure of how rapidly the feedback is adjusted, is also set to 0.
- the tissue on which the dosage forms are tested is secured in the Mucoadhesive Rig; the rig is then completely immersed in a 600 mL Pyrex beaker containing 375 niL of PBS. The tissue is maintained at approximately 37 0 C for the duration of the test; no stirring is used as the machine can detect the oscillations from the stir bar.
- hydrophilic polymers In the past, two classes of polymers have shown useful bioadhesive properties, hydrophilic polymers and hydrogels.
- carboxylic groups e.g., poly[acrylic acid]
- hydrogels In the large class of hydrophilic polymers, those containing carboxylic groups (e.g., poly[acrylic acid]) exhibit the best bioadhesive properties. It is thus expected that polymers with the highest concentrations of carboxylic groups are preferred materials for bioadhesion on soft tissues.
- carboxylic groups e.g., poly[acrylic acid]
- the most promising polymers were sodium alginate, carboxymethylcellulose, hydroxymethylcellulose and methylcellulose. Some of these materials are water-soluble, while others are hydrogels.
- Rapidly bioerodible polymers such as poly[lactide-co-glycolide], polyanhydrides, and polyorthoesters, whose carboxylic groups are exposed on the external surface as their smooth surface erodes, are suitable for bioadhesive drug delivery systems.
- polymers containing labile bonds such as polyanhydrides and polyesters, are well known for their hydrolytic reactivity. Their hydrolytic degradation rates can generally be altered by simple changes in the polymer backbone.
- Representative natural polymers suitable for the present invention include proteins (e.g., hydrophilic proteins), such as zein, modified zein, casein, gelatin, gluten, serum albumin, or collagen, and polysaccharides such as cellulose, dextrans, polyhyaluronic acid, polymers of acrylic and methacrylic esters and alginic acid. These are generally less suitable for use in bioadhesive coatings due to higher levels of variability in the characteristics of the final products, as well as in degradation following administration.
- Synthetically modified natural polymers include alkyl celluloses, hydroxyalkyl celluloses, cellulose ethers, cellulose esters, and nitrocelluloses.
- Representative synthetic polymers for use in bioadhesive coatings include polyphosphazines, poly(vinyl alcohols), polyamides, polycarbonates, polyalkylenes, polyacrylamides, polyalkylene glycols, polyalkylene oxides, polyalkylene terephthalates, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyurethanes and copolymers thereof.
- polymers suitable for use in the invention include, but are not limited to, methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxymethyl cellulose, cellulose triacetate, cellulose sulfate sodium salt, poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), poly(octadecyl acrylate) polyethylene, polypropylene, poly(ethylene glycol), poly(ethylene
- bioerodible polymers for use in bioadhesive coatings include polylactides, polyglycolides and copolymers thereof, poly(ethylene terephthalate), poly(butyric acid), poly(valeric acid), poly(lactide-co- caprolactone), poly[lactide-co-glycolide], polyanhydrides (e.g., poly(adipic anhydride)), polyorthoesters, chitosan, chitin, hyaluronic acid, hyaluronan, Carbopols, Corplex polymers, Polycarbophils-Cysteine (Thiomers), Chitosan-Thioglycolic acid copolymers, ⁇ oly(methacrylic acid-grafted-ethylene glycol), poly (methyl vinyl ether-co-malic anhydride), cholestyramine (Duolite AP- 143), sucralfate and gliadin blends and copolymers thereof.
- Polyanhydrides are particularly suitable for use in bioadhesive delivery systems because, as hydrolysis proceeds, causing surface erosion, more and more carboxylic groups are exposed to the external surface.
- polylactides erode more slowly by bulk erosion, which is advantageous in applications where it is desirable to retain the bioadhesive coating for longer durations.
- polymers that have high concentrations of carboxylic acid are preferred.
- the high concentrations of carboxylic acids can be attained by using low molecular weight polymers (MW of 2000 or less), because low molecular weight polymers contain a high concentration of carboxylic acids at the end groups.
- the polymers listed above can be obtained from sources such as Sigma Chemical Co., St. Louis, Mo., Polysciences, Warrenton, Pa., Aldrich, Milwaukee, Wis., Fluka, Ronkonkoma, N. Y., and BioRad, Richmond, Calif., or can alternatively be synthesized from monomers obtained from these suppliers using standard techniques.
- the synthetic polymer is typically selected from polyamides, polycarbonates, polyalkylenes, polyalkylene glycols, polyalkylene oxides, polyalkylene terephthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyurethanes, polystyrene, polymers of acrylic and methacrylic esters, polylactides, poly(butyric acid), poly(valeric acid), poly(lactide-co-glycolide), polyanhydrides, polyorthoesters, poly(fumaric acid), poly(maleic acid), and blends and copolymers of thereof.
- the synthetic polymer is poly(fumaric-co-sebacic) anhydride.
- polymers suitable for use as bioadhesive polymeric coatings are polymers having a hydrophobic backbone with at least one hydrophobic group pendant from the backbone.
- Suitable hydrophobic groups are groups that are generally non-polar. Examples of such hydrophobic groups include alkyl, alkenyl and alkynyl groups. Preferably, the hydrophobic groups are selected to not interfere and instead to enhance the bioadhesiveness of the polymers.
- a further group of polymers suitable for use as bioadhesive polymeric coatings are polymers having a hydrophobic backbone with at least one hydrophilic group pendant from the backbone.
- Suitable hydrophilic groups are groups that are capable of hydrogen bonding to another functional group.
- Example of such hydrophilic groups include negatively charged groups such as carboxylic acids, sulfonic acids and phosponic acids, positively charged groups such as (protonated) amines and neutral, polar groups such as amides and imines.
- the hydrophilic groups are selected to not interfere and instead to enhance the bioadhesiveness of the polymers.
- the hydrophilic groups can be either directly attached to a hydrophobic polymer backbone or attached through a spacer group.
- a spacer group is an alkylene group, particularly a C 1 -C 8 alkyl group such as a C 2 -Cg alkyl group.
- Preferred compounds containing one or more hydrophilic groups include amino acids (e.g., phenyalanine, tyrosine and derivatives thereof) and amine-containing carbohydrates (sugars) such as glucosamine.
- Polymers can be modified by increasing the number of carboxylic groups accessible during biodegradation, or on the polymer surface.
- the polymers can also be modified by binding amino groups to the polymer.
- the polymers can be modified using any of a number of different coupling chemistries available in the art to covalently attach ligand molecules with bioadhesive properties to the surface-exposed molecules of the polymeric microspheres.
- any positively charged ligand such as polyethyleneimine or polylysine
- a polymer may improve bioadhesion due to the electrostatic attraction of the cationic groups coating the beads to the net negative charge of the mucus.
- Any ligand with a high binding affinity for mucin could also be covalently linked to most polymers with the appropriate chemistry, such as with carbodiimidazole (CDI), and be expected to influence the binding to the gut.
- CDI carbodiimidazole
- polyclonal antibodies raised against components of mucin or else intact mucin, when covalently coupled to a polymer, would provide for increased bioadhesion.
- antibodies directed against specific cell surface receptors exposed on the lumenal surface of the intestinal tract would increase the residence time when coupled to polymers using the appropriate chemistry.
- the ligand affinity need not be based only on electrostatic charge, but other useful physical parameters such as solubility in mucin or specific affinity to carbohydrate groups.
- any of the natural components of mucin in either pure or partially purified form to the polymers would increase the solubility of the polymer in the mucin layer.
- useful ligands would include but not be limited to the following: sialic acid, neuraminic acid, n-acetyl-neuraminic acid, n-glycolylneuraminic acid, 4-acetyl-n- acetylneuraminic acid, diacetyl-n-acetylneuraminic acid, glucuronic acid, iduronic acid, galactose, glucose, mannose, fucose, any of the partially purified fractions prepared by chemical treatment of naturally occurring mucin, e.g., mucoproteins, mucopolysaccharides and mucopolysaccharide-protein complexes, and antibodies immunoreactive against proteins or sugar structure on the mucosal surface.
- polyamino acids containing extra pendant carboxylic acid side groups such as polyaspartic acid and polyglutamic acid
- the polyamino chains would increase bioadhesion by means of chain entanglement in mucin strands as well as by increased carboxylic charge.
- polymers such as those named above, having a metal compound incorporated therein have a further improved ability to adhere to tissue surfaces, such as mucosal membranes.
- the metal compound incorporated into the polymer can be, for example, a water-insoluble metal oxide.
- Metal compounds which can be incorporated into polymers to improve their bioadhesive properties preferably are water-insoluble metal compounds, such as water- insoluble metal oxides and metal hydroxides, which are capable of becoming incorporated into and associated with a polymer to thereby improve the bioadhesiveness of the polymer.
- a water-insoluble metal compound is defined as a metal compound with little or no solubility in water, for example, less than about 0.0 to 0.9 mg/ml.
- the water-insoluble metal compounds can be derived from a wide variety of metals, including, but not limited to, calcium, iron, copper, zinc, cadmium, zirconium and titanium.
- the water insoluble metal compound preferably is a metal oxide or hydroxide. Water insoluble metal compounds of multivalent metals are preferred.
- Representative metal oxides suitable for use in the compositions described herein include cobalt (I) oxide (CoO), cobalt (II) oxide (Co 2 O 3 ), selenium oxide (SeO 2 ), chromium (IV) oxide (CrO 2 ), manganese oxide (MnO 2 ), titanium oxide (TiO 2 ), lanthanum oxide (La 2 O 3 ), zirconium oxide (ZrO 2 ), silicon oxide (SiO 2 ), scandium oxide (Sc 2 O 3 ), beryllium oxide (BeO), tantalum oxide (Ta 2 O 5 ), cerium oxide (CeO 2 ), neodymium oxide (Nd 2 O 3 ), vanadium oxide (V 2 Os), molybdenum oxide (Mo 2 O 3 ), tungsten oxide (WO), tungsten trioxide (WO 3 ), samarium oxide (Sm 2 O 3 ), europium oxide (Eu 2 O 3 ), gadolinium oxide (Gd 2
- oxides include barium oxide (BaO), calcium oxide (CaO), nickel oxide (III) (Ni 2 O 3 ), magnesium oxide (MgO), iron (II) oxide (FeO) 5 iron (III) oxide (Fe 2 O 3 ), copper oxide (II) (CuO), cadmium oxide (CdO), and zirconium oxide (ZrO 2 ).
- Preferred properties defining the metal compound include: (a) substantial insolubility in aqueous environments, such as acidic or basic aqueous environments (such as those present in the gastric lumen); and (b) ionizable surface charge at the pH of the aqueous environment.
- the water-insoluble metal compounds can be incorporated into the polymer by one of the following mechanisms: (a) physical mixtures which result in entrapment of the metal compound; (b) ionic interaction between metal compound and polymer; (c) surface modification of the polymers which would result in exposed metal compound on the surface; and (d) coating techniques such as fluidized bed, pan coating, or any similar methods known to those skilled in the art, which produce a metal compound enriched layer on the surface of the device.
- nanoparticles or microparticles of the water-insoluble metal compound are incorporated into the polymer.
- the metal compound is provided as a fine particulate dispersion of a water-insoluble metal oxide which is incorporated throughout the polymer or at least on the surface of the polymer which is to be adhered to a tissue surface.
- the metal compound also can be incorporated in an inner layer of the polymer and exposed only after degradation or else dissolution of a "protective" outer layer.
- a tablet core containing a polymer and metal may be covered with an enteric coating designed to dissolve when exposed to intestinal fluid. The metal compound-enriched core then is exposed and become available for binding to GI mucosa.
- Fine metal oxide particles can be produced for example by micronizing a metal oxide by mortar and pestle treatment to produce particles ranging in size, for example, from 10.0 to 300 ran.
- the metal oxide particles can be incorporated into the polymer, for example, by dissolving or dispersing the particles into a solution or dispersion of the polymer.
- metal compounds which are incorporated into polymers to improve their bioadhesive properties can be metal compounds which are already approved by the FDA as either food or pharmaceutical additives, such as zinc oxide.
- Suitable polymers which can be used and into which the metal compounds can be incorporated include soluble and water-insoluble, and biodegradable and nonbiodegradable polymers, including hydrogels, thermoplastics, and homopolymers, copolymers and blends of natural and synthetic polymers, provided that they have the requisite fracture strength when mixed with a metal compound.
- representative polymers which can be used in conjunction with a metal compound include hydrophilic polymers, such as those containing carboxylic groups, including polyacrylic acid.
- Bioerodible polymers including polyanhydrides, poly(hydroxy acids) and polyesters, as well as blends and copolymers thereof also can be used.
- bioerodible poly(hydroxy acids) and copolymers thereof which can be used include poly(lactic acid), poly(glycolic acid), poly(hydroxy-butyric acid), poly(hydroxyvaleric acid), poly(caprolactone), poly(lactide-co- caprolactone), and poly(lactide-co-glycolide).
- Polymers containing labile bonds such as polyanhydrides and polyorthoesters, can be used optionally in a modified form with reduced hydrolytic reactivity.
- Positively charged hydrogels, such as chitosan, and thermoplastic polymers, such as polystyrene also can be used.
- Representative natural polymers which also can be used include proteins, such as zein, modified zein, casein, gelatin, gluten, serum albumin, or collagen, and polysaccharides such as dextrans, polyhyaluronic acid and alginic acid.
- Representative synthetic polymers include polyphosphazenes, polyamides, polycarbonates, polyacrylamides, polysiloxanes, polyurethanes and copolymers thereof. Celluloses also can be used. As defined herein the term "celluloses" includes naturally occurring and synthetic celluloses, such as alkyl celluloses, cellulose ethers, cellulose esters, hydroxyalkyl celluloses and nitrocelluloses.
- Exemplary celluloses include ethyl cellulose, methyl cellulose, carboxymethyl cellulose, hydroxymethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose triacetate and cellulose sulfate sodium salt.
- Polymers of acrylic and methacrylic acids or esters and copolymers thereof can be used.
- Representative polymers which can be used include poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), and poly(octadecyl acrylate).
- polymers which can be used include polyalkylenes such as polyethylene and polypropylene; polyarylalkylenes such as polystyrene; poly(alkylene glycols), such as poly(ethylene glycol); poly(alkylene oxides), such as poly(ethylene oxide); and poly(alkylene terephthalates), such as poly(ethylene terephthalate).
- polyvinyl polymers can be used, which, as defined herein includes polyvinyl alcohols, polyvinyl ethers, polyvinyl esters and polyvinyl halides.
- Exemplary polyvinyl polymers include poly(vinyl acetate), polyvinyl phenol and polyvinylpyrrolidone.
- Water soluble polymers can also be used.
- suitable water soluble polymers include polyvinyl alcohol, polyvinylpyrrolidone, methyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose and polyethylene glycol, copolymers of acrylic and methacrylic acid esters, and mixtures thereof. Water insoluble polymers also can be used.
- Suitable water insoluble polymers include ethylcellulose, cellulose acetate, cellulose propionate (lower, medium or -higher molecular weight), cellulose acetate propionate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose triacetate, poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), poly(octadecyl acrylate), poly(ethylene), poly(ethylene) low density, poly(ethylene) high density, poly(propylene), poly(ethylene oxide), poly(ethylene terephthalate), poly(vinyl isobutyl ether),
- a water insoluble polymer and a water soluble polymer are used together, such as in a mixture.
- Such mixtures are useful in controlled drug release formulations, wherein the release rate can be controlled by varying the ratio of water soluble polymer to water insoluble polymer.
- Polymers varying in viscosity as a function of temperature or shear or other physical forces also may be used.
- Poly(oxyalkylene) polymers and copolymers such as poly(ethylene oxide)-poly(propylene oxide) (PEO-PPO) or poly(ethylene oxide)-poly(butylene oxide) (PEO-PBO) copolymers, and copolymers and blends of these polymers with polymers such as poly(alpha-hydroxy acids), including but not limited to lactic, glycolic and hydroxybutyic acids, polycaprolactones, and polyvalerolactones, can be synthesized or commercially obtained.
- polyoxyalkylene copolymers are described in U.S. Patent Nos.
- Polyoxyalkylene copolymers are sold, for example, by BASF under the trade name PLURONICSTM. These materials are applied as viscous solutions at room temperature or lower which solidify at the higher body temperature. Other materials with this behavior are known in the art, and can be utilized as described herein. These include KLUCELTM (hydroxypropyl cellulose), and purified konjac glucomannan gum. Other suitable polymers are polymeric lacquer substances based on acrylates and/or methacrylates, commonly called EUDRAGITTM polymers (sold by Rohm America, Inc.).
- EUDRAGITTM polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent.
- EUDRAGITTM RL and EUDRAGITTM RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films, whereas EUDRAGITTM RL is freely permeable and EUDRAGITTM RS is slightly permeable, independent of pH.
- the permeability of EUDRAGITTM L is pH dependent.
- EUDRAGITTM L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester. It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable.
- thermoreversible polymers include natural gel-forming materials such as agarose, agar, furcellaran, beta-carrageenan, beta-l,3-glucans such as curdlan, gelatin, or polyoxyalkylene containing compounds, as described above.
- specific examples include thermosetting biodegradable polymers for in vivo use described in U.S. Patent No. 4,938,763, the contents of which are incorporated herein by reference.
- Polymers with enhanced bioadhesive properties are provided by incorporating anhydride monomers or oligomers into one of the polymers listed above by dissolving, dispersing, or blending, as taught by U.S. Patent Nos. 5,955,096 and 6,156,348, the contents of which are incorporated herein by reference.
- the polymers may be used to form drug delivery systems which have improved ability to adhere to tissue surfaces, such as mucosal membranes.
- the anhydride oligomers are formed from organic diacid monomers, preferably the diacids normally found in the Krebs glycolysis cycle.
- Anhydride oligomers which enhance the bioadhesive properties of a polymer have a molecular weight of about 5000 or less, typically between about 100 and 5000 Daltons, or include 20 or fewer diacid units linked by anhydride linkages and terminating in an anhydride linkage with a carboxylic acid monomer.
- the oligomer excipients can be blended or incorporated into a wide range of hydrophilic and hydrophobic polymers including proteins, polysaccharides and synthetic biocompatible polymers, including those described above.
- anhydride oligomers may be combined with metal oxide particles, such as those described above, to improve bioadhesion even more than with the organic additives alone.
- Organic dyes because of their electronic charge and hydrophobicity or hydrophilicity, can either increase or decrease the bioadhesive properties of polymers when incorporated into the polymers.
- anhydride oligomer refers to a diacid or polydiacid linked by anhydride bonds, and having carboxy end groups linked to a monoacid such as acetic acid by anhydride bonds.
- the anhydride oligomers have a molecular weight less than about 5000, typically between about 100 and 5000 Daltons, or are defined as including between one to about 20 diacid units linked by anhydride bonds.
- the diacids are those normally found in the Krebs glycolysis cycle.
- the anhydride oligomer compounds have high chemical reactivity.
- the oligomers can be formed in a reflux reaction of the diacid with excess acetic anhydride.
- the excess acetic anhydride is evaporated under vacuum, and the resulting oligomer, which is a mixture of species which include between about one to twenty diacid units linked by anhydride bonds, is purified by recrystallizing, for example, from toluene or other organic solvents.
- the oligomer is collected by filtration, and washed, for example, in ethers.
- the reaction produces anhydride oligomers of mono and poly acids with terminal carboxylic acid groups linked to each other by anhydride linkages.
- the anhydride oligomer is hydrolytically labile. As analyzed by gel permeation chromatography, the molecular weight may be, for example, on the order of 200-400 for fumaric acid oligomer (FAPP) and 2000-4000 for sebacic acid oligomer (SAPP).
- FAPP fumaric acid oligomer
- SAPP sebacic acid oligomer
- the anhydride bonds can be detected by Fourier transform infrared spectroscopy by the characteristic double peak at 1750 cm “1 and 1820 cm “1 , with a corresponding disappearance of the carboxylic acid peak normally at 1700 cm "1 .
- the oligomers may be made from diacids described for example in U.S. Patent Nos. 4,757,128, 4,997,904 and 5,175,235, the disclosures of which are incorporated herein by reference.
- monomers such as sebacic acid, bis(p- carboxy-phenoxy)propane, isophathalic acid, fumaric acid, maleic acid, adipic acid or dodecanedioic acid may be used.
- Organic dyes because of their electronic charge and hydrophilicity or hydrophobicity, may alter the bioadhesive properties of a variety of polymers when incorporated into the polymer matrix or bound to the surface of the polymer.
- a partial listing of dyes that affect bioadhesive properties include, but are not limited to: acid fuchsin, alcian blue, alizarin red s, auramine o, azure a and b, Bismarck brown y, brilliant cresyl blue aid, brilliant green, carmine, cibacron blue 3GA, congo red, cresyl violet acetate, crystal violet, eosin b, eosin y, erythrosin b, fast green fcf, giemsa, hematoylin, indigo carmine, Janus green b, Jenner's stain, malachite green oxalate, methyl blue, methylene blue, methyl green, methyl violet 2b,
- Polymers having an aromatic group which contains one or more hydroxyl groups grafted onto them or coupled to individual monomers are also suitable for use in the bioadhesive coatings of the invention.
- Such polymers can be biodegradable or nonbiodegradable polymers.
- the polymer can be hydrophobic.
- the aromatic group is catechol or a derivative thereof and the polymer contains reactive functional groups.
- the polymer is a polyanhydride and the aromatic compound is the catechol derivative DOPA.
- the molecular weight of the suitable polymers and percent substitution of the polymer with the aromatic group may vary greatly.
- the degree of substitution varies based on the desired adhesive strength, it may be as low as 10%, 25% or 50%, or up to 100% substitution.
- at least 50% of the monomers in the polymeric backbone are substituted with at least one aromatic group.
- the resulting polymer has a molecular weight ranging from about 1 to 2,000 kDa.
- the polymer that forms that backbone of the bioadhesive material can be a biodegradable polymer.
- biodegradable polymers include synthetic polymers such as poly hydroxy acids, such as polymers of lactic acid and glycolic acid, polyanhydrides, poly(ortho)esters, polyesters, polyurethanes, poly(butyric acid), poly(valeric acid), poly(caprolactone), poly(hydroxybutyrate), poly(lactide-co-glycolide) and poly(lactide- cocaprolactone), and natural polymers such as alginate and other polysaccharides, collagen and chemical derivatives thereof (substitutions, additions of chemical groups, for example, alkyl, alkylene, hydroxylations, oxidations, and other modifications routinely made by those skilled in the art), albumin and other hydrophilic proteins, zein and other prolamines and hydrophobic proteins, copolymers and mixtures thereof. In general, these materials degrade either by enzymatic hydrolysis or exposure
- Suitable polymers can formed by first coupling the aromatic compound to the monomer and then polymerizing.
- the monomers may be polymerized to form a polymer backbone, including biodegradable and non-biodegradable polymers.
- Suitable polymer backbones include, but are not limited to, polyanhydrides, polyamides, polycarbonates, polyalkylenes, polyalkylene oxides such as polyethylene glycol, polyalkylene terephthalates such as poly(ethylene terephthalate), polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyethylene, polypropylene, poly(vinyl acetate), poly(vinyl chloride), polystyrene, polyvinyl halides, polyvinylpyrrolidone, polyhydroxy acids, polysiloxanes, polyurethanes and copolymers thereof, alkyl cellulose, hydroxyalkyl celluloses, cellulose ethers, cellulose esters, nitrocellulloses,
- a suitable polymer backbone can be a known bioadhesive polymer that is hydrophilic or hydrophobic.
- Hydrophilic polymers include CARBOPOLTM, polycarbophil, cellulose esters, and dextran.
- Non-biodegradable polymers especially hydrophobic polymers are also suitable as polymer backbones.
- preferred non-biodegradable polymers include ethylene vinyl acetate, poly(methacrylic acid), copolymers of maleic anhydride with other unsaturated polymerizable monomers, poly(butadiene maleic anhydride), polyamides, copolymers and mixtures thereof and dextran, cellulose and derivatives thereof.
- Hydrophobic polymer backbones include polyanhydrides, poly(ortho)esters, and polyesters such as polycaprolactone.
- the polymer is sufficiently hydrophobic that it is not readily water soluble, for example the polymer should be soluble up to less than about 1% w/w in water, preferably about 0.1% w/w in water at room temperature or body temperature.
- the polymer is a polyanhydride, such as a poly(butadiene maleic anhydride) or another copolymer of maleic anhydride.
- Polyanhydrides may be formed from dicarboxylic acids as described in U.S. Patent No. 4,757,128 to Domb et al, incorporated herein by reference.
- Suitable diacids include aliphatic dicarboxylic acids, aromatic dicarboxylic acids, aromatic-aliphatic dicarboxylic acid, combinations of aromatic, aliphatic and aromatic-aliphatic dicarboxylic acids, aromatic and aliphatic heterocyclic dicarboxylic acids, and aromatic and aliphatic heterocyclic dicarboxylic acids in combination with aliphatic dicarboxylic acids, aromatic-aliphatic dicarboxylic acids, and aromatic dicarboxylic acids of more than one phenyl group.
- Suitable monomers include sebacic acid (SA), fumaric acid (FA), bis(p-carboxyphenoxy)propane (UP), isophthalic acid (IPh), and dodecanedioic acid (DD).
- a wide range of molecular weights are suitable for the polymer that forms the backbone of the bioadhesive material.
- the molecular weight may be as low as about 200 Da (for oligomers) up to about 2,000 kDa.
- the polymer has a molecular weight of at least 1,000 Da, more preferably at least 2,000 Da, most preferably the polymer has a molecular weight of up to 20 kDa or up to 200 kDa.
- the molecular weight of the polymer may be up to 2,000 kDa.
- the range of substitution on the polymer varies greatly and depends on the polymer used and the desired bioadhesive strength. For example, a butadiene maleic anhydride copolymer that is 100% substituted with DOPA will have the same number of DOPA molecules per chain length as a 67% substituted ethylene maleic anhydride copolymer.
- the polymer has a percentage substitution ranging from 10% to 100%, preferably ranging from 50% to 100%.
- the polymers and copolymers that form the backbone of the bioadhesive material include reactive functional groups that interact with the functional groups on the aromatic compound.
- the polymer or monomer that forms the polymeric backbone contains accessible functional groups that easily react with molecules contained in the aromatic compounds, such as amines and thiols.
- the polymer contains amino reactive moieties, such as aldehydes, ketones, carboxylic acid derivatives, cyclic anhydrides, alkyl halides, aryl azides, isocyanates, isothiocyanates, succinimidyl esters or a combination thereof.
- the aromatic compound containing one or more hydroxyl groups is catechol or a derivative thereof.
- the aromatic compound is a polyhydroxy aromatic compound, such as a trihydroxy aromatic compound (e.g., phloroglucinol) or a multihydroxy aromatic compound (e.g., tannin).
- the catechol derivative may contain a reactive group, such as an amino, thiol, or halide group.
- the preferred catechol derivative is 3,4-dihydroxyphenylalanine (DOPA), which contains a primary amine. Tyrosine, the immediate precursor of DOPA, which differs only by the absence of one hydroxyl group in the aromatic ring, can also be used. Tyrosine is capable of conversion (e.g., by hydroxylation) to the DOPA form.
- a particularly preferred aromatic compound is an amine-containing aromatic compound, such as an amine-containing catechol derivative (e.g., dopamine).
- Two general methods are used to form the polymer product.
- a compound containing an aromatic group which contains one or more hydroxyl groups is grafted onto a polymer.
- the polymeric backbone is a biodegradable polymer.
- the aromatic compound is coupled to individual monomers and then polymerized.
- Any chemistry which allows for the conjugation of a polymer or monomer to an aromatic compound containing one or more hydroxyl groups can be used, for example, if the aromatic compound contains an amino group and the monomer or polymer contains an amino reactive group, this modification to the polymer or monomer is performed through a nucleophilic addition or a nucleophilic substitution reaction, such as a Michael-type addition reaction, between the amino group in the aromatic compound and the polymer or monomer. Additionally, other procedures can be used in the coupling reaction.
- carbodiimide and mixed anhydride based procedures form stable amide bonds between carboxylic acids or phosphates and amino groups
- bifunctional aldehydes react with primary amino groups
- bifunctional active esters react with primary amino groups
- divinylsulfone facilitates reactions with amino, thiol, or hydroxy groups.
- L-DOPA is grafted to maleic anhydride copolymers by reacting the free amine in L-DOPA with the maleic anhydride bond in the copolymer.
- polymers can be used as the backbone of the bioadhesive material, as described above. Additional representative polymers include 1:1 random copolymers of maleic anhydride with ethylene, vinyl acetate, styrene, or butadiene. In addition, a number of other compounds containing aromatic rings with hydroxy substituents, such as tyrosine or derivatives of catechol, can be used in this reaction. In another embodiment, the polymers are prepared by conjugate addition of a compound containing an aromatic group that is attached to an amine to one or more monomers containing an amino reactive group. In a preferred method, the monomer is an acrylate or the polymer is acrylate.
- the monomer can be a diacrylate such as 1,4-butanediol diacrylate, 1,3 -propanediol diacrylate, 1,2-ethanediol diacrylate, 1,6- hexanediol diacrylate, 2,5-hexanediol diacrylate or 1,3 -propanediol diacrylate.
- diacrylate such as 1,4-butanediol diacrylate, 1,3 -propanediol diacrylate, 1,2-ethanediol diacrylate, 1,6- hexanediol diacrylate, 2,5-hexanediol diacrylate or 1,3 -propanediol diacrylate.
- the monomer and the compound containing an aromatic group are each dissolved in an organic solvent (e.g., THF, CH 2 Cl 2 , methanol, ethanol, CHCl 3 , hexanes, toluene, benzene, CCl 4 , glyme, diethyl ether, etc.) to form two solutions.
- an organic solvent e.g., THF, CH 2 Cl 2 , methanol, ethanol, CHCl 3 , hexanes, toluene, benzene, CCl 4 , glyme, diethyl ether, etc.
- the molecular weight of the synthesized polymer can be controlled by the reaction conditions (e.g., temperature, starting materials, concentration, solvent, etc.) used in the synthesis.
- a monomer such as 1,4-phenylene diacrylate or 1,4-butanediol diacrylate having a concentration of 1.6 M
- DOPA or another primary amine containing aromatic molecule are each dissolved in an aprotic solvent such as DMF or DMSO to form two solutions.
- the solutions are mixed to obtain a 1:1 molar ratio between the diacrylate and the amine group and heated to 56 0 C to form a bioadhesive material.
- Hydrophobic polymers such as polyesters, poly (anhydrides), ethyl cellulose, even if possibly non-adhesive on their own, may nevertheless be made bioadhesive simply by physically mixing the hydrophobic polymers with one or more suitable compounds (such as catechols or derivatives L-DOPA, D-DOPA, dopamine, or carbidopa, etc.) to create "bioadhesive compositions.”
- suitable compounds such as catechols or derivatives L-DOPA, D-DOPA, dopamine, or carbidopa, etc.
- metal oxides may also be used for this purpose.
- the molecular weight of the bioadhesive polymers and percent substitution of the polymers with residues of the compounds disclosed may vary greatly.
- the degree of substitution varies based on the desired adhesive strength, it may be as low as 10%, 20%, 25%, 50%, or up to 100% substitution.
- at least 50% of the repeat units in the polymeric backbone are substituted with at least one residue.
- 75-95% of the residues in the backbone are substituted with at least one residue.
- on average 100% of the repeat units in the polymeric backbone are substituted with at least one residue.
- the resulting bioadhesive polymer typically has a molecular weight ranging from about 1 to 2,000 kDa, such as 1 to 1,000 kDa, 10 to 1,000 kDa or 100 to 1,000 kDa.
- Polymers used in bioadhesive compositions typically have the same range of molecular weights.
- there is typically no covalent bond formed between the compounds and the polymer in the bioadhesive compositions i.e., the polymer does not chemically react with the compound, although hydrogen bonds, ionic bonds and/or van der Waals interactions can occur).
- Suitable polymers for use in bioadhesive compositions are described above.
- the polymer itself may not be bioadhesive, but the polymer can be bioadhesive ⁇ e.g., a polymer with hydrogen bond-forming pendant groups).
- the polymer is a hydrophobic polymer such as a poly(lactone), e.g., poly(caprolactone).
- bioadhesive compositions of the invention typically a polymer and a suitable compound are dissolved in a compatible solvent and mixed together. The solvent is then evaporated, preferably at a controlled temperature and rate of removal. Alternatively or in combination with general evaporation, the bioadhesive composition can be spray dried or dried at room temperature.
- a mixture of a polymer and a suitable compound are melted at or slightly above the melting point of the polymer, typically while being mixed.
- Both the polymer and the suitable compound should be selected such that they are chemically stable ⁇ e.g., do not decompose, do not become oxidized) at the melting point temperature. After the composition has re-solidified, it can be milled in order to obtain particles of the desired size.
- the subject bioadhesive compositions can also be prepared by dry mixing of a polymer and a suitable compound, provided that the suitable compound is sufficiently distributed throughout the composition.
- additional components can be added to the mixture prior to dissolution, melting and/or mixing.
- the additional components are preferably stable under the conditions the mixture is exposed to.
- active agents should be stable at the melting point temperature if that method is employed.
- the weight ratio of polymer to the suitable compound in a bioadhesive composition can be selected to give the desired amount of bioadhesion.
- the weight ratio of polymer to compound is 9:1 to 1:9, such as 3:l to 1:3 or 2:1 to 1:2.
- the weight ratio is 9 : 1 to 1 : 1 , 3 : 1 to 1 : 1 or 2 : 1 to 1 : 1.
- the suitable compounds may be used as agents to render the hydrophobic polymers bioadhesive, and/or be used as active ingredients in the pharmaceutical composition to be delivered to the patient.
- the total carbidopa dosage may be adjusted to account for the release of carbidopa from the bioadhesive material.
- the dosage of total levodopa or precursor thereof may be adjusted elsewhere in, for example, the relevant portion or sub- portions of the IR or CR (controlled release, e.g., zero-order release rate portion).
- L-dopa may be used to achieve a significant amount of release (e.g., more or less immediate release) from the polymers.
- L- or D-Dopa may be used such that the polymer is still adhesive, but the release of L- or D-Dopa from the bioadhesive polymer is less significant compared to the levodopa or precursors thereof in IR, and/or one or more other portions or sub-portions of the subject dosage form.
- Preferred bioadhesive coatings do not appreciably swell upon hydration, such that they do not substantially inhibit or block movement (e.g., of ingested food) through the gastrointestinal tract, as compared to the polymers disclosed by Duchene et at Generally, polymers that do not appreciably swell upon hydration include one or more hydrophobic regions, such as a polymethylene region (e.g., (CH 2 ) n , where n is 4 or greater). The swelling of a polymer can be assessed by measuring the change in volume when the polymer is exposed to an aqueous solution. Polymers that do not appreciably swell upon hydration expand in volume by 50% or less when fully hydrated.
- such polymers expand in volume by less than 25%, less than 20%, less than 15%, less than 10% or less than 5%.
- the bioadhesive coatings are mucophilic.
- a polymer that does not appreciably swell upon hydration can be mixed with a polymer that does swell (e.g., CARBOPOLTM, poly(acrylic acid), provided that the amount of swelling in the polymer does not substantially interfere with bioadhesiveness.
- the bioadhesive polymeric coating consists of two layers, an inner bioadhesive layer that does not substantially swell upon hydration and an outer bioadhesive layer that is readily hydratable and optionally bioerodable, such as one comprised of CARBOPOLTM.
- the bioadhesive polymers discussed above can be mixed with one or more plasticizers or thermoplastic polymers. Such agents typically increase the strength and/or reduce the brittleness of polymeric coatings.
- plasticizers include dibutyl sebacate, polyethylene glycol, triethyl citrate, dibutyl adipate, dibutyl fumarate, diethyl phthalate, ethylene oxide-propylene oxide block copolymers such as PLURONICTM F68 and di(sec-butyl) fumarate.
- thermoplastic polymers examples include polyesters, poly(caprolactone), polylactide, poly(lactide-co- glycolide), methyl methacrylate ⁇ e.g., EUDRAGITTM), cellulose and derivatives thereof such as ethyl cellulose, cellulose acetate and hydroxypropyl methyl cellulose (HPMC) and large molecular weight polyanhydrides.
