WO2007136506A2 - Polyolefin solution polymerization process and polymer - Google Patents
Polyolefin solution polymerization process and polymer Download PDFInfo
- Publication number
- WO2007136506A2 WO2007136506A2 PCT/US2007/010070 US2007010070W WO2007136506A2 WO 2007136506 A2 WO2007136506 A2 WO 2007136506A2 US 2007010070 W US2007010070 W US 2007010070W WO 2007136506 A2 WO2007136506 A2 WO 2007136506A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- bis
- phenyl
- oxoyl
- phenoxy
- Prior art date
Links
- NZIBATAMHTYNNK-UHFFFAOYSA-N CONCCC1CC1 Chemical compound CONCCC1CC1 NZIBATAMHTYNNK-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F10/00—Homopolymers and copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F10/00—Homopolymers and copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
- C08F10/02—Ethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/04—Polymerisation in solution
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F210/00—Copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond
- C08F210/16—Copolymers of ethene with alpha-alkenes, e.g. EP rubbers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65908—Component covered by group C08F4/64 containing a transition metal-carbon bond in combination with an ionising compound other than alumoxane, e.g. (C6F5)4B-X+
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F4/00—Polymerisation catalysts
- C08F4/42—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors
- C08F4/44—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides
- C08F4/60—Metals; Metal hydrides; Metallo-organic compounds; Use thereof as catalyst precursors selected from light metals, zinc, cadmium, mercury, copper, silver, gold, boron, gallium, indium, thallium, rare earths or actinides together with refractory metals, iron group metals, platinum group metals, manganese, rhenium technetium or compounds thereof
- C08F4/62—Refractory metals or compounds thereof
- C08F4/64—Titanium, zirconium, hafnium or compounds thereof
- C08F4/659—Component covered by group C08F4/64 containing a transition metal-carbon bond
- C08F4/65912—Component covered by group C08F4/64 containing a transition metal-carbon bond in combination with an organoaluminium compound
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L7/00—Compositions of natural rubber
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S526/00—Synthetic resins or natural rubbers -- part of the class 520 series
- Y10S526/905—Polymerization in presence of transition metal containing catalyst in presence of hydrogen
Definitions
- Catalyst compositions based on well defined donor ligand containing metal complexes referred to as post-metal locene complexes have been shown to give products having better comonomer incorporation and narrow molecular weight distribution.
- these catalysts often have poor high temperature stability and suffer from poor catalytic efficiencies, especially at elevated polymerization temperatures.
- Examples of one type of the foregoing post metallocene catalysts are disclosed in USP 6,827,976, where Group 3-6 or Lanthanide metal complexes, preferably Group 4 metal complexes, of bridged divalent aromatic ligands containing a divalent Lewis base chelating group are disclosed.
- Higher solution reaction temperatures are particularly desired for olefin polymerizations in order to improve operating efficiency and to produce long chain branching in the resulting polymer.
- Long chain branching in olefin polymers is believed to result in one embodiment from the incorporation of vinyl terminated polymers, generated in situ by ⁇ -hydride elimination that results in vinyl group formation in growing polymer chains. These processes are benefited by use of high reaction temperatures and high monomer conversion conditions.
- catalyst compositions capable of incorporating long chain branching such as for example reincorporation of in situ produced vinyl terminated polymer, under the foregoing extreme reaction conditions is highly desired.
- certain metal complexes may be employed in a solution polymerization process to prepare high molecular weight ethylene containing interpolymers containing relatively large quantities of long chain branching therein at high olefin conversions if certain process conditions are observed.
- the resulting polymer products possess desirable properties such as better flexibility, reduced density (greater comonomer incorporation) and improved processability (less energy required for extrusion, reduced melt fracture and reduction of surface imperfections or "sharkskin" formation).
- This unique combination of polymer properties is also attainable by use of low molar ratios (200 or less, preferably 100 or less, more preferably 80 or less, based on zirconium) of an alkylalumoxane cocatalyst or a trialkylaluminum- modified alumoxane cocatalyst.