- the plasticizers and/or thermoplastic polymers are mixed with a bioadhesive polymer to achieve the desired properties.
- the proportion of plasticizers and thermoplastic polymers, when present, is from 0.5% to 40% by weight.
- the bioadhesive polymer coating in a dry packaged form of a tablet, is a hardened shell.
- a tablet or a drug eluting device can have one or more coatings in addition to the bioadhesive polymeric coating. These coatings and their thickness can, for example, be used to control where in the gastrointestinal tract the bioadhesive coating becomes exposed. In one example, the additional coating prevents the bioadhesive coating from contacting the mouth, esophagus, and stomach. In another example, the additional coating remains intact until reaching the small intestine ⁇ e.g., an enteric coating).
- coatings include methylmethacrylates, zein, cellulose acetate, cellulose phthalate, HMPC, sugars, enteric polymers, gelatin and shellac. Premature dissolution of a tablet in the mouth can be prevented with hydrophilic polymers such as HPMC or gelatin.
- Coatings used in tablets of the invention typically include a pore former, such that the coating is permeable to the drug.
- exemplary pore formers include: sugar, mannitol, HPC (hydroxypropyl cellulose), HPMC, dendrites, NaCl, etc.
- Tablets and drug eluting devices of the invention can be coated by a wide variety of methods. Suitable methods include compression coating, coating in a fluidized bed or a pan, enrobing, and hot melt (extrusion) coating, etc. Such methods are well known to those skilled in the art.
- compositions, derivatives, precursors, additional components that can be used with the subject pramipexole compositions, dosage forms, methods of making and using, etc., axe adaptable or directly useable with the instant invention, and are thus expressly incorporated herein by reference.
- MIRAPEX ® 0.125 mg tablets were enteric-coated manually to produce the enteric polymer-coated MIRAPEX ® 0.125 mg tablets. Specifically, tablets were hand-dipped using forceps in 10% w/v coating solution of EUDRAGIT L 100 in acetone, so as to achieve a final weight gain of 5-20% w/w.
- Example 3 Pharmacokinetic evaluation ofMirapex 0.125 mg Tablet administered in three times a day dosing regimen
- This examples describes pharmacokinetic data for MIRAPEX (pramipexole dihydrochloride) tablets 0.125 mg; (lot#511-047) to be dosed at the 0, 8, and 16 hour time point (Lot# 511-047) in the fed state of the dog model.
- MIRAPEX pramipexole dihydrochloride
- PK studies in beagle dogs have been conducted with single dose of Mirapex 0.125 mg tablet, and this dose has been well tolerated by the beagle dog model.
- the subject pramipexole extended release capsule formulation contains 0.375 mg of pramipexole. This study was conducted to compare the bioavailability of a commercially available immediate release formulation to the subject ER formulation containing equivalent amount of the active drug.
- pramipexole extended release (XR) formulation which contains multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric-coated capsule.
- Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (Mfl.01) with OPADRY ® Clear as a binder.
- the placebo core pellets were prepared using low shear granulation, extrusion and spheronization techniques. Following is the composition used for preparing placebo core pellets.
- the placebo pellets were dried in oven at 5O 0 C for 17 hours to achieve a desired moisture level of 0.3% w/w. These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves. The particles retained on screen 14 and 16 were used for subsequent pramipexole layering process.
- Pramipexole layered pellets were subsequently coated in Vector Mini Fluid Bed Drier (Mfl.01) with rate controlling polymer composition containing ethylcellulose (ETHOCEL ® 10 cps.) to achieve a final weight gain of 8.3% w/w and over coated with bioadhesive polymer SPHEROMERTM III polymer (5.3%). These pellets were then encapsulated in a size 1 gelatin capsule and tested for release profile in USP II dissolution apparatus. The pellets were also evaluated for their in vivo performance in beagle dogs (see Figure 5).
- the following is the unit dose composition used for preparing pramipexole extended release capsule.
- Applicants produced a pramipexole extended release (XR) formulation, which contains multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric-coated capsule.
- Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (MfLOl) with OPADRY ® Clear as a binder.
- MfLOl Vector Mini Fluid Bed Drier
- OPADRY ® Clear a binder.
- the placebo core pellets were prepared using low shear granulation, extrusion and spheronization techniques. Following is the composition used for preparing placebo core pellets.
- the placebo pellets were dried in oven at 50 0 C for 17 hours to achieve a desired moisture level of 0.3% w/w. These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves. The particles retained on screen 14 and 16 were used for subsequent pramipexole layering process.
- Pramipexole layered pellets were subsequently coated in a Vector Mini Fluid Bed Drier (Mfl.01) with a rate controlling polymer composition containing ethylcellulose (ETHOCEL ® 10 cps.) to achieve a final weight gain of 8.3% w/w
- pellets were then encapsulated in a size 1 gelatin capsule and tested for release profile in USP II dissolution apparatus. The pellets were also evaluated for their in vivo performance in beagle dogs (See Figure 6).
- unit dose composition used for preparing pramipexole extended release capsule.
- Extended release capsules from Example 4A were further coated with an enteric coating composition, Acryl-EZETM White in a pan coater (O'Hara). Specifically, about 10% w/v solution of Acryl-EZETM White was prepared in ethanol and sprayed on pramipexole extended release (XR) capsule so as to achieve a final weight gain of 12% w/w. These delayed extended release (DXR) capsules were then tested for release profile in USP II dissolution apparatus, and were also evaluated for their in vivo performance in beagle dogs.
- an enteric coating composition Acryl-EZETM White in a pan coater (O'Hara).
- XR pramipexole extended release
- enteric-coated pramipexole delayed extended release (DXR) capsule showed a two hour delay in acidic environment, followed by a slower release of pramipexole at pH 6.8.
- Example 6 Pramipexole ER, 0.375 mg Tablets [5 % coating (80 parts Surelease® + 20 parts OPADRY®) Formulation
- An extended release matrix dosage form according to the International Publication No. WO 2004/010999A1 (Lee et at, the entire contents of which is incorporated herein by reference) was prepared as a comparator formulation.
- the pramipexole matrix based controlled release formulation given in Example 5 of WO 2004/010999A1 (incorporated herein by reference) was prepared according to the process disclosed therein, with the exception that pramipexole was layered onto the lactose particles to achieve its uniform dispersion.
- the in vitro release rate of the assembled formulation in phosphate buffer (pH 6.8) using the USP Apparatus II at 50 rpm was similar to that disclosed in Lee.
- immediate release MIRAPEX ® tablets exhibited rapid initial absorption of pramipexole and dramatic fluctuations in pramipexole concentration, while the extended release formulations resulted in a lag time, followed by extended absorption up to 16 hours.
- the bioavailability estimate of the extended release capsule formulation was about 50% when compared to MIRAPEX ® tablets given in a repeated manner.
- the bioavailability of Lee matrix formulation was even lower than the multiparticulate-based system.
- decreased bioavailability was observed for the extended release formulation, this can be primarily attributed to short residence time in GI tract. Since there are known differences between humans and dogs in terms of motility, bacterial metabolism and GI transit time, the above extended release formulation is expected to show higher extent of absorption in humans, as the half life of pramipexole is longer in humans.
- Type A Three formulations of pramipexole extended release capsule, Type A, Type B and Type C, are provided for clinical testing.
- These formulations are once daily extended release (XR) pramipexole formulations containing multiparticulates encapsulated in enteric coated gelatin capsules.
- XR once daily extended release
- These formulations are similar with respect to the active substance to the existing formulation for Mirapex ® tablets, e.g., pramipexole dihydrochloride monohydrate is the active substance in all the formulations.
- the dose levels are different.
- Each Mirapex ® tablet contains 0.125 mg of pramipexole dihydrochloride monohydrate, and is dosed three times a day with a total drug substance level of 0.375 mg (0.125mg x 3).
- the currently available immediate release pramipexole formulation ⁇ e.g., Mirapex ®
- the subject formulations contain 0.375 mg of active substance, and are suitable for once-daily administration. They are delayed, extended release oral dosage forms that will maintain effective plasma pramipexole levels to produce a therapeutic effect over approximately 24 hours when administered to patients in need, and should result in diminished incidence and decreased intensity of pramipexole's unwanted side effects.
- the pramipexole extended release capsules, Type A, Type B and Type C use 0.375 mg of pramipexole dihydrochloride monohydrate as their active ingredient.
- the level used is within the limits specified in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations 25 th Edition, 2005, (Orange book). All excipients utilized in these formulations are within or below the listed levels for orally administered products.
- the subject extended release formulation was conceptualized for once-daily administration with improved bioavailability, patient compliance and tolerability. It was to provide a lag time with slow absorption followed by steady plasma levels over an extended duration.
- the objectives of subject formulation approach were to slow down or delay the rapid absorption of pramipexole that has been correlated with the major adverse effects of Mirapex ® tablets, while maintaining the effective plasma concentration over a 24 hours period.
- An enteric polymer coating was applied on the capsule not to allow the drug to get released in the stomach.
- pramipexole extended release capsules, Type A, Type B and Type C differ either in the level of the rate controlling polymer, ethylcellulose, or bioadhesive coating composition containing Spheromer III.
- the manufacture of pramipexole multiparticulate extended release capsules Type A, Type B and Type C involves the following steps.
- Mannitol and microcrystalline cellulose are blended with hydroxypropyl cellulose in a blender.
- the dry blend is granulated with purified water using a low shear mixer.
- the granules are extruded in a double roller extruder using a 1.5 mm screen and the moisture content measured.
- the extrudate is spheronized in an extruder.
- the pellets are dried in a dryer until the desired moisture content is achieved.
- the dried pellets are checked for size distribution.
- the placebo core pellets are tested for appearance, moisture content and particle size distribution.
- the placebo core pellets are coated with a solution of OPADRY® Clear (YS- 1-19025-A) and pramipexole in the fluidized bed coater until the target weight gain is achieved.
- the pramipexole layered pellets are film coated with a polymer solution comprised of Ethocel 10 cps and dibutyl sebacate in the fluidized bed coater until the target weight gain is achieved.
- the target weight gain is 8.3% w/w for Type A and Type B and 12% w/w of the original pramipexole mannitol pellets for formulation Type C.
- the resulting extended release Type B pellets are film coated with a coating solution of SPHEROMERTM III and Poloxamer 188 (Lutrol® F68) in the fluidized bed coater until the target weight gain of 5.3% w/w is achieved.
- the pellets are then tested for appearance, identification, content assay, residual solvent and in vitro dissolution.
- the resulting capsules are applied with a gelatin band at the seam and coated with Acryl-EzeTM White (93018509) enteric coating in the pan coater until the target weight gain is attained.
- the pellets are then tested for appearance, identification, content uniformity, content assay, impurities and dissolution.
- pramipexole delayed and extended release (XR) formulation containing multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric coated hard gelatin capsule was formulated.
- Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (Mfl.Ol) with OPADRY ® Clear as a binder.
- Mfl.Ol Vector Mini Fluid Bed Drier
- OPADRY ® Clear a binder.
- the placebo core pellets were prepared using low shear granulation, extrusion and spheronization technique. Following is the composition used for preparing placebo core pellets.
- the placebo pellets were dried in oven at 50 0 C for 12-17 hours to achieve a desired moisture level of 1% w/w. These pellets were then screened through size 10, 12, 14, 16 andl8 mesh sieves and the particles retained on screen 14 and 16 were used for subsequent pramipexole layering process, pramipexole layered pellets were subsequently coated in Vector Mini Fluid Bed Drier (Mfl.01) with rate controlling polymer composition containing ethylcellulose (Ethocel ® 10 cps.) to achieve a final weight gain of 8.3% w/w. These pellets were then encapsulated in a size 2 gelatin capsule.
- Vector Mini Fluid Bed Drier Mfl.01
- rate controlling polymer composition containing ethylcellulose Ethocel ® 10 cps.
- the amount of pramipexole was calculated using a weighted calibration curve.
- the linearity curve was obtained using pramipexole standards ranging from 100 pg/mL to 25 ng/mL.
- AU standards were prepared in Harlan Dog plasma. In order to assure accuracy throughout the experiment, quality control (QC) samples were injected every 12 samples. The QC standards were prepared in dog plasma at known concentrations of ⁇ 1.0 ng/mL and ⁇ 10.0 ng/mL.
- Figures 9A-C represent the pramipexole plasma concentration vs. time graph.
- the area under the plasma pramipexole vs. time curve (AUC), maximum concentration (C max ), time to maximum concentration (T max ) were calculated and are indicated in Table 6.
- Table 6 PK parameters comparing different 0.375 mg Pramipexole Delayed Extended Release Multiparticulate Formulations with Mirapex® tablets, 0.375 mg (0.125 mg X 3)
- Example 8 Pramipexole ER, 0.375 mg Tablets [5 % coating (80 parts Surelease® + 20 parts OPADRY®) Formulation in Fasted condition
- This example describes pharmacokinetic data for pramipexole 0.375 mg Tablet (Lot #509-060) coated with OPADRY ® / Surelease ® in beagle dogs under fasted condition.
- Pramipexole was layered on sugar spheres and compressed into a tablet along with other excipients as listed below. A pharmacokinetic study was performed earlier in the fed conditions using the same formulation; however this study was done under fasted conditions. The data obtained from this study can be used to compare against the subject pramipexole extended release formulations which contain 0.375 mg of pramipexole dihydrochloride.
- Pramipexole ER 0.375 mg Tablets coated with 80 parts Surelease ® + 20 parts
- Figure 1OA shows the in vitro dissolution profile obtained using USP II apparatus.
- the dissolution was performed in phosphate buffer pH 6.8 for 24 hours.
- the Surelease ® coated tablets formulated in house show an identical profile to the innovator's sustained release formulation.
- Figure 10 B is the comparison of above example with Pramipexole Delayed Extended Release Capsule, Type B, 0.375mg described in example 5. Both the formulations demonstrate a similar extent of absorption.
- Figure 11 is the comparison of pramipexole ER Formulations with Mirapex (0.125) mg tablets administered in three times a day dosing regimen.
- Pramipexole multiparticulates from Example 7 were fed to beagle dogs in order to evaluate their pharmacokinetic performance and were later on compared to Mirapex 0.125 mg tablets administered in three times a day dosing regimen and Pramipexole ER, 0.375 mg tablets (Example 8).
- Mirapex (0.125) mg tablets administered in three times a day dosing regimen exhibited rapid initial absorption of pramipexole, whereas pramipexole ER formulations showed an extended absorption with just once a day administration.
- the bioavailability estimate of the extended release formulations was about 50% when compared to Mirapex ® tablets given in a repeated manner.
- Pramipexole was initially layered on placebo core pellets (1.1— 1.4 mm dia.) with Opadry ® Clear as a binder using a Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert.
- the placebo core pellets were prepared using low-shear granulation, extrusion and spheronization technique.
- the following table provides the weight and composition of placebo core pellets.
- the placebo pellets were dried in an oven at 5O 0 C to achieve a desired moisture level of 1% (w/w). These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves and the particles retained on screen size 14 and 16 were used for subsequent pramipexole layering process. Pramipexole layered pellets were subsequently coated in the Vector MFL.Ol Micro Batch Fluid Bed System, equipped with a Wurster insert, with a release rate-controlling polymer composition containing ethylcellulose to achieve a weight gain of 8.3% (w/w), and then coated with bioadhesive SpheromerTM III polymer to a weight gain of 5.3% (w/w).
- the unit dose composition of a pramipexole 0.375 mg extended-release pellet formulation is given below.
- bioadhesive pramipexole pellets may be optionally top-coated with bioadhesive SpheromerTM I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
- Levodopa, carbidopa, and levodopa-carbidopa pellets were produced with granulation-extrusion-spheronization and fluid bed drying.
- the production processes included the following: (1) Weighing levodopa or carbidopa, or both levodopa and carbidopa, optionally a bioadhesive polymer composition, and pharmaceutically acceptable excipients.
- the granulation fluids were mainly selected from a group consisting of purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro- alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated solvent or in a ketone.
- step (3) Extruding the wet granulation from step (3) through the screen of a screen-type extruder, Caleva Model 20 (or Model 25) Extruder, operating at 10-20 rpm, and forming breakable wet strands, the extrudate.
- the screen aperture was 0.8, 1, or 1.5 mm.
- levodopa pellets Three identical sub-lots of levodopa pellets (sub-lots # 511-068, 511-069, and 511- 070) were prepared in accordance with the method described in Example 1. The weight and composition of pellets of the sub-lot # 511-068 are given in the following table.
- Levodopa was blended with inactive excipients for 5 min.
- the levodopa-excipients blend was then granulated by spraying purified water while mixing at low shear. The granulation was blended for an additional 5 min and then extruded through a 1.5 mm screen of a Caleva extruder, model 25, operating at 15 rpm.
- the extrudate was spheronized in a Caleva spheronizer, model 250, operating at 1000 rpm for 5 min.
- the spheronized pellets were dried in an ACT (Applied Chemical Technology) fluidized bed drier at 104°F ⁇ 4°F for 75 min.
- the dried pellets were screened and particles with diameters ranging from 1 mm to 2 mm were selected for future experimentation.
- the screened pellets of the three sub-lots were blended in a GlobePharma Maxiblend Blender equipped with an 8-qt stainless steel V-shell.
- Example 12 Film coating of Levodopa-Carbidopa Pellets with Bioadhesive Polymer, SpheromerTMIII, Lot # 510-098
- Levodopa, carbidopa, and levodopa-carbidopa pellets were film-coated with a bioadhesive polymeric composition, SpheromerTM III.
- Bioadhesive SpheromerTM III and optionally a functional polymer, or a non-functional polymer, and optionally pharmaceutically acceptable excipients were dissolved in methanol.
- the film coating was performed in a fluidized bed coater, Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert, operating at an inlet air flow rate of 100-300 lpm (liter per minute) and an inlet air temperature of 35°C ⁇ 2°C.
- pellets were pre-warmed at 35 0 C for 2-5 min and after film-coating were post-dried at 3O 0 C for 15-30 min.
- pellets were coated in a Fluid Air Model 5 fluid bed processor, equipped with a Wurster insert, operating at an inlet air flow rate of 70 cfm (cubic foot per minute) and an inlet air temperature of 35 0 C.
- the pellets were pre-warmed at 4O 0 C for 5-7 min and after film-coating were post-dried at 35 0 C for 30 min.
- Methyl alcohol is removed during the coating/drying process.
- Example 13 Film coating of Levodopa Pellets with Bioadhesive Polymer, SpheromerTM III, and Hydroxypropylcellulose (HPC-SSL), Lot # 511-092
- Carbidopa granules were produced with low shear granulation method consisting of the following processes:
- the granulation fluid was mainly selected from purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, an alcohol, a hydro-alcoholic mixture, or an alcoholic or hydro-alcoholic solution of a polymeric composition.
- step (3) Drying the granulation from step (3) in a fluidized bed drier, Vector MFL.Ol Micro Batch Fluid Bed System, operating at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of 5O 0 C.
- a fluidized bed drier Vector MFL.Ol Micro Batch Fluid Bed System
- the granulation from step (3) was dried in a Precision gravity oven, operating at 5O 0 C, for 8-24 h.
- Levodopa pellets (lot # 510-095), SpheromerTM Ill-coated levodopa-carbidopa pellets (lot # 510-098), HPC-SSL/SpheromerTM Ill-coated levodopa pellets (lot # 511-092), and carbidopa granules (lot # 511-101) were encapsulated in 00-size hard gelatin capsules. Each capsule contained 200 mg levodopa and 50 mg carbidopa anhydrous. The composition of multiparticulates in each capsule formulation is given below.
- Pramipexole extended-release pellets lot # 601-048 (from Example 9), containing 0.375 mg pramipexole, and levodopa-carbidopa immediate/controlled-release multiparticulates, lot # 510-099 (from Example 15), containing 200 mg levodopa and 50 mg carbidopa, were co-encapsulated in two-piece hard gelatin capsules. These capsules were sealed at the junction of cap and body using an aqueous gelatin solution and then coated with 1.6% (w/w) Opadry ® Clear (YS-1-19025-A).
- the Opadry-coated capsules were top-coated with an enteric coating composition, Acryl-EZETM White, in a pan coater (O'Hara Technologies Labcoat System).
- the capsules were sprayed with a 10% (w/v) solution of Acryl-EZE White in ethanol and water mixture (90: 10 v/v) so as to achieve a final weight gain of 5-12% (w/w).
- bioadhesive pramipexole and levodopa-carbidopa pellets may be optionally top- coated with bioadhesive SpheromerTM I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
- Pramipexole extended-release pellets lot # 601-048 (from Example 9) containing 0.375 mg pramipexole were encapsulated in a size 2 hard shell gelatin capsule. These capsules were sealed at the junction of cap and body using an aqueous gelatin solution and coated with 1.6 % Opadry ® Clear (YS-1-19025-A). The Opadry-coated capsules were then coated with an enteric coating composition, Acryl-EZETM White, in a pan coater (O'Hara Technologies Labcoat System). ). The capsules were sprayed with a 10% (w/v) solution of Acryl-EZETM White in ethanol and water mixture (90:10 v/v) so as to achieve a final weight gain of 5-12% (w/w).
- the unit dose composition of a pramipexole 0.375 mg delayed/extended-release capsule formulation is given below.
- Pramipexole delayed/extended-release pellets lot # 601-056 (from Example 17), containing 0.375 mg pramipexole, and levodopa-carbidopa immediate-controlled-release multiparticulates, lot # 510-099 (from Example 15), containing 200 mg levodopa and 50 mg carbidopa, were co-encapsulated in two-piece hard gelatin capsules.
- bioadhesive pramipexole and levodopa-carbidopa pellets may be optionally top- coated with bioadhesive SpheromerTM I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
Abstract
The present invention is directed to pharmaceutical compositions that allow for once- daily dosage forms of pramipexole. The proposed delayed / extended release dosage form in a single dosage form is equivalent to the immediate release three-time daily regimen, and upon administration, provides average steady state blood levels of pramipexole. A once-a-day administration of pramipexole is advantageous over the thrice-a-day administration in terms of both patient compliance and reduced adverse events, thus providing better treatment of the conditions for which the pramipexole is indicated.
Description
Delayed Release or Extended-Delayed Release Dosage Forms of Pramipexole
Reference to Related Application
This application claims the benefit of the filing date of U.S. Provisional Application Serial No. 60/693,602, entitled "IMPROVED DOSAGE FORMS FOR MOVEMENT DISORDER TREATMENT," and filed on June 23, 2005. The teachings of the entire referenced application are incorporated herein by reference.
Background of the Invention
A movement disorder is a neurological disturbance that involves one or more muscles or muscle groups. Movement disorders affect a significant portion of the population, causing disability as well as distress. Movement disorders include Parkinson's disease, Huntington's chorea, progressive supranuclear palsy, Wilson's disease, Tourette's syndrome, epilepsy, tardive dyskinesia, and various chronic tremors, tics and dystonias. Different clinically observed movement disorders can be traced to the same or similar areas of the brain. For example, abnormalities of basal ganglia (a large cluster of cells deep in the hemispheres of the brain) are postulated as a causative factor in diverse movement disorders.
Parkinson's disease (PD) is a common disabling disease of old age affecting about one percent of the population over the age of 60 in the United States. The incidence of Parkinson's disease increases with age and the cumulative lifetime risk of an individual developing the disease is about 1 in 40. It is a progressive neurodegenerative disorder of the extra pyramidal nervous system, and is associated with the depletion of dopamine from cells in the corpus striatum. The disease affects the mobility and control of the skeletal muscular system. Its characteristic features include resting tremor, bradykinetic movements, rigidity and postural change. A perceived pathophysiological cause of Parkinson's disease is progressive destruction of dopamine-producing cells in the basal ganglia which comprise the pars compartum of the substantia nigra, basal nuclei located in the brain stem. Loss of dopamineric neurons results in a relative excess of acetylcholine. See Jellinger, Post Mortem Studies in Parkinson 's Disease - Is It Possible to Detect Brain Areas For Specific Symptoms? J. Neural. Transm. 56(Supp): 1-29:1999. Parkinson's disease often begins with mild limb stiffness and infrequent tremors and progresses over a period often or more years to frequent tremors and memory impairment, to uncontrollable tremors and dementia.
The management of early Parkinson's disease must employ strategies that slow or halt the progression of the disease and reduce the risk of eventual motor complications. Continuous dopaminergic stimulation early in the disease is the therapeutic goal for treatment of patients with Parkinson disease. Pramipexole is a non-ergot potent dopamine receptor agonist and has been shown to be clinically effective in treating Parkinson's patients in early PD. It has preferential affinity for D3 receptor within the D2 subfamily of dopamine receptors and inhibits dopamine synthesis and release. It has been found effective as a monotherapy as well as an adjunct to levodopa therapy in patients with advance disabilities. Pramipexole is marketed as immediate release tablets by Pfizer under the trade name MIRAPEX®.
Tardive Dyskinesia (TD) is a chronic disorder of the nervous system, characterized by involuntary, irregular rhythmic movements of the mouth, tongue, and facial muscles. The upper extremities also may be involved. These movements may be accompanied, to a variable extent, by other involuntary movements and movement disorders. These include rocking, writhing, or twisting movements of the trunk (tardive dystonia), forcible eye closure (tardive blepharospasm), an irresistible impulse to move continually (tardive akathisia), jerking movements of the neck (tardive spasmodic torticollis), and disrupted respiratory movements (respiratory dyskinesia). The vast majority of TD cases are caused by the prolonged use of antipsychotic drugs (neuroleptics). A relatively small number are caused by the use of other medications, such as metoclopramide, that, like neuroleptics, block dopamine receptors. TD often manifests or worsens in severity after neuroleptic drug therapy is discontinued. Resumption of neuroleptic therapy will temporarily suppress the involuntary movements, but may aggravate them in the long run.
TD affects approximately 15-20% of patients treated with neuroleptic drugs (Khot et al., Neuroleptics and Classic Tardive Dyskinesia, in Lang AE, Werner WJ (eds.): Drug Induced Movement Disorders, Futura Publishing Co., 1992, pp 121-166). Therefore, the condition affects hundreds of thousands of people in the United States alone. The cumulative incidence of TD is substantially higher in women, in older people, and in those being treated with neuroleptics for conditions other than schizophrenia, such as bipolar disorder (manic- depressive illness) (see, e.g., Hayashi et al, Clin. Neuropharmacol. 19: 390, 1996; Jeste et al., Arck Gen. Psychiatry 52: 756, 1995). Unlike the acute motor side effects of neuroleptic drugs, TD does not respond in general to antiparkinson drugs (Decker et al, New Eng. J Med. Oct. 7, p. 861, 1971).
Focal Dystonias (FD) are a class of related movement disorders involving the intermittent sustained contraction of a group of muscles. The prevalence of focal dystonias in one US county was estimated as 287 per million (Monroe County Study); this suggests that at least 70,000 people are affected in the US alone. The spasms of focal dystonia can last many seconds at a time, causing major disruption of the function of the affected area. Some of the focal dystonias are precipitated by repetitive movements; writer's cramp is the best known example. Focal dystonia can involve the face (e.g., blepharospasm, mandibular dystonia), the neck (torticollis), the limbs (e.g., writer's cramp), or the trunk. Dystonia can occur spontaneously or can be precipitated by exposure to neuroleptic drugs and other dopamine receptor blockers (tardive dystonia). No systemic drug therapy is generally effective, but some drugs give partial relief to some patients. Those most often prescribed are anticholinergics, baclofen, benzodiazepines, and dopamine agonists and antagonists. The most consistently effective treatment is the injection of botulinum toxin into affected muscles.
The various focal dystonias tend to respond to the same drugs (Chen, Clin. Orthop. June 102-6, 1998; Esper et al, Term. Med. 90: 18-20, 1997; De Mattos et al., Arq Neuropsiquiatr 54: 30-6, 1996) This suggests that a new treatment helpful for one focal dystonia would be likely to be helpful for another. Furthermore, the common symptoms, signs, and responses to medication of spontaneous (idiopathic) dystonia and neuroleptic- induced dystonia suggest that an effective treatment for a drug-induced focal dystonia will be effective for the same dystonia occurring spontaneously.
A tic is an abrupt repetitive movement, gesture, or utterance that often mimics a normal type of behavior. Motor tics include movements such as eye blinking, head jerks or shoulder shrugs, but can vary to more complex purposive-appearing behaviors such as facial expressions of emotion or meaningful gestures of the arms and head. In extreme cases, the movement can be obscene (copropraxia) or self-injurious. Phonic or vocal tics range from throat clearing sounds to complex vocalizations and speech, sometimes with coprolalia (obscene speech) (Leckman et al, supra). Tics are irregular in time, though consistent regarding the muscle groups involved. Characteristically, they can be suppressed for a short time by voluntary effort.
Tics are estimated to affect 1% to 13% of boys and 1% to 11% of girls, the male- female ratio being less than 2 to 1. Approximately 5% of children between the ages of 7 and 11 years are affected with tic behavior (Leckman et al, Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997). The estimated prevalence of multiple tics with vocalization, e.g.,
Tourette's syndrome, varies among different reports, ranging from 5 per 10,000 to 5 per 1,000.
Gilles de Ia Tourette syndrome (TS) is the most severe tic disorder. Tourette's syndrome is 3-4 times more common in boys than girls and 10 times more common in children and adolescents than in adults (Leckman et al., Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997; Esper et al, Tenn. Med. 90: 18-20, 1997). Patients with TS have multiple tics, including at least one vocal (phonic) tic. TS becomes apparent in early childhood with the presentation of simple motor tics, for example, eye blinking or head jerks. Initially, tics may come and go, but in time tics become persistent and severe, and begin to have adverse effects on the child and the child's family. Phonic tics manifest, on average, 1 to 2 years after the onset of motor tics. By the age of 10, most children have developed an awareness of the premonitory urges that frequently precede a tic. Such premonitions may enable the individual to voluntary suppress the tic, yet premonition unfortunately adds to the discomfort associated with having the disorder. By late adolescence/early adulthood, tic disorders can improve significantly in certain individuals. However, adults who continue to suffer from tics often have particularly severe and debilitating symptoms. (Leckman et al., Neuropsychiatry of the Bas. Gang 20(4): 839-861, 1997).
Although the present day pharmacopeia offers a variety of agents to treat movement disorders, none of these agents can prevent or cure these conditions. Many treatments focus on eliminating or at least alleviating certain symptoms of the disorder. Furthermore, the most effective treatments are often associated with intolerable side effects.
For example, in the case of Parkinson's Disease, pramipexole therapy is usually associated with a number of undesirable side effects, and patient compliance is a significant obstacle for effective treatment.
Specifically, the majority of the patients taking MIRAPEX® are middle-aged and quite active. MIRAPEX® tablets often have to be given three times a day in equally divided doses. Therefore, compliance is a major problem with patients.
A second problem for the multiple dose regimen is that the daily "peak and trough" blood levels produced by multiple daily doses result in fluctuating stimulation of the dopaminergic neurons. These fluctuations may contribute to the pathogenesis of the motor complications in Parkinson disease. Commonly occurring adverse effects associated with MIRAPEX® include nausea, vomiting / emesis, weakness, dizziness, fainting, agitation, confusion, hallucinations, muscle twitching, uncontrollable movements, a tingling sensation, chest pain, insomnia, somnolence, decreased appetite, dry mouth, sweating, headache,
constipation and gastric intestinal complications. Episodes of sudden uncontrollable somnolence have been reported in 22% of PD patients receiving pramipexole in a dose related manner. Other side effects of pramipexole have been reported to include orthostatic hypotension, the incidence of which is dose- and peak-related. There are also reports of subjects on pramipexole medication experiencing increased somnolence, in particular "sleep attacks." Such attacks involve a subject falling asleep while engaged in activities of daily living, including operation of a motor vehicle, sometimes resulting in accidents. In addition, compulsive gambling behaviors (see Dodd et al, Pathological Gambling Caused by Drugs Used to Treat Parkinson Disease, Archives of Neurology vol. 62, Sept. 2005; Driver- Dunckley et al., Pathological Gambling Associated With Dopamine Agonist Therapy in Parkinson's Disease, Neurology, Vol. 61, August 2003), excessive shopping, overeating, and hypersexuality have also been linked to MIRAPEX® treatment.
Although the main indication of MIRAPEX® is PD treatment, MIRAPEX® is also used in lower doses to treat other movement disorders such as restless legs syndrome {e.g. , 0.125 mg daily versus 4.5 mg daily for Parkinson's). It is also being used off-label to treat depression as well as some sleep disorders.
Thus, the currently available immediate release pramipexole formulation is not ideal as it is associated with poor patient compliance as well as treatment-emergent side effects that lead to poor patient tolerance. Therefore, there remains a clear-cut need for new treatments and improved dosage forms for various movement disorders, such as using pramipexole in alleviating at least one adverse effect associated with the treatment of Parkinson's disease.
Summary of the Invention
The pharmacokinetics of pramipexole is linear, with plasma concentrations increasing proportionate with increase in dosage. Pramipexole is rapidly absorbed, reaching peak concentration in approximately 2 hours. The absolute bioavailability of pramipexole is greater than 90%. It undergoes little presystemic metabolism and is excreted virtually unchanged in the urine. However, it is difficult to reach the upper limit of the dose range using the currently available immediate release formulation. This is partly because both gastrointestinal and CNS side effects are more frequent during the initial ascending phase of the plasma profile. Nausea was primarily reported at the moment of peak pramipexole plasma levels, and increased with increase in dose. Data from other studies suggest that pramipexole
may induce a locally mediated nausea via gastric irritation as rapid onset of the nausea was observed prior to achieving peak plasma levels.
The present invention is directed to pramipexole pharmaceutical compositions that allow for highly controlled, delayed administration, preferably once-daily administration that release pramipexole over an extended period of time. The delayed / extended release dosage form is preferably at least equivalent in effectiveness to the conventional immediate release, three-time daily regimen, and provides average steady-state blood levels of pramipexole over a course of treatment. A delayed and/or once-a-day administration of pramipexole is advantageous over thrice-a-day administration in terms of patient compliance and reduced adverse events, thus providing better treatment of the conditions for which the pramipexole is indicated.
In one aspect, the invention provides an oral delayed immediate release (DIR or DR) or delayed extended release (DXR) dosage form that provides continuous and stable delivery of pramipexole over extended duration and maintains the desired therapeutic effects, while minimizing, if not eliminating, the undesired side effects and with improved patient compliance.
Preferably, pramipexole and/or its prodrug(s) and/or stereoisomers are released at a rate that results in reduction in the frequency or severity of at least one adverse effect associated with pramipexole therapy. In certain embodiments, the dosage form releases pramipexole and/or its prodrug and/or stereoisomer at a rate that results in reduction in the frequency or severity of at least one adverse event associated with current pramipexole therapies, or allows for a more convenient dosing regimen than current therapies.
Thus one aspect of the invention provides a delayed-release (DR) pramipexole pharmaceutical composition in an orally deliverable form, comprising an enteric coating, a pramipexole core, and one or more pharmaceutically acceptable carriers and excipients, wherein the enteric coating reduces or substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
In certain embodiments, pramipexole is first released and/or absorbed in intestine.
In certain embodiments, the enteric coating delays the release of pramipexole by at least about 1.5 - 2 hours, or 2-3 hours after ingestion.
In certain embodiments, the enteric coating is selected from: cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate, hydroxypropyl methylcellulose succinate, cellulose acetate succinate,
cellulose acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of methylmethacrylic acid and methyl methacrylate, copolymer of methyl acrylate, methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and maleic anhydride (Gantrez ES series), ethyl methyacrylate-methylmethacrylate- chlorotrimethylammonium ethyl acrylate copolymer, natural resins such as zein, shellac and copal collophorium, carboxymethyl ethylcellulose, Spheromer III (dopa-functionalized poly(butadiene-co-maleic acid), Spherics, Inc.), Spheromer IV (carbidopa-functionalized poly(butadiene-co-maleic acid), Spherics, Inc.), co-polymerized methacrylic acid / methacrylic acid methyl esters selected from: EUDRAGIT® L12.5, LlOO, EUDRAGIT® S12.5, SlOO, EUDRAGIT® L30D55, EUDRAGIT® FS30D, EUDRAGIT® L100-55, EUDRAGIT® SlOO (Rohm Pharma), KOLLICOAT® MAE30D and 30DP (BASF), ESTACRYL® 30D (Eastman Chemical), AQUATERIC® and AQUACOAT® CPD30 (FMC)), Acryl-EZETM White, or equivalents thereof.
In certain embodiments, the enteric coating becomes soluble at above pH 4.5, such as around pH 5.5-6.8.
In certain embodiments, the pramipexole pharmaceutical composition comprises one or more pramipexole salts, derivatives and/or stereoisomers.
In certain embodiments, the pramipexole salt is pramipexole dihydrochloride monohydrate.
In certain embodiments, the pramipexole core is formulated as an immediate release (IR) composition.
In certain embodiments, the pramipexole core is formulated as an extended release (XR) composition.
In certain embodiments, the XR composition is prepared by coating pramipexole- layered inert pellets with a release-controlling polymer.
In certain embodiments, the release-controlling polymer is ethylcellulose-based.
In certain embodiments, the release-controlling polymer is selected from: EUDRAGIT® RL; EUDRAGIT® RS; cellulose derivatives selected from: ethylcellulose aqueous dispersions (AQUACOAT®, SURELEASE®), hydroxyethyl cellulose, hydroxypropyl cellulose, or hydroxypropyl methylcellulose; polyvinylpyrrolidone; polyvinylpyrrolidone / vinyl acetate copolymer; OPADRY®, or equivalents thereof.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition is formulated to provide an effective dose over at least 4 - 24 hours after administration to the patient.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition is formulated to provide an effective plasma level over at least 8 - 16 hours after administration to the patient.
In certain embodiments, the effective plasma level at a 0.375 mg dose is about 50 - 400 ρg/mL for Parkinson's Disease treatment. The effective plasma levels may increase with an increase in dose levels, such as to 800-1800 pg/mL or even 400-4000 pg/mL.
In certain embodiments, the pramipexole core comprises an XR portion and an IR portion.
In certain embodiments, the XR portion and the IR portion are both present as multiparticulate beads / pellets embedded within an inactive dissolvable / disintegratable matrix.
In certain embodiments, the XR portion and the IR portion are each present as a section of the pramipexole core.
In certain embodiments, the XR portion is partially or completely covered by a rate- controlling coating that controls the release rate of the XR portion.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition is formulated as a once-a-day composition.
In certain embodiments, the once-a-day composition contains about 0.375 mg, 0.5 mg, 1.0 mg, 1.5 mg, 3.0 mg, or 4.5 mg of pramipexole dihydrochloride monohydrate, or equivalent thereof.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition further comprises a bioadhesive layer that adheres to the lower GI tract.
In certain embodiments, the bioadhesive layer comprises polymeric materials selected from polyamides, polyalkylene glycols, polyalkylene oxides, polyvinyl alcohols, polyvinylpyrrolidone, polyglycolides, polyurethanes, polymers of acrylic and methacrylic esters, polylactides, poly(butyric acid), polyanhydrides, polyorthoesters, poly(fumaric acid), poly(maleic acid), polycarbonates, polyalkylenes, polyalkylene terephthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polysiloxanes, polystyrene, poly(lactide-co-glycolide), chitosan, chitin, hyaluronic acid, hyaluronan, Carbopols, Corplex polymers, Polycarbophils-Cysteine (Thiomers), Chitosan-Thioglycolic acid copolymers, poly(methacrylic acid-grafted-ethylene glycol), poly (methyl vinyl ether-co-malic anhydride), cholestyramine (Duolite AP- 143), sucralfate, gliadin, blends and copolymers thereof.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition, upon administration to an individual, reduces or eliminates at least one
undesirable side-effect selected from: nausea, emesis, insomnia, hallucination, somnolence, constipation, and gastric and/or intestinal complication as compared to treatment with a thrice-daily immediate release composition of the same overall dosage.
In certain embodiments, the nausea or emesis results from a locally mediated gastric iπitation.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition provides substantially the same bioavailability and/or maximum blood concentration (Cmaχ) compared to an immediate-release pramipexole pharmaceutical composition of the same dosage without the enteric coating.
In certain embodiments, the delayed and/or extended release pharmaceutical composition provides a substantially reduced degree of fluctuation in plasma levels compared to an immediate release pharmaceutical composition of the pramipexole of the same dose administered three times daily.
In certain embodiments, the delayed and/or extended release pharmaceutical composition is associated with reduced side effects (e.g., nausea, vomiting) compared to an immediate release pharmaceutical composition of the pramipexole of the same dose administered three times daily.
In certain embodiments, the delayed-release pramipexole pharmaceutical composition is suitable for human treatment, or for veterinary treatment of a non-human mammal.
Another aspect of the invention provides a method of preparing a pramipexole pharmaceutical composition, comprising coating a formulation comprising pramipexole with an enteric coating that reduces or substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
Another aspect of the invention provides a method of treating Parkinson's Disease in an individual, comprising administering to the individual a delayed-release pramipexole pharmaceutical composition as set forth above.
Embodiments described herein are contemplated to be combined with each other embodiments as appropriate. Embodiments described in detail under one aspect of the invention may be equally applicable for the other aspects of the invention.
Brief Description of the Drawings
Figures IA - IJ are schematic drawings (not to scale) illustrating cross-sectional views of exemplary designs for the subject delivery device.
Figure 2 shows the results of an exemplary experiment comparing concentration of MIRAPEX® 0.125 mg tablets over time in fed and fasted Beagle dogs.
Figure 3 shows the results of an exemplary experiment comparing enteric-coated MIRAPEX® 0.125 mg tablets and plain MIRAPEX® 0.125 mg tablets in fasted Beagle dogs.
Figure 4 shows the dissolution profile of pramipexole 0.375 mg Extended-Release (ER) and delayed extended release (DER) formulations.
Figure 5 shows the results of comparing pramipexole 0.375 mg Extended Release (ER) and Delayed Extended Release (DER) formulations in fasted Beagles.
Figure 6 shows the results of comparing pramipexole 0.375 mg Extended Release multiparticulate-based capsules and matrix-based tablet formulations, with 0.375 mg MIRAPAX® Tablets Given in a Three-In-a-Day (TID) Dosing Regimen (0.125 mg x 3) in fed Beagles.
Figure 7 shows dissolution profiles of pramipexole 0.375 mg Delayed Extended- Release formulations in phosphate buffer pH = 6.8 using USP II apparatus.
Figure 8 shows a comparison between pramipexole 0.375 mg Extended Release Multiparticulate-based capsules formulations vs. 0.375mg Mirapex tablets given in a TID dosing regimen (0.125 mg x 3) in beagles.
Figures 9A-C show the mean human pramipexole plasma concentration comparing pramipexole 0.375 mg extended release multiparticulate formulations with Mirapex® tablets, 0.375 mg (0.125 mg x 3).
Figure 1OA is the in vitro dissolution profile of pramipexole extended release tablet formulation, 0.375 mg [5% coating (80 parts Surelease and 20 parts OPADRY), obtained using a USP II apparatus.
Figure 1OB shows the in vivo PK performance of a pramipexole extended release tablet, 0.375 mg [5% coating (80 parts Surelease + 20 parts OPADRY)] and pramipexole extended release capsules, 0.375mg [8.3 % Ethocel and 5% Spheromer III coated) in fasted beagle dogs.
Figure 11 is a comparison of pramipexole ER Formulations with Mirapex (0.125) mg tablets administered in three times a day dosing regimen.
Figure 12 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
Figure 13 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
.Figure 14 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
Figure 15 is a schematic drawing (not to scale) illustrating a cross-sectional view of one design of the subject delivery device.
Detailed Description of Invention
/. Overview
In general, the present invention relates to pharmaceutical compositions and methods for the treatment of disorders for which pramipexole is administered. Such disorders include some sleep disorders and certain movement disorders (such as Parkinson's disease and restless legs syndrome, etc.). The present invention also relates to the methods for protecting neural cells. The pharmaceutical compositions and methods of the invention relate to the use of pramipexole either alone or in combination with other active agents or pharmaceutical compositions suitable for the treatment of such diseases or the prevention or inhibition of diseases using pramipexole as a neuroprotectant.
In certain embodiments, the invention relates to particular pramipexole dosage forms (e.g., a delayed release, preferably once-a-day dosage form) that provide release profiles that are effective for the intended therapeutic use (e.g., ameliorating or overcoming symptoms of a movement disorder, such as Parkinson's disease), while reducing or avoiding at least one undesirable side-effect associated with conventional pramipexole treatment.
While not wishing to be bound by any particular theory, it is believed that releasing pramipexole in the upper GI tract may cause local irritation and lead to at least one undesirable side effect of pramipexole treatment, including emesis and nausea. Thus, according to one aspect of the invention, one or more undesirable side effects traditionally associated with administering pramipexole to an individual can be alleviated or even eliminated by delaying the release of pramipexole until the drug is in the lower GI tract, such as in the intestine (e.g., the small intestine, the colon, and/or the rectum). Also according to the instant invention, one way to reduce or eliminate the release of pramipexole in the stomach is to utilize an enteric coating, such that pramipexole is not substantially released in the acidic environment of the stomach. Once inside the intestine, where the local pH environment based on the fed or fasted states varies between 4.5 and 7.4, pramipexole is released either as an immediate release (IR) dosage form or as an extended release (XR)
dosage form, or a mixture thereof. Since such dosage forms are also delayed-release dosage forms, they are referred to as delayed immediate release (DIR or DR) or delayed extended release (DXR) dosage forms, respectively.
In a preferred embodiment, the DXR dosage form achieves the therapeutic benefit of the conventional thrice-a-day pramipexole regimen and yet is administered as a single daily administration, e.g., which contains substantially the same total dosage of pramipexole, yet releases the drug in a controlled manner and over an extended period of time, thus improving patient compliance, and alleviating and/or eliminating the undesirable daily "peak and trough" blood levels produced by multiple daily doses, and the associated fluctuating stimulation of the dopaminergic neurons.
In certain embodiments, the dosage form may include bioadhesive layers that adhere to the lower GI tract, such as intestinal walls, to prolong the release of pramipexole in the lower GI tract. The bioadhesive layer may be inside or outside the enteric coating. In the former case, the presence of the bioadhesive layer (e.g., as a partial coating that is continuous or discontinuous) preferably does not substantially impede the release of pramipexole. In the latter case, the presence of the bioadhesive layer {e.g., as a partial coating that is continuous or discontinuous) does not substantially impede the degradation of the enteric layer in the neutral pH environment of the intestine.
Pramipexole is not acid-sensitive, and thus need not be protected from the relative acidic environment of the upper GI tract per se. Indeed, in certain embodiments, pramipexole needs to be delivered to the lower GI tract, for example, for targeted treatment of certain colon diseases. However, the present invention is based in part on the unexpected discovery that by by-passing the release of pramipexole in the upper GI tract (e.g., the stomach) it is possible to avoid one or more undesirable side effects typically associated with pramipexole treatments, such as nausea and/or emesis.
The compositions of the present invention maybe in the form of, among others, a granule, tablet (including matrix or osmotic), pellet, powder, sachet, capsule, gel, dispersion, solution or suspension. The only requirement is that the dosage forms be composed in such a manner as to achieve the profiles set forth herein.
In vivo profiles for pramipexole that provide the appropriate blood (or, more particularly, plasma) concentration levels over time in order to meet the therapeutic requirements for once daily administration were provided in the present invention. These profiles are such that the mean blood pramipexole levels provide an effective amount of the drug for the treatment of such conditions as PD or other related movement disorders, yet
below levels that induce adverse side effects typically associated with spikes in the plasma concentration that follow the multiple administration of fast / immediate release formulations. In addition, by-passing the release of pramipexole in the upper GI tract, such as in the stomach, avoids side effects such as nausea and/or emesis.
Thus, with the present invention, it was found that an effective blood pramipexole concentration at relatively steady state could be achieved by formulating pramipexole in several inventive dosage forms. These dosage forms are in the form of a delayed release, an immediate release, an extended release, or combination thereof.
Another aspect of the invention provides a method for making the pharmaceutical compositions with one or more features as described above.
Another aspect of the invention provides a method for using the pharmaceutical compositions with one or more features as described above, in treating a movement disorder, such as Parkinson's disease.
Another aspect of the invention provides the use of a pharmaceutical composition with one or more features as described above in manufacturing medicaments for the treatment of a movement disorder, such as Parkinson's disease.
The subject preparations and methods can be used as part of treatments for human and/or other animal subjects. In addition to humans, other animal subjects to which the invention is applicable extend to both domestic animals and livestock, raised either as laboratory animals, pets or zoo animals, or for commercial purposes. Examples are rodents such as mice, rats, hamsters, or rabbits; dogs; cats; cattle; horses; sheep; hogs; and goats.
Certain general features of the invention are further elaborated in the sections below.
//. Definitions
For convenience, certain terms employed in the specification, examples, and appended claims are collected here. All other terms have their ordinary meanings as understood by a skilled artisan.
As used herein, "about" means within the pharmaceutically acceptable limits found in the United States Pharmacopeia (USP-NF 21), 2003 Annual Edition, or available at the USP website, for amount of active pharmaceutical ingredients. With respect to blood levels, "about" means within FDA acceptable guidelines.
The term "water-soluble" herein means having solubility of at least about 10 mg/ml. Unless otherwise specified, "solubility" herein means solubility in water at 20-25 0C at any physiologically acceptable pH, for example at any pH in the range of about 4 to about 8. In
the case of a salt, reference herein to solubility in water pertains to the salt, not to the free base form of pramipexole.
"Solid fraction" is the ratio of absolute to apparent density of a compact of the starch. A "compact" herein is a compressed tablet, prepared for example on a tablet press, consisting only of a sample of starch for which it is desired to measure tensile strength. A "solid fraction representative of the tablet" is a solid fraction selected to be similar to the solid fraction of tablets prepared according to the invention. Typically a solid fraction of about 0.75 to about 0.85, illustratively 0.8, will be selected.
The term "orally deliverable" herein means suitable for oral, including peroral and intra-oral (e.g., sublingual or buccal) administration, but tablets of the present invention are adapted primarily for peroral administration, i.e., for swallowing, typically whole (or, in certain embodiments, broken), with the aid of water or other drinkable fluid.
A "subject" herein is an animal of any species, preferably mammalian, most preferably human. Conditions and disorders in a subject for which a particular agent is said herein to be "indicated" are not restricted to conditions and disorders for which the agent has been expressly approved by a regulatory authority, but also include other conditions and disorders known or believed by a physician to be amenable to treatment with the agent.
"Treatment" herein embraces prophylactic treatment unless the context requires otherwise.
The term "adrenergic" refers to neurotransmitters or neuromodulators chemically related to adrenaline (epinephrine) or to neurons which release such adrenergic mediators. Examples are dopamine, norepinephrine, and epinephrine. Such agents are also referred to as catecholamines, which are derived from the amino acid tyrosine.
As used herein "catechol moiety" refers to a moiety with the following generic structure:
In certain embodiments of the invention, a polymer may be functionalized by covalently attaching catechol moieties or compounds comprising catechol moieties. Alternatively, a compound comprising a catechol moiety may be blended with a polymer to form a simple mixture with no covalent association between the catechol moieties and the polymer.
The term "catecholamines" refers to neurotransmitters that have a catechol ring (e.g., a 3, 4-dihydroxylated benzene ring). Examples are dopamine, norepinephrine, and epinephrine.
The term "cholinergic" refers to neurotransmitters or neuromodulators chemically related to choline or to neurons which release such cholinergic mediators.
The term "dopaminergic" refers to neurotransmitters or neuromodulators chemically related to dopamine or to neurons which release such dopaminergic mediators.
The term "dopamine" refers to an adrenergic neurotransmitter, as is known in the art.
The term "ED50" means the dose of a drug which produces 50% of its maximum response or effect.
An "effective amount" of, e.g., a movement disorder pharmaceutical composition, with respect to the subject method of treatment, refers to an amount of the pharmaceutical composition in a preparation which, when applied as part of the subject dosage regimen brings about the desired correction / suppression of the movement disorder (e.g., dyskinesis and/or bradykinesis) according to clinically acceptable standards.
The term "LD50" means the dose of a drug which is lethal in 50% of test subjects.
The term "lethal therapeutic index" refers to the therapeutic index of a drug defined as
The term "metabolites" refers to active derivatives produced upon introduction of a compound into a biological milieu, such as a patient.
A "patient," "individual," or "subject" to be treated by the subject method can mean either a human or non-human animal.
The term "prevent," "preventing," or "prevention" as used herein means reducing the probability / risk of developing a condition in a subject (e.g., a human), or delaying the onset of a condition in the subject, or lessening the severity of one or more symptoms of a condition (e.g., a movement disorder) that may develop in the subject, or any combination thereof.
The term "prodrug" is intended to encompass compounds which, under physiologic conditions, are converted into the therapeutically active agents of the present invention. A common method for making a prodrug is to include one or more selected moieties which are hydrolyzed under physiologic conditions to reveal the desired molecule. In other embodiments, the prodrug is converted by an enzymatic activity of the host animal.
The phrase "protecting group" as used herein means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations. Examples of such protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively. The field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991).
The term "SeU5o" means the dose of a drug which is produces a particular side-effect in 50% of test subjects.
The term "side-effect therapeutic index" refers to the therapeutic index of a drug defined as SeDso/EDso.
The term "statistically significant" as used herein means that the obtained results are not likely to be due to chance fluctuations at the specified level of probability. The two most commonly specified levels of significance are 0.05 (p=0.05) and 0.01 (p=0.01). The level of significance equal to 0.05 and 0.01 means that the probability of error is 5 out of 100 and 1 out of 100, respectively.
The term "treat," "treating," or "treatment" as used herein means to counteract a medical condition (e.g., a movement disorder) to the extent that the medical condition is improved according to clinically acceptable standard(s). For example, "to treat a movement disorder" means to improve the movement disorder or relieve symptoms of the particular movement disorder in a patient, wherein the improvement and relief are evaluated with a clinically acceptable standardized test (e.g., a patient self-assessment scale) and/or an empirical test (e.g., PET scan).
The term "Cmax" as used herein means maximum plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
The term "Cmin" as used herein means minimum plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
The term "Cavg" as used herein means average plasma concentration of pramipexole achieved by the ingestion of the composition of the invention or the t.i.d comparator (Mirapex IR tablets). Cavg is calculated by AUC over a 24 hours intervals divided by 24.
The term "Tmax" as used herein means the time to achieve maximum plasma
concentrations produced by ingestion of of the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
The term "AUC" as used herein means the area under the plasma concentration-time curve, as calculated by the trapezoidal rule over the 24 hour interval for all the formulations.
The term "Degree of Fluctuation (DFL)" as used herein is expressed as :
DFL = (Cmax - Cmin)/Cavg
produced by ingestion of the the composition of the invention or the t.i.d comparator (Mirapex IR tablets).
As used in this application, the term "Cmin" and "trough levels" should be considered synonyms. Likewise, "Cmax" and "peak levels" should be considered synonyms.
III. Dosage Forms
The effective ingredient of the various dosage forms of the invention is pramipexole. Pramipexole (formula I below) is a dopamine D2 receptor agonist useful in treatment of Parkinson's disease and complications associated therewith. Pramipexole as its dihydrochloride salt is commercially available in the United States as MIRAPEX® tablets of Pharmacia & Upjohn / Pfizer. These are marketed as immediate-release tablets in 0.125 mg, 0.25 mg, 0.5 mg, 1.0 mg and 1.5 mg strengths, designed for thrice-a-day oral administration of a single tablet each to provide a daily dose of 0.375 to 4.5 mg. See Physicians' Desk Reference 57th edition (2003), 2768-2772. Doses herein are expressed in amounts of pramipexole dihydrochloride monohydrate unless otherwise specified; e.g., 1.0 mg pramipexole dihydrochloride monohydrate is equivalent to about 0.7 mg pramipexole base. Other salt forms can be readily converted from the amount of pramipexole base contained therein.
It should be understood that mention of pramipexole or a salt thereof herein embraces racemates, enantiomers, polymorphs, hydrates and solvates thereof. Pramipexole (I) is used preferably in the form of its S-enantiomer, (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)-
benzothiazole. A preferred salt of pramipexole is the dihydrochloride salt, most preferably in the form of the monohydrate. Pramipexole compositions of the invention are preferably suitable for administration no more than once daily. Such compositions are useful in treatment of any CNS condition or disorder for which pramipexole has therapeutic utility, but especially Parkinson's disease and complications associated therewith.
Pramipexole and its salts useful herein can be prepared by processes known per se, including processes disclosed in patents and other literature pertaining to pramipexole.
The amount of the pramipexole salt present in a composition of the invention is sufficient to provide a daily dose in one to a small plurality, for example one to about 4, of tablets to be administered at one time. Preferably the full daily dose is delivered in a single tablet. An amount of pramipexole salt, expressed as pramipexole dihydrochloride monohydrate equivalent, of about 0.1 to about 10 mg per tablet, or about 0.05% to about 5% by weight of the composition, will generally be suitable. Preferably an amount of about 0.2 to about 6 mg, more preferably an amount of about 0.3 to about 5 mg, per tablet is present. Although many examples of this application use 0.375 mg pramipexole dihydrochloride monohydrate for Beagle dogs, specific dosage amounts per tablet contemplated herein include about 0.375, 0.5, 0.75, 1.0, 1.5, 3.0 and 4.5 mg pramipexole dihydrochloride monohydrate.
A. Immediate Release (IR) Composition
By "immediate release composition" is meant a dosage form that is formulated to release substantially all the active ingredient on administration with no enhanced, delayed or extended release effect. Such a composition may be in the form of a pellet (a term used interchangeably with "bead" or "beadlet" herein). The immediate release pellet can serve as a precursor to an extended or delayed release pellet, or be used with an extended or delayed release pellet.
The non-active ingredients and processes for preparing such immediate release pellets are well known in the art, and the present invention is not limited in these respects. See, for example, Remington's Pharmaceutical Sciences, 18th Edition, A. Gennaro, Ed., Mack Pub. Co. (Easton, Pa. 1990), Chapters 88-91, the entireties of which are hereby incorporated by reference.
For instance, an immediate release pellet can be prepared by mixing pramipexole with a bulking agent. Additionally, one can add disintegrating agents, antiadherents and glidants to the formulation.
Bulking agents employable in these compositions may be chosen from, among others: microcrystalline cellulose, for example, AVICEL® (FMC Corp.) or EMCOCEL® (Mendell Inc.), which also has binder properties; dicalcium phosphate, for example, EMCOMPRESS® (Mendell Inc.); calcium sulfate, for example, COMPACTROL® (Mendell Inc.); and starches, for example, Starch 1500; and polyethylene glycols (CARBOWAX®). Such bulking agents are typically present in the range of about 5% to about 75% (w/w), with a preferred range of about 25% to about 50% (w/w).
Suitable disintegrants include, but are not limited to: crosslinked sodium carboxymethyl cellulose (AC-DI-SOL®), sodium starch glycolate (EXPLOTAB®, PRIMO JEL®) and crosslinked polyvinylpolypyrrolidone (PLASONE-XL®). Disintegrants are used to facilitate disintegration of the pellet upon administration and are typically present in an amount of about 3% to about 15% (w/w), with a preferred range of about 5% to about 10% (w/w).
Antiadherents and glidants employable in such formulations can include talc, cornstarch, silicon dioxide, sodium lauryl sulfate, colloidal silica dioxide, and metallic stearates, among others.
In addition, the immediate release composition may contain one or more binders to give the pellets cohesiveness. Such binders are well known in the art, and include such substances as microcrystalline cellulose, polyvinyl pyrrolidone, starch, maltrin, methylcellulose, hydroxypropyl methylcellulose, carboxymethyl cellulose, sucrose solution, dextrose solution, acacia, tragacanth and locust bean gum, which may be applied wet. The binding agent may be present in the composition in an amount of from about 0.2 wt % to about 40 wt %, preferably from about 5 wt % to about 30 wt %, or from about 10 wt % to about 15 wt %.
The pellets can be made by, for example, simple granulation such as wet granulation or dry granulation, followed by sieving; extrusion and marumerization (spheronization); rotogranulation; or any agglomeration process that results in a pellet of reasonable size and robustness. For extrusion and marumerization, the drug and other additives are granulated by addition of a binder solution. The wet mass is passed through an extruder equipped with a certain size screen, and the extrudates are spheronized in a marumerizer. The resulting pellets are dried and sieved for further applications.
One may also use high-shear granulation, wherein the drug and other additives are dry-mixed and then the mixture is wetted by addition of a binder solution in a high shear-
granulator/mixer. The granules are kneaded after wetting by the combined actions of mixing and milling. The resulting granules or pellets are dried and sieved for further applications.
Alternatively, and preferably, the immediate release beadlets or pellets are prepared by solution or suspension layering, whereby a drug solution or dispersion, with or without a binder and optionally an anti-tacking agent such as talc, is sprayed onto a core or starting seed (either prepared or a commercially available product) in a fluid bed processor or other suitable equipment. The cores or starting seeds can be, for example, sugar spheres or spheres made from microcrystalline cellulose. The binder in the formula can be present in amounts ranging from about 0% to about 5% by weight, and preferably about 0.5% to about 2% by weight. The amount of anti-tacking agent used can be from about 0% to about 5%, preferably about 0.5% to about 2% by weight. The drug thus is coated on the surface of the starting seeds. The drug may also be layered onto the drug-containing pellets described above, if desired. Following drug layering, the resulting drug-loaded pellets are dried for further applications.
A protective layer, or overcoating, may be desired to ensure that the drug-loaded pellets do not aggregate during processing or upon storage. The protective coating layer may be applied immediately outside the core, either a drug-containing core or a drug-layered core, by conventional coating techniques such as pan coating or fluid bed coating using solutions of polymers in water or suitable organic solvents or by using aqueous polymer dispersions. OPADRY®, OPADRY II® (Colorcon) and corresponding color and colorless grades from Colorcon can be used to protect the pellets from being tacky and provide colors to the product. Different anhydride-based polymers (e.g., sebacic/fumaric copolymers such as Spheromer I or Spheromer II from Spherics, Inc.) may also be used as protective layer. The suggested levels of protective or color coating are from about 1% to about 6%, preferably about 2% to about 3% (w/w). In certain embodiments, many ingredients can be incorporated into the overcoating formula, for example to provide a quicker immediate release, such as plasticizers: acetyltriethyl citrate, triethyl citrate, acetyltributyl citrate; dibutylsebacate, triacetin, polyethylene glycols, propylene glycol and the others; lubricants: talc, colloidal silica dioxide, magnesium stearate, calcium stearate, titanium dioxide, magnesium silicate, and the like.
In certain embodiments, the immediate release composition may be prepared as an uncoated tablet, or a tablet core prior to coating, comprising starch and a hydrophilic polymer acting as a matrix for a water-soluble drug or prodrug requires to have a certain minimum hardness in order to be able to resist breakage and/or attrition due to mechanical stresses
imposed during a high-speed tableting operation (including all steps up to and including filling of the tablets into containers). The minimum acceptable hardness will depend on a number of factors, including the severity of the mechanical stresses, but is typically at least about 20 SCU, preferably at least about 22 SCU, more preferably at least about 24 SCU (about 17 kp).
Hardness can be increased by increasing the compression force applied by the tablet press, but only up to a certain level. At least in the case of tablets as described herein, above a certain compression force, further increases in compression force give little or no further increase in tablet hardness. There is, in other words, a maximum hardness achievable by compression of a particular starch / hydrophilic polymer / active agent composition. A starch providing a maximum hardness inadequate to withstand the mechanical stresses of a highspeed tableting operation is unsuitable for the present purpose. Certain pregelatinized starches provide a maximum hardness of 20 SCU or less; these are starches having low tensile strength (0.1 kN cm"2 or less). Even if a maximum hardness of at least about 20 SCU is achievable, with a starch of low tensile strength it may be achievable only by use of extremely high compression forces. A requirement for such forces reduces speed and efficiency and increases cost of a tableting operation and is undesirable for these reasons.
The immediate release pellets are contemplated as being used in combination with extended release pellets and/or delayed release pellets in a single dosage form, and/or being modified to generate extended release (XR) pellets, delayed release (DR) pellets, and/or delayed and extended release (DXR) pellets in a single dosage form.
B. Delayed Release Composition (DR)
The delayed-release component has a coat applied to the surface of the active pellet that delays the release of the drug from the pellet after administration for a certain period of time. This delayed release can be accomplished by applying a coating of enteric materials.
"Enteric materials" are polymers that are substantially insoluble in the acidic environment of the stomach, but are predominantly soluble in intestinal fluids at various specific pH's, such as pH 4.5 or higher. The enteric materials are non-toxic, pharmaceutically acceptable polymers, and include, for example, cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate, hydroxypropyl methylcellulose succinate, cellulose acetate succinate, cellulose acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of methylmethacrylic acid and
methyl methacrylate, copolymer of methyl acrylate, methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and maleic anhydride (Gantrez ES series), ethyl methyacrylate-methylmethacrylate-chlorotrimethylammonium ethyl acrylate copolymer, natural resins such as zein, shellac and copal coUophorium, carboxymethyl ethylcellulose, Spheromer III, Spheromer IV, co-polymerized methacrylic acid/methacrylic acid methyl esters such as, for instance, materials known under the trade name EUDRAGIT® L12.5, LlOO, or EUDRAGIT® S12.5, SlOO, and several commercially available enteric dispersion systems (e.g., EUDRAGIT® L30D55, EUDRAGIT® FS30D, EUDRAGIT® L100-55, EUDRAGIT® SlOO (Rohm Pharma), KOLLICOAT® MAE30D and 30DP (BASF), ESTACRYL® 30D (Eastman Chemical), AQUATERIC® and AQUACOAT® CPD30 (FMC)), Acryl-EZE™ White, etc.
The foregoing is merely a list of possible enteric coating materials, but one of skill in the art would appreciate that there are other such materials that would meet the objectives of the present invention of providing for a delayed release profile, including tailoring release based on the ambient pH environment, temporal considerations and/or other factors.
These coating materials can be employed in coating the surfaces in a range of from about 1.0% (w/w) to about 50% (w/w) of the pellet composition. Preferably, these coating materials are in the range of from about 10-20% (w/w). The pellets may be coated in a fluidized bed apparatus or pan coating, for example, in a conventional manner.
With the enteric-coated pellets, there is no substantial release of pramipexole in the acidic stomach environment of below about pH 4.5. The pramipexole becomes available when the pH-sensitive enteric layer dissolves at a higher pH in the GI tract, after a certain delayed time, or after the unit passes through the stomach. The preferred delay time is in the range of about 0.5 to about 6 hours, but more preferable is about 0.5 to about 4 hours.
For example, certain DR pellets may be coated with EUDRAGIT® L30D-55, which dissolves at about pH 5.5-6.0, i.e., in the upper intestines. In other embodiments, the DR pellets may be coated with EUDRAGIT® FS30D, which dissolves at about pH 7.0, i.e., in the lower intestine and colon.
As a variation of this embodiment, the XR pellet described above may be additionally coated with the enteric material to generate delayed and extended release (DXR) pellets. Such a dosage form is delayed release until the drug reaches non-acidic environment, such as the upper and/or lower intestine,1 and thereupon releasing drugs over an extended period of time.
C. Extended Release Composition (XR)
Pramipexole extended release pellets can be prepared in many different ways to achieve an extended release profile.
For example, in certain embodiments, the subject pramipexole XR pellets can be prepared by coating drug layered inert pellets with release-controlling polymers. First, the inert pellet is coated with the drug layer, or a drug loaded granule is prepared, as described above. Then the active (drug loaded) pellet is coated with a release-controlling polymeric membrane. The release-controlling coating layer may be applied immediately outside the core (such as a drug-containing core or a drug-layered core), by conventional coating techniques, such as pan coating or fluid bed coating, using solutions of polymers in water or suitable organic solvents, or by using aqueous polymer dispersions. As an alternative embodiment, the release controlling membrane can separate additional drug layers on the core; for instance, after coating with the release controlling substance, another drug layer can be applied, which is followed by another release controlling layer, etc. Suitable materials for the release-controlling layer include EUDRAGIT® RL, EUDRAGIT® RS, cellulose derivatives such as ethylcellulose aqueous dispersions (AQUACOAT®, SURELEASE®), hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, polyvinylpyrrolidone, polyvinylpyrrolidone/vinyl acetate copolymer, OPADRY®), and the like. The thickness of the coating affects the release profile, and so this parameter can be used to customize the profile. The suggested coating levels are from about 1% to about 40%, about 5% to about 30% (w/w), or about 20% or about 25% in other embodiments.
For example, for pramipexole salts of high water solubility as specified herein, a hydrophilic polymer matrix core can be inadequate to provide sustained release of sufficiently long duration to permit once daily administration. It is believed that such salts are readily leached out of the hydrophilic matrix when contacted by an aqueous medium such as gastrointestinal fluid. Thus in certain embodiments, it is desirable to further slow the process of drug release by providing a release-controlling coating around the tablet to produce an extended-release (XR) tablet. Such a coating may comprise a hydrophobic or water-insoluble polymer component such as ethylcellulose together with a hydrophilic or water-soluble pore- forming component such as HPMC. In addition, where tablets are to be subjected to an additional process step after compression, in particular a coating step, exposure to mechanical stresses is also greatly increased.
Where a starch is used having a tensile strength of at least about 0.15 kN cm"2, preferably at least about 0.175 kN cm"2, more preferably at least about 0.2 kN cm""2, at a solid fraction representative of the tablet (e.g., about 0.75 to about 0.85), the composition is found
to be especially suited to a high-speed tableting operation that includes a step of coating the tablet with a release-controlling layer.
Alternatives to ethylcellulose and HPMC as components of a release coating layer include other cellulosic polymers {e.g., methylcellulose, hydroxypropylcellulose, hydroxyethylcellulose, carboxymethylcellulose sodium, cellulose esters such as cellulose acetate, etc.), polyvinyl acetate, polyvinyl pyrrolidone, polymers and copolymers of acrylic acid and methacrylic acid and esters thereof, polyethylene glycol, carrageenan and other gums, etc.
A release-controlling layer, if present, typically constitutes about 1% to about 15%, preferably about 2.5% to about 10%, by weight of the tablet as a whole. The hydrophobic or water-insoluble component, preferably comprising ethylcellulose, typically constitutes about 1% to about 10%, preferably about 2% to about 7%, by weight of the tablet as a whole. The pore-forming component, preferably comprising HPMC, is typically present in an amount of about 5% to about 50%, preferably about 10% to about 40%, by weight of the water- insoluble or hydrophobic component.
The coating, if present, can optionally contain additional pharmaceutically acceptable excipients such as plasticizers, dyes, etc. Illustratively, a release-controlling layer in an amount of about 2.5% to about 5% by weight of the tablet core (i.e., the tablet weight excluding the coating) comprises an ethylcellulose-based material {e.g., SURELEASE® of Colorcon) and an HPMC-based pore-forming material (e.g., OPADRY® of Colorcon) in a weight ratio of about 3: 1 to about 4: 1. A release-controlling layer or coating is preferably applied at a relatively uniform thickness to provide even control of release rate of the pramipexole.
Alternatively or in addition, the sustained-release tablet of the invention comprises a nonfunctional coating. A nonfunctional coating can comprise a polymer component, for example HPMC, optionally with other ingredients, for example one or more plasticizers, colorants, etc. The term "nonfunctional" in the present context means having no substantial effect on release properties of the tablet, and does not imply that the coating serves no useful purpose. For example, such a coating can impart a distinctive appearance to the tablet, provide protection against attrition during packaging and transportation, improve ease of swallowing, and/or have other benefits. A nonfunctional coating should be applied in an amount sufficient to provide complete coverage of the tablet. Typically an amount of about 1% to about 10%, more typically an amount of about 2.5% to about 5%, by weight of the tablet as a whole, will be found suitable.