- the polymers can be prepared under high temperature, high conversion conditions at high catalyst efficiencies.
- the present invention is particularly advantageous for use under continuous solution polymerization conditions wherein a reaction mixture comprising a metal complex, an activating cocatalyst or cocatalyst mixture, optionally a chain transfer agent, and at least one C 2 . 2 o ⁇ -olefin is continuously added to a reactor operating under solution polymerization conditions, and polymer product is continuously or semi-continuously removed therefrom.
- the invention is used to prepare copolymers of ethylene and at least one C 3 . 20 ⁇ -olefin, preferably ethylene and at least one 0 3 . 3 ⁇ -olefin, having improved processability.
- the invention is particularly suitable for production of resins that are used in the insulation layer of electrical wires and cables, particularly in medium and high voltage applications, films and extruded objects having improved surface appearance and reduced energy consumption in their preparation.
- references to the Periodic Table of the Elements herein shall refer to the Periodic Table of the Elements, published and copyrighted by CRC Press, Inc., 2003. Also, any references to a Group or Groups shall be to the Group or Groups reflected in this Periodic Table of the Elements using the IUPAC system for numbering groups. Unless stated to the contrary, implicit from the context, or customary in the art, all parts and percents are based on weight and all test methods are current as of the filing date hereof.
- compositions claimed herein through use of the term “comprising” may include any additional additive, adjuvant, or compound whether polymeric or otherwise, unless stated to the contrary.
- hexane includes all isomers of hexane individually or collectively.
- compound and “complex” are used interchangeably herein to refer to organic-, inorganic- and organometal compounds.
- atom refers to the smallest constituent of an element regardless of ionic state, that is, whether or not the same bears a charge or partial charge or is bonded to another atom.
- heteroatom refers to an atom other than carbon or hydrogen.
- Preferred heteroatoms include: F, Cl, Br, N, O, P, B, S, Si, Sb, Al, Sn, As, Se and Ge.
- amorphous refers to a polymer lacking a crystalline melting point as determined by differential scanning calorimetry (DSC) or equivalent technique.
- hydrocarbyl refers to univalent substituents containing only hydrogen and carbon atoms, including branched or unbranched, saturated or unsaturated, cyclic, polycyclic or noncyclic species. Examples include alkyl-, cycloalkyl-, alkenyl-, alkadienyl-, cycloalkenyl-, cycloalkadienyl-, aryl-, and alkynyl- groups. “Substituted hydrocarbyl” refers to a hydrocarbyl group that is substituted with one or more nonhydrocarbyl substituent groups.
- heteroatom containing hydrocarbyl or “heterohydrocarbyl” refer to univalent groups in which at least one atom other than hydrogen or carbon is present along with one or more carbon atom and one or more hydrogen atoms.
- heterocarbyl refers to groups containing one or more carbon atoms and one or more heteroatoms, but no hydrogen atoms.
- the bond between the carbon atom and any heteroatom as well as the bonds between any two heteroatoms may be a single or multiple covalent bond or a coordinating or other donative bond.
- an alkyl group substituted with a heterocycloalkyl-, aryl- substituted heterocycloalkyl-, heteroaryl-, alkyl- substituted heteroaryl-, alkoxy-, aryloxy-, dihydrocarbylboryl-, dihydrocarbylphosphino-, dihydrocarbylamino-, trihydr ⁇ carbylsilyK hydrocarbylthio-, or hydrocarbylseJeno- group is within the scope of the term heteroalkyl.
- specific heteroalkyl groups include cyanomethyl-, benzoylmethyl-, (2- pyridyl)methyl-, and trifluoromethyl- groups.
- aromatic refers to a polyatomic, cyclic, conjugated ring system containing (4 ⁇ +2) ⁇ -electrons, wherein ⁇ is an integer greater than or equal to 1.
- fused as used herein with respect to a ring system containing two or more polyatomic, cyclic rings means that with respect to at least two rings thereof, at least one pair of adjacent atoms is included in both rings.
- aryl refers to a monovalent aromatic substituent which may be a single aromatic ring or multiple aromatic rings which are fused together, linked covalentJy, or linked to a common group such as a methylene or ethylene moiety. Examples of aromatic ring(s) include phenyl, naphthyl, anthracenyl, and biphenyl, among others.
- Substituted aryl refers to an aryl group in which one or more hydrogen atoms bound to any carbon is replaced by one or more functional groups such as alkyl, substituted alkyl, cycloalkyl, substituted cycloalkyl, heterocycloalkyl, substituted heterocycloalkyl, halogen, alkylhalos (for example, CF 3 ), hydroxy, amino, phosphido, alkoxy, amino, thio, nitro, and both saturated and unsaturated cyclic hydrocarbons which are fused to the aromatic ring(s), linked covalently or linked to a common group such as a methylene or ethylene moiety.
- functional groups such as alkyl, substituted alkyl, cycloalkyl, substituted cycloalkyl, heterocycloalkyl, substituted heterocycloalkyl, halogen, alkylhalos (for example, CF 3 ), hydroxy, amino, phosphido, alkoxy
- the common linking group may also be a carbonyl as in benzophenone, or oxygen as in diphenylether, or nitrogen as in diphenylamine.
- Embodiments of the invention provide a new solution process for making olefin polymers using a catalyst composition comprising a transition metal complex at high temperature, high catalyst efficiency and high monomer conversion, wherein the produced polymers comprise increased long chain branching content.
- the produced polymers are of high molecular weight (I 2 ⁇ 5.0) and possess I 10 //I 2 ⁇ 10 with a narrow molecular weight distribution (Mw/Mn ⁇ 3.0), thereby indicating the presence of long chain branching.
- Such polymers are suitably employed where improved extrusion performance is desired, such as in molding and extrusion grades of polymer especially for film, foam or wire and cable insulating applications.
- polymer refers to a macromolecular compound prepared by polymerizing one or more monomers.
- a polymer refers to homopolymers, copolymers, terpolymers, interpolymers, and so on.
- interpolymer is used herein interchangeably with the term copolymer to refer to polymers incorporating in polymerized form at least two copolymerizable monomers, or incorporating long chain branching as a result of chain termination/olefin formation reactions in situ, and reincorporation of the in situ formed olefin. Accordingly, copolymers may result from the polymerization of a single monomer, under the correct operating conditions.
- the least prevalent monomer in the resulting copolymer or interpolymer is generally referred to by the term "comonomer".
- the chain length of the resulting long chain branches referred to above is consequently longer than the carbon length resulting from polymerization of any deliberately added comonomer, and in particular, is longer than 6 carbons for ethylene/1 -octene copolymers.
- the presence of long chain branching may also be detected by the increased shear sensitivity of the polymer, as disclosed in EP-A-608,369, and elsewhere, or determined by Melt Index Ratio (MIR), a ratio of polymer melt viscosities measured under differing loads, especially I2)/ ⁇ 2 -
- MIR Melt Index Ratio
- the process described herein may be employed to prepare any olefin interpolymer, especially copolymers of ethylene with one or more C 3-2O olefins, and optionally one or more G ⁇ o diolefms, and, especially, ethylene/propylene, ethylene/1-butene, ethylene/ 1-hexene, ethylene/4- methyl- 1 -pentene, ethylene/styrene, ethylene/propylene/styrene, and ethylene/ 1 -octene copolymers, as well as copolymers of ethylene, propylene and a non-conjugated diene, for example, EPDM interpolymers.
- any olefin interpolymer especially copolymers of ethylene with one or more C 3-2O olefins, and optionally one or more G ⁇ o diolefms, and, especially, ethylene/propylene, ethylene/1-butene, ethylene/ 1-hexen
- Polymerization conditions generally refer to temperature, pressure, monomer content (including comonomer concentration), catalyst concentration, cocatalyst concentration, monomer conversion, or other conditions that influence the properties of the resulting polymer.