Uncoated tablets and cores of coated tablets of the invention can optionally contain one or more pharmaceutically acceptable excipients in addition to the starch and hydrophilic polymer components described above. Such excipients include without limitation glidants and lubricants. Other conventional excipients known in the art can also be included. A glidant can be used to improve powder flow properties prior to and during tableting and to reduce caking. Suitable glidants include colloidal silicon dioxide, magnesium trisilicate, powdered cellulose, starch, talc, tribasic calcium phosphate and the like. In certain embodiments, colloidal silicon dioxide is included as a glidant in an amount up to about 2%, preferably about 0.2% to about 0.6%, by weight of the tablet. A lubricant can be used to enhance release of a tablet from apparatus on which it is formed, for example by preventing adherence to the face of an upper punch ("picking") or lower punch ("sticking"). Suitable lubricants include magnesium stearate, calcium stearate, canola oil, glyceryl palmitostearate, hydrogenated vegetable oil, magnesium oxide, mineral oil, poloxamer, polyethylene glycol, polyvinyl alcohol sodium benzoate, sodium lauryl sulfate, sodium stearyl fumarate, stearic acid, talc, hydrogenated vegetable oil, zinc stearate and the like. In certain embodiments, magnesium stearate is included as a lubricant in an amount of about 0.1 % to about 1.5%, preferably about 0.3% to about 1%, by weight of the tablet.
In another embodiment, the coated extended-release (XR) tablets of pramipexole dihydrochloride of the invention are prepared using the composition shown in the table below:
Composition of coated tablets
Tablet cores were prepared as following: all ingredients except the lubricant (magnesium stearate) were screened to remove lumps and were blended thoroughly in a low- shear mixer operating at 24 rpm for 10-30 minutes. The lubricant was then screened into the mixer and the materials were blended for a further 2-5 minutes. The resulting lubricated mixture was compressed into 350 mg tablets using a GlobePharma Manual Tablet Compaction Machine.
In an alternative embodiment, pramipexole was layered onto the lactose particles to achieve its uniform dispersion.
A coating solution was prepared as follows: OPADRY® HPMC-based material in an amount of 8.002 g was added to 171.735 g water and mixed for 45 minutes to provide an HPMC mixture. Next, 128.032 g SURELEASE® ethylcellulose-based material was added to the HPMC mixture and mixed for an additional 30 minutes to provide a coating solution.
Coating to a 5% total weight gain and curing of the coated tablets were performed as following: the coating solution was applied to the tablet cores in an amount providing a 5% weight gain. The resulting coated tablets were cured using a 12 inch (about 30 cm) Vector LCDS or 24 inch (about 60 cm) Thomas Accela-Coata coating pan for about 15 minutes at a bed temperature of at least about 700C. After curing, temperature was ramped down over a period of about 8 minutes to an exhaust temperature of about 45°C.
The extended release pellets typically contain the same amount of total pramipexole used for thrice-a-day conventional treatment, e.g., about 0.375 mg for the 0.125 mg regimen.
IV. Exemplary Delivery Devices
A. General Considerations
As noted previously herein, the compositions of the present invention can be in a number of different forms, such as tablets, powders, suspensions, solutions, etc. The composition is preferably in pellet/beadlet form, which can be incorporated into hard gelatin or other kinds of capsules, either with additional excipients, or alone.
The dosage formulations described herein, e.g., the cores of tablets and drug eluting devices of the invention, may contain one or more excipients, carriers or diluents. These excipients, carriers or diluents can be selected, for example, to control the disintegration rate of a tablet or drug eluting device to fit the desired release profile according to the instant invention. In addition, the one or more carriers (additives) and/or diluents may be pharmaceutically acceptable.
The phrase "pharmaceutically acceptable carrier" as used herein means a pharmaceutically acceptable material, composition or vehicle, such as a liquid or solid filter, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting the subject regulators from one organ, or portion of the body, to another organ, or portion of the body. Each carrier must be "acceptable" in the sense of being compatible with the other ingredients of the formulation and not injurious to the patient. Some examples of materials which can serve as pharmaceutically acceptable carriers include (1) sugars, such as lactose, glucose and sucrose; (2) starches, such as corn starch and potato starch; (3) cellulose, and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; (4) powdered tragacanth; (5) malt; (6) gelatin; (7) talc; (8) excipients, such as cocoa butter and suppository waxes; (9) oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10) glycols, such as propylene glycol; (11) polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; (12) esters such as ethyl oleate and ethyl laurate; (13) agar; (14) buffering agents, such as magnesium hydroxide and aluminum hydroxide; (15) alginic acid; (16) pyrogen-free water; (17) isotonic saline; (18) Ringer's solution; (19) ethyl alcohol; (20) phosphate buffer solutions; and (21) other non-toxic compatible substances employed in pharmaceutical formulations.
Typical excipients to be added to a capsule formulation include, but are not limited to: fillers such as microcrystalline cellulose, soy polysaccharides, calcium phosphate dihydrate, calcium sulfate, lactose, sucrose, sorbitol, or any other inert filler. In addition, there can be flow aids such as fumed silicon dioxide, silica gel, magnesium stearate, calcium stearate or any other materials that impart good flow properties. A lubricant can also be added if desired, such as polyethylene glycol, leucine, glyceryl behenate, magnesium stearate or calcium stearate.
The formulations can conveniently be presented in unit dosage form and can be prepared by any of the methods well known in the art of pharmacy. All methods include bringing into association the drug with the carrier or diluent which constitutes one or more accessory ingredients. In general, the formulations are prepared by uniformly and intimately bringing into association the agent with the carriers and then, if necessary, dividing the product into unit dosages thereof. It will be understood by those skilled in the art that any vehicle or carrier conventionally employed and which is inert with respect to the active agent, and preferably does not interfere with bioadhesion in embodiments employing a bioadhesive coating, may be utilized for preparing and administering the pharmaceutical compositions of the present invention. Illustrative of such vehicles and carriers are those described, for
example, in Remington's Pharmaceutical Sciences, 18th ed. (1990), the disclosure of which is incorporated herein by reference.
Examples of carriers and diluents include pharmaceutically accepted hydrogels such as alginate, chitosan, methylmethacrylates, cellulose and derivatives thereof (microcrystalline cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, carboxymethylcellulose, ethylcellulose), agarose and POVIDONE™, kaolin, magnesium stearate, starch, lactose, sucrose, density-controlling agents such as barium sulfate and oils, dissolution enhancers such as aspartic acid, citric acid, glutamic acid, tartartic acid, sodium bicarbonate, sodium carbonate, sodium phosphate, glycine, tricine, Tromethamine, and TRIS.
The excipients, carriers or diluents can also be selected to control the time until a dosage form detaches from a mucosal membrane. In particular, the addition of one or more disintegrating agents will reduce the time until a tablet or drug eluting device detaches. Alternatively or in combination with the disintegrating agents, an agent that interferes with the mucosa-tablet / device adhesion can be used to control the time until detachment occurs.
As set out above, certain components, such as pramipexole, of the present pharmaceutical compositions may contain a basic functional group, such as amino or alkylamino, and are thus capable of forming pharmaceutically acceptable salts with pharmaceutically acceptable acids. The term "pharmaceutically acceptable salts" in this respect, refers to the relatively non-toxic, inorganic and organic acid addition salts of compounds of the present invention. These salts can be prepared in situ during the final isolation and purification of the compounds of the invention, or by separately reacting a purified compound of the invention in its free base form with a suitable organic or inorganic acid, and isolating the salt thus formed. Representative salts include but are not limited to following: 2-hydroxyethanesulfonate, 2-naphthalenesulfonate, 3-hydroxy-2-naphthoate, 3- phenylpropionate, acetate, adipate, alginate, amsonate, aspartate, benzenesulfonate, benzoate, besylate, bicarbonate, bisulfate, bitartrate, borate, butyrate, calcium edetate, camphorate, camphorsulfonate, camsylate, carbonate, citrate, clavulariate, cyclopentanepropionate, digluconate, dodecylsulfate, edetate, edisylate, estolate, esylate, ethanesulfonate, fumarate, gluceptate, glucoheptanoate, gluconate, glutamate, glycerophosphate, glycollylarsanilate, hemisulfate, heptanoate, hexafluorophosphate, hexanoate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroiodide, hydroxynaphthoate, iodide, isothionate, lactate, lactobionate, laurate, laurylsulphonate, malate, maleate, mandelate, mesylate, methanesulfonate, methylbromide, methylnitrate, methylsulfate, mucate, naphthylate, napsylate, nicotinate, nitrate, N-methylglucamine ammonium salt, oleate, oxalate, palmitate,
pamoate, pantothenate, pectinate, persulfate, phosphate, phosphate/diphosphate, picrate, pivalate, polygalacturonate, propionate, p-toluenesulfonate, salicylate, stearate, subacetate, succinate, sulfate, sulfosaliculate, suramate, tannate, tartrate, teoclate, thiocyanate, tosylate, triethiodide, undecanoate, and valerate salts, and the like. (See, for example, Berge et ah, "Pharmaceutical Salts", J. Pharm. ScL 66: 1-19, 1977).
In certain embodiments, the pharmaceutically acceptable salts of compounds, such as pramipexole, include the conventional non-toxic salts of the compounds, e.g., from non-toxic organic or inorganic acids. Particularly suitable are salts of weak acids. For example, such conventional non-toxic salts include those derived from inorganic acids such as hydrochloric, hydrobromic, hydriodic, cinnamic, gluconic, sulfuric, sulfamic, phosphoric, nitric, and the like; and the salts prepared from organic acids such as acetic, propionic, succinic, glycolic, stearic, lactic, maleic, tartaric, citric, ascorbic, palmitic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicyclic, sulfanilic, 2-acetoxybenzoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulfonic, oxalic, isothionic, and the like.
In other cases, the components of formulations of the present invention may contain one or more acidic functional groups and, thus, are capable of forming pharmaceutically acceptable salts with pharmaceutically acceptable bases. The term "pharmaceutically acceptable salts" in these instances refers to the relatively non-toxic, inorganic and organic base addition salts of compounds of the present invention. These salts can likewise be prepared in situ during the final isolation and purification of the compounds, or by separately reacting the purified compound in its free acid form with a suitable base, such as the hydroxide, carbonate or bicarbonate of a pharmaceutically acceptable metal cation, with ammonia, or with a pharmaceutically acceptable organic primary, secondary or tertiary amine. Representative alkali or alkaline earth salts include the lithium, sodium, potassium, calcium, and magnesium salts and the like. Representative organic amines useful for the formation of base addition salts include ethylamine, diethylamine, ethylenediamine, tromethamin, ethanolamine, diethanolamine, piperazine and the like. (See, for example, Berge et al, supra).
Wetting agents, emulsifiers and lubricants, such as sodium lauryl sulfate and magnesium stearate, as well as coloring agents, release agents, coating agents, sweetening, flavoring and perfuming agents, preservatives and antioxidants can also be present in the compositions.
Pharmaceutically acceptable antioxidants may also be included. Examples of pharmaceutically acceptable antioxidants include: (1) water soluble antioxidants, such as
ascorbic acid, cysteine nyαrocnionde, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like; (2) oil-soluble antioxidants, such as ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin, propyl gallate, alpha- tocopherol, and the like; and (3) metal chelating agents, such as citric acid, ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid, phosphoric acid, and the like.
In certain embodiments, the disintegration time of a composition (e.g., XR or DXR) may be formulated to effect a substantially zero-order release, over a period of 2, 4, 6, 8, 12, or 24 hours, for instance.
In certain embodiments, multiparticulate capsules are preferred because they provide an increased surface area as opposed to a tablet or matrix, and thus allow for better release profiles and bioavailability.
However, the pellets described above can be incorporated into a tablet, in particular by incorporation into a tablet matrix, which rapidly disperses the particles after ingestion. In order to incorporate these particles into such a tablet, a filler/binder must be used in the tableting process that will not allow the destruction of the pellets during the tableting process. Materials that are suitable for this purpose include, but are not limited to, microcrystalline cellulose (AVICEL®), soy polysaccharide (EMCOSOY®), pre-gelatinized starches (STARCH® 1500, NATIONAL® 1551), and polyethylene glycols (CARBOWAX®). These materials should be present in the range of about 5%-75% (w/w), and preferably between about 25%-50% (w/w).
In addition, disintegrants may be added to the tablets in order to disperse the beads once the tablet is ingested. Suitable disintegrants include, but are not limited to: crosslmked sodium carboxymethyl cellulose (AC-DI-SOL®), sodium starch glycolate (EXPLOTAB®, PRIMOJEL®), and crosslinked polyvinylpolypyrrolidone (Plasone-XL). These materials should be present in the range of about 3%-15% (w/w), with a preferred range of about 5%- 10% (w/w).
Lubricants may also be added to assure proper tableting, and these can include, but are not limited to: magnesium stearate, calcium stearate, stearic acid, polyethylene glycol, leucine, glyceryl behenate, and hydrogenated vegetable oil. These lubricants should be present in amounts from about 0.1%- 10% (w/w), with a preferred range of about 0.3%-3.0% (w/w).
Tablets are formed, for example, as follows. The pellets are introduced into a blender along with AVICEL®, disintegrants and lubricant, mixed for a set number of minutes to provide a homogeneous blend which is then put in the hopper of a tablet press with which
tablets are compressed. The compression force used is adequate to form a tablet; however, it is not sufficient to fracture the beadlets or coatings.
The subject once-a-day dosage forms of pramipexole typically contain the same total amount of therapeutically effective amount of pramipexole that is administered to a patient during a conventional pramipexole treatment. For example, for the treatment of PD, one conventional thrice-a-day regimen comprises 3 times a day of 0.125 mg pramipexole each (other dosages are available as 0.5, 0.75, 1.0, 1.5, 3.0, and 4.5 mg, etc.). Thus for this embodiment, the total amount of pramipexole for PD treatment is about 0.375 mg (3 x 0.125 mg) in the subject once-a-day dosage forms. However, in certain embodiments, the total amount of pramipexole used may be adjusted upward or downward by, for example, 5-30%, or 10-20%, etc., depending on specific patient's age, weight, gender, race, health condition, and other considerations.
In certain embodiments, the subject pharmaceutical composition is formulated for variable dosing, such as customized dosing for individual patients.
In addition, more than one type of drugs can be present in a tablet or a drug eluting device of the invention, e.g., for combination therapy with other pharmaceutical compositions effective for treating PD or other movement disorders (see below). The drugs can be evenly distributed throughout a medicament or can be heterogeneously distributed in a medicament, such that one drug is fully or partially released before a second drug. See different embodiments of the drug devices and/or layering in other parts of this specification.
Dosage forms of the invention typically weigh at least about 50 mg. Dosage forms (such as the various shell designs of the invention) can also weigh at least 100 mg, at least 150 mg, at least 250 mg, at least 500 mg, or at least 1000 mg, etc.
Dosage forms of the invention typically measure at least 2 mm in one direction. For example, dosage forms can measure at least 5 mm, at least 10 mm, at least 15 mm or at least 20 mm in one direction. Typically, the diameter of the dosage forms is 2 to 40 mm, preferably 10 to 30 mm such as 20 to 26 mm. Mini-tablets have a diameter of 2 mm to about 5 mm. Such dosage forms can measure at least 2 mm, at least 5 mm, at least 10 mm, at least 15 mm or least 20 mm in a second direction and, optionally, a third direction. Preferably, the dosage form is of a size that facilitates swallowing by a subject.
The volume of a typical dosage form of the invention is at least 0.008 mL, at least 0.01 mL, at least 0.05 mL, at least 0.1 mL, at least 0.125 mL, at least 0.2 mL, at least 0.3 mL, at least 0.4 mL or at least 0.5 mL.
Dosage forms of the invention may be a tablet that can be of any suitable size and shape, for example, round, oval polygonal or pillow-shaped, and optionally bear nonfunctional surface markings. Especially in the case of coated tablets, they are preferably designed to be swallowed whole and are therefore typically not provided with a breaking score. Tablets of the invention can be packaged in a container, e.g., accompanied by a package insert providing pertinent information such as, for example, dosage and administration information, contraindications, precautions, drug interactions and adverse reactions.
To produce a dosage form that can release at least two or three drugs at two or three different rates, and with preprogrammed delays, special dosage forms are used. For example, in the embodiments of the invention wherein different dosage forms of pramipexole {e.g., IR, DR, XR, DXR, etc.) axe designed to be released concomitantly, the drugs may be formulated as bilayer (or other multilayer) tablets or shells (e.g., stacked layer of cakes, each may represent an independent formulation). Alternatively, the drugs may be formulated as a tablet within a tablet or bead (not limited to two nested layers). Optionally, a bioadhesive layer may be coated over part or all of a gel capsule (or other forms of delivery device) to enhance the stay of the device within a certain area of the GI tract, such as the intestine.
B. Exemplary Delivery Devices / Forms
In certain embodiments, the drugs may be formulated into a core tablet held in a recessed fashion within an annular ring of drug material. Such a dosage form is described in U.S. patent application Ser. No. 10/419,536 entitled "Dosage Form with a Core Tablet of Active Ingredient Sheathed in a Compressed Angular Body of Powder or Granular Material, and Process and Tooling for Producing It," filed on Apr. 21, 2003 and Ser. No. 10/379,338 entitled "Controlled Release Dosage Forms," filed on Mar. 3, 2003 and are incorporated herein by reference. This design may be used for many embodiments of the subject dosage forms. For example, the outer annular ring is formulated for either immediate release (IR) or extended release (XR) delivery for a desired amount of time. The inner core(s) of the dosage form may be released after a delay which may be formulated for the desired release profile. The enteric coating covers the tablet to delay drug delivery until the tablet enters a non-acidic environment.
Other embodiments of the invention use the dosage form described in U.S. patent application Ser. No. 10/191,298 entitled "Drug Delivery System for Zero-order, Zero-Order Biphasic, Ascending or Descending Drug Delivery," filed on JuI. 10, 2002, incorporated
herein by reference. The dopamine transport inhibitor may be formulated in the tablet mantle and released at the desired rate after a delay. The pramipexole composition may be formulated in the expanding plug and released at the desired rate upon entry into the intestine.
Another embodiment of this invention may be achieved by formulating each of the drugs as pellets / beads, each with its own release profile and delay where applicable, and delivering the mixture of the pellets (e.g., IR, DR, DXR, etc.) in a shell using methods commonly known in the art. Furthermore, the proportions of the different types of pellets / beads may be altered or customized by a skilled artisan {e.g., qualified physician or pharmacologist), based on an individual patient's characteristics, such as weight, age, gender, ethnicity, and/or specific genetic backgrounds. Such customization may be effected with the aid of, or automatically executed by a computer program based on relevant parameters such as those described above.
In certain embodiments, the drug-releasing beads are characterized by a dissolution profile wherein 0 to 20% (e.g., 1-20%) of the beads undergo dissolution and release the drug in 0 to 2 hours, 20 to 40% undergo dissolution and release the drug in 2 to 4 hours, 40 to 60% exhibit dissolution and release in 4 to 6 hours, 60 to 80% in 6 to 8 hours, and 80 to 100% in 8 to 10 hours or longer. The drug-releasing beads can include a central composition or core comprising a drug and pharmaceutically acceptable composition forming ingredients including a lubricant, antioxidant, and buffer. The beads comprise increasing doses of drug, for example, 0.1 mg, 0.2 mg, 0.5 mg, and so forth to a high dose. For sustained release embodiments, the beads may be coated with a release rate-controlling polymer that can be selected utilizing the dissolution profile disclosed above. The manufacture of the beads can be adapted from, for example, Liu et ah, Inter. J. ofPharm. 112: 105-116, 1994; Liu et at, Inter. J. ofPharm. 112: 117-124, 1994; Pharm. ScL, by Remington, 14th Ed. pp. 1626-1628 (1970); Fincher et al., J. Pharm. Set 57: 1825-1835, 1968; and U.S. Pat. No. 4,083,949.
Certain embodiments of the subject beads or pellets are described in more detail below.
Some specific tablets or gel capsules designed are described below for illustration purpose. These designs are by no means limiting, and a skilled artisan can readily envision other equivalent designs based on the general teachings described herein.
In one example, as shown in the schematic drawing of Figure IA (not necessarily to scale), the tablet is a longitudinally compressed tablet. The core of the tablet is a slow- eroding active core 1 with pramipexole and other pharmaceutical excipients. The side of the core is coated with a bioadhesive polymer layer 4, while the two ends of the core are coated
with an insoluble plug 2 and an enteric polymer plug 3, respectively. The enteric polymer plug 3 will only dissolve in a relatively higher pH environment (e.g., about pH 4.5 and higher), such as those found in intestine or colon. Once the enteric polymer layer 3 is dissolved, the active core 1 starts to release its contents. The bioadhesive layer 4 is selectively adhesive to intestine or colon, such that the content of the active core 1 may be released over a prolonged period of time.
Figure IB shows a slight variation of the device depicted in Figure IA, in that the insoluble plug 2 in replaced by a second enteric polymer plug 2. According to this embodiment, both enteric polymer plugs 2 will dissolve in relatively higher pH environments, either substantially simultaneously, or at different time, such that the rate of release from the slow-eroding active core 1 may be regulated.
Figure 1C shows yet another alternative embodiment, in that the slow eroding active core 1 in Figure IA becomes two consecutive layers - an immediate release active core layer
2, followed by a slow-eroding active core layer 1. As a result, once the enteric polymer plug 4 is dissolved, the immediate release layer 2 provides a rapid drug release, which is maintained by more sustained drug release from the slow-eroding active core 1.
Figure ID shows a schematic (not necessarily to scale) drawing of another embodiment of the delivery device containing multiparticulate beads / pellets. The multiparticulate dosage form combines two types of pellets - the immediate release pellets 1 and the controlled release active pellets (DR or XR) 2 — both embedded in an appropriate matrix of excipients {e.g., HPMC, MCC, lactose). The matrix is inside a hard gelatin capsule
3, which in turn is coated by enteric material 4. This type of dosage form will provide multiple pulses of drug release, with the effect being a more or less sustained blood level of drug within the acceptable range. The release is delayed by the enteric coating 4 in order to by-pass the upper GI tract.
With this combination, the IR pellets are designed to provide an effective blood level soon after the start of the drug release, which is subsequently maintained by the DR and/or XR combinations. The DR portion provides an immediate release after a delay. IfXR pellets are also used, the XR portion provides an extended release profile that maintains the effective blood level of pramipexole throughout the remaining course of the day. The total dose of pramipexole in this composition is usually no greater than 0.375 mg. The IR pellets may comprise 1/3 (or 0.125 mg) of the total, while the remaining 2/3 is provided by the DR and/or XR.
A similar effect may be achieved by a device as depicted in Figure IE, where an enteric coating 3 covers an inner core with two (asymmetric) portions - the immediate release active layer (IR) 1 and the controlled release active layer (DR or XR) 2. The ratio of IR to DR / XR may be anywhere between 1 : 10 to 2: 1. In a preferred embodiment, the ratio may be 1 :2.
In Figure IF, the complex core of Figure IE is replaced with a uniform slow-eroding or non-eroding active matrix core 1, from which pramipexole is released after the enteric coating 2 is dissolved.
Figure IG presents yet another embodiment, wherein an enteric coating 4 delays the release its drug contents. Upon degradation of the enteric coating 4, the immediate release active core 1 is quickly dissolved, effectively splitting the core into two halves of controlled- release active core 2, each coated by a layer of rate-controlling coating 3 at surfaces not in contact with the immediate release active core 1. Thus the release of the drug content from the controlled-release active core 2 is only through the rate-controlling coating 3 (comparatively slow) before the immediate release active core 1 is dissolved. The rate gradually increases as the immediate release active core 1 dissolves, exposing more surface area of the two controlled-release active cores 2 not coated by the rate-controlling coating 3. Release profile may be controlled by, for example, the amount of the immediate release active core 1, the thickness and material of the rate-controlling coating 3, the geometric shape / surface area of the controlled-release active core 2 directly in contact with the immediate release active core 1, etc.
In Figure IH, the active core 1 is substantially covered by a layer of semi-permeable coating 3, which contains one or more small openings / orifices 2. The outermost portion of the whole device is further coated with a layer of delayed-release coating / enteric coating 4. Once coating 4 is dissolved, the orifice(s) is exposed, allowing direct release of the active core 1 through the orifice(s) 2. Different release profiles may be obtained, for example, by controlling the number and/or size of the orifice(s) 2, the thickness and/or material of the semi-permeable coating 3.
An alternative embodiment is shown in Figure II. Although the enteric coating outside the semi-permeable coating 5 is not shown, the enteric coating may be added in certain embodiments. For the depicted embodiment in Figure II, the core comprises three layers, with the middle layer being the active core 1. Underneath the active core 1 is a push layer 2 that will swell after the tablet comes into contact with body fluid and when the fluid enter the tablet through the semi-permeable coating 5. Above the active core is a delayed- release layer 3 having access to one or more orifice(s) for drug release. The swelling push
layer 2 will cause first the delayed-release layer 3, and then the active core 1 to be released through the orifice 4. The delayed release layer may also have the IR component or an immediate release component in between the delayed release and slow release core layer.
In yet another embodiment (Figure IJ), the immediate release beads / pellets 1 and the controlled-release (XR and/or DR and/or DXR) beads / pellets 2 are embedded within the enteric polymer material 3 as multiparticulate beads / pellets. The enteric polymer material 3 may additionally comprise compression enhancers or fillers, or any other materials described herein that are customarily used in tablet production.
Alternatively, the IR portion of the dosage form is formulated as a matrix for embedding one or more other portions of the same dosage form (DR, XR, DXR, etc.). The IR may be coated by enteric layer to avoid release in upper GI tract. Each controlled release portion (DR, XR, DXR, etc.) is optionally coated by a bioadhesive coat and/or a delayed release coat. Each CR portion may be formed as microparticles {e.g. , beads) suspended in the first portion {e.g., IR portion) matrix. The disintegration of the matrix leads to the release of the embedded microparticles, which may re-adhere to the gut or other tissues (if coated by bioadhesive layer), and provided for sustained release.
Figure 12 features yet another configuration of the delivery device, in which a drug portion 1201 is sandwiched between two adhesive layers 1202 {e.g., a layered cross section) or inside one continuous adhesive layer 1202 {e.g., configured as a filled tube). SPHEROMER™ I Ip(FASA)] and SPHEROMER™ III are exemplary such bioadhesive layers. The portion / layer can (but need not) be substantially flat. In certain embodiments, there are two substantially flat adhesive layers 1202 sandwiching one drug layer 1201. Components of the drug can be either released from surfaces not in contact with the adhesive parts 1202, and/or through the adhesive materials if such materials are at least partially permeable.
In certain embodiments, an immediate release portion IR 1203 may be present, and is coated over all or a part of the adhesive layer 1202. In certain embodiments, the rapid dissolution of the IR portion exposes a drug surface not in contact with the adhesive material. In another embodiment, the dissolution of the IR portion does not substantially change the release rate of the drug portion. This multilayer configuration is finally applied with an enteric or delayed release coating.
Figure 13 features yet another configuration of the subject delivery device, which may be used in general to deliver any kind of drugs (or prodrugs/metabolic precursors thereof, etc). It should be understood that the subject delivery devices (such as the one
described in Figure 13), dosage form, and methods of making and using are not limited to these specific exemplary drug compositions described herein.
Thus according to this aspect of the invention, any drug to be delivered (e.g., pramipexole), optionally including a bioadhesive polymer composition, and/or pharmaceutically acceptable excipients, may be formulated using the subject granulation- extrusion-spheronization process into multiparticulate pellets, which in turn may be dispersed in certain matrix materials, or simply encapsulated in capsules, e.g., according to the various embodiments disclosed above.
Specifically, appropriate amounts of the different ingredients are first weighed and mixed.
Suitable excipients for use in the subject granulation-extrusion-spheronization process include: Starcap-1500, starch-1500, and glycerine monostearate. In certain embodiments, the mixture is substantially free of microcrystalline cellulose.
In an exemplary embodiment, about 30-90%, about 40-85%, or about 50-80% (v/v) of the mixture (and the pellets formed therefrom) is effective ingredient (e.g., drug composition), rather than excipients or polymers. Such loadings can be achieved using any drug or combination of drugs that are suitably cohesive, plastic, and engage in hydrogen bonding. Pramipexole is an example of such drugs, though others will be known to or can be easily identified by those of skill in the art.
These different ingredients can then be blended together in any suitable device, such as a planetary type mixer (e.g., Hobart Mixer with a 5-qt mixing bowl, operating at the speed setting #1, for about 5-15 min.). Optionally, the blending process is done in small volume to reduce any possible loss of the ingredients due to their non-specific adherence to the blending device. The blending step is typically done to ensure the formation of a uniform dry mix of the ingredients, typically over a period of, e.g., 5-15 min.
The dry mix is then granulated, e.g., under low shear with a granulation fluid, so as to form a wet granulation. Granulation fluids may be purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro-alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated "solvent or in a ketone, etc. or any suitable mixture thereof
In certain embodiments, the granulation process is conducted in a small volume, such as in a 500-mL cylindrical vessel.
In certain embodiments, the granulation process is conducted with manual mixing, or conducted mechanically, e.g., in a planetary type mixer (such as a Hobart Mixer with a 5-qt mixing bowl). If the Hobart Mixer is used, it can be operated at its speed setting #1, depending on the batch size. Other types of mechanical mixers may also be used, with their respective appropriate settings, to achieve substantially the same result.
Once the wet granulation is formed, it is extruded through the screen of a screen-type extruder. In certain embodiments, a Caleva Model 20 (or Model 25) Extruder may be used, operating at 10-20 rpm, and forming breakable wet strands ("the extrudate"). The screen aperture may be set at 0.8, 1 , or 1.5 mm. Other types of extruders may be used to achieve substantially the same result.
The extrudate is then spheronized in a spheronizer. For example, a Caleva Model 250 spheronizer equipped with a 2.5-mm spheronization plate may be used, which may be operated at a speed of about 1000-2000 rpm, typically for 5-10 min., in order to form spheronized pellets. Other types of spheronizer may be used to achieve substantially the same result.
The spheronized pellets are then dried. The drying may be conducted in a fluidized bed drier, such as a Vector MFL.01 Micro Batch Fluid Bed System. If the Vector drier is used, it may be operated at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of about 50 0C. Alternatively, the pellets may be dried in an ACT (Applied Chemical Technology) fluidized bed drier, operating at an inlet air flow rate of 140- 150 fpm (foot per minute) and an inlet air temperature of 1040F. Other types of driers may also be used to achieve substantially the same result. Depending on the specific type of drugs / compositions, the drying temperature for a drier similar to the Vector drier may be between 35-700C, or 40-65 0C, or 45-6O0C, or 45-55 0C, etc. The drying temperature for a drier similar to the ACT drier may be between 70-1400F, or 80-130 0F, or 90-1200F, or 100-110 0F, etc.
In yet another embodiment, the spheronized pellets may be dried in an oven, such as a Precision gravity oven, operating at about 500C, for 4-48 hrs, or 8-24 hrs. Depending on the specific type of drugs / compositions, the oven drying temperature for a drier similar to the Precision gravity oven may be between 35-700C, or 40-65 0C, or 45-6O0C, or 45-55 0C, etc.
The dried pellets are then screened and/or classified. This can be done by using a stack of sieves, such as stainless steel sieves U.S. standard mesh sizes 8, 10, 12, 14, 16, 18, 20, 25, 30, 40, 45, or 60, etc., and using a mechanical sieve shaker (e.g., W.S. Tyler Sieve Shaker Ro-Tap Rx-29, operated for 5 min.). Particle size and distribution of pellet formulations can then be analyzed, and the classified pellets ranging from 0.25 mm (mesh #
60) to 2 mm (mesh # 10) may be selected for use or future formulation, such as additional film coating or other experimentation.
In certain embodiments, the selected pellets may be film-coated, e.g., with a delayed- release coating (such as an entaric coating), a controlled-release (CR) coating, a bioadhesive polymeric composition, and/or a dispersion-promoting coating, etc.
For example, the pellet core may be optionally surrounded by a CR coating, such as polymeric substance based on acrylates and/or methacrylates, e.g., a EUDRAGIT™ polymer (sold by Rohm America, Inc.). Specific EUDRAGIT™ polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent. For example, EUDRAGIT™ RL, EUDRAGIT™ NE, and EUDRAGIT™ RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films. EUDRAGIT™ RL is freely permeable and EUDRAGIT™ RS is slightly permeable, independent of pH. In contrast, the permeability of EUDRAGIT™ L is pH dependent. EUDRAGIT™ L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester. It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable. If desired, two or more types of polymeric substances may be mixed for use as the CR coating. Other polymers suitable for CR coatings, such as ethyl cellulose and cellulose acetate, can also be used in the CR coating. In certain embodiments, the CR coating may comprise one or more suitable polymers, such as a combination of two or more of the polymers discussed above.
Optionally, the pellets may also be coated by a bioadhesive polymeric composition. The adhesive material may facilitate the adhesion of the pellets to a desired surface, such as a preferred GI tract surface. For example, the pellets / beads may be coated by a top-layer of a bioadhesive polymer such as SPHEROMER™ I [p(FASA)], SPHEROMER™ II, SPHEROMER™ III, SPHEROMER™ IV, or mixtures thereof.
In certain embodiments, the functions of a CR coating and bioadhesive coating can be combined in a single layer by using a mixture of polymers including a bioadhesive polymer and a polymer suitable for controlled release, i.e., a single layer may be both the CR layer and the bioadhesive layer of a particle.
Optionally, the pellets can also be film-coated with an additional layer of a so-called "non-functional polymer," such as OPADRY™ II, EUDRAGIT™ E, AcrylEZE™, hydroxypropylmethyl cellulose, hydroxypropyl cellulose, polyvinyl alcohol,
polyvinylacetate, polyanhydride, etc. This layer may serve as a dispersion-promoting coating that inhibits clumping and aggregation of the particles during dispersion. In embodiments wherein the pellets are further compressed with excipients to form tablets, this layer is preferably sufficiently strong or resilient to remain substantially intact during the compression process. This layer may also be protected by including a cushioning material among the excipients of the tablet matrix.
The coating material (such as bioadhesive polymers and/or functional / nonfunctonal polymers) maybe dissolved in an appropriate solvent, such as methylene chloride {e.g., for SPHEROMER™ I), methanol (e.g., for SPHEROMER™ III), a binary mixture of methanol and methylene chloride (e.g., for SPHEROMER™ I and SPHEROMER™ III), methanol or a binary mixture of ethanol and water (3:1 v/v) (e.g., for SPHEROMER™ IV), or methanol, ethanol, or isopropanol, or their binary mixture with acetone (e.g., functional or nonfunctional polymer).
The film coating may be performed in a fluidized bed coater, such as a Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert, operating at an inlet air flow rate of 100-300 lpm (liters per minute), and an inlet air temperature of about 25-45 0C, or about 30-40 0C, depending on the specific drugs and coatings (e.g., 25-300C for SPHEROMER™ I-coated pramipexole; about 35 0C for SPHEROMER™ Ill-coated pramipexole, etc.). If the Vector System is used, the pellets may be pre- warmed at 350C for 2-5 min., and after film-coating, post-dried at about 30 0C for about 15-30 min.
Alternatively, pellets may be coated in a fluid bed processor, such as a Fluid Air Model 5 fluid bed processor equipped with a Wurster insert, operating at an inlet air flow rate of about 70 cfm (cubic foot per minute) and an inlet air temperature of about 35 0C. For this type of fluid bed processor, the pellets may be pre-warmed at 40 0C for 5-7 min., and after film-coating, post-dried at about 35 0C for about 30 min.
Other types of coaters may also be used to achieve substantially the same result.
Different lots of the same pellets produced using the subject method may optionally be mixed, e.g., by using a blender (such as a GlobePharma Maxiblend Blender equipped with an 8-qt stainless steel V-shell).
In certain embodiments, different types of pellets may be mixed. For example, some pellets may have no coating other than a core comprising the effective ingredients. Other pellets, such as those identically made, may have additionally been coated by one or more types of coatings, e.g., bioadhesive coating, delayed-release coating, controlled-release coating, and/or dispersion-promoting coating, etc.