- high molecular weight polymers may be prepared having relatively high comonomer incorporation with high catalyst activities, low cocatalyst usage and high I 1 0/I 2 or MIR.
- activities (based on weight of polymer to weight of transition metal) greater than 0.5 g/ ⁇ g, preferably greater than 0.55 g/ ⁇ g, and even greater than 0.6 g/ ⁇ g are possible.
- melt index I2, 110 or I 21 , measured, for example, according to ASTM D-1238 may be employed as an indication of molecular weight.
- melt index is inversely related to the molecular weight of the polymer. The higher the molecular weight, the lower the melt index, although the relationship is not necessarily linear.
- One embodiment of this invention entails a process and the resulting polymer product which process comprises contacting ethylene and one or more C 3-2O ⁇ -olefins in a solution polymerization process.
- the present invented process is particularly advantageous for use under polymerization conditions wherein a reaction mixture comprising metal complex, activating cocatalyst, ethylene, and at least one C 3 . 2 o ⁇ -olefin comonomer (or the individual components thereof) is continuously or intermittently added to a reactor operating under solution polymerization conditions, optionally in the additional presence of a chain transfer agent, and polymerized product is continuously or semi-continuously removed therefrom.
- This process can consist of: 1) Polymerizing ethylene and one or more C 3 .
- the present metal complexes are capable of producing polymers of extremely high molecular weight under a variety of polymerization conditions, and catalyst efficiencies of greater than 0.5 g po iy m er/ ⁇ gme t ai, t thereby allowing the use of a chain transfer agent to control molecular weight without sacrificing molecular weight distribution or long chain branching content.
- a sufficient quantity of chain transfer agent is preferably used so that a substantial decrease in molecular weight (>10 percent) occurs compared to a comparative polymerization without the use of chain transfer agent.
- the chain transfer agent is hydrogen, at least 0.01 mol percent (based on ethylene) is used, and a maximum of about 2 mol percent is used.
- very low density (high comonomer content) polymers can be prepared with high levels of chain transfer agents, while still affording polymers with high I 1 0//I 2 . optionally using low levels of alumoxane activators.
- use of high levels of chain transfer agent and high levels of comonomer with conventional catalysts results in production of increased levels of non- polymerizable end-groups, thereby resulting in a reduction of long chain branch formation and production of polymers having lower I M JH I -
- the metal complexes are activated in various ways to yield catalyst compounds having a vacant coordination site that will coordinate, insert, and polymerize addition polymerizable monomers, especially olefin(s).
- activator or "cocatalyst” is defined to be any compound or component or method which can activate the metal complex in the foregoing manner.
- suitable activators include Lewis acids, non-coordinating ionic activators, ionizing activators, organometal compounds, and combinations of the foregoing substances capable of converting the neutral metal complex to a catalytically active species.
- catalyst activation may involve formation of a cationic, partially cationic, or zwitterionic species, by means of proton transfer, oxidation, or other suitable activation process. It is to be understood that the present invention is operable and fully enabled regardless of whether or not such an identifiable cationic, partially cationic, or zwitterionic species actually results during the activation process, also interchangeably referred to herein as an "ionization” process or "ionic activation process".
- Ionizing cocatalysts may contain an active proton, or some other cation associated with, but not coordinated to or only loosely coordinated to, an anion of the ionizing compound.
- Such compounds are described in European publications EP-A-570982, EP-A-520732, EP-A-495375, EP- A-500944, EP-A-277 003 and EP-A-277004, and U.S. Patents: 5,153, 157, 5,198,401, 5,066,741, 5,206, 197, 5,241 ,025, 5,384,299 and 5,502, 124.
- ammonium cation containing salts especially those containing trihydrocarbyl- substituted ammonium cations containing one or two C 10 - 4 o alkyl groups, especially methylbts(octadecyl)- ammonium- and methylbis(tetradecyl)-ammonium- cations and a non-coordinating anion, especially a tetrakis(perfluoro)arylborate anion, especially tetrakis(pentafluorophenyl)borate.