In certain embodiments, pellets produced using the methods of the invention may be encapsulated in capsules, such as hard gelatin capsules or pullulan capsules (NPcaps™), each with a predetermined amount of effective ingredients.
In certain embodiments, pellets produced using the methods of the invention may be dispersed in a matrix material to assist the delivery of the effective ingredients of the pellets. There are at least two preferred configurations according to this embodiment of the invention.
Figure 13 shows a schematic drawing (not to scale) of one such configuration. In Figure 19, the active components 1301 (such as the pellets produced using the subject method, which are not necessarily round in shape) are embedded / dispersed within an inactive material or carrier matrix 1302. The carrier matrix 1302 can rapidly disintegrate, e.g., dissolve substantially completely (superdisintegrant) within about 15 minutes, 10 minutes, 8 minutes, 7 minutes, 6 minutes, 5 minutes, 3 minutes, 2 minutes, or about 1 minute or less.
The inactive material 1302 may additionally comprise one or more cushioning material(s) dispersed throughout, e.g., sufficient to protect the active components 1301 when preparing the delivery device, by substantially absorbing the impact of compacting, and/or reducing friction on the surface of the particles 1301 (to prevent damaging the substructure of the particles, see below).
The particles 1301 may be in any suitable size and shape (rods, beads, or other regular or irregular shapes). In certain embodiments, the particles are beads with a diameter of less than about 2 mm, about 1.5 mm, about 1 mm, about 0.8 mm, about 0.5 mm, about 0.3 mm, or about 0.1 mm. In certain embodiments, for pellets with pramipexole as effective ingredient, the pellet size is about 0.8 - 1 mm. Particles are formulated to these sizes in order to enable high drug loading when needed.
As described above, particles 1301 may have substructures, such as various coating layers surrounding a drug / prodrug core. Although the following describes the substructures using a bead with pramipexole as effective ingredient, it is an illustrative example only, and the description also applies to other shapes of particles with other effective ingredients.
The core by itself may be an immediate release portion, or may have release- controlling components (e.g., CR portion), and preferably, the core is made by extrusion, such as the granulation-extrusion-spheronization process. The core is optionally surrounded by a CR coating, such as polymeric substance based on acrylates and/or methacrylates, e.g., a EUDRAGIT™ polymer (sold by Rohm America, Inc.). Specific EUDRAGIT™ polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent. For example, EUDRAGIT™ RL, EUDRAGIT™ NE, and
EUDRAGIT™ RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films. EUDRAGIT™ RL is freely permeable and EUDRAGIT™ RS is slightly permeable, independent of pH. In contrast, the permeability of EUDRAGIT™ L is pH dependent. EUDRAGIT™ L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester. It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable. If desired, two or more types of polymeric substances may be mixed for use as the CR coating. Other polymers suitable for CR coatings, such as ethyl cellulose and cellulose acetate, can be used in the CR coating. The CR coating may comprise one or more suitable polymers, such as a combination of two or more of the polymers discussed above.
Optionally, the CR coating is itself coated by a layer of adhesive material that facilitates the adhesion of the particles / beads to a desired surface, such as a preferred GI tract surface. Various suitable adhesive materials are described herein above. For example, the pellets / beads may be coated by a top-layer of a bioadhesive polymer such as SPHEROMER™ I [p(FASA)], SPHEROMER™ III, SPHEROMER™ IV, or mixtures thereof. In certain embodiments, the functions of a CR coating and bioadhesive coating can be combined in a single layer by using a mixture of polymers including a bioadhesive polymer and a polymer suitable for controlled release, i.e., a single layer may be both the CR layer and the bioadhesive layer of a particle.
Optionally, pellets can be further film-coated with an additional layer of a so-called "non-functional polymer" such as OPADRY™ II, EUDRAGIT™ E, Acryloeze™, hydroxypropylmethyl cellulose, hydroxypropyl cellulose, polyvinyl alcohol, polyvinylacetate, polyanhydride, etc. This layer may serve as a dispersion-promoting coating that inhibits clumping and aggregation of the particles during dispersion. In embodiments wherein the pellets are further compressed with excipients to form tablets, this layer is preferably sufficiently strong or resilient to remain substantially intact during the compression process. This layer may also be protected by including a cushioning material among the excipients of the tablet matrix.
Optionally, an IR portion is included in the particle, such as over the dispersion- promoting coating, or between the dispersion-promoting coating and the adhesive layer, etc.
In an alternative embodiment, particles 1301 are not embedded within the inactive material 1302, but are instead disposed loose in a capsule that dissolves and releases the particles in the GI tract.
Figure 14 features yet another embodiment of the delivery device, in which particles described herein above (e.g., with respect to Figure 19) are embedded within a slow eroding material 1401 (e.g., that gradually erodes over 30 minutes, 45 minutes, 1 hr, 2 hrs, 4 hrs, 6 hrs, or longer). At least a portion of the eroding material 1401 is covered by an IR portion 1402, which disintegrates relatively rapidly to expose a surface of eroding material 1401. A portion of the slow eroding material 1401 is also optionally covered by a passive polymer support layer and/or an adhesive material 1403 as described herein above. In certain embodiments, the IR portion 1402 may be disposed on the adhesive layer 1403 instead of the eroding material 1401 as depicted.
According to a related aspect of the invention, any drug to be delivered (e.g., pramipexole), optionally including a bioadhesive polymer composition, and/or pharmaceutically acceptable excipients, may also be formulated as a multilayer tablet.
Specifically, different ingredients (such as those described above) are weighed and mixed. These ingredients, possibly with the exception of any lubricants, can then be blended together in any suitable device, such as an end-over-end ATR rotator (e.g., model RKVS), or a planetary type mixer (e.g., Hobart Mixer). Optionally, the blending process is done in small volume to reduce any possible loss of the ingredients due to their non-specific adherence to the blending device. The blending step is typically done to ensure the formation of a uniform dry mix of the ingredients, typically over a period of, e.g., 5-15 min.
The dry mix is then granulated, e.g., under low shear with a granulation fluid, so as to form a wet granulation. Granulation fluids may be purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro-alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated solvent or in a ketone, etc.
In certain embodiments, the granulation process is conducted in a small volume, such as in a 500-mL cylindrical vessel.
In certain embodiments, the granulation process is conducted with manual mixing, or conducted mechanically, e.g., in a planetary type mixer (such as a Hobart Mixer with a 5-qt mixing bowl). If the Hobart Mixer is used, it can be operated at its speed setting #1,
depending on the batch size. Other types of mechanical mixers may also be used, with their respective appropriate settings, to achieve substantially the same result.
Once the wet granulation is formed, it is dried. In certain embodiments, the wet granulation is dried in an oven (e.g., a Precision gravity oven, operating at about 50 0C, for 8- 24 hrs; or similar appropriate conditions for other types of ovens). Alternatively, the granulation may be dried in a fluidized bed drier, such as a Vector MFL.01 Micro Batch Fluid Bed System, operating at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of about 500C. The drying temperature is generally around 50 0C. However, depending on different types of drugs / compositions, the temperature may be 35- 70 0C, or 40-65 0C, or 45-600C, or 45-55 0C, etc.
The dried granulation is then grinded, e.g., by using a pestle in a mortar, optionally followed by sieving the ground material, e.g., through an appropriate-sized screen (such as a U.S. Std. mesh # 60 screen), depending on the desired size of the granules.
At this point, the sieved granulation may be blended with a lubricant. In certain embodiments, the blending is conducted using an end-over-end ATR rotator (e.g., model RKVS). In certain embodiments, the blending is conducted using a planetary type mixer (e.g., Hobart Mixer, operating at the speed setting #1, for 5-15 min.). As a result, a uniformly lubricated dry mix is formed, which is then ready for compression.
Optionally, before compression, the lubricated dry mix may be passed through a sieve or screen, e.g., a U.S. Std. mesh # 60 screen.
Different components of the pharmaceutical composition (e.g., the effective ingredients, any bioadhesive polymers, or other coatings, etc.) may be prepared as a mixture or separately using the subject methods. Once the dry mixes are formed, they can be compressed into single layer or multilayer tablets. For example, the lubricated dry mix may be pressed into tablets, such as by using a single-station manual tablet press (e.g., GlobePharma Manual Tablet Compaction Machine MTCM-I, equipped with adequate die and punch set). If the GlobePharma machine is used, tablets may be prepared, e.g., at a pressure ranging from 250 to 4000 pounds per square inch (psi), and a compression time of, e.g., 1 to 4 seconds. Other machines may also be used to achieve substantially the same result.
Alternatively, in certain embodiments, tablets may be produced with wet granulation of active ingredients followed by direct compression.
In certain embodiments, multilayer tablets may be produced, with each layer comprising a different ingredient. In these embodiments, a single-station manual tablet press
(e.g., GlobePharma Manual Tablet Compaction Machine MTCM-I, equipped with adequate die and punch set) may be used in several steps to produce the multilayer tablets. For example, for a bilayer tablet, the compression process may include:
(1) adding the first layer blend into the die cavity, optionally followed by manually tapping it using a stainless steel spatula;
(2) adding the second layer blend into the die cavity;
(3) pre-compressing the two layers together, e.g., at a pressure ranging from 250 to 500 pounds per square inch (psi) and a compression time of, e.g., 1 to 5 seconds.
(4) compressing the pre-compacted layers together, e.g., at a pressure ranging from 1000 to 4000 pounds per square inch (psi) and a compression time of, e.g., 1 to 4 seconds.
The process can be repeated or modified if more than two layers of ingredients are to be used.
In certain embodiments, the tablet can be made with a pre-compressed insert with effective ingredients. Such pre-compressed inserts may be produced with direct compression. The same press machine may be used for this process. For example, if using the GlobePharma Manual Tablet Compaction Machine MTCM-I machine, tablet inserts may be prepared, e.g., at a pressure ranging from 500 to 1000 pounds per square inch (psi), and a compression time of, e.g., 1 to 2 seconds. Other machines may also be used to achieve substantially the same result. The pre-compressed insert may be used as one of the layers {e.g., the second layer) in the tablet, or embedded in the middle of another layer {e.g., the second layer).
Optionally, the tablets may be coated with one or more coating compositions, such as in the form of successive layers. The coating compositions may include bioadhesive layers, delayed release layers, controlled-release layers, and/or other functional / non-functional polymers etc. (supra). For example, tablets may be film-coated for this purpose, using a pan coater (e.g., O'Hara Labcoat, operating at an inlet air flow rate of about 60 cfrn (cubic foot per minute) and an inlet air temperature of about 35 0C). The tablets may be pre-warmed at 35 0C for 5-10 min., and after film coating, may be post-dried at about 30 0C for about 15-30 min. Other coaters may also be used to achieve substantially the same result.
Figure 15 features yet another embodiment of the delivery device, in which particles 1500 described herein above (e.g., with respect to Figure 13) are disposed on the surface of a bioadhesive film 1501. The film may optionally be dried or cured, e.g., without disrupting the particle adhesion. The film may then be folded and placed in a capsule 1502 for
administration to a patient. If needed the capsule containing the active containing bioadhesive film is coated with delayed release coating to allow the film to adhere to the proximal part of the GI tract. If needed, the film may first be folded or cut to a suitable shape or size. Once administered to a patient, the capsule releases the film, which then rehydrates (if necessary) and adheres to a mucosal surface, allowing the particles spreaded and adhered thereto to release the active components.
Additional details of the granulation-extrusion-spheronization process are described (with examples) in the co-pending U.S. application entitled "IMPROVED DOSAGE FORMS FOR MOVEMENT DISORDER TREATMENT," filed on June 23, 2006 (the teachings of the entire referenced application are incorporated herein by reference).
These various embodiments are only a sample of numerous possible configurations to deliver the subject dosage forms. Other variations may be readily envisioned based on the principals and teachings of the instant specification. For example, various other drug-eluting devices are described in U.S. Patent Nos. 4,290,426, 5,256,440, 5,378,475, 5,773,019 and 6,797,283, the contents of which are incorporated herein by reference.
In these and other embodiments of the invention, the various bioadhesive coatings that can be used are described in detail in the section below.
Many of the different embodiments described above may be implemented by using rechargeable or biodegradable devices. Various slow release polymeric devices have been developed and tested in vivo in recent years for the controlled delivery of drugs. A variety of biocompatible polymers (including hydrogels), including both biodegradable and non- degradable polymers, can be used to form an implant for the sustained release of a subject pharmaceutical composition at a particular target site. The biodegradable polymers undergo chemical decomposition to form soluble monomers or soluble polymer units. The biodegradation of polymers usually involves chemically or enzymatically catalyzed hydrolysis. Representative biodegradable polymers comprise a member selected from biodegradable poly(amides), poly(amino acids), poly(esters), poly(lactic acid), poly(glycolic acid), poly(orthoesters), poly(anhydrides), biodegradable poly(dehydropyrans), and poly(dioxinones). The polymers are known to the art in Controlled Release of Drugs, by Rosoff, Ch. 2, pp. 53-95 (1989); and in U.S. Pat. Nos. 3,811,444; 3,962,414; 4,066,747; 4,070,347; 4,079,038; and 4,093,709.
In certain embodiments, representative dosage forms include hydrogel matrix containing a plurality of tiny pills or other particles. The hydrogel matrix comprises a hydrophilic polymer, such as selected from a polysaccharide, agar, agarose, natural gum,
alkali alginate including sodium alginate, carrageenan, fucoidan, furcellaran, laminaran, hypnea, gum arabic, gum ghatti, gum karaya, gum tragacanth, locust bean gum, pectin, aniylopectin, gelatin and a hydrophilic colloid. The hydrogel matrix comprises a plurality of tiny pills or particles (such as 4 to 50), each tiny pill or particle may comprise a different portion of the subject pramipexole compositions (e.g., IR, XR, DR, DXR, etc.). Representative of wall-forming materials include a triglyceryl ester selected from glyceryl tristearate, glyceryl monostearate, glyceryl dipalmitate, glyceryl laureate, glyceryl didecenoate and glyceryl tridecenoate. Other wall forming materials comprise polyvinyl acetate phthalate, methylcellulose phthalate, and microporous vinyl olefins. Procedures for manufacturing tiny pills are disclosed in U.S. Pat. Nos. 4,434,153; 4,721,613; 4,853,229; 2,996,431; 3,139,383 and 4,752,470, which are incorporated by reference herein.
In still other embodiments, the invention employs a dosage form comprising a polymer that releases a drug by diffusion, flux through pores, or by rupture of a polymer matrix. The dosage form matrix can be made by procedures known to the polymer art. An example of providing a dosage form comprises blending a pharmaceutically acceptable carrier, like polyethylene glycol, with a known dose of the subject pharmaceutical composition, and adding it to a silastic medical grade elastomer with a cross-linking agent, like stannous octanoate, followed by casting in a mold. The step is repeated for each successive layer. The system is allowed to set, e.g., for 1 hour, to provide the dosage form. Representative polymers suitable for manufacturing the dosage form include olefin and vinyl polymers, condensation polymers, carbohydrate polymers, and silicon polymers as represented by poly(ethylene), poly(propylene), polyvinyl acetate), poly(methyl acrylate), poly(isobutyl methacrylate), poly(alginate), poly(amide), and poly(silicone). The polymers and manufacturing procedures are known in Polymers, by Coleman et al, Vol. 31, pp. 1187- 1230 (1990); Drug Carrier Systems, by Roerdink et ah, Vol. 9, pp. 57-109 (1989); Adv. Drug Delivery Rev., by Leong et al, Vol. 1, pp. 199-233 (1987); Handbook of Common Polymers, Compiled by Roff et at, (1971) published by CRC Press; and U.S. Pat. No. 3,992,518.
V. Combination Therapy
In a further embodiment, a composition of the invention is administered in combination therapy with one or more additional drugs or prodrugs. The term "combination therapy" herein means a treatment regimen wherein the agent provided by the composition of the invention and a second agent are administered individually or together, sequentially or simultaneously, in such a way as to provide a beneficial effect from co-action of these
therapeutic agents. Such beneficial effect can include, but is not limited to, pharmacokinetic or pharmacodynamic co-action of the therapeutic agents. Combination therapy can, for example, enable administration of a lower dose of one or both agents than would normally be administered during monotherapy, thus decreasing risk or incidence of adverse effects associated with higher doses. Alternatively, combination therapy can result in increased therapeutic effect at the normal dose of each agent in monotherapy.
Compositions of the invention can be especially suited to combination therapies, particularly where the second agent is one that is, or can be, administered once daily. There are significant advantages in patient convenience and compliance where both components of a combination therapy can be administered at the same time and with the same frequency. This is especially true in the case of geriatric patients or those suffering memory impairment.
When administered simultaneously, the two components of the combination therapy can be administered in separate dosage forms or in coformulation, i.e., in a single dosage form, When administered sequentially or in separate dosage forms, the second agent can be administered by any suitable route and in any pharmaceutically acceptable dosage form, for example by a route and/or in a dosage form other than the present composition. In a preferred embodiment, both components of the combination therapy are formulated together in a single dosage form.
The second components of the subject combination therapy, e.g., drugs useful for the treatment Parkinson's disease and other movement disorders, include L-dopa, selegiline, apomorphine and anticholinergics. L-dopa (levo-dihydroxy-phenylalanine) is a dopamine precursor which can cross the blood-brain barrier and be converted to dopamine in the brain. Unfortunately, L-dopa has a short half life in the body and it is typical after long use (z. e. , after about 4-5 years) for the' effect of L-dopa to become sporadic and unpredictable, resulting in fluctuations in motor function, dyskinesias and psychiatric side effects. Additionally, L-dopa can cause B vitamin deficiencies to arise. The gastrointestinal absorption of orally administered levodopa depends on the gastrointestinal transit rates as absorption occurs primarily in the proximal third of the intestine (duodenum/jejunum) and not in the stomach (Rivera-Calimlim et al. Europ. J. Clin. Invest. 1, 1313-1320, 1971). Therefore a delayed release dosage form containing levodopa/carbidopa or levodopa/carbidopa/entacapone with pramipexole will allow the levodopa to be released in the target proximal intestine region and release levodopa is a sustained manner similar to enteral infusion of levodopa.
Selegiline (Deprenyl, Eldepryl) has been used as an alternative to L-dopa, and acts by reducing the breakdown of dopamine in the brain. Unfortunately, selegiline becomes ineffective after about nine months of use. Apomorphine, a dopamine receptor agonist, has been used to treat Parkinson's disease, although is causes severe vomiting when used on its own, as well as skin reactions, infection, drowsiness and some psychiatric side effects.
Systemically administered anticholinergic drugs (such as benzhexol and orphenedrine) have also been used to treat Parkinson's disease and act by reducing the amount of acetylcholine produced in the brain and thereby redress the dopamine/acetylcholine imbalance present in Parkinson's disease. Unfortunately, about 70% of patients taking systemically administered anticholinergics develop serious neuropsychiatric side effects, including hallucinations, as well as dyskinetic movements, and other effects resulting from wide anticholinergic distribution, including vision effects, difficulty swallowing, dry mouth, and urine retention. See e.g. Playfer, Parkinson's Disease, Postgrad Med J 13: 257-264, 1997 and Nadeau, Parkinson's Disease, J Am GerSoc 45: 233- 240, 1997.
Newer drug refinements and developments include direct-acting dopamine agonists, slow-release L-dopa formulations, inhibitors of the dopamine degrading enzymes catechol-O- methyltransferase (COMT) and monoamine oxidase B (MAO-B), and dopamine transport blockers. These treatments enhance central dopaminergic neurotransmission during the early stages of Parkinson's disease, ameliorate symptoms associated with Parkinson's disease, and temporarily improve the quality of life. However, despite improvements in the use of L-dopa for treating Parkinson's disease, the benefits accorded by these dopaminergic therapies are temporary, and their efficacy declines with disease progression. In addition, these treatments are accompanied by severe adverse motor and mental effects, most notably dyskinesias at peak dose and "on-off ' fluctuations in drug effectiveness (Poewe and Granata, in Movement Disorders. Neurological Principles and Practice (Watts and Koller, eds) McGraw-Hill, New York, 1997; and Marsden and Parkes, Lancet 1: 345-349, 1977). No drug treatments are currently available that lessen the progressive pace of nigrostriatal degeneration, postpone the onset of illness, or that substantively slow disability (Shoulson, supra).
Other methods for the treatment of Parkinson's disease involve neurosurgical intervention, such as thalamotomy, pallidotomy, and deep brain stimulation. The thalamic outputs of the basal ganglia are an effective lesion target for the control of tremor (i.e., thalamotomy). Thalamotomy destroys part of the thalamus, a brain region involved in movement control. Unilateral stereotactic thalamotomy has proven to be effective for
controlling contralateral tremor and rigidity, but carries a risk of hemiparesis. Bilateral thalamotomy carries an increased risk of speech and swallowing disorders resulting.
Stereotactic pallidotomy, surgical ablation of part of the globus pallidus (a basal ganglia), has also be used with some success. Pallidotomy is performed by inserting a wire probe into the globus pallidus and heating the probe to destroy nearby tissue. Pallidotomy is most useful for the treatment of peak-dose diskinesias and for dystonia that occurs at the end of a dose.
Aside from surgical resection, deep brain stimulation, high frequency stimulating electrodes placed in the ventral intermedialis nucleus, has been found to suppress abnormal movements in some cases. A variety of techniques exist to permit precise location of a probe, including computed tomography and magnetic resonance imaging. Unfortunately, the akinesia, speech and gait disorder symptoms of Parkinson's disease are little helped by these surgical procedures, all of which result in destructive brain lesions. Despite the development of modem imaging and surgical techniques to improve the effectiveness of these neurosurgical interventions for the treatment of Parkinson's disease tremor symptoms, the use of neurosurgical therapies is not widely applicable. For example, thalamotomy does not alleviate the akinetic symptoms which are the major functional disability for many people suffering from Parkinson's disease (Marsden et al., Adv. Neurol. 74: 143-147, 1997).
Therapeutic methods aimed at controlling suspected causative factors associated with Parkinson's disease {e.g., therapies which control oxidative stress and excitotoxicity) have also been developed. Clinical trials have shown that administration of antioxid^tive agents vitamin E and deprenyl provided little or no neuroprotective function (Shoulson et at, Ann. Neurol. 43: 318-325, 1998). Glutamate-receptor blockers and neuronal nitric oxide synthase (NOS) inhibitors have been proposed as therapies for Parkinson's disease; however, no experimental results from human studies have yet been published (Rodriguez, Ann. Neurol. 44: S175-S188, 1998).
The use of neurotrophic factors to stimulate neuronal repair, survival, and growth in Parkinson's disease has also been studied, particularly the use of glial cell line-derived neurotrophic factor (GDNF). Although GDNF protein protects some dopamine neurons from death, it is difficult to supply GDNF protein to the brain. Furthermore, the use of such protein therapies in general is problematic, since protein molecules show rapid in vivo degradation, are unable to penetrate the blood-brain barrier, and must be directly injected into the ventricles of the patient's brain (Palfϊ et al, Soc. Neurosci. Abstr. 24: 41, 1998; Hagg, Exp. Neurol. 149: 183-192, 1998; and Dunnett and Bjorklund, supra). Other neurotrophic factors
which may have therapeutic value have been proposed based on in vitro and animal model systems, including neurturin, basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), neurotrophins 3 and 4/5, ciliary neurotrophic factor and transforming growth factor β (TGF-β). However, the effectiveness of these therapies in humans remains unknown. At present, no single chemical compound or peptide has been reported to completely protect dopamine neurons from death by tropic factor withdrawal or neurotoxin exposure.
Cell replacement therapies have also received much attention as potential methods for treating Parkinson's disease (Freed et al, Arch. Neurol. 47: 505-512, 1990; Freed et al.,N. Engl. J. Med. 327: 1549-1555, 1992; Lindvall et al, Science 247: 574-577, 1990; Spencer et al, N. Engl J. Med. 321: 1541-1548, 1992; Widner et al, N. Engl J. Med. 327: 1556-1563, 1992; Lindvall, NeuroReport 8: iii-x, 1997; Olanow et al., Adv. Neurol. 74: 249-269, 1997; and Lindvall, Nature Biotechn. 17: 635-636, 1999). These neural grafting therapies use dopamine supplied from cells implanted into the striatum as a substitute for nigrostriatal dopaminergic neurons that have been lost due to neurodegeneration. Although animal models and preliminary human clinical studies have shown that cell replacement therapies may be useful in the treatment of Parkinson's disease, the failure of the transplanted neurons to survive in the striatum is a major impediment in the development of cell replacement therapies.
Various sources of dopaminergic neurons for use in the transplantation process have been tried in animal experiments, including the use of mesencephalic dopamine neurons obtained from human embryo cadavers, immature neuronal precursor cells (i.e., neuronal stem cells), dopamine secreting non-neuronal cells, terminally differentiated teratocarcinoma- derived neuronal cell lines (Dunnett and Bjorkland, supra), genetically modified cells (Raymon et al., Exp. Neurol. 144: 82-91, 1997; and Kang, Mov. Dis. 13: 59-72, 1998), cells from cloned embryos (Zawada et al, Nature Medicine 4: 569-573, 1998) and xenogenic cells (Bjorklund et al, Nature 298: 652-654, 1982; Huffaker et al, Exp. Brain Res. 77: 329-336, 1989; Galpem et al, Exp. Neurol 140: 1-13, 1996; Deacon et al, Nature Med. 3: 350-353, 1997; and Zawada et al, Nature Med. 4: 569-573, 1998). Nonetheless, in current grafting protocols, no more than 5-20% of the transplanted dopamine neurons survive.
Additional therapies are also available, such as physical therapy, occupational therapy, or speech / language therapy. Exercise, diet, nutrition, patient/caregiver education, and psychosocial interventions have also been shown to have a positive effect on the mental and/or physical state of a person suffering from Parkinson's disease.
Various methods of evaluating Parkinson's disease in a patient include Hoehn and Yahr Staging of Parkinson's Disease, Unified Parkinson Disease Rating Scale (UPDRS), and Schwab and England Activities of Daily Living Scale.
A person suffering from Parkinson's disease should avoid contraindicated and potentially contraindicated drugs such as antipsychotic drugs, Haloperidol (Haldol), Perphenazine (Trilafon), Chlorpromazine (Thorazine), Trifluoperazine (Stelazine), Flufenazine (Prolixin, Permitil) Thiothixene (Navane), Thioridazine (Mellaril); antidepressant drug, combination of Perphenazine and Amitriptyline (Triavil); anti-vomiting drugs, Prochlorperazine (Compazine), Metoclopramide (Reglan, Maxeran), Thiethylperazine (Torecan), Reserpine (Serpasil), Tetrabenazine (Nitoman); blood pressure drug, Alpha- methyldopa (Aldomet); anti-seizure drug, Phenytoin (Dilantin); mood stabilizing drug, lithium; and anti-anxiety drug, Buspirone (Buspar).
In certain embodiments, the method includes administering, conjointly with the subject pharmaceutical composition, one or more of other therapeutic compositions useful for the treatment of diseases, for which pramipexole is indicated for. For example, in the case of treating Parkinson's Disease and certain movement disorders, pramipexole may be coadministered with a dopamine precursor, a dopaminergic agent, a dopaminergic and anticholinergic agent, an anti-chόlinergic agent, a dopamine agonist, a MAO-B (monoamine oxidase B) inhibitor, a COMT (catechol 0-methyltransferase) inhibitor, a muscle relaxant, a sedative, an anticonvulsant agent, a dopamine reuptake inhibitor, a dopamine blocker, a β- blocker, a carbonic anhydrase inhibitor, a narcotic agent, a GABAergic agent, or an alpha antagonist.
In certain embodiments, the subject packages, preparations, pharmaceutical compositions, and methods for the treatment of movement disorders further comprise one or more therapeutic agents for treating Parkinson's disease selected from a dopamine precursor, such as L-dopa; a dopaminergic agent, such as Levodopa-carbidopa (SINEMET®, SINEMET CR®) or Levodopa-benserazide (PROLOPA®, MADOPAR®, MADOPAR HBS®); a dopaminergic and anti-cholinergic agent, such as amantadine (SYMMETRYL®, SYMADINE®); an anti-cholinergic agent, such as trihexyphenidyl (ARTANE®), benztropine (COGENTIN®), ethoproprazine (PARSITAN®), or procyclidine (KEMADRIN®); a dopamine agonist, such as apomorphine, bromocriptine (PARLODEL®), cabergoline (DOSTINEX®), lisuride (DOPERGINE®), pergolide (PERMAX®), or ropinirole (REQUIP®); a MAO-B (monoamine oxidase B) inhibitor, such as selegiline or deprenyl (ATAPRYL®, CARBEX®, ELDEPRYL®); a COMT (catechol 0-methyltransferase) inhibitor, such as CGP-
28014, tolcapone (TASMAR®) or entacapone (COMTAN®); or other therapeutic agents, such as baclofen (LIORESAL®), domperidone (MOTILIUM®), fludrocortisone (FLORINEF®), midodrine (AMATINE®), oxybutynin (DITROPAN®), propranolol (INDERAL®, INDERAL- LA®), clonazepam (RIVOTRIL®), or yohimbine.
US20030045539 (incoφorated herein by reference) discloses a combination treatment of cabergoline and pramipexole provided concurrently to a patient suffering from various central nervous system diseases, and in particular for the treatment of Parkinson's Disease (PD). The initial dose of cabergoline is administered to the patient at a dose of 0.5 to 1 mg/patient/day and is adjusted upward at weekly intervals to a therapeutic dosage of 2, 4, 6, 8 or 10 mg/patient/day and where the initial dose of pramipexole is started at 0.375 mg/patient/day and is adjusted upward every 5 to 7 days to a therapeutic dosage of 3, 4, 5, 6, or 7 mg/patient/day. At least one portion of the subject pharmaceutical composition may additional comprises cabergoline and pramipexole for treating Parkinson's disease.
US20040166159 (incorporated herein by reference) discloses a pharmaceutical dosage forms having immediate and controlled release properties that contain an aromatic amino acid decarboxylase (AAAD) inhibitor (such as carbidopa), levodopa, and optionally a catechol-0-methyltransferase (COMT) inhibitor, for the treatment of medical conditions associated with reduced dopamine levels in a patient's brain. The dosage form may comprise up to about 1000 mg, or about 20-500 mg, about 50-500 mg, or about 100-200 mg of COMT inhibitor. The COMT inhibitor may be contained only within the immediate release component, or only within the sustained release component, or both. The COMT inhibitor may be CGP-28014, entacapone, or tolcapone. The dosage form may further comprise one or more drugs such as anticholinergics, beta 2-agonists, cyclooxygenase-2 (COX-2) inhibitors, dopamine receptor agonists, monoamine oxidase (MAO) inhibitors, opiate delta receptor agonists, opiate delta receptor antagonists, and N-methyl-D-aspartate (NMDA) antagonists. The dosage form may further comprise one or more drugs selected from albuterol, alpha- lipoic acid, amantadine, andropinirole, apomorphine, baclofen, biperiden, benztropine, bromocriptine, budipine, cabergoline, clozapine, deprenyl, dextromethorphan, dihydroergokryptine, dihydrolipoic acid, eliprodil, eptastigmine, ergoline, formoterol, galanthamine, lazabemide, lysuride, mazindol, memantine, mofegiline, orphenadrine, pergolide, pirbuterol, propentofylline, procyclidine, rasagiline, remacemide, riluzole, rimantadine, ropinirole, salmeterol, selegiline, spheramine, terguride, and trihexyphenidyl.
Similarly, other movement disorders may also be treated with similar methods and suitable pharmaceutical compositions, such as the ones described below.
For example, in certain embodiments of the packages, preparations, compositions, and methods for the treatment of a movement disorder, the invention further comprises one or more therapeutic agents for treating dystonia selected from an anti-cholinergic agent, such as trihexyphenidyl (ARTANE®), benztropine (COGENTIN®), ethoproprazine (PARSITAN®), or procyclidine (KEMADRIN®); a dopaminergic agent, such as Levodopa-carbidopa (SINEMET®, SINEMET CR®) or Levodopa-benserazide (PROLOPA®, MADOPAR®, MADOPAR HBS®); a muscle relaxant, such as baclofen (LIORESAL®); a sedative, such as Clonazepam (RIVOTRIL®); an anticonvulsant agent, such as carbamazepine (TEGRETOL®); a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN®); or a dopamine blocker, such as haloperidol (HALDOL®).
In certain embodiments of the packages, preparations, compositions, and methods for the treatment of a movement disorder, the invention further comprises one or more therapeutic agents for treating tremor selected from a /3-blocker, such as propranolol (INDERAL®, INDERAL-LA®); an anticonvulsant agent, such as primidone (MYSOLINE®); or a carbonic anhydrase inhibitor, such as acetalzolamide (DIAMOX®) or methazolamide (NEPTAZANE®).
In certain embodiments of the packages, preparations, compositions, and methods for the treatment of a movement disorder, the invention further comprises one or more therapeutic agents for treating myoclonus selected from a sedative, such as clonazepam (RIVOTRIL®); or an anticonvulsant agent, such as valproic acid (EPIVAL®).
In certain embodiments of the packages, preparations, compositions, and methods for the treatment of a movement disorder, the invention further comprises one or more therapeutic agents for treating chorea selected from a dopamine blocker, such as haloperidol (HALDOL®); or a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN®).
In certain embodiments of the packages, preparations, compositions, and methods for the treatment of a movement disorder, the invention further comprises one or more therapeutic agents for treating restless leg syndrome selected from a dopaminergic, such as Levodopa-carbidopa (SINEMET®, SINEMET CR®) or Levodopa-benserazide (PROLOPA®, MADOPAR®, MADOPAR HBS®); a sedative, such as clonazepam (RIVOTRIL®); a dopamine agonists, such as bromocriptine (PARLODEL®), pergolide (PERMAX®), or ropinirole (REQUIP®); a narcotic agent, such as codeine (TYLENOL # 3®); or a GABAergic agent, such as gabapentin (NEURONTIN®).
In certain embodiments of the subject packages, preparations, compositions, and methods for the treatment of movement disorders, the invention further comprises one or
more therapeutic agents for treating tics selected from a sedative, such as clonazepam (RIVOTRIL®); an alpha antagonist, such as clonidine (CATAPRESS®); a dopamine reuptake inhibitor, such as tetrabenazine (NITOMAN®); or a dopamine blocker, such as haloperidol (HALDOL®) or perphenazine.
In certain embodiments, the method includes administering, conjointly with the pharmaceutical composition, one or more of physical therapy, occupational therapy, or speech/language therapy.
An agent to be administered conjointly with a subject compound may be formulated together with a subject compound as a single pharmaceutical preparation, e.g., as a pill or other medicament including both agents, or may be administered as a separate pharmaceutical preparation.
Another aspect of the invention provides a packaged pharmaceutical composition, comprising the subject pharmaceutical composition in an amount sufficient to treat or prevent a movement disorder in a patient, which may additionally include a pharmaceutically acceptable carrier, and instructions (written and/or pictorial) describing the use of the formulation for treating the patient, wherein the patient suffers from ataxia, corticobasal ganglionic degeneration (CBGD), dyskinesia, dystonia, tremors, hereditary spastic paraplegia, Huntington's disease, multiple system atrophy, myoclonus, Parkinson's disease, progressive supranuclear palsy, restless legs syndrome, Rett syndrome, spasticity, Sydenham's chorea, other choreas, athetosis, ballism, stereotypy, tardive dyskinesia/dystonia, tics, Tourette's syndrome, olivopontocerebellar atrophy (OPCA), diffuse Lewy body disease, hemibalismus, hemi-facial spasm, restless leg syndrome, Wilson's disease, stiff man syndrome, akinetic mutism, psychomotor retardation, painful legs moving toes syndrome, a gait disorder, a drug-induced movement disorder, or other movement disorder.
In certain preferred embodiments, the movement disorder is Parkinson's disease.
VI. Exemplary Uses of the Dosage Forms
In various embodiments, the present invention contemplates modes of treatment and/or prophylaxis {e.g., treating or preventing the development of symptoms in high-risk populations), which utilize one or more of the subject dosage forms for decreasing or overcoming the defects in a movement disorder patient. The improvement and/or restoration of mental or physical state in an organism has positive behavioral, social, and psychological consequences.