- the cation may comprise a mixture of hydrocarbyl groups of differing lengths.
- the protonated ammonium cation derived from the commercially available long-chain amine comprising a mixture of two Ci 4 , Ci ⁇ or Q 8 alkyl groups and one methyl group.
- Such amines are available from Chemtura Corp., under the trade name KemamineTM T9701, and from Akzo- Nobel under the trade name ArmeenTM M2HT.
- a most preferred ammonium salt activator is methyldi(Ci 4-2 oalkyl)ammonium tetrakis(pentafluorophenyl)borate.
- ionizing ionic compounds not containing an active proton but capable of forming active catalyst compositions are also contemplated for use herein, and are described in EP-A-426637, EP-A- 573403 and U.S. Patent 5,387,568.
- strong Lewis acids especially tris(perfluoro)aryl borane compounds, such as tris(pentafluorophenyl)borane, which are capable of abstraction of a ligand groups, especially a hydrocarbyl ligand, thereby forming a non-coordinating counter anion for the cationic derivative of the metal complex.
- a class of cocatalysts comprising non-coordinating anions generically referred to as expanded anions, further disclosed in U. S. Patent 6,395,671 , may be suitably employed to activate the metal complexes of the present invention for olefin polymerization.
- these cocatalysts (illustrated by those having imidazolide, substituted imidazoHde, imidazolinide, substituted imidazolinide, benzimidazolide, or substituted benzimidazolide anions) may be depicted as follows:
- A* + is a cation, especially a proton containing cation, and preferably is a trihydrocarbyl ammonium cation containing one or two Cio- 40 alkyl groups, especially a methyldi(C] 4 .
- R 4 independently each occurrence, is hydrogen or a halo, hydrocarbyl, halocarbyl, halohydrocarbyl, silylhydrocarbyl, or silyl, (including mono-, di- and tri(hydrocarbyl)silyl) group of up to 30 atoms not counting hydrogen, preferably Ci -20 alkyl, and
- J*' is tris(pentafluorophenyl)borane or tris(pentafluorophenyl)alumane).
- catalyst activators include trihydrocarbylammonium- salts, especially, methyldi(Ci4- 2 oalkyl)arnrnonium- salts of: bis(tris(pentafluorophenyl)borane)imidazolide, bis(tris(pentafluorophenyl)borane)-2-undecylimidazoIide, bis(tris( ⁇ entafluorophenyI)borane)-2-heptadecylimidazolide, bis(tris( ⁇ entafluorophenyl)borane)-
- activators are also contemplated by the invention, for example, alumoxanes and ionizing activators in combinations, see for example, EP-A-O 573120, PCT publications WO 94/07928 and WO 95/14044 and US Patents 5, 153, 157 and 5,453,410.
- WO 98/09996 describes activating catalyst compounds with perchlorates, periodates and iodates, including their hydrates.
- WO 99/18135 describes the use of organoboroaluminum activators.
- EP-A-781299 describes using a silylium salt in combination with a non-coordinating compatible anion.
- activators or methods for activating a catalyst compound are described in for example, U. S. Patents 5,849,852, 5,859, 653, 5,869,723, EP-A-615981, and PCT publication WO 98/32775.
- Another suitable class of organometal activators or cocatalysts are alumoxanes, also referred to as alkylaluminoxanes.
- Alumoxanes are well known activators for use with metallocene type catalyst compounds to prepare addition polymerization catalysts.
- methods for preparing alumoxanes and modified alumoxanes non-limiting examples of which are described in U.S.
- Preferred alumoxanes are Lewis acid modified alumoxanes, especially tri(C 3 .
- alkylaluminum modified methylalumoxane including tri(isobutyl)aluminum modified methalumoxane, available commercially as MMAO-3A or tri(n-octyl)aluminum modified methalumoxane, available commercially as MMAO- 12, from Akzo Nobel, Inc.