For example, Parkinson's disease is the second most common neurodegenerative disorder, affecting nearly 1 million people in North America. The disease is characterized by symptoms such as muscle rigidity, tremor and bradykinesia. Early studies of Parkinson's disease showed unusual inclusions in the cytoplasm of neurons (i.e., Lewy bodies), occurring predominantly in the substantia nigra, which innervate the striatal region of the forebrain. Although Lewy bodies were also found in other neurodegenerative conditions, the presence of Lewy bodies in Parkinson's disease is accompanied by cell loss in the substantia nigra. This cell loss is considered to be the defining pathological feature of Parkinson's disease.
Epidemiological studies have reported geographic variation in Parkinson's disease incidence, leading to the search for environmental factors (Olanow and Tatton, Ann. Rev. Neurosci. 22: 123-144, 1998). The recent discovery that l-methyl-4-phenyl-l,2,3,6- tetrahydropyridine (MPTP) toxin causes a Parkinson's-like syndrome indistinguishable from the idiopathic disease suggests that Parkinson's disease may be caused by environmental factors {e.g., toxins and causative agents). (See e.g., Langston, Ann. Neurol. 44: S45-S52, 1998).
Recent research has also identified genes associated with Parkinson's disease (Mizuno et al, Biomed. Pharmacother. 53(3): 109-116, 1999; Dunnett and Bjorklund, Nature 399 (6738 Suppl): A32-A39, 1999); namely, the α-synuclein gene (Polymeropouos et al, Science 276: 2045-2047, 1997), the parkin gene (Kitada et al, Nature 392: 605-608, 1998), and the UCH-Ll thiol protease gene (Leroy et al, Nature 395: 451-452, 1998). Although additional chromosomal loci associated with the disease state have been identified, these chromosomal loci have not been analyzed at the molecular level. At present, the biochemical roles played by these gene products in both normal cells and in diseased neurons remain ambiguous, and no gene therapy protocols involving their use have been developed.
Furthermore, Parkinson's disease is associated with the progressive loss of dopamine neurons in the ventral mesencephalon of the substantia nigra (Shoulson, Science 282: 1072- 1074, 1998), which innervates the major motor-control center of the forebrain, the striatum. Although a gradual decline in the number of neurons and dopamine content of the basal ganglia is normally associated with increasing age, progressive dopamine loss is pronounced in people suffering from Parkinson's disease, resulting in the appearance of symptoms when about 70-80% of striatal dopamine and 50% of nigral dopamine neurons are lost (Dunnett and Bjorklund, supra). This loss of dopamine-producing neurons resulting in a dopamine deficiency is believed to be responsible for the motor symptoms of Parkinson's disease.
Although the cause of dopaminergic cell death remains unknown, it is believed that dopaminergic cell death is affected by a combination of necrotic and apoptotic cell death. Mechanisms and signals responsible for the progressive degeneration of nigral dopamine neurons in Parkinson's disease have been proposed (Olanow et al, Ann. Neurol. 44: Sl -S 196, 1998), and include oxidative stress (from the generation of reactive oxygen species), mitochondrial dysfunction, excitotoxicity, calcium imbalance, inflammatory changes and apoptosis as contributory and interdependent factors in Parkinson's disease neuronal cell death.
Apoptosis {i.e., programmed cell death) plays a fundamental role in the development of the nervous system (Oppenheim, Ann. Rev. Neurosci. 14: 453-501, 1991), and accelerated apoptosis is believed to underlie many neurodegenerative diseases, including Parkinson's disease (Barinaga, Science 281: 1303-1304, 1998; Mochizuki et al, J. Neurol. Set 137: 120- 123, 1996; and Oo et al, Neuroscience 69: 893-901, 1995). In living systems, apoptotic death can be initiated by a variety of external stimuli, and the biochemical nature of the intracellular apoptosis effectors is at least partially understood.
VII. Controlled Release / Bioadhesive Layer
According to the instant invention, the subject dosage form is administered orally to the lower gastrointestinal (GI) tract. Thus, it is desirable that the subject drug delivery system adhere to the lining of the appropriate viscus, such that its contents can be delivered as a function of proximity and duration of contact.
An orally ingested product can adhere to either the epithelial surface or the mucus lining of the GI tract. For the delivery of bioactive substances, it can be advantageous to have a polymeric drug delivery device adhere to the epithelium or to the mucous layer. Bioadhesion in the GI tract may proceed in two stages: (1) viscoelastic deformation at the point of contact of the synthetic material into the mucus substrate, and (2) formation of bonds between the adhesive synthetic material and the mucus or the epithelial cells. In general, adhesion of polymers to tissues may be achieved by (i) physical or mechanical bonds, (ii) primary or covalent chemical bonds, and/or (iii) secondary chemical bonds {e.g., ionic). Physical or mechanical bonds can result from deposition and inclusion of the adhesive material in the crevices of the mucus or the folds of the mucosa. Secondary chemical bonds, contributing to bioadhesive properties, consist of dispersive interactions {e.g., van der Waals interactions) and stronger specific interactions, which include hydrogen bonds. The
hydrophilic functional groups primarily responsible for forming hydrogen bonds are the hydroxyl and the carboxylic groups.
"Bioadhesion" is defined as the ability of a material to adhere to a biological tissue for an extended period of time. Bioadhesion is one solution to the problem of inadequate residence time resulting from intestinal peristalsis, and from displacement by ciliary movement. For sufficient bioadhesion to occur, an intimate contact must exist between the bioadhesive and the receptor tissue, the bioadhesive must penetrate into the crevice of the tissue surface and/or mucus, and mechanical, electrostatic, or chemical bonds must form. Polycarbophils and acrylic acid polymers usually have the best adhesive properties. Duchene et al, in Drug Dev. bid. Pharm., 14:283-318, 1988, reviewed the pharmaceutical and medical aspects of bioadhesive systems for drug delivery (incorporated herein by reference). These bioadhesive systems may be adapted for use in the instant invention. Other bioadhesive systems that may be adapted for use in the instant application are described in WO 93/21906; Smart et al, J. Pharm. Pharmacol. 36: 295-299, 1984; Gurney et al, Biomaterials 5: 336- 340, 1984; Park et al, "Alternative Approaches to Oral Controlled Drug Delivery: Bioadhesives and In-Situ Systems," in J. M. Anderson and S. W. Kim, Eds., "Recent Advances in Drug Delivery " Plenum Press, New York, 1984, pp. 163-183; Mikos et al.,J. Colloid Interface Sci. 143: 366-373, 1991; and Lehr et al., J. Controlled ReI 13: 51-62, 1990, all incorporated herein by reference.
In certain embodiments, the subject dosage forms having increased lower gastrointestinal retention time. For purposes of this invention, intestinal residence time is the time required for a dosage form to transit through the intestine to the pyloric sphincter. For example, a dosage form of the invention has an intestinal residence time of at least 3 hours, at least 4 hours, at least 6 hours, at least 8 hours or at least 12 hours. The dosage forms of the invention may have an increased retention time in the small and/or large intestine, or in the area of the gastrointestinal tract that absorbs the drug contained in the dosage form. For example, dosage forms of the invention can be retained in the small intestine (or one or two portions thereof, selected from the duodenum, the jejunum and the ileum) for at least 6 hours, at least 8 hours or at least 12 hours, such as from 16 to 18 hours.
Certain polymers for use in the subject invention are described in more details below.
Polymers
Suitable bioadhesive polymeric coatings are disclosed in U.S. Patent Nos. 6,197,346, 6,217,908 and 6,365,187 (the contents of which are incorporated herein by reference), and
include soluble and insoluble, biodegradable and nonbiodegradable polymers. These can be hydrogels or thermoplastics, homopolymers, copolymers or blends, and/or natural or synthetic polymers. The preferred polymers are synthetic polymers, with controlled synthesis and degradation characteristics. Particularly preferred polymers are anhydride copolymers of fumaric acid and sebacic acid (P(FA:SA)), which have exceptionally good bioadhesive properties when administered to the GI tract. Examples of P(FA: SA) copolymers include those having a 1:99 to 99:1 ratio of fumaric acid to sebacic acid, such as 5:95 to 75:25, for example, 10:90 to 60:40 or at least 15:85 to 25:75. Specific examples of such copolymers have a 20:80 or a 50:50 ratio of fumaric acid to sebacic acid.
Polymers used in dosage forms of the invention produce a bioadhesive interaction (fracture strength) of at least 100 N/m2 (10 mN/cm ) when applied to the mucosal surface of rat intestine. The fracture strength of the dosage forms is advantageously at least 250 N/m2, at least 500 N/m2 or at least 1000 N/m2. For example, the fracture strength of a polymer- containing dosage form can be from 100 to 500 N/m2. The forces described herein refer to measurements made upon rat intestinal mucosa, unless otherwise stated. The same adhesive measurements made on a different species of animal will differ from those obtained using rats. This difference is attributed to both compositional and geometrical variations in the mucous layers of different animal species as well as cellular variations in the mucosal epithelium. However, the data shows that the same general trends prevail no matter what animal is studied {i.e., P(FA: SA) produces stronger adhesions than polylactic acid (PLA) in rats, sheep, pigs, etc.). For example, the fracture strength of dosage forms of the invention on rat intestine is generally at least 125 N/m2, such as at least 150 N/m2, at least 250 N/m2, at least 500 N/m2 or at least 1000 N/m2.
The fracture strength of a dosage form can be measured according to the methods disclosed by Duchene et at Briefly, the dosage form is attached on one side to a tensile tester and is contacted with a testing surface (e.g., a mucosal membrane) on the opposite surface. The tensile tester measures the force required to displace the dosage form from the testing surface. Common tensile testers include a Texture Analyzer and the Instron tensile tester.
In the preferred method for mucoadhesive testing, dosage forms are pressed using flat-faced tooling, 0.3750" (9.525 mm) in diameter. Dosage form weight will depend on composition; in most cases, the dosage forms have a final weight of 200 mg. These dosage forms are then glued to a plastic 10 mm diameter probe using a common, fast-drying cyanoacrylate adhesive. Once the dosage forms are firmly adhered to the probe, the probe is
attached to the Texture Analyzer. The Texture Analyzer is fitted with a 1 kg load cell for maximum sensitivity. The following settings are used:
The Test and Post-Test Speeds are as low as the instrument will allow, to ensure a maximum number of data points captured. The Pre-Test speed is used only until the probe encounters the Trigger Force; i.e., prior to contacting the tissue.
The Proportional, Integral, and Differential Gain are set to 0. These settings, when optimized, maintain the system at the Applied Force for the duration of the Contact Time. With soft tissue as a substrate, however, the probe and dosage form are constantly driven into the deformable surface. This results in visible damage to the tissue. Thus, the probe and dosage form are allowed to relax gradually from the Applied Force by setting these parameters to 0. The tracking speed, which is a measure of how rapidly the feedback is adjusted, is also set to 0.
The tissue on which the dosage forms are tested is secured in the Mucoadhesive Rig; the rig is then completely immersed in a 600 mL Pyrex beaker containing 375 niL of PBS. The tissue is maintained at approximately 370C for the duration of the test; no stirring is used as the machine can detect the oscillations from the stir bar.
In the past, two classes of polymers have shown useful bioadhesive properties, hydrophilic polymers and hydrogels. In the large class of hydrophilic polymers, those containing carboxylic groups (e.g., poly[acrylic acid]) exhibit the best bioadhesive properties. It is thus expected that polymers with the highest concentrations of carboxylic groups are preferred materials for bioadhesion on soft tissues. In other studies, the most promising polymers were sodium alginate, carboxymethylcellulose, hydroxymethylcellulose and methylcellulose. Some of these materials are water-soluble, while others are hydrogels.
Rapidly bioerodible polymers such as poly[lactide-co-glycolide], polyanhydrides, and polyorthoesters, whose carboxylic groups are exposed on the external surface as their smooth
surface erodes, are suitable for bioadhesive drug delivery systems. In addition, polymers containing labile bonds, such as polyanhydrides and polyesters, are well known for their hydrolytic reactivity. Their hydrolytic degradation rates can generally be altered by simple changes in the polymer backbone.
Representative natural polymers suitable for the present invention include proteins (e.g., hydrophilic proteins), such as zein, modified zein, casein, gelatin, gluten, serum albumin, or collagen, and polysaccharides such as cellulose, dextrans, polyhyaluronic acid, polymers of acrylic and methacrylic esters and alginic acid. These are generally less suitable for use in bioadhesive coatings due to higher levels of variability in the characteristics of the final products, as well as in degradation following administration. Synthetically modified natural polymers include alkyl celluloses, hydroxyalkyl celluloses, cellulose ethers, cellulose esters, and nitrocelluloses.
Representative synthetic polymers for use in bioadhesive coatings include polyphosphazines, poly(vinyl alcohols), polyamides, polycarbonates, polyalkylenes, polyacrylamides, polyalkylene glycols, polyalkylene oxides, polyalkylene terephthalates, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyurethanes and copolymers thereof. Other polymers suitable for use in the invention include, but are not limited to, methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxymethyl cellulose, cellulose triacetate, cellulose sulfate sodium salt, poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), poly(octadecyl acrylate) polyethylene, polypropylene, poly(ethylene glycol), poly(ethylene oxide), poly (ethylene terephthalate), poly(vinyl acetate), polyvinyl chloride, polystyrene, polyvinyl pyrrolidone, and polyvinylphenol. Representative bioerodible polymers for use in bioadhesive coatings include polylactides, polyglycolides and copolymers thereof, poly(ethylene terephthalate), poly(butyric acid), poly(valeric acid), poly(lactide-co- caprolactone), poly[lactide-co-glycolide], polyanhydrides (e.g., poly(adipic anhydride)), polyorthoesters, chitosan, chitin, hyaluronic acid, hyaluronan, Carbopols, Corplex polymers, Polycarbophils-Cysteine (Thiomers), Chitosan-Thioglycolic acid copolymers, ρoly(methacrylic acid-grafted-ethylene glycol), poly (methyl vinyl ether-co-malic anhydride), cholestyramine (Duolite AP- 143), sucralfate and gliadin blends and copolymers thereof.
Polyanhydrides are particularly suitable for use in bioadhesive delivery systems because, as hydrolysis proceeds, causing surface erosion, more and more carboxylic groups are exposed to the external surface. However, polylactides erode more slowly by bulk erosion, which is advantageous in applications where it is desirable to retain the bioadhesive coating for longer durations. In designing bioadhesive polymeric systems based on polylactides, polymers that have high concentrations of carboxylic acid are preferred. The high concentrations of carboxylic acids can be attained by using low molecular weight polymers (MW of 2000 or less), because low molecular weight polymers contain a high concentration of carboxylic acids at the end groups.
The polymers listed above can be obtained from sources such as Sigma Chemical Co., St. Louis, Mo., Polysciences, Warrenton, Pa., Aldrich, Milwaukee, Wis., Fluka, Ronkonkoma, N. Y., and BioRad, Richmond, Calif., or can alternatively be synthesized from monomers obtained from these suppliers using standard techniques.
When the bioadhesive polymeric coating is a synthetic polymer coating, the synthetic polymer is typically selected from polyamides, polycarbonates, polyalkylenes, polyalkylene glycols, polyalkylene oxides, polyalkylene terephthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyurethanes, polystyrene, polymers of acrylic and methacrylic esters, polylactides, poly(butyric acid), poly(valeric acid), poly(lactide-co-glycolide), polyanhydrides, polyorthoesters, poly(fumaric acid), poly(maleic acid), and blends and copolymers of thereof. Preferably, the synthetic polymer is poly(fumaric-co-sebacic) anhydride.
Another group of polymers suitable for use as bioadhesive polymeric coatings are polymers having a hydrophobic backbone with at least one hydrophobic group pendant from the backbone. Suitable hydrophobic groups are groups that are generally non-polar. Examples of such hydrophobic groups include alkyl, alkenyl and alkynyl groups. Preferably, the hydrophobic groups are selected to not interfere and instead to enhance the bioadhesiveness of the polymers.
A further group of polymers suitable for use as bioadhesive polymeric coatings are polymers having a hydrophobic backbone with at least one hydrophilic group pendant from the backbone. Suitable hydrophilic groups are groups that are capable of hydrogen bonding to another functional group. Example of such hydrophilic groups include negatively charged groups such as carboxylic acids, sulfonic acids and phosponic acids, positively charged groups such as (protonated) amines and neutral, polar groups such as amides and imines.
Preferably, the hydrophilic groups are selected to not interfere and instead to enhance the bioadhesiveness of the polymers. The hydrophilic groups can be either directly attached to a hydrophobic polymer backbone or attached through a spacer group. Typically, a spacer group is an alkylene group, particularly a C1-C8 alkyl group such as a C2-Cg alkyl group. Preferred compounds containing one or more hydrophilic groups include amino acids (e.g., phenyalanine, tyrosine and derivatives thereof) and amine-containing carbohydrates (sugars) such as glucosamine.
Polymers can be modified by increasing the number of carboxylic groups accessible during biodegradation, or on the polymer surface. The polymers can also be modified by binding amino groups to the polymer. The polymers can be modified using any of a number of different coupling chemistries available in the art to covalently attach ligand molecules with bioadhesive properties to the surface-exposed molecules of the polymeric microspheres.
The attachment of any positively charged ligand, such as polyethyleneimine or polylysine, to a polymer may improve bioadhesion due to the electrostatic attraction of the cationic groups coating the beads to the net negative charge of the mucus. The mucopolysaccharides and mucoproteins of the mucin layer, especially the sialic acid residues, are responsible for the negative charge coating. Any ligand with a high binding affinity for mucin could also be covalently linked to most polymers with the appropriate chemistry, such as with carbodiimidazole (CDI), and be expected to influence the binding to the gut. For example, polyclonal antibodies raised against components of mucin or else intact mucin, when covalently coupled to a polymer, would provide for increased bioadhesion. Similarly, antibodies directed against specific cell surface receptors exposed on the lumenal surface of the intestinal tract would increase the residence time when coupled to polymers using the appropriate chemistry. The ligand affinity need not be based only on electrostatic charge, but other useful physical parameters such as solubility in mucin or specific affinity to carbohydrate groups.
The covalent attachment of any of the natural components of mucin in either pure or partially purified form to the polymers would increase the solubility of the polymer in the mucin layer. The list of useful ligands would include but not be limited to the following: sialic acid, neuraminic acid, n-acetyl-neuraminic acid, n-glycolylneuraminic acid, 4-acetyl-n- acetylneuraminic acid, diacetyl-n-acetylneuraminic acid, glucuronic acid, iduronic acid, galactose, glucose, mannose, fucose, any of the partially purified fractions prepared by chemical treatment of naturally occurring mucin, e.g., mucoproteins, mucopolysaccharides
and mucopolysaccharide-protein complexes, and antibodies immunoreactive against proteins or sugar structure on the mucosal surface.
The attachment of polyamino acids containing extra pendant carboxylic acid side groups, such as polyaspartic acid and polyglutamic acid, may also increase bioadhesiveness. The polyamino chains would increase bioadhesion by means of chain entanglement in mucin strands as well as by increased carboxylic charge.
Polymer-Metal Complexes
As disclosed in U.S. Patent Nos. 5,985,312, 6,123,965 and 6,368,586, the contents of which are incorporated herein by reference, polymers, such as those named above, having a metal compound incorporated therein have a further improved ability to adhere to tissue surfaces, such as mucosal membranes. The metal compound incorporated into the polymer can be, for example, a water-insoluble metal oxide. The incorporation of metal compounds into a wide range of different polymers, even those that are not normally bioadhesive, improves their ability to adhere to tissue surfaces such as mucosal membranes.
Metal compounds which can be incorporated into polymers to improve their bioadhesive properties preferably are water-insoluble metal compounds, such as water- insoluble metal oxides and metal hydroxides, which are capable of becoming incorporated into and associated with a polymer to thereby improve the bioadhesiveness of the polymer. As defined herein, a water-insoluble metal compound is defined as a metal compound with little or no solubility in water, for example, less than about 0.0 to 0.9 mg/ml.
The water-insoluble metal compounds can be derived from a wide variety of metals, including, but not limited to, calcium, iron, copper, zinc, cadmium, zirconium and titanium. The water insoluble metal compound preferably is a metal oxide or hydroxide. Water insoluble metal compounds of multivalent metals are preferred. Representative metal oxides suitable for use in the compositions described herein include cobalt (I) oxide (CoO), cobalt (II) oxide (Co2O3), selenium oxide (SeO2), chromium (IV) oxide (CrO2), manganese oxide (MnO2), titanium oxide (TiO2), lanthanum oxide (La2O3), zirconium oxide (ZrO2), silicon oxide (SiO2), scandium oxide (Sc2O3), beryllium oxide (BeO), tantalum oxide (Ta2O5), cerium oxide (CeO2), neodymium oxide (Nd2O3), vanadium oxide (V2Os), molybdenum oxide (Mo2O3), tungsten oxide (WO), tungsten trioxide (WO3), samarium oxide (Sm2O3), europium oxide (Eu2O3), gadolinium oxide (Gd2O3), terbium oxide (Tb4O7), dysprosium oxide (Dy2O3), holmium oxide (Ho2O3), erbium oxide (Er2O3), thulium oxide (Tm2O3), ytterbium oxide (Yb2O3), lutetium oxide (Lu2O3), aluminum oxide (Al2O3), indium oxide (InO3), germanium oxide (GeO2), antimony oxide (Sb2O3), tellurium oxide (TeO2), nickel
oxide (NiO), and zinc oxide (ZnO). Other oxides include barium oxide (BaO), calcium oxide (CaO), nickel oxide (III) (Ni2O3), magnesium oxide (MgO), iron (II) oxide (FeO)5 iron (III) oxide (Fe2O3), copper oxide (II) (CuO), cadmium oxide (CdO), and zirconium oxide (ZrO2).
Preferred properties defining the metal compound include: (a) substantial insolubility in aqueous environments, such as acidic or basic aqueous environments (such as those present in the gastric lumen); and (b) ionizable surface charge at the pH of the aqueous environment.
The water-insoluble metal compounds can be incorporated into the polymer by one of the following mechanisms: (a) physical mixtures which result in entrapment of the metal compound; (b) ionic interaction between metal compound and polymer; (c) surface modification of the polymers which would result in exposed metal compound on the surface; and (d) coating techniques such as fluidized bed, pan coating, or any similar methods known to those skilled in the art, which produce a metal compound enriched layer on the surface of the device. In certain embodiments, nanoparticles or microparticles of the water-insoluble metal compound are incorporated into the polymer.
In certain embodiments, the metal compound is provided as a fine particulate dispersion of a water-insoluble metal oxide which is incorporated throughout the polymer or at least on the surface of the polymer which is to be adhered to a tissue surface. The metal compound also can be incorporated in an inner layer of the polymer and exposed only after degradation or else dissolution of a "protective" outer layer. For example, a tablet core containing a polymer and metal may be covered with an enteric coating designed to dissolve when exposed to intestinal fluid. The metal compound-enriched core then is exposed and become available for binding to GI mucosa.
Fine metal oxide particles can be produced for example by micronizing a metal oxide by mortar and pestle treatment to produce particles ranging in size, for example, from 10.0 to 300 ran. The metal oxide particles can be incorporated into the polymer, for example, by dissolving or dispersing the particles into a solution or dispersion of the polymer.
Advantageously, metal compounds which are incorporated into polymers to improve their bioadhesive properties can be metal compounds which are already approved by the FDA as either food or pharmaceutical additives, such as zinc oxide.
Suitable polymers which can be used and into which the metal compounds can be incorporated include soluble and water-insoluble, and biodegradable and nonbiodegradable polymers, including hydrogels, thermoplastics, and homopolymers, copolymers and blends of natural and synthetic polymers, provided that they have the requisite fracture strength when
mixed with a metal compound. In additional to those listed above, representative polymers which can be used in conjunction with a metal compound include hydrophilic polymers, such as those containing carboxylic groups, including polyacrylic acid. Bioerodible polymers including polyanhydrides, poly(hydroxy acids) and polyesters, as well as blends and copolymers thereof also can be used. Representative bioerodible poly(hydroxy acids) and copolymers thereof which can be used include poly(lactic acid), poly(glycolic acid), poly(hydroxy-butyric acid), poly(hydroxyvaleric acid), poly(caprolactone), poly(lactide-co- caprolactone), and poly(lactide-co-glycolide). Polymers containing labile bonds, such as polyanhydrides and polyorthoesters, can be used optionally in a modified form with reduced hydrolytic reactivity. Positively charged hydrogels, such as chitosan, and thermoplastic polymers, such as polystyrene also can be used.
Representative natural polymers which also can be used include proteins, such as zein, modified zein, casein, gelatin, gluten, serum albumin, or collagen, and polysaccharides such as dextrans, polyhyaluronic acid and alginic acid. Representative synthetic polymers include polyphosphazenes, polyamides, polycarbonates, polyacrylamides, polysiloxanes, polyurethanes and copolymers thereof. Celluloses also can be used. As defined herein the term "celluloses" includes naturally occurring and synthetic celluloses, such as alkyl celluloses, cellulose ethers, cellulose esters, hydroxyalkyl celluloses and nitrocelluloses. Exemplary celluloses include ethyl cellulose, methyl cellulose, carboxymethyl cellulose, hydroxymethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose triacetate and cellulose sulfate sodium salt.
Polymers of acrylic and methacrylic acids or esters and copolymers thereof can be used. Representative polymers which can be used include poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), and poly(octadecyl acrylate).
Other polymers which can be used include polyalkylenes such as polyethylene and polypropylene; polyarylalkylenes such as polystyrene; poly(alkylene glycols), such as poly(ethylene glycol); poly(alkylene oxides), such as poly(ethylene oxide); and poly(alkylene terephthalates), such as poly(ethylene terephthalate). Additionally, polyvinyl polymers can be used, which, as defined herein includes polyvinyl alcohols, polyvinyl ethers, polyvinyl esters
and polyvinyl halides. Exemplary polyvinyl polymers include poly(vinyl acetate), polyvinyl phenol and polyvinylpyrrolidone.
Water soluble polymers can also be used. Representative examples of suitable water soluble polymers include polyvinyl alcohol, polyvinylpyrrolidone, methyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose and polyethylene glycol, copolymers of acrylic and methacrylic acid esters, and mixtures thereof. Water insoluble polymers also can be used. Representative examples of suitable water insoluble polymers include ethylcellulose, cellulose acetate, cellulose propionate (lower, medium or -higher molecular weight), cellulose acetate propionate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose triacetate, poly(methyl methacrylate), poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), poly(hexyl methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), poly(octadecyl acrylate), poly(ethylene), poly(ethylene) low density, poly(ethylene) high density, poly(propylene), poly(ethylene oxide), poly(ethylene terephthalate), poly(vinyl isobutyl ether), poly(vinyl acetate), poly(vinyl chloride), polyurethanes, and mixtures thereof. In certain embodiments, a water insoluble polymer and a water soluble polymer are used together, such as in a mixture. Such mixtures are useful in controlled drug release formulations, wherein the release rate can be controlled by varying the ratio of water soluble polymer to water insoluble polymer.
Polymers varying in viscosity as a function of temperature or shear or other physical forces also may be used. Poly(oxyalkylene) polymers and copolymers such as poly(ethylene oxide)-poly(propylene oxide) (PEO-PPO) or poly(ethylene oxide)-poly(butylene oxide) (PEO-PBO) copolymers, and copolymers and blends of these polymers with polymers such as poly(alpha-hydroxy acids), including but not limited to lactic, glycolic and hydroxybutyic acids, polycaprolactones, and polyvalerolactones, can be synthesized or commercially obtained. For example, polyoxyalkylene copolymers are described in U.S. Patent Nos. 3,829,506, 3,535,307, 3,036,118, 2,979,578, 2,677,700 and 2,675,619. Polyoxyalkylene copolymers are sold, for example, by BASF under the trade name PLURONICS™. These materials are applied as viscous solutions at room temperature or lower which solidify at the higher body temperature. Other materials with this behavior are known in the art, and can be utilized as described herein. These include KLUCEL™ (hydroxypropyl cellulose), and purified konjac glucomannan gum.
Other suitable polymers are polymeric lacquer substances based on acrylates and/or methacrylates, commonly called EUDRAGIT™ polymers (sold by Rohm America, Inc.). Specific EUDRAGIT™ polymers can be selected having various permeability and water solubility, which properties can be pH dependent or pH independent. For example, EUDRAGIT™ RL and EUDRAGIT™ RS are acrylic resins comprising copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups, which are present as salts and give rise to the permeability of the lacquer films, whereas EUDRAGIT™ RL is freely permeable and EUDRAGIT™ RS is slightly permeable, independent of pH. In contrast, the permeability of EUDRAGIT™ L is pH dependent. EUDRAGIT™ L is an anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester. It is insoluble in acids and pure water, but becomes increasingly soluble in a neutral to weakly alkaline solution by forming salts with alkalis. Above pH 5.0, the polymer becomes increasingly permeable.
Polymer solutions that are liquid at an elevated temperature but solid or gelled at body temperature can also be utilized. A variety of thermoreversible polymers are known, including natural gel-forming materials such as agarose, agar, furcellaran, beta-carrageenan, beta-l,3-glucans such as curdlan, gelatin, or polyoxyalkylene containing compounds, as described above. Specific examples include thermosetting biodegradable polymers for in vivo use described in U.S. Patent No. 4,938,763, the contents of which are incorporated herein by reference.
Polymer Blends with Monomers and/or Oligomers
Polymers with enhanced bioadhesive properties are provided by incorporating anhydride monomers or oligomers into one of the polymers listed above by dissolving, dispersing, or blending, as taught by U.S. Patent Nos. 5,955,096 and 6,156,348, the contents of which are incorporated herein by reference. The polymers may be used to form drug delivery systems which have improved ability to adhere to tissue surfaces, such as mucosal membranes. The anhydride oligomers are formed from organic diacid monomers, preferably the diacids normally found in the Krebs glycolysis cycle. Anhydride oligomers which enhance the bioadhesive properties of a polymer have a molecular weight of about 5000 or less, typically between about 100 and 5000 Daltons, or include 20 or fewer diacid units linked by anhydride linkages and terminating in an anhydride linkage with a carboxylic acid monomer.
The oligomer excipients can be blended or incorporated into a wide range of hydrophilic and hydrophobic polymers including proteins, polysaccharides and synthetic
biocompatible polymers, including those described above. In certain embodiments, anhydride oligomers may be combined with metal oxide particles, such as those described above, to improve bioadhesion even more than with the organic additives alone. Organic dyes, because of their electronic charge and hydrophobicity or hydrophilicity, can either increase or decrease the bioadhesive properties of polymers when incorporated into the polymers.
As used herein, the term "anhydride oligomer" refers to a diacid or polydiacid linked by anhydride bonds, and having carboxy end groups linked to a monoacid such as acetic acid by anhydride bonds. The anhydride oligomers have a molecular weight less than about 5000, typically between about 100 and 5000 Daltons, or are defined as including between one to about 20 diacid units linked by anhydride bonds. In certain embodiments, the diacids are those normally found in the Krebs glycolysis cycle. The anhydride oligomer compounds have high chemical reactivity.
The oligomers can be formed in a reflux reaction of the diacid with excess acetic anhydride. The excess acetic anhydride is evaporated under vacuum, and the resulting oligomer, which is a mixture of species which include between about one to twenty diacid units linked by anhydride bonds, is purified by recrystallizing, for example, from toluene or other organic solvents. The oligomer is collected by filtration, and washed, for example, in ethers. The reaction produces anhydride oligomers of mono and poly acids with terminal carboxylic acid groups linked to each other by anhydride linkages.
The anhydride oligomer is hydrolytically labile. As analyzed by gel permeation chromatography, the molecular weight may be, for example, on the order of 200-400 for fumaric acid oligomer (FAPP) and 2000-4000 for sebacic acid oligomer (SAPP). The anhydride bonds can be detected by Fourier transform infrared spectroscopy by the characteristic double peak at 1750 cm"1 and 1820 cm"1, with a corresponding disappearance of the carboxylic acid peak normally at 1700 cm"1.
In certain embodiments, the oligomers may be made from diacids described for example in U.S. Patent Nos. 4,757,128, 4,997,904 and 5,175,235, the disclosures of which are incorporated herein by reference. For example, monomers such as sebacic acid, bis(p- carboxy-phenoxy)propane, isophathalic acid, fumaric acid, maleic acid, adipic acid or dodecanedioic acid may be used.
Organic dyes, because of their electronic charge and hydrophilicity or hydrophobicity, may alter the bioadhesive properties of a variety of polymers when incorporated into the polymer matrix or bound to the surface of the polymer. A partial listing of dyes that affect bioadhesive properties include, but are not limited to: acid fuchsin, alcian blue, alizarin red s,
auramine o, azure a and b, Bismarck brown y, brilliant cresyl blue aid, brilliant green, carmine, cibacron blue 3GA, congo red, cresyl violet acetate, crystal violet, eosin b, eosin y, erythrosin b, fast green fcf, giemsa, hematoylin, indigo carmine, Janus green b, Jenner's stain, malachite green oxalate, methyl blue, methylene blue, methyl green, methyl violet 2b, neutral red, Nile blue a, orange II, orange G, orcein, paraosaniline chloride, phloxine b, pyronin b and y, reactive blue 4 and 72, reactive brown 10, reactive green 5 and 19, reactive red 120, reactive yellow 2,3, 13 and 86, rose bengal, safranin, Sudan III and IV, Sudan black B and toluidine blue.
Polymers Functionalized with Hydroxy-Substituted Aromatic Groups
Polymers having an aromatic group which contains one or more hydroxyl groups grafted onto them or coupled to individual monomers are also suitable for use in the bioadhesive coatings of the invention. Such polymers can be biodegradable or nonbiodegradable polymers. The polymer can be hydrophobic. Preferably, the aromatic group is catechol or a derivative thereof and the polymer contains reactive functional groups. Typically, the polymer is a polyanhydride and the aromatic compound is the catechol derivative DOPA. These materials display bioadhesive properties superior to conventional bioadhesives used in therapeutic and diagnostic applications.
The molecular weight of the suitable polymers and percent substitution of the polymer with the aromatic group may vary greatly. The degree of substitution varies based on the desired adhesive strength, it may be as low as 10%, 25% or 50%, or up to 100% substitution. Generally, at least 50% of the monomers in the polymeric backbone are substituted with at least one aromatic group. Preferably, about 100% of the monomers in the polymeric backbone are substituted with at least one aromatic group. The resulting polymer has a molecular weight ranging from about 1 to 2,000 kDa.
The polymer that forms that backbone of the bioadhesive material can be a biodegradable polymer. Examples of preferred biodegradable polymers include synthetic polymers such as poly hydroxy acids, such as polymers of lactic acid and glycolic acid, polyanhydrides, poly(ortho)esters, polyesters, polyurethanes, poly(butyric acid), poly(valeric acid), poly(caprolactone), poly(hydroxybutyrate), poly(lactide-co-glycolide) and poly(lactide- cocaprolactone), and natural polymers such as alginate and other polysaccharides, collagen and chemical derivatives thereof (substitutions, additions of chemical groups, for example, alkyl, alkylene, hydroxylations, oxidations, and other modifications routinely made by those
skilled in the art), albumin and other hydrophilic proteins, zein and other prolamines and hydrophobic proteins, copolymers and mixtures thereof. In general, these materials degrade either by enzymatic hydrolysis or exposure to water in vivo and by surface or bulk erosion. The foregoing materials may be used alone, as physical mixtures (blends), or as co-polymers.