- alumoxane(s) or modified alumoxane(s) as an activator or as a tertiary component in the invented process. That is, the compound may be used alone or in combination with other activators, either neutral or ionic, such as tri(alkyl)ammonium tetrakis(pentafluorophenyl)borate compounds, trisperfluoroaryl compounds, polyhalogenated heteroborane anions as disclosed in WO 98/43983, and combinations thereof.
- the amount of alumoxane employed is generally less than that necessary to effectively activate the metal complex when employed alone.
- Suitable alumoxanes include polymeric or oligomeric alumoxanes, especially methylalumoxane (MAO) as well as Lewis acid- modified alumoxanes, especially trihydrocarbylaluminum-, halogenated tri(hydrocarbyl)aluminum- or halogenated tri(hydrocarbyl)boron- modified alumoxanes, having from 1 to 10 carbons in each hydrocarbyl or halogenated hydrocarbyl group.
- Such activating cocatalysts are previously disclosed in USP' s 6,214,760, 6, 160,146, 6,140,521, and 6,696,379, and elsewhere.
- Preferred Lewis acid- modified alumoxane compounds are tri(i-butyl)aluminum- modified methalumoxane and tri(n- octyl)aluminum- modified methalumoxane containing from 10 to 30, preferably 15 to 25 mole percent i-butyl content and 10 to 20, preferably 12 to 18 mole percent n-octyl contents, respectively, said molar percents based on total alkyl ligand content.
- the alumoxane or Lewis acid- modified alumoxane activator is preferably utilized in molar ratios cocatalyst.catalyst from 20-200, more preferably from 20-150, and most preferably from 20-80.
- the present zirconium complexes can achieve reduced levels of cocatalyst byproducts in the resulting polymer along with long chain branch formation in the resulting polymer. This in turn allows the polymers to be employed in demanding applications that have been previously unsuited for ethylene/ ⁇ -olefin interpolymers, such as wire and cable electrical insulation and extrusion forming process for profiles, pipes, and other applications, while retaining good flexibility and processing properties.
- Multiple reactor polymerization processes are suitably employed in the present invention. Examples include such systems as are disclosed in USP 3,914,342, among others.
- the multiple reactors can be operated in series or in parallel, with at least one catalyst composition according to the present invention employed in at least one of the reactors.
- One or both reactors may also contain at least two catalysts which have different comonomer incorporation capability and/or different molecular weight capability.
- a relatively high molecular weight product (M w from 100,000 to over 1 ,000,000, more preferably 200,000 to 500,000) is formed while in the second reactor a product of a relatively low molecular weight (M w 2,000 to 300,000) is formed. Both of these reactor products can have similar or different densities.
- the final product is a mixture of the two reactor effluents which are combined prior to devolatilization to result in a uniform mixing of the two polymer products.
- the molecular weight of the products from both reactors is nearly the same but the densities vary to the extent that one of the reactors produces a polymer with density in the range of 0.865-0.895, while the other reactor produces polymer with a different density in the range of 0.885-0.950.
- the reactors are connected in series, that is, the effluent from the first reactor is charged to the second reactor and fresh monomer, solvent and hydrogen is optionally added to the second reactor. Reactor conditions are adjusted such that the weight ratio of polymer produced in the first reactor to that produced in the second reactor is ideally in the range from 20:80 to 80:20.
- the foregoing dual reactor process is capable of producing polymers having broadened molecular weight distribution or polydispersity index (PDI).
- Preferred polymers made in the foregoing manner have PDI from 2.8 to 10.0, more preferably from 3.0 to 7.0.
- the high molecular weight component contains higher quantities of comonomer (lower density) than the low molecular weight component.
- one of the reactors in the polymerization process including the first of two reactors operating in series, contains a heterogeneous Ziegler-Natta catalyst or a chromium containing catalyst, such as one of the numerous such catalysts known in the art.
- Ziegler-Natta catalysts include, but are not limited to, titanium-based catalysts supported on MgCl 2 , and additionally comprise compounds of aluminum containing at least one aluminum-alkyl bond.