Suitable polymers can formed by first coupling the aromatic compound to the monomer and then polymerizing. In this example, the monomers may be polymerized to form a polymer backbone, including biodegradable and non-biodegradable polymers. Suitable polymer backbones include, but are not limited to, polyanhydrides, polyamides, polycarbonates, polyalkylenes, polyalkylene oxides such as polyethylene glycol, polyalkylene terephthalates such as poly(ethylene terephthalate), polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyethylene, polypropylene, poly(vinyl acetate), poly(vinyl chloride), polystyrene, polyvinyl halides, polyvinylpyrrolidone, polyhydroxy acids, polysiloxanes, polyurethanes and copolymers thereof, alkyl cellulose, hydroxyalkyl celluloses, cellulose ethers, cellulose esters, nitrocellulloses, polymers of acrylic and methacrylic esters, methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxy- propyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxylethyl cellulose, cellulose triacetate, cellulose sulfate sodium salt, and polyacrylates such as poly(methyl methacrylate), poly(ethylmethacrylate), poly(butylmethacrylate), poly(isobutylmethacrylate), poly(hexylmethacrylate), poly(isodecylmethacrylate), poly(lauryl methacrylate), ρoly(phenyl methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl acrylate), poly(octadccyl acrylate).
A suitable polymer backbone can be a known bioadhesive polymer that is hydrophilic or hydrophobic. Hydrophilic polymers include CARBOPOL™, polycarbophil, cellulose esters, and dextran.
Non-biodegradable polymers, especially hydrophobic polymers are also suitable as polymer backbones. Examples of preferred non-biodegradable polymers include ethylene vinyl acetate, poly(methacrylic acid), copolymers of maleic anhydride with other unsaturated polymerizable monomers, poly(butadiene maleic anhydride), polyamides, copolymers and mixtures thereof and dextran, cellulose and derivatives thereof.
Hydrophobic polymer backbones include polyanhydrides, poly(ortho)esters, and polyesters such as polycaprolactone. Preferably, the polymer is sufficiently hydrophobic that it is not readily water soluble, for example the polymer should be soluble up to less than about 1% w/w in water, preferably about 0.1% w/w in water at room temperature or body
temperature. In the most preferred embodiment, the polymer is a polyanhydride, such as a poly(butadiene maleic anhydride) or another copolymer of maleic anhydride. Polyanhydrides may be formed from dicarboxylic acids as described in U.S. Patent No. 4,757,128 to Domb et al, incorporated herein by reference. Suitable diacids include aliphatic dicarboxylic acids, aromatic dicarboxylic acids, aromatic-aliphatic dicarboxylic acid, combinations of aromatic, aliphatic and aromatic-aliphatic dicarboxylic acids, aromatic and aliphatic heterocyclic dicarboxylic acids, and aromatic and aliphatic heterocyclic dicarboxylic acids in combination with aliphatic dicarboxylic acids, aromatic-aliphatic dicarboxylic acids, and aromatic dicarboxylic acids of more than one phenyl group. Suitable monomers include sebacic acid (SA), fumaric acid (FA), bis(p-carboxyphenoxy)propane (UP), isophthalic acid (IPh), and dodecanedioic acid (DD).
A wide range of molecular weights are suitable for the polymer that forms the backbone of the bioadhesive material. The molecular weight may be as low as about 200 Da (for oligomers) up to about 2,000 kDa. Preferably the polymer has a molecular weight of at least 1,000 Da, more preferably at least 2,000 Da, most preferably the polymer has a molecular weight of up to 20 kDa or up to 200 kDa. The molecular weight of the polymer may be up to 2,000 kDa.
The range of substitution on the polymer varies greatly and depends on the polymer used and the desired bioadhesive strength. For example, a butadiene maleic anhydride copolymer that is 100% substituted with DOPA will have the same number of DOPA molecules per chain length as a 67% substituted ethylene maleic anhydride copolymer. Typically, the polymer has a percentage substitution ranging from 10% to 100%, preferably ranging from 50% to 100%.
The polymers and copolymers that form the backbone of the bioadhesive material include reactive functional groups that interact with the functional groups on the aromatic compound.
It is desirable that the polymer or monomer that forms the polymeric backbone contains accessible functional groups that easily react with molecules contained in the aromatic compounds, such as amines and thiols. In a preferred embodiment, the polymer contains amino reactive moieties, such as aldehydes, ketones, carboxylic acid derivatives, cyclic anhydrides, alkyl halides, aryl azides, isocyanates, isothiocyanates, succinimidyl esters or a combination thereof.
Preferably, the aromatic compound containing one or more hydroxyl groups is catechol or a derivative thereof. Optionally, the aromatic compound is a polyhydroxy
aromatic compound, such as a trihydroxy aromatic compound (e.g., phloroglucinol) or a multihydroxy aromatic compound (e.g., tannin). The catechol derivative may contain a reactive group, such as an amino, thiol, or halide group. The preferred catechol derivative is 3,4-dihydroxyphenylalanine (DOPA), which contains a primary amine. Tyrosine, the immediate precursor of DOPA, which differs only by the absence of one hydroxyl group in the aromatic ring, can also be used. Tyrosine is capable of conversion (e.g., by hydroxylation) to the DOPA form. A particularly preferred aromatic compound is an amine-containing aromatic compound, such as an amine-containing catechol derivative (e.g., dopamine).
Two general methods are used to form the polymer product. In one example, a compound containing an aromatic group which contains one or more hydroxyl groups is grafted onto a polymer. In this example, the polymeric backbone is a biodegradable polymer. In a second example, the aromatic compound is coupled to individual monomers and then polymerized.
Any chemistry which allows for the conjugation of a polymer or monomer to an aromatic compound containing one or more hydroxyl groups can be used, for example, if the aromatic compound contains an amino group and the monomer or polymer contains an amino reactive group, this modification to the polymer or monomer is performed through a nucleophilic addition or a nucleophilic substitution reaction, such as a Michael-type addition reaction, between the amino group in the aromatic compound and the polymer or monomer. Additionally, other procedures can be used in the coupling reaction. For example, carbodiimide and mixed anhydride based procedures form stable amide bonds between carboxylic acids or phosphates and amino groups, bifunctional aldehydes react with primary amino groups, bifunctional active esters react with primary amino groups, and divinylsulfone facilitates reactions with amino, thiol, or hydroxy groups.
The aromatic compounds are grafted onto the polymer using standard techniques to form the bioadhesive material. In one example, L-DOPA is grafted to maleic anhydride copolymers by reacting the free amine in L-DOPA with the maleic anhydride bond in the copolymer.
A variety of different polymers can be used as the backbone of the bioadhesive material, as described above. Additional representative polymers include 1:1 random copolymers of maleic anhydride with ethylene, vinyl acetate, styrene, or butadiene. In addition, a number of other compounds containing aromatic rings with hydroxy substituents, such as tyrosine or derivatives of catechol, can be used in this reaction.
In another embodiment, the polymers are prepared by conjugate addition of a compound containing an aromatic group that is attached to an amine to one or more monomers containing an amino reactive group. In a preferred method, the monomer is an acrylate or the polymer is acrylate. For example, the monomer can be a diacrylate such as 1,4-butanediol diacrylate, 1,3 -propanediol diacrylate, 1,2-ethanediol diacrylate, 1,6- hexanediol diacrylate, 2,5-hexanediol diacrylate or 1,3 -propanediol diacrylate. In an example of the coupling reaction, the monomer and the compound containing an aromatic group are each dissolved in an organic solvent (e.g., THF, CH2Cl2, methanol, ethanol, CHCl3, hexanes, toluene, benzene, CCl4, glyme, diethyl ether, etc.) to form two solutions. The resulting solutions are combined, and the reaction mixture is heated to yield the desired polymer. The molecular weight of the synthesized polymer can be controlled by the reaction conditions (e.g., temperature, starting materials, concentration, solvent, etc.) used in the synthesis.
For example, a monomer, such as 1,4-phenylene diacrylate or 1,4-butanediol diacrylate having a concentration of 1.6 M, and DOPA or another primary amine containing aromatic molecule are each dissolved in an aprotic solvent such as DMF or DMSO to form two solutions. The solutions are mixed to obtain a 1:1 molar ratio between the diacrylate and the amine group and heated to 560C to form a bioadhesive material.
Bioadhesive Polymer Blends
Hydrophobic polymers, such as polyesters, poly (anhydrides), ethyl cellulose, even if possibly non-adhesive on their own, may nevertheless be made bioadhesive simply by physically mixing the hydrophobic polymers with one or more suitable compounds (such as catechols or derivatives L-DOPA, D-DOPA, dopamine, or carbidopa, etc.) to create "bioadhesive compositions." Similarly, metal oxides may also be used for this purpose.
The molecular weight of the bioadhesive polymers and percent substitution of the polymers with residues of the compounds disclosed may vary greatly. The degree of substitution varies based on the desired adhesive strength, it may be as low as 10%, 20%, 25%, 50%, or up to 100% substitution. On average, at least 50% of the repeat units in the polymeric backbone are substituted with at least one residue. In one particular embodiment, 75-95% of the residues in the backbone are substituted with at least one residue. In another particular embodiment, on average 100% of the repeat units in the polymeric backbone are substituted with at least one residue. The resulting bioadhesive polymer typically has a molecular weight ranging from about 1 to 2,000 kDa, such as 1 to 1,000 kDa, 10 to 1,000 kDa or 100 to 1,000 kDa. Polymers used in bioadhesive compositions typically have the same range of molecular weights.
Unlike the bioadhesive polymers described above, there is typically no covalent bond formed between the compounds and the polymer in the bioadhesive compositions (i.e., the polymer does not chemically react with the compound, although hydrogen bonds, ionic bonds and/or van der Waals interactions can occur).
Suitable polymers for use in bioadhesive compositions are described above. Typically, the polymer itself may not be bioadhesive, but the polymer can be bioadhesive {e.g., a polymer with hydrogen bond-forming pendant groups). Preferably, the polymer is a hydrophobic polymer such as a poly(lactone), e.g., poly(caprolactone).
To form the bioadhesive compositions of the invention, typically a polymer and a suitable compound are dissolved in a compatible solvent and mixed together. The solvent is then evaporated, preferably at a controlled temperature and rate of removal. Alternatively or in combination with general evaporation, the bioadhesive composition can be spray dried or dried at room temperature.
In another example, a mixture of a polymer and a suitable compound are melted at or slightly above the melting point of the polymer, typically while being mixed. Both the polymer and the suitable compound should be selected such that they are chemically stable {e.g., do not decompose, do not become oxidized) at the melting point temperature. After the composition has re-solidified, it can be milled in order to obtain particles of the desired size.
The subject bioadhesive compositions can also be prepared by dry mixing of a polymer and a suitable compound, provided that the suitable compound is sufficiently distributed throughout the composition.
In each of the above methods, additional components can be added to the mixture prior to dissolution, melting and/or mixing. The additional components are preferably stable under the conditions the mixture is exposed to. In particular, active agents should be stable at the melting point temperature if that method is employed.
The weight ratio of polymer to the suitable compound in a bioadhesive composition can be selected to give the desired amount of bioadhesion. Typically, the weight ratio of polymer to compound is 9:1 to 1:9, such as 3:l to 1:3 or 2:1 to 1:2. For example, when the polymer is predominant component, the weight ratio is 9 : 1 to 1 : 1 , 3 : 1 to 1 : 1 or 2 : 1 to 1 : 1.
In the subject methods and pharmaceutical compositions, the suitable compounds (such as L-DOPA, D-DOPA, dopamine, or carbidopa, etc.) may be used as agents to render the hydrophobic polymers bioadhesive, and/or be used as active ingredients in the pharmaceutical composition to be delivered to the patient. Thus, in certain embodiments, if carbidopa is used as part of the bioadhesive layer (for example, as the bioadhesive material
on the shell of Figure 5, or as the layer to coat the core comprising the second zero-order release portion), the total carbidopa dosage may be adjusted to account for the release of carbidopa from the bioadhesive material.
Similarly, in certain embodiments, when L- or D-dopa is used as the suitable compound to render the hydrophobic polymer bioadhesive, the dosage of total levodopa or precursor thereof may be adjusted elsewhere in, for example, the relevant portion or sub- portions of the IR or CR (controlled release, e.g., zero-order release rate portion).
In certain embodiments, a higher proportion of L-dopa (or D-Dopa) may be used to achieve a significant amount of release (e.g., more or less immediate release) from the polymers. In other embodiments, L- or D-Dopa may be used such that the polymer is still adhesive, but the release of L- or D-Dopa from the bioadhesive polymer is less significant compared to the levodopa or precursors thereof in IR, and/or one or more other portions or sub-portions of the subject dosage form. Coatings
Preferred bioadhesive coatings do not appreciably swell upon hydration, such that they do not substantially inhibit or block movement (e.g., of ingested food) through the gastrointestinal tract, as compared to the polymers disclosed by Duchene et at Generally, polymers that do not appreciably swell upon hydration include one or more hydrophobic regions, such as a polymethylene region (e.g., (CH2)n, where n is 4 or greater). The swelling of a polymer can be assessed by measuring the change in volume when the polymer is exposed to an aqueous solution. Polymers that do not appreciably swell upon hydration expand in volume by 50% or less when fully hydrated. Preferably, such polymers expand in volume by less than 25%, less than 20%, less than 15%, less than 10% or less than 5%. Even more preferably, the bioadhesive coatings are mucophilic. A polymer that does not appreciably swell upon hydration can be mixed with a polymer that does swell (e.g., CARBOPOL™, poly(acrylic acid), provided that the amount of swelling in the polymer does not substantially interfere with bioadhesiveness.
In certain embodiments, the bioadhesive polymeric coating consists of two layers, an inner bioadhesive layer that does not substantially swell upon hydration and an outer bioadhesive layer that is readily hydratable and optionally bioerodable, such as one comprised of CARBOPOL™.
The bioadhesive polymers discussed above can be mixed with one or more plasticizers or thermoplastic polymers. Such agents typically increase the strength and/or reduce the brittleness of polymeric coatings. Examples of plasticizers include dibutyl
sebacate, polyethylene glycol, triethyl citrate, dibutyl adipate, dibutyl fumarate, diethyl phthalate, ethylene oxide-propylene oxide block copolymers such as PLURONIC™ F68 and di(sec-butyl) fumarate. Examples of thermoplastic polymers include polyesters, poly(caprolactone), polylactide, poly(lactide-co- glycolide), methyl methacrylate {e.g., EUDRAGIT™), cellulose and derivatives thereof such as ethyl cellulose, cellulose acetate and hydroxypropyl methyl cellulose (HPMC) and large molecular weight polyanhydrides. The plasticizers and/or thermoplastic polymers are mixed with a bioadhesive polymer to achieve the desired properties. Typically, the proportion of plasticizers and thermoplastic polymers, when present, is from 0.5% to 40% by weight.
In certain embodiments, the bioadhesive polymer coating, in a dry packaged form of a tablet, is a hardened shell.
A tablet or a drug eluting device can have one or more coatings in addition to the bioadhesive polymeric coating. These coatings and their thickness can, for example, be used to control where in the gastrointestinal tract the bioadhesive coating becomes exposed. In one example, the additional coating prevents the bioadhesive coating from contacting the mouth, esophagus, and stomach. In another example, the additional coating remains intact until reaching the small intestine {e.g., an enteric coating).
Examples of coatings include methylmethacrylates, zein, cellulose acetate, cellulose phthalate, HMPC, sugars, enteric polymers, gelatin and shellac. Premature dissolution of a tablet in the mouth can be prevented with hydrophilic polymers such as HPMC or gelatin.
Coatings used in tablets of the invention typically include a pore former, such that the coating is permeable to the drug. Exemplary pore formers include: sugar, mannitol, HPC (hydroxypropyl cellulose), HPMC, dendrites, NaCl, etc.
Tablets and drug eluting devices of the invention can be coated by a wide variety of methods. Suitable methods include compression coating, coating in a fluidized bed or a pan, enrobing, and hot melt (extrusion) coating, etc. Such methods are well known to those skilled in the art.
All the above compositions, derivatives, precursors, additional components that can be used with the subject pramipexole compositions, dosage forms, methods of making and using, etc., axe adaptable or directly useable with the instant invention, and are thus expressly incorporated herein by reference.
Examples:
Having described the invention with reference to certain preferred embodiments, other embodiments will become apparent to one skilled in the art from consideration of the specification. The invention is further defined by reference to the following examples describing in detail the preparation of the composition and methods of use of the invention. It will be apparent to those skilled in the art that many modifications, both to materials and methods, may be practiced without departing from the scope of the invention.
Example 1 Pharmacokinetic Studies for Mirapex® Tablets
Preliminary pharmacokinetic studies were conducted in beagle dogs to evaluate the performance of MIRAPEX® tablets. The purpose of these studies was to determine the performance and limitations of MIRAPEX® tablets.
The results showed that the pharmacokinetic (PK) performance of MIRAPEX® 0.125 mg tablets in fed and fasted beagles was identical, and food intake did not affect the bioavailability of pramipexole (see Figure 2 and Table 1). These results were in accordance to those reported for humans.
However, all six fasted dogs had emesis within 1 hour of dosing, while no emesis was observed in fed dogs. Emesis was mainly attributed to rapid dissolution of tablets with an immediate release of pramipexole, triggering a locally mediated nausea via gastric irritation.
Table 1: Pharmacokinetic Performance of MIRAPEX® 0.125 mg Tablets in Fed and Fasted Beagle Dogs
Example 2 Pramipexole Delayed Release (DR) Tablets
Based on the PK data above, it was determined that an effective formulation of pramipexole with reduced peak plasma levels, and a lag time should reduce the adverse effects.
An additional study was performed to evaluate the performance of MIRAPEX® 0.125 mg tablets, plain, as well as MIRAPEX® 0.125 mg tablets, coated with an enteric polymer, EUDRAGIT L 100 in fasted dogs. In this experiment, MIRAPEX® 0.125 mg tablets were enteric-coated manually to produce the enteric polymer-coated MIRAPEX® 0.125 mg tablets. Specifically, tablets were hand-dipped using forceps in 10% w/v coating solution of EUDRAGIT L 100 in acetone, so as to achieve a final weight gain of 5-20% w/w.
The results of one exemplary experiment are shown in Figure 3 and Table 2.
Table 2: PK Performance and Emesis Response of Enteric-Coated MIRAPEX® 0.125 mg Tablets and Plain MIRAPEX® 0.125 mg Tablets in Fasted Beagle Dogs
The experiments showed that the enteric-coated MIRAPEX tablets remained intact in the gastric pH of the stomach and did not release the drug. The time to achieve maximum plasma concentration (Tmax) was increased by about 1.5 - 2 hours without affecting the extent of absorption. Importantly, none of the dogs involved in this study showed emesis, demonstrating that the modified delayed-release tablets substantially eliminates one side- effect associated with the conventional pramipexole treatment.
Example 3: Pharmacokinetic evaluation ofMirapex 0.125 mg Tablet administered in three times a day dosing regimen
This examples describes pharmacokinetic data for MIRAPEX (pramipexole dihydrochloride) tablets 0.125 mg; (lot#511-047) to be dosed at the 0, 8, and 16 hour time point (Lot# 511-047) in the fed state of the dog model.
Previously, PK studies in beagle dogs have been conducted with single dose of Mirapex 0.125 mg tablet, and this dose has been well tolerated by the beagle dog model. The subject pramipexole extended release capsule formulation contains 0.375 mg of pramipexole.
This study was conducted to compare the bioavailability of a commercially available immediate release formulation to the subject ER formulation containing equivalent amount of the active drug.
Table 3: PK Performance of MIRAPEX® 0.125 mg Tablets three times a day in Beagle Dogs
Example 4A Preparation of the Pramipexole Extended Release (XR) Formulation
Based on the above results, Applicants produced pramipexole extended release (XR) formulation, which contains multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric-coated capsule. Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (Mfl.01) with OPADRY® Clear as a binder.
The placebo core pellets were prepared using low shear granulation, extrusion and spheronization techniques. Following is the composition used for preparing placebo core pellets.
* Evaporated during drying processΛ
The placebo pellets were dried in oven at 5O0C for 17 hours to achieve a desired moisture level of 0.3% w/w. These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves. The particles retained on screen 14 and 16 were used for subsequent pramipexole layering process.
Pramipexole layered pellets were subsequently coated in Vector Mini Fluid Bed Drier (Mfl.01) with rate controlling polymer composition containing ethylcellulose (ETHOCEL® 10 cps.) to achieve a final weight gain of 8.3% w/w and over coated with bioadhesive polymer SPHEROMER™ III polymer (5.3%). These pellets were then encapsulated in a size 1 gelatin capsule and tested for release profile in USP II dissolution apparatus. The pellets were also evaluated for their in vivo performance in beagle dogs (see Figure 5).
The following is the unit dose composition used for preparing pramipexole extended release capsule.
Unit Dose Composition of Pramipexole Extended Release Capsule
* evaporated during processing
Example 4B Preparation of the Pramipexole Extended Release (XR) Formulation
Based on the above results, Applicants produced a pramipexole extended release (XR) formulation, which contains multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric-coated capsule. Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (MfLOl) with OPADRY® Clear as a binder.
The placebo core pellets were prepared using low shear granulation, extrusion and spheronization techniques. Following is the composition used for preparing placebo core pellets.
* Evaporated during drying process.
The placebo pellets were dried in oven at 500C for 17 hours to achieve a desired moisture level of 0.3% w/w. These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves. The particles retained on screen 14 and 16 were used for subsequent pramipexole layering process.
Pramipexole layered pellets were subsequently coated in a Vector Mini Fluid Bed Drier (Mfl.01) with a rate controlling polymer composition containing ethylcellulose (ETHOCEL® 10 cps.) to achieve a final weight gain of 8.3% w/w
These pellets were then encapsulated in a size 1 gelatin capsule and tested for release profile in USP II dissolution apparatus. The pellets were also evaluated for their in vivo performance in beagle dogs (See Figure 6).
The following is the unit dose composition used for preparing pramipexole extended release capsule.
Unit Dose Composition of Spherics' Pramipexole Multiparticulates Coated with 8.3% Ethylcellulose.
* evaporate during processing
Example 5 Preparation of the Pramipexole Delayed and Extended Release Formulation
Extended release capsules from Example 4A were further coated with an enteric coating composition, Acryl-EZE™ White in a pan coater (O'Hara). Specifically, about 10% w/v solution of Acryl-EZE™ White was prepared in ethanol and sprayed on pramipexole extended release (XR) capsule so as to achieve a final weight gain of 12% w/w. These delayed extended release (DXR) capsules were then tested for release profile in USP II dissolution apparatus, and were also evaluated for their in vivo performance in beagle dogs.
The following is the unit dose composition of pramipexole Delayed Extended Release (DXR) capsule.
Unit Dose Composition of the Pramipexole Delayed Extended Release Capsule
* evaporate during processing
The enteric-coated pramipexole capsules were tested for dissolution profiles using USP II apparatus. Initially, the dissolution was performed in 0.1 N HCL media, pH = 1.2 for 2 hours, followed by phosphate buffer pH = 6.8 for 22 hours.
As shown in Figure 4, enteric-coated pramipexole delayed extended release (DXR) capsule showed a two hour delay in acidic environment, followed by a slower release of pramipexole at pH 6.8.
Example 6: Pramipexole ER, 0.375 mg Tablets [5 % coating (80 parts Surelease® + 20 parts OPADRY®) Formulation
An extended release matrix dosage form according to the International Publication No. WO 2004/010999A1 (Lee et at, the entire contents of which is incorporated herein by reference) was prepared as a comparator formulation. The pramipexole matrix based controlled release formulation given in Example 5 of WO 2004/010999A1 (incorporated
herein by reference) was prepared according to the process disclosed therein, with the exception that pramipexole was layered onto the lactose particles to achieve its uniform dispersion. The in vitro release rate of the assembled formulation in phosphate buffer (pH 6.8) using the USP Apparatus II at 50 rpm was similar to that disclosed in Lee.
A study was conducted in fed beagle dogs to evaluate the performance of: (1) extended release multiparticulate-based capsule formulation, (2) matrix-based tablet formulation, both containing 0.375 mg pramipexole administered as a single dose, and (3) MIRAPEX® tablets (0.125 mg) administered in three-times-a-day dosing regimen.
As shown in Figure 6, immediate release MIRAPEX® tablets exhibited rapid initial absorption of pramipexole and dramatic fluctuations in pramipexole concentration, while the extended release formulations resulted in a lag time, followed by extended absorption up to 16 hours.
As shown in Table 4 below, the bioavailability estimate of the extended release capsule formulation was about 50% when compared to MIRAPEX® tablets given in a repeated manner. The bioavailability of Lee matrix formulation was even lower than the multiparticulate-based system. Although in the preliminary study in beagle dogs, decreased bioavailability was observed for the extended release formulation, this can be primarily attributed to short residence time in GI tract. Since there are known differences between humans and dogs in terms of motility, bacterial metabolism and GI transit time, the above extended release formulation is expected to show higher extent of absorption in humans, as the half life of pramipexole is longer in humans.
Table 4: MIRAPEX® tablets, 0.375 mg (0.125 mg X 3) vs. Pramipexole 0.375 mg Extended Release Multiparticulate and Matrix Based Formulations
Example 7: Pramipexole Delayed and Extended Release Capsule Formulations
Applicants have conducted a human pharmacokinetic study to evaluate three subject pramipexole extended release capsules (known as "Type A," "Type B" and "Type C" formulations) against the approved reference product Mirapex® tablets (manufactured and marketed by Boehringer Ingelheim Pharmaceuticals, Inc., and marketed by Pfizer) listed in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations 25th Edition, 2005.
Three formulations of pramipexole extended release capsule, Type A, Type B and Type C, are provided for clinical testing. These formulations are once daily extended release (XR) pramipexole formulations containing multiparticulates encapsulated in enteric coated gelatin capsules. These formulations are similar with respect to the active substance to the existing formulation for Mirapex® tablets, e.g., pramipexole dihydrochloride monohydrate is the active substance in all the formulations. However, the dose levels are different. Each Mirapex® tablet contains 0.125 mg of pramipexole dihydrochloride monohydrate, and is dosed three times a day with a total drug substance level of 0.375 mg (0.125mg x 3). The currently available immediate release pramipexole formulation {e.g., Mirapex®) is not ideal, as it is associated with poor patient compliance as well as treatment-emergent side effects that lead to poor patient tolerance.
The subject formulations contain 0.375 mg of active substance, and are suitable for once-daily administration. They are delayed, extended release oral dosage forms that will maintain effective plasma pramipexole levels to produce a therapeutic effect over approximately 24 hours when administered to patients in need, and should result in diminished incidence and decreased intensity of pramipexole's unwanted side effects.
The pramipexole extended release capsules, Type A, Type B and Type C, use 0.375 mg of pramipexole dihydrochloride monohydrate as their active ingredient. The level used is within the limits specified in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations 25th Edition, 2005, (Orange book). All excipients utilized in these formulations are within or below the listed levels for orally administered products.
The subject extended release formulation was conceptualized for once-daily administration with improved bioavailability, patient compliance and tolerability. It was to provide a lag time with slow absorption followed by steady plasma levels over an extended duration. The objectives of subject formulation approach were to slow down or delay the rapid absorption of pramipexole that has been correlated with the major adverse effects of Mirapex® tablets, while maintaining the effective plasma concentration over a 24 hours period. An enteric polymer coating was applied on the capsule not to allow the drug to get released in the stomach.
The pramipexole extended release capsules, Type A, Type B and Type C, described below differ either in the level of the rate controlling polymer, ethylcellulose, or bioadhesive coating composition containing Spheromer III. Typically, the manufacture of pramipexole multiparticulate extended release capsules Type A, Type B and Type C involves the following steps.
1. Manufacture of placebo core pellets.
2. Manufacture of pramipexole extended release pellets Type A, Type B and Type C.
3. Manufacture of the enteric coated pramipexole extended release capsules Type A, Type B and Type C.
A brief description of the typical manufacturing process and in-process controls are detailed below.
1. Preparation of Placebo Core Pellets:
Mannitol and microcrystalline cellulose are blended with hydroxypropyl cellulose in a blender.
The dry blend is granulated with purified water using a low shear mixer.
The granules are extruded in a double roller extruder using a 1.5 mm screen and the moisture content measured.
The extrudate is spheronized in an extruder.
The pellets are dried in a dryer until the desired moisture content is achieved.
The dried pellets are checked for size distribution. The placebo core pellets are tested for appearance, moisture content and particle size distribution.
2. Preparation of Pramipexole XR Pellets for Types A, B and C
The placebo core pellets are coated with a solution of OPADRY® Clear (YS- 1-19025-A) and pramipexole in the fluidized bed coater until the target weight gain is achieved.
The pramipexole layered pellets are film coated with a polymer solution comprised of Ethocel 10 cps and dibutyl sebacate in the fluidized bed coater until the target weight gain is achieved. The target weight gain is 8.3% w/w for Type A and Type B and 12% w/w of the original pramipexole mannitol pellets for formulation Type C.
The resulting extended release Type B pellets are film coated with a coating solution of SPHEROMER™ III and Poloxamer 188 (Lutrol® F68) in the fluidized bed coater until the target weight gain of 5.3% w/w is achieved. The pellets are then tested for appearance, identification, content assay, residual solvent and in vitro dissolution.
3. Encapsulation and Enteric coating of Drug Product for Types A, B and C Gelatin capsules are manually filled with pramipexole extended release pellets for either Type A, Type B and Type C.
The resulting capsules are applied with a gelatin band at the seam and coated with Acryl-Eze™ White (93018509) enteric coating in the pan coater until the target weight gain is attained. The pellets are then tested for appearance, identification, content uniformity, content assay, impurities and dissolution.
Specifically, pramipexole delayed and extended release (XR) formulation containing multiparticulate beads containing 0.375 mg of pramipexole encased in an enteric coated hard gelatin capsule was formulated. Pramipexole was initially layered on placebo core pellets (1.1- 1.4 mm) using Vector Mini Fluid Bed Drier (Mfl.Ol) with OPADRY® Clear as a binder.
The placebo core pellets were prepared using low shear granulation, extrusion and spheronization technique. Following is the composition used for preparing placebo core pellets.
* Evaporated during drying process.
The placebo pellets were dried in oven at 500C for 12-17 hours to achieve a desired moisture level of 1% w/w. These pellets were then screened through size 10, 12, 14, 16 andl8 mesh sieves and the particles retained on screen 14 and 16 were used for subsequent pramipexole layering process, pramipexole layered pellets were subsequently coated in Vector Mini Fluid Bed Drier (Mfl.01) with rate controlling polymer composition containing ethylcellulose (Ethocel® 10 cps.) to achieve a final weight gain of 8.3% w/w. These pellets were then encapsulated in a size 2 gelatin capsule. These capsules were sealed at the junction of cap and body using an aqueous gelatin solution and later on coated with 1.6 % OPADRY® Clear (YS-1-19025-A). These OPADRY coated capsules were later on coated with an enteric coating composition, Acryl-EZE ' White in a pan coater (O'Hara). 10% w/v solution of Acryl-EZE White was prepared in ethanol and water (90: 10) and sprayed on pramipexole extended release capsule so as to achieve a final weight gain of 12% w/w. These delayed extended release capsules were then tested for release profile in USP II dissolution apparatus and were also evaluated for their in vivo performance in beagle dogs. Following is the unit dose composition used for preparing pramipexole delayed extended release capsules Type A, Type B and Type C.
Unit Dose Composition of the Pramipexole Delayed Extended Release Capsule, Type A,
0.375 mg
* evaporate during processing
Unit Dose Composition of the Pramipexole Delayed Extended Release Capsule, Type B,
0.375 mg
Unit Dose Composition of the Pramipexole Delayed Extended Release Capsule, Type C,
0.375 mg
In vitro dissolution Profile of Pramipexole Formulations
The enteric-coated pramipexole capsules were tested for dissolution profiles using USP II apparatus. Initially, the dissolution was performed in 0.1 N HCL media, pH = 1.2 for 2 hours and later on in phosphate buffer at pH = 6.8 for 24 hours.
No drug was released from the three enteric coated pramipexole Formulations in 0.1 N HCL. Mirapex® tablets dissolved in 10 minutes in phosphate buffer pH 6.8. Figure 7 shows the dissolution profile of the three enteric coated pramipexole formulations in phosphate buffer at pH 6.8.
Pharmacokinetic Evaluation of Pramipexole Formulations in Beagles:
A study was conducted in fasted beagle dogs to evaluate the performance of 0.375 mg pramipexole delayed and extended release multiparticulate based capsule formulations (Types A, B and C) administered as a single dose and Mirapex tablets, (0.125) mg administered in three times a day dosing regimen. As shown in Figure 8, immediate release Mirapex® tablets exhibited rapid initial absorption of pramipexole, while the extended release formulation resulted in a lag time, followed by extended absorption up to 16 hours. As shown in Table 5, the bioavailability estimate of the extended release capsule formulations
was about 50% when compared to Mirapex® tablets given in a repeated manner. Although in the preliminary study in beagle dogs decreased bioavailability was observed for the extended release formulation, this can be primarily attributed to short residence time in beagle GI tract. There are known differences between humans and dogs in terms of motility, bacterial metabolism and GI transit time.
Table 5: Mirapex® tablets, 0.375 mg (0.125 mg X 3) vs. Pramipexole 0.375 mg Extended Release Multiparticulate Formulations
Pilot Singe-dose Pharmacokinetic Study Comparing 0.375 mg Pramipexole Extended/Delayed Release Multiparticulate Formulations with Mirapex® tablets, 0.375 mg (0.125 mg X 3) in Humans
A single-dose, crossover study comparing the pharmacokinetics and tolerability of comparing pramipexole 0.375 mg extended release multiparticulate formulations with Mirapex® tablets, 0.375 mg (0.125 mg X 3) in 12 healthy volunteers was carried out. Each subject received a single dose of each of the formulations in random order under the fasted conditions (a light breakfast was given 60 minutes after the dosing) and plasma levels of pramipexole were measured using LC/MS/MS. The LC-MS/MS method employed injection of plasma samples diluted 1:1 with dichloroacetic acid containing an internal standard (that was not observed in the dog plasma blank sample) at a concentration of 20 ng/mL. The amount of pramipexole was calculated using a weighted calibration curve. The linearity curve was obtained using pramipexole standards ranging from 100 pg/mL to 25 ng/mL. AU standards were prepared in Harlan Dog plasma. In order to assure accuracy throughout the
experiment, quality control (QC) samples were injected every 12 samples. The QC standards were prepared in dog plasma at known concentrations of ~1.0 ng/mL and ~10.0 ng/mL.
The doses were separated by 1-week washout periods. Figures 9A-C represent the pramipexole plasma concentration vs. time graph. The area under the plasma pramipexole vs. time curve (AUC), maximum concentration (Cmax), time to maximum concentration (Tmax) were calculated and are indicated in Table 6.
Table 6: PK parameters comparing different 0.375 mg Pramipexole Delayed Extended Release Multiparticulate Formulations with Mirapex® tablets, 0.375 mg (0.125 mg X 3)
* One of the subjects in Type A testing showed a Cmax of about 1650 pg/mL vs. the average Cmax of 424 pg/mL
Example 8: Pramipexole ER, 0.375 mg Tablets [5 % coating (80 parts Surelease® + 20 parts OPADRY®) Formulation in Fasted condition
This example describes pharmacokinetic data for pramipexole 0.375 mg Tablet (Lot #509-060) coated with OPADRY® / Surelease® in beagle dogs under fasted condition.
Pramipexole was layered on sugar spheres and compressed into a tablet along with other excipients as listed below. A pharmacokinetic study was performed earlier in the fed
conditions using the same formulation; however this study was done under fasted conditions. The data obtained from this study can be used to compare against the subject pramipexole extended release formulations which contain 0.375 mg of pramipexole dihydrochloride.
Pramipexole ER, 0.375 mg Tablets coated with 80 parts Surelease® + 20 parts
OPADRY®
Figure 1OA shows the in vitro dissolution profile obtained using USP II apparatus. For the above formulation, the dissolution was performed in phosphate buffer pH 6.8 for 24 hours. The Surelease® coated tablets formulated in house show an identical profile to the innovator's sustained release formulation.
Figure 10 B is the comparison of above example with Pramipexole Delayed Extended Release Capsule, Type B, 0.375mg described in example 5. Both the formulations demonstrate a similar extent of absorption.
Figure 11 is the comparison of pramipexole ER Formulations with Mirapex (0.125) mg tablets administered in three times a day dosing regimen.
Pramipexole multiparticulates from Example 7 (Type A, Type B or Type C) were fed to beagle dogs in order to evaluate their pharmacokinetic performance and were later on compared to Mirapex 0.125 mg tablets administered in three times a day dosing regimen and Pramipexole ER, 0.375 mg tablets (Example 8). Mirapex (0.125) mg tablets administered in three times a day dosing regimen exhibited rapid initial absorption of pramipexole, whereas pramipexole ER formulations showed an extended absorption with just once a day administration. The bioavailability estimate of the extended release formulations was about
50% when compared to Mirapex® tablets given in a repeated manner. Although in the preliminary study in beagle dogs decreased bioavailability was observed for the extended release formulation, this can be primarily attributed to short residence time in GI tract. There are known differences between humans and dogs in terms of motility, bacterial metabolism and GI transit time. The above extended release formulation is expected to show higher extent of absorption in humans as the half life of pramipexole is longer in humans.
Example 9 Preparation of Pramipexole Extended-Release Pellet Formulation, Lot # 601-048
An extended-release pellet formulation of pramipexole was prepared to be combined with an immediate- and controlled-release multiparticulate formulation of levodopa- carbidopa. Pramipexole was initially layered on placebo core pellets (1.1— 1.4 mm dia.) with Opadry® Clear as a binder using a Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert.
The placebo core pellets were prepared using low-shear granulation, extrusion and spheronization technique. The following table provides the weight and composition of placebo core pellets.
Weight and Composition of Placebo Core Pellets
* Evaporated during drying process.
The placebo pellets were dried in an oven at 5O0C to achieve a desired moisture level of 1% (w/w). These pellets were then screened through size 10, 12, 14, 16 and 18 mesh sieves and the particles retained on screen size 14 and 16 were used for subsequent pramipexole layering process.
Pramipexole layered pellets were subsequently coated in the Vector MFL.Ol Micro Batch Fluid Bed System, equipped with a Wurster insert, with a release rate-controlling polymer composition containing ethylcellulose to achieve a weight gain of 8.3% (w/w), and then coated with bioadhesive Spheromer™ III polymer to a weight gain of 5.3% (w/w).
The unit dose composition of a pramipexole 0.375 mg extended-release pellet formulation is given below.
Unit Dose Composition of Pramipexole 0.375 mg Extended-Release Pellet Formulation
The bioadhesive pramipexole pellets may be optionally top-coated with bioadhesive Spheromer™ I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
Example 10 Production of Levodopa, Carbidopa, and Levodopa-Carbidopa Pellets with Granulation-Extrusion-Spheronization and Fluid Bed Drying
Levodopa, carbidopa, and levodopa-carbidopa pellets were produced with granulation-extrusion-spheronization and fluid bed drying. The production processes included the following:
(1) Weighing levodopa or carbidopa, or both levodopa and carbidopa, optionally a bioadhesive polymer composition, and pharmaceutically acceptable excipients.
(2) Blending levodopa or carbidopa, or both levodopa and carbidopa, and optionally a bioadhesive polymer composition, with pharmaceutically acceptable excipients in a planetary type mixer, Hobart Mixer with a 5-qt mixing bowl, operating at the speed setting #1, for 5-15 min, forming a dry mix.
(3) Granulating the dry mix from step (2) under low shear with a granulation fluid, forming a wet granulation. The granulation fluids were mainly selected from a group consisting of purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, a pharmaceutically acceptable alcohol, a ketone or a chlorinated solvent, a hydro-alcoholic mixture, an alcoholic or hydro- alcoholic solution of a polymeric composition, a solution of a polymeric composition in a chlorinated solvent or in a ketone.
(4) Extruding the wet granulation from step (3) through the screen of a screen-type extruder, Caleva Model 20 (or Model 25) Extruder, operating at 10-20 rpm, and forming breakable wet strands, the extrudate. The screen aperture was 0.8, 1, or 1.5 mm.
(5) Spheronizing the extrudate from step (4) in a spheronizer, Caleva Model 250, equipped with a 2.5-mm spheronization plate, operating at 1000-2000 rpm for 5-10 min, and forming spheronized pellets.
(6) Drying the spheronized pellets from step (5) in a fluidized bed drier, Vector MFL.01 Micro Batch Fluid Bed System, operating at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of 5O0C. Alternatively, pellets were dried either in an ACT (Applied Chemical Technology) fluidized bed drier or in a conventional Precision oven. The ACT fluidized bed drier was operated at an inlet air flow rate of 140-150 fpm (foot per minute) and an inlet air temperature of 1040F. The oven was set at 5O0C.
(7) Screening and classifying the dried pellets from step (6) through a stack of stainless steel sieves, U.S. standard mesh sizes 8, 10, 12, 14, 16, 18, 20, 25, 30, 40, 45, and 60 using a mechanical sieve shaker, W.S. Tyler Sieve Shaker Ro-Tap Rx-29, operated for 5 min. The particle size and distribution of pellet formulations were analyzed, and classified pellets ranging from 0.25 mm (mesh # 60) to 2 mm (mesh # 10) were selected for future film coating or other experimentation.
Levodopa, carbidopa, and levodopa-carbidopa pellets were produced with granulation-extrusion-spheronization and oven drying. The production processes included the steps 1 to 5 and 7 of Example 1 but the spheronized pellets were dried in a Precision gravity oven, operating at 5O0C, for 8-24 h.
Example 11 Production of Levodopa Pellets with Granulation-Extrusion-Spheronization, Lot # 510-095
Three identical sub-lots of levodopa pellets (sub-lots # 511-068, 511-069, and 511- 070) were prepared in accordance with the method described in Example 1. The weight and composition of pellets of the sub-lot # 511-068 are given in the following table. Levodopa was blended with inactive excipients for 5 min. The levodopa-excipients blend was then granulated by spraying purified water while mixing at low shear. The granulation was blended for an additional 5 min and then extruded through a 1.5 mm screen of a Caleva extruder, model 25, operating at 15 rpm. The extrudate was spheronized in a Caleva spheronizer, model 250, operating at 1000 rpm for 5 min. The spheronized pellets were dried in an ACT (Applied Chemical Technology) fluidized bed drier at 104°F±4°F for 75 min. The dried pellets were screened and particles with diameters ranging from 1 mm to 2 mm were selected for future experimentation. The screened pellets of the three sub-lots were blended in a GlobePharma Maxiblend Blender equipped with an 8-qt stainless steel V-shell.
Weight and Composition of Levodopa Pellets, Sub-lot # 511-068
Example 12 Film coating of Levodopa-Carbidopa Pellets with Bioadhesive Polymer, Spheromer™III, Lot # 510-098
Levodopa, carbidopa, and levodopa-carbidopa pellets were film-coated with a bioadhesive polymeric composition, Spheromer™ III. Bioadhesive Spheromer™ III and optionally a functional polymer, or a non-functional polymer, and optionally pharmaceutically acceptable excipients, were dissolved in methanol. The film coating was performed in a fluidized bed coater, Vector MFL.01 Micro Batch Fluid Bed System, equipped with a Wurster insert, operating at an inlet air flow rate of 100-300 lpm (liter per minute) and an inlet air temperature of 35°C±2°C. The pellets were pre-warmed at 350C for 2-5 min and after film-coating were post-dried at 3O0C for 15-30 min. Alternatively, pellets were coated in a Fluid Air Model 5 fluid bed processor, equipped with a Wurster insert, operating at an inlet air flow rate of 70 cfm (cubic foot per minute) and an inlet air temperature of 350C. The pellets were pre-warmed at 4O0C for 5-7 min and after film-coating were post-dried at 350C for 30 min.
Composition of Spheromer™ III Coating Solution, Lot # 511-098
Example 13 Film coating of Levodopa Pellets with Bioadhesive Polymer, Spheromer™ III, and Hydroxypropylcellulose (HPC-SSL), Lot # 511-092
One thousand grams of levodopa pellets, lot # 510-095, were film-coated in a Fluid Air Model 5 fluid bed processor, equipped with a Wurster insert, in accordance with the method described in Example 12. The composition of the coating solution is given below. Spheromer™ III and Hydroxypropylcellulose (HPC-SSL) were dissolved in methanol and sprayed onto the fluidized pellets to obtain a 12% weight gain on pellets.
Composition of Spheromer™ III/Hydroxypropylcellulose (HPC-SSL) Coating Solution, Lot # 511-092
* Methyl alcohol is removed during the coating/drying process.
Example 14 Production ofCarbidopa Granules with Low Shear Granulation, Lot # 511- 101
Carbidopa granules were produced with low shear granulation method consisting of the following processes:
(1) Weighing carbidopa, optionally a bioadhesive polymer composition, and pharmaceutically acceptable excipients.
(2) Blending carbidopa, and optionally a bioadhesive polymer composition, with pharmaceutically acceptable excipients in a planetary type mixer, Hobart Mixer, operating at the speed setting #1, for 5-15 min, forming a dry mix.
(3) Granulating the dry mix from step (2) under low shear with a granulation fluid, forming a wet granulation. The granulation fluid was mainly selected from purified water, an aqueous solution of a mineral or organic acid, an aqueous solution of a polymeric composition, an alcohol, a hydro-alcoholic mixture, or an alcoholic or hydro-alcoholic solution of a polymeric composition.
(4) Drying the granulation from step (3) in a fluidized bed drier, Vector MFL.Ol Micro Batch Fluid Bed System, operating at an inlet air flow rate of 100-300 lpm (liters per minute) and an inlet air temperature of 5O0C. Alternatively, the granulation from step (3) was dried in a Precision gravity oven, operating at 5O0C, for 8-24 h.
(5) Screening and classifying the dried granules from step (4) through a stack of stainless steel sieves, U.S. standard mesh sizes 20 and 60, using a mechanical sieve shaker, W. S. Tyler Sieve Shaker Ro-Tap Rx-29, operated for 5 min. Particle size and distribution of granular formulations were analyzed, and classified granules ranging from 0.25 mm (mesh # 60) to 0.85 mm (mesh # 20) were selected for future experimentation.
The weight and composition of granules are given below. Carbidopa was blended with inactive excipients for 5 min. The carbidopa-excipients blend was then granulated by spraying purified water while mixing at low shear. The granulation was blended for an additional 5 min and then dried in a Precision gravity oven at 5O0C for 8 - 48 hours. The dried granules were screened and particles smaller than 0.85 mm were selected for future experimentation.
Weight and Composition of Carbidopa Granules, Lot # 511-101
Example 15 Preparation of Levodopa-Carbidopa 200 mg/50 mg Multiparticulate Capsules, Lots # 510-099 & 510-100
Levodopa pellets (lot # 510-095), Spheromer™ Ill-coated levodopa-carbidopa pellets (lot # 510-098), HPC-SSL/Spheromer™ Ill-coated levodopa pellets (lot # 511-092), and carbidopa granules (lot # 511-101) were encapsulated in 00-size hard gelatin capsules. Each capsule contained 200 mg levodopa and 50 mg carbidopa anhydrous. The composition of multiparticulates in each capsule formulation is given below.
Composition (mg) of Multiparticulate Capsule Formulations, Lot # 510-099 & 510-100
Example 16 Preparation of Combined Pramipexole 0.375 mg Extended-Release Pellets and Levodopa-Carbidopa 200 mg/50 mg Immediate/Controlled-Release Multiparticulates as a Delayed-Release Capsule Formulation
Pramipexole extended-release pellets, lot # 601-048 (from Example 9), containing 0.375 mg pramipexole, and levodopa-carbidopa immediate/controlled-release multiparticulates, lot # 510-099 (from Example 15), containing 200 mg levodopa and 50 mg carbidopa, were co-encapsulated in two-piece hard gelatin capsules. These capsules were sealed at the junction of cap and body using an aqueous gelatin solution and then coated with 1.6% (w/w) Opadry® Clear (YS-1-19025-A). The Opadry-coated capsules were top-coated with an enteric coating composition, Acryl-EZE™ White, in a pan coater (O'Hara Technologies Labcoat System). The capsules were sprayed with a 10% (w/v) solution of Acryl-EZE White in ethanol and water mixture (90: 10 v/v) so as to achieve a final weight gain of 5-12% (w/w).
The bioadhesive pramipexole and levodopa-carbidopa pellets may be optionally top- coated with bioadhesive Spheromer™ I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
Example 17 Preparation of Pramipexole 0.375 mg Delayed/Extended-Release Capsule Formulation, Lot # 601-056
Pramipexole extended-release pellets, lot # 601-048 (from Example 9) containing 0.375 mg pramipexole were encapsulated in a size 2 hard shell gelatin capsule. These capsules were sealed at the junction of cap and body using an aqueous gelatin solution and coated with 1.6 % Opadry® Clear (YS-1-19025-A). The Opadry-coated capsules were then
coated with an enteric coating composition, Acryl-EZE™ White, in a pan coater (O'Hara Technologies Labcoat System). ). The capsules were sprayed with a 10% (w/v) solution of Acryl-EZE™ White in ethanol and water mixture (90:10 v/v) so as to achieve a final weight gain of 5-12% (w/w).
The unit dose composition of a pramipexole 0.375 mg delayed/extended-release capsule formulation is given below.
Unit Dose Composition of Pramipexole 0.375 mg Delayed/Extended-Release Capsule Formulation
Example 18 Preparation of Combined Pram ipexole 0.375 mg Delayed/Extended-Release Pellets and Levodopa-Carbidopa 200 mg/50 mglmmediate/Controlled-Release Multiparticulates as a Capsule Formulation
Pramipexole delayed/extended-release pellets, lot # 601-056 (from Example 17), containing 0.375 mg pramipexole, and levodopa-carbidopa immediate-controlled-release multiparticulates, lot # 510-099 (from Example 15), containing 200 mg levodopa and 50 mg carbidopa, were co-encapsulated in two-piece hard gelatin capsules.
The bioadhesive pramipexole and levodopa-carbidopa pellets may be optionally top- coated with bioadhesive Spheromer™ I polymer, a hypromellose polymer, a hydroxypropylcellulose polymer, or a polyvinyl alcohol polymer to a weight gain of 2-5% (w/w).
Equivalents
Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents to the specific embodiments of the invention described herein. Such equivalents are intended to be encompassed by the following claims.
All patents, publications, and other references cited above are hereby incorporated by reference in their entirety.
Claims
1. A delayed-release (DR) pramipexole pharmaceutical composition in an orally deliverable form, comprising an enteric coating, a pramipexole core, and pharmaceutically acceptable carriers and excipients, wherein the enteric coating substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
2. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein pramipexole is first released and/or absorbed in intestine.
3. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the enteric coating delays the release of pramipexole by at least about 1.5 - 2 hours.
4. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the enteric coating is selected from: cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate, hydroxypropyl methylcellulose succinate, cellulose acetate succinate, cellulose acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of methylmethacrylic acid and methyl methacrylate, copolymer of methyl acrylate, methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and maleic anhydride (Gantrez ES series), ethyl methyacrylate-methylmethacrylate- chlorotrimethylammonium ethyl acrylate copolymer, natural resins such as zein, shellac and copal collophorium, carboxymethyl ethylcellulose, Spheromer III, Spheromer IV, co-polymerized methacrylic acid / methacrylic acid methyl esters selected from: EUDRAGIT® L12.5, LlOO, EUDRAGIT® S12.5, SlOO, EUDRAGIT® L30D55, EUDRAGIT® FS30D, EUDRAGIT® Ll 00-55, EUDRAGIT® SlOO (Rohm Pharma), KOLLICOAT® MAE30D and 30DP (BASF), ESTACRYL® 3OD (Eastman Chemical), AQUATERIC® and AQUACOAT® CPD30 (FMC)), Acryl-EZE™ White, or equivalents thereof.
5. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the enteric coating becomes soluble around pH 6.8.
6. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the pramipexole pharmaceutical composition comprises a pramipexole salt.
7. The delayed-release pramipexole pharmaceutical composition of claim 6, wherein the .pramipexole salt is pramipexole dihydrochloride monohydrate.
8. The delayed-release pramipexole pharmaceutical composition of claim 1 , wherein the pramipexole core is formulated as an immediate release (IR) composition.
9. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the pramipexole core is formulated as an extended release (XR) composition.
10. The delayed-release pramipexole pharmaceutical composition of claim 9, wherein the XR composition is prepared by coating pramipexole-layered inert pellets with a release-controlling polymer.
11. The delayed-release pramipexole pharmaceutical composition of claim 10, wherein the release-controlling polymer is ethylcellulose-based.
12. The delayed-release pramipexole pharmaceutical composition of claim 10, wherein the release-controlling polymer is selected from: EUDRAGIT® RL; EUDRAGIT® RS; cellulose derivatives selected from: ethylcellulose aqueous dispersions (AQUACO AT®, SURELEASE®), hydroxyethyl cellulose, hydroxypropyl cellulose, or hydroxypropyl methylcellulose; polyvinylpyrrolidone; polyvinylpyrrolidone / vinyl acetate copolymer; OPADRY®, or equivalents thereof.
13. The delayed-release pramipexole pharmaceutical composition of claim 9, which is formulated to provide an effective dose over at least 4 - 20 hours or 8 - 16 hours after administration to the patient.
14. The delayed-release pramipexole pharmaceutical composition of claim 13, wherein the effective dose is about 800 - 1800 pg/mL for Parkinson's Disease treatment.
15. The delayed-release pramipexole pharmaceutical composition of claim 1, wherein the pramipexole core comprises an XR portion and an IR portion.
16. The delayed-release pramipexole pharmaceutical composition of claim 15, wherein the XR portion and the IR portion are both multiparticulate beads / pellets embedded within an inactive dissolvable / disintegratable matrix.
17. The delayed-release pramipexole pharmaceutical composition of claim 15, wherein the XR portion and the IR portion are each a symmetric or asymmetric portion of the pramipexole core.
18. The delayed-release pramipexole pharmaceutical composition of claim 15, wherein the XR portion is partially or completely covered by a rate-controlling coating that controls the release rate of the XR portion.
19. The delayed-release pramipexole pharmaceutical composition of claim 1 , which is formulated as a once-a-day composition.
20. The delayed-release pramipexole pharmaceutical composition of claim 19, wherein the once-a-day composition contains about 0.375 mg, 0.5 mg, 1.0 mg, 1.5 mg, 3.0 mg, or 4.5 mg of pramipexole dihydrochloride monohydrate, or equivalent thereof.
21. The delayed-release pramipexole pharmaceutical composition of claim 1 , further comprising a bioadhesive layer that selectively adheres to the lower GI tract.
22. The delayed-release pramipexole pharmaceutical composition of claim 21 , wherein the bioadhesive layer comprises polymeric materials selected from polyamides, polyalkylene glycols, polyalkylene oxides, polyvinyl alcohols, polyvinylpyrrolidone, polyglycolides, polyurethanes, polymers of acrylic and methacrylic esters, polylactides, poly(butyric acid), polyanhydrides, polyorthoesters, poly(fumaric acid), poly(maleic acid), polycarbonates, polyalkylenes, polyalkylene terephthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polysiloxanes, polystyrene, poly(lactide-co-glycolide), blends and copolymers thereof.
23. The delayed-release pramipexole pharmaceutical composition of claim 1, which, upon administering to an individual, does not induce at least one undesirable side-effect selected from: nausea, emesis, insomnia, hallucination, somnolence, constipation, and gastric and/or intestinal complication at a severity induced by administration of an immediate-release formulation of the same dosage.
24. The delayed-release pramipexole pharmaceutical composition of claim 23, wherein the nausea or emesis results from a locally mediated gastric irritation triggered by the immediate release formulation.
25. The delayed-release pramipexole pharmaceutical composition of claim 1 , which has substantially the same bioavailability and/or maximum blood concentration (Cmax) compared to a pramipexole pharmaceutical composition of equivalent dosage without the enteric coating.
26. The delayed-release pramipexole pharmaceutical composition of claim 1, which is suitable for human administration, or for veterinary treatment of a non-human mammal.
27. A method of preparing a pramipexole pharmaceutical composition, comprising coating pramipexole with an enteric coating that substantially eliminates the release and/or absorption of pramipexole in the upper gastrointestinal (GI) tract.
28. A method of treating Parkinson's Disease in an individual, comprising administering to the individual a delayed-release pramipexole pharmaceutical composition of claim 1.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06773930A EP1901721A1 (en) | 2005-06-23 | 2006-06-23 | Delayed release or extended-delayed release dosage forms of pramipexole |
| PCT/US2006/024665 WO2007002518A1 (en) | 2005-06-23 | 2006-06-23 | Delayed release or extended-delayed release dosage forms of pramipexole |
| CA002613107A CA2613107A1 (en) | 2005-06-23 | 2006-06-23 | Delayed release or extended-delayed release dosage forms of pramipexole |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69360205P | 2005-06-23 | 2005-06-23 | |
| US60/693,602 | 2005-06-23 | ||
| PCT/US2006/024665 WO2007002518A1 (en) | 2005-06-23 | 2006-06-23 | Delayed release or extended-delayed release dosage forms of pramipexole |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007002518A1 true WO2007002518A1 (en) | 2007-01-04 |
Family
ID=38556398
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/024665 WO2007002518A1 (en) | 2005-06-23 | 2006-06-23 | Delayed release or extended-delayed release dosage forms of pramipexole |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP1901721A1 (en) |
| CA (1) | CA2613107A1 (en) |
| WO (1) | WO2007002518A1 (en) |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007056570A2 (en) * | 2005-11-07 | 2007-05-18 | Teva Pharmaceutical Industries Ltd. | Levodopa compositions |
| WO2007090882A2 (en) * | 2006-02-10 | 2007-08-16 | Boehringer Ingelheim International Gmbh | Pharmaceutical extended release compositions comprising pramipexole |
| WO2008145252A1 (en) * | 2007-05-25 | 2008-12-04 | Boehringer Ingelheim International Gmbh | Pharmaceutical formulation comprising pramipexole |
| US7695734B2 (en) | 2004-08-13 | 2010-04-13 | Boehringer Ingelheim International Gmbh | Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof |
| WO2010115267A1 (en) * | 2009-04-09 | 2010-10-14 | Purdue Pharma | Once-daily oral ir/cr pramipexole formulation |
| US20100291201A1 (en) * | 2009-05-14 | 2010-11-18 | Cerovene, Inc. | Coated pharmaceutical capsule dosage form |
| WO2011148243A1 (en) | 2010-05-24 | 2011-12-01 | Lupin Limited | Extended release formulation of pramipexole |
| EP2448411A1 (en) * | 2009-07-02 | 2012-05-09 | Supernus Pharmaceuticals, Inc. | A method of treatment of a neurological disorder |
| WO2012140604A1 (en) * | 2011-04-15 | 2012-10-18 | Sandoz Ag | Stable formulations of pramipexole hydrochloride |
| US8399016B2 (en) | 2002-07-25 | 2013-03-19 | Boehringer Ingelheim International Gmbh | Sustained-release tablet composition of pramipexole |
| EP2588106A2 (en) * | 2010-07-02 | 2013-05-08 | Hyundai Pharm Co., Ltd. | Sustained-release pharmaceutical composition containing pramipexole or pharmaceutically acceptable salt thereof with improved stability |
| US8557283B2 (en) | 2007-12-28 | 2013-10-15 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US8715728B2 (en) | 2004-08-13 | 2014-05-06 | Boehringer Ingelheim International Gmbh | Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof |
| US8969417B2 (en) | 2008-06-06 | 2015-03-03 | Pharmatwob Ltd. | Pharmaceutical compositions for treatment of Parkinsons disease |
| WO2015054302A1 (en) | 2013-10-07 | 2015-04-16 | Impax Laboratories, Inc. | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| EP2305742B1 (en) * | 2009-10-01 | 2017-02-22 | Symrise AG | Spherical core-shell-particle |
| WO2017091739A1 (en) * | 2015-11-23 | 2017-06-01 | Grace Therapeutics Llc | Topical film-forming spray |
| US9895422B2 (en) | 2009-06-05 | 2018-02-20 | Veroscience Llc | Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders |
| WO2020139571A1 (en) * | 2018-12-27 | 2020-07-02 | Chase Therapeutics Corporation | Domperidone compositions and methods for treating depression |
| WO2020191229A1 (en) * | 2019-03-20 | 2020-09-24 | Lyndra, Inc. | Capsules and capsule coatings for gastric residence dosage forms |
| US10987313B2 (en) | 2013-10-07 | 2021-04-27 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109316466B (en) * | 2017-07-31 | 2022-04-05 | 深圳翰宇药业股份有限公司 | Pramipexole dihydrochloride sustained-release preparation and preparation method thereof |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030045539A1 (en) * | 2000-11-06 | 2003-03-06 | Baltazar Gomez-Mancilla | Cabergoline and pramipexole: new uses and combinations |
| WO2003053402A1 (en) * | 2001-12-20 | 2003-07-03 | Pharmacia Corporation | Zero-order sustained released dosage forms and method of making the same |
| US20030152627A1 (en) * | 2001-01-31 | 2003-08-14 | Thomas Beckert | Multi-particulate form of medicament, comprising at least two differently coated forms of pellet |
| WO2004010982A1 (en) * | 2002-07-25 | 2004-02-05 | Pharmacia Corporation | Method of preparing solid dosage forms coated in two layers comprising a water-insoluble polymer and a water-soluble pore former |
| US20040166159A1 (en) * | 2002-05-29 | 2004-08-26 | Chien-Hsuan Han | Pharmaceutical dosage forms having immediate and controlled release properties that contain an aromatic amino acid decarboxylase inhibitor and levodopa |
| WO2004087175A1 (en) * | 2003-04-04 | 2004-10-14 | Pharmacia Corporation | Oral extended release compressed tablets of multiparticulates |
| WO2004091585A1 (en) * | 2003-04-16 | 2004-10-28 | Synthon B.V. | Orally disintegrating tablets |
| WO2005014562A1 (en) * | 2003-07-25 | 2005-02-17 | Synthon B.V. | Pramipexole acid addition salts |
| WO2005079748A2 (en) * | 2004-02-13 | 2005-09-01 | Lacer, S.A. | Pharmaceutical preparation for sustained release of a pharmaceutically active ingredient |
| WO2006015943A2 (en) * | 2004-08-13 | 2006-02-16 | Boehringer Ingelheim International Gmbh | Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof, method for manufacturing the same and use thereof |
-
2006
- 2006-06-23 CA CA002613107A patent/CA2613107A1/en not_active Abandoned
- 2006-06-23 WO PCT/US2006/024665 patent/WO2007002518A1/en active Application Filing
- 2006-06-23 EP EP06773930A patent/EP1901721A1/en not_active Withdrawn
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030045539A1 (en) * | 2000-11-06 | 2003-03-06 | Baltazar Gomez-Mancilla | Cabergoline and pramipexole: new uses and combinations |
| US20030152627A1 (en) * | 2001-01-31 | 2003-08-14 | Thomas Beckert | Multi-particulate form of medicament, comprising at least two differently coated forms of pellet |
| WO2003053402A1 (en) * | 2001-12-20 | 2003-07-03 | Pharmacia Corporation | Zero-order sustained released dosage forms and method of making the same |
| US20040166159A1 (en) * | 2002-05-29 | 2004-08-26 | Chien-Hsuan Han | Pharmaceutical dosage forms having immediate and controlled release properties that contain an aromatic amino acid decarboxylase inhibitor and levodopa |
| WO2004010982A1 (en) * | 2002-07-25 | 2004-02-05 | Pharmacia Corporation | Method of preparing solid dosage forms coated in two layers comprising a water-insoluble polymer and a water-soluble pore former |
| WO2004087175A1 (en) * | 2003-04-04 | 2004-10-14 | Pharmacia Corporation | Oral extended release compressed tablets of multiparticulates |
| WO2004091585A1 (en) * | 2003-04-16 | 2004-10-28 | Synthon B.V. | Orally disintegrating tablets |
| WO2005014562A1 (en) * | 2003-07-25 | 2005-02-17 | Synthon B.V. | Pramipexole acid addition salts |
| WO2005079748A2 (en) * | 2004-02-13 | 2005-09-01 | Lacer, S.A. | Pharmaceutical preparation for sustained release of a pharmaceutically active ingredient |
| WO2006015943A2 (en) * | 2004-08-13 | 2006-02-16 | Boehringer Ingelheim International Gmbh | Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof, method for manufacturing the same and use thereof |
Cited By (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8399016B2 (en) | 2002-07-25 | 2013-03-19 | Boehringer Ingelheim International Gmbh | Sustained-release tablet composition of pramipexole |
| US7695734B2 (en) | 2004-08-13 | 2010-04-13 | Boehringer Ingelheim International Gmbh | Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof |
| US8377977B2 (en) | 2004-08-13 | 2013-02-19 | Boehringer Ingelheim International Gmbh | Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof |
| US8715728B2 (en) | 2004-08-13 | 2014-05-06 | Boehringer Ingelheim International Gmbh | Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof |
| WO2007056570A2 (en) * | 2005-11-07 | 2007-05-18 | Teva Pharmaceutical Industries Ltd. | Levodopa compositions |
| WO2007056570A3 (en) * | 2005-11-07 | 2007-07-05 | Teva Pharma | Levodopa compositions |
| WO2007090882A2 (en) * | 2006-02-10 | 2007-08-16 | Boehringer Ingelheim International Gmbh | Pharmaceutical extended release compositions comprising pramipexole |
| WO2007090882A3 (en) * | 2006-02-10 | 2007-12-13 | Boehringer Ingelheim Int | Pharmaceutical extended release compositions comprising pramipexole |
| WO2008145252A1 (en) * | 2007-05-25 | 2008-12-04 | Boehringer Ingelheim International Gmbh | Pharmaceutical formulation comprising pramipexole |
| US9533046B2 (en) | 2007-12-28 | 2017-01-03 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US9463246B2 (en) | 2007-12-28 | 2016-10-11 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US9089607B2 (en) | 2007-12-28 | 2015-07-28 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US9901640B2 (en) | 2007-12-28 | 2018-02-27 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US9089608B2 (en) | 2007-12-28 | 2015-07-28 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US8557283B2 (en) | 2007-12-28 | 2013-10-15 | Impax Laboratories, Inc. | Controlled release formulations of levodopa and uses thereof |
| US9259418B2 (en) | 2008-06-06 | 2016-02-16 | Pharmatwo B Ltd | Pharmaceutical compositions for treatment of Parkinson's disease |
| US8969417B2 (en) | 2008-06-06 | 2015-03-03 | Pharmatwob Ltd. | Pharmaceutical compositions for treatment of Parkinsons disease |
| WO2010115267A1 (en) * | 2009-04-09 | 2010-10-14 | Purdue Pharma | Once-daily oral ir/cr pramipexole formulation |
| US20100291201A1 (en) * | 2009-05-14 | 2010-11-18 | Cerovene, Inc. | Coated pharmaceutical capsule dosage form |
| US20120244216A1 (en) * | 2009-05-14 | 2012-09-27 | Shah Manish S | Coated pharmaceutical capsule dosage form |
| US10688155B2 (en) | 2009-06-05 | 2020-06-23 | Veroscience Llc | Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders |
| US9895422B2 (en) | 2009-06-05 | 2018-02-20 | Veroscience Llc | Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders |
| EP2448411A4 (en) * | 2009-07-02 | 2012-11-28 | Supernus Pharmaceuticals Inc | A method of treatment of a neurological disorder |
| EP2448411A1 (en) * | 2009-07-02 | 2012-05-09 | Supernus Pharmaceuticals, Inc. | A method of treatment of a neurological disorder |
| EP2305742B1 (en) * | 2009-10-01 | 2017-02-22 | Symrise AG | Spherical core-shell-particle |
| JP2013526601A (en) * | 2010-05-24 | 2013-06-24 | ルピン・リミテッド | Sustained release formulation of pramipexole |
| WO2011148243A1 (en) | 2010-05-24 | 2011-12-01 | Lupin Limited | Extended release formulation of pramipexole |
| EP2588106A4 (en) * | 2010-07-02 | 2014-01-01 | Hyundai Pharm Co Ltd | Sustained-release pharmaceutical composition containing pramipexole or pharmaceutically acceptable salt thereof with improved stability |
| EP2588106A2 (en) * | 2010-07-02 | 2013-05-08 | Hyundai Pharm Co., Ltd. | Sustained-release pharmaceutical composition containing pramipexole or pharmaceutically acceptable salt thereof with improved stability |
| WO2012140604A1 (en) * | 2011-04-15 | 2012-10-18 | Sandoz Ag | Stable formulations of pramipexole hydrochloride |
| EP3782614A1 (en) * | 2013-10-07 | 2021-02-24 | Impax Laboratories, LLC | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US11357733B2 (en) | 2013-10-07 | 2022-06-14 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US10098845B2 (en) | 2013-10-07 | 2018-10-16 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US10292935B2 (en) | 2013-10-07 | 2019-05-21 | Impax Laboratories, Inc. | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US11666538B2 (en) | 2013-10-07 | 2023-06-06 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US10688058B2 (en) | 2013-10-07 | 2020-06-23 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| EP3054929A4 (en) * | 2013-10-07 | 2017-05-17 | Impax Laboratories, Inc. | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US11622941B2 (en) | 2013-10-07 | 2023-04-11 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| WO2015054302A1 (en) | 2013-10-07 | 2015-04-16 | Impax Laboratories, Inc. | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US10973769B2 (en) | 2013-10-07 | 2021-04-13 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| US10987313B2 (en) | 2013-10-07 | 2021-04-27 | Impax Laboratories, Llc | Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof |
| WO2017091739A1 (en) * | 2015-11-23 | 2017-06-01 | Grace Therapeutics Llc | Topical film-forming spray |
| AU2016359706B2 (en) * | 2015-11-23 | 2022-07-28 | Acasti Pharma U.S., Inc. | Topical film-forming spray |
| CN113329747A (en) * | 2018-12-27 | 2021-08-31 | 才思治疗公司 | Domperidone compositions and methods for treating depression |
| WO2020139571A1 (en) * | 2018-12-27 | 2020-07-02 | Chase Therapeutics Corporation | Domperidone compositions and methods for treating depression |
| WO2020191229A1 (en) * | 2019-03-20 | 2020-09-24 | Lyndra, Inc. | Capsules and capsule coatings for gastric residence dosage forms |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1901721A1 (en) | 2008-03-26 |
| CA2613107A1 (en) | 2007-01-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007002518A1 (en) | Delayed release or extended-delayed release dosage forms of pramipexole | |
| US9744137B2 (en) | Topiramate compositions and methods of enhancing its bioavailability | |
| US20070148238A1 (en) | Dosage forms for movement disorder treatment | |
| US20100316712A1 (en) | Pharmaceutical compositions for treatment of parkinson's disease and related disorders | |
| US20080131492A1 (en) | Dosage forms for movement disorder treatment | |
| RU2385712C2 (en) | Controlled-release formulation | |
| US20100226855A1 (en) | Rate-Controlled Oral Dosage Formulations | |
| ES2606463T3 (en) | Combination of levodopa / carbidopa immediate release and controlled release dosage forms | |
| TWI495491B (en) | Compressible-coated pharmaceutical compositions and tablets and methods of manufacture | |
| US20150272888A1 (en) | Misuse preventative, controlled release formulation | |
| US20080260819A1 (en) | Sustained release compositions of drugs | |
| JP6846343B2 (en) | Methods and compositions for the treatment of attention deficit disorder in particular | |
| JP2015098477A (en) | Compositions comprising weakly basic drugs and controlled-release dosage forms | |
| US20130259941A1 (en) | Controlled release dosage forms | |
| JP2004521910A (en) | Tramadol | |
| US8642078B2 (en) | Coated formulations for tolterodine | |
| US7235258B1 (en) | Sustained-release formulations for treating CNS-mediated disorders | |
| US20090028935A1 (en) | Carvedilol forms, compositions, and methods of preparation thereof | |
| AU1969501A (en) | Sustained-release formulations for treating cns-mediated disorders | |
| Surti et al. | Colonic drug delivery systems as multiunit potential: therapeutic strategies and opportunities | |
| TW201813643A (en) | Enteric-coated medicinal composition of duloxetine and method of making the same | |
| AU2005202728A1 (en) | Sustained-release formulations for treating CNS-mediated disorders | |
| EP2886110A1 (en) | Multi-layered, multiple unit pharmaceutical compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| ENP | Entry into the national phase |
Ref document number: 2613107 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006773930 Country of ref document: EP |