- Suitable Ziegler-Natta catalysts and their preparation include, but are not limited to, those disclosed in USP' s 4,612,300, 4,330,646, and 5,869,575.
- Suitable chromium based catalysts are those disclosed in USP's 4,981 ,927, 4,835,219, 4,564,660, 4,173,548, 3,953,413, and elsewhere.
- Single reactor, multiple catalyst processes are also useful in the present invention.
- two or more catalysts are introduced into a single reactor at the high monomer conversion conditions that are herein disclosed, wherein each catalyst inherently produces different polyolefin copolymers.
- a relatively high molecular weight product (M w from 100,000 to over 1 ,000,000, more preferably 200,000 to 500,000) is formed from one catalyst while a product of a relatively low molecular weight (M w 2,000 to 300,000) is formed from the other catalyst.
- M w relatively high molecular weight product
- M w 2,000 to 300,000 a relatively low molecular weight
- Both of these catalyst compositions can have similar or different comonomer incorporation ability, at least one of which comprises a metal complex as set forth herein.
- the resulting polymer will have properties dependant on the ratio of the two catalysts that are employed in the single reactor. Suitable combinations of polymer molecular weight, comonomer incorporation ability, processes, and ratios of catalysts for such products are disclosed in USP 6,924,342.
- the second catalyst composition may comprise a metal complex as herein disclosed, a metallocene or other ⁇ -bonded Iigand group containing metal complex (including constrained geometry metal complexes), or a polyvalent heteroatom Iigand group containing metal complex, especially polyvalent pyridylamine or imidizolylamine based complexes and tetradendate oxygen-ligated biphenylphenol based Group 4 metal complexes.
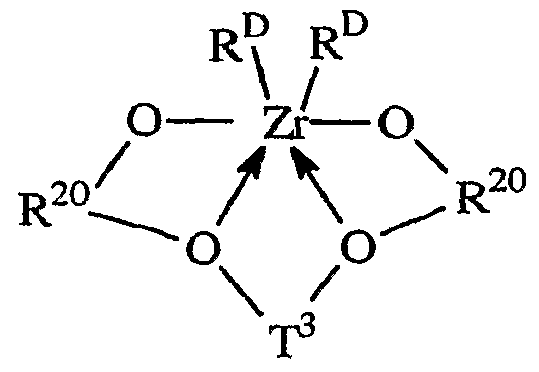
- Suitable metal complexes for use according to the present invention include compounds corresponding to the formula:
- R 20 independently each occurrence is a arylene- or inertly substituted arylene- group of from 6 to 20 atoms not counting hydrogen or any atoms of any substituent, said group being substituted at the position adjacent to the oxyl-metal bond with a cyclic Iigand, said cyclic Iigand containing from 6 to 30 atoms not counting hydrogen;
- T 3 is a divalent hydrocarbon or silane group having from 1 to 20 atoms not counting hydrogen, or an inertly substituted derivative thereof;
- R D independently each occurrence is a monovalent Iigand group of from 1 to 20 atoms, not counting hydrogen, or two R D groups together are a divalent Iigand group of from 1 to 20 atoms, not counting hydrogen.
- such complexes correspond to the formula:
- Ar 2 independently each occurrence is phenylene or an alkyl-, aryl-, alkoxy- or amino- substituted phenylene group of from 6 to 20 atoms not counting hydrogen or any atoms of any substituent, and further substituted at the position adjacent to the oxyl-metal bond with a polycycHc aryl group containing from 6 to 30 atoms not counting hydrogen;
- T 3 is a divalent hydrocarbon bridging group of from 2 to 20 atoms not counting hydrogen, preferably a divalent substituted or unsubstituted C 3 . ⁇ aliphatic, cycloaliphatic, or bis(alkylene)- substituted cycloaliphatic group;
- R D independently each occurrence is a monovalent ligand group of from I to 20 atoms, not counting hydrogen, or two R D groups together are a divalent Hgand group of from 1 to 40 atoms, not counting hydrogen.
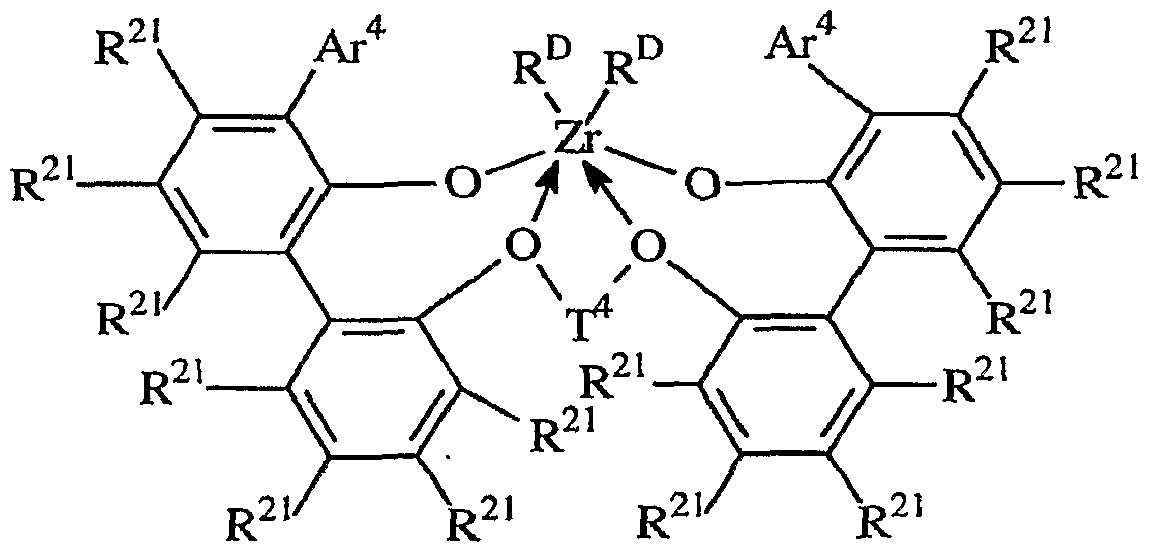
- metal complexes suitable for use herein include compounds of the formula:
- Ar 4 independently each occurrence is C&. 2 0 aryl or inertly substituted derivatives thereof, especially 3,5-di(isopropyl)phenyl, 3,5-di(isobutyl)phenyl, dibenzo-lH-pyrrole-1-yl, naphthyl, anthracen-5-yl, 1 ,2,3,4,6,7,8,9-octahydroanthracen-5-yI;
- T* independently each occurrence is a propylene- 1,3-diyl group, a cyclohexan-1 ,2-diyl group, a bis(alkylene)cyclohexan-l ,2-diyl group, a cyclohexen-4,5-diyl group, or an inertly substituted derivative thereof;
- R 21 independently each occurrence is hydrogen, halo, hydrocarbyl, trihydrocarbylsilyl, trihydrocarbylsilylhydrocarbyl, alkoxy or amino of up to 50 atoms not counting hydrogen;
- R D independently each occurrence is halo or a hydrocarbyl or trihydrocarbylsilyl group of up to 20 atoms not counting hydrogen, or 2 R D groups together are a divalent hydrocarbylene, hydrocarbadiyl or trihydrocarbylsilyl group of up to 40 atoms not counting hydrogen.
- Especially preferred metal complexes are compounds of the formula:
- Ar independently each occurrence, is dibenzo-lH- ⁇ yrrole-1-yl or anthracen-5-yl
- R 21 independently each occurrence is hydrogen, halo, hydrocarbyl, trihydrocarbylsilyl, trihydrocarbylsilylhydrocarbyl, alkoxy or amino of up to 50 atoms not counting hydrogen;
- T 4 is propan-l ,3-diyl, cyclohexanediyl, cyclohexen-4,5-diyl, or bis(methylene)cyclohexan- l ,2-diyl;
- R D independently each occurrence is halo or a hydrocarbyl or trihydrocarbylsilyl group of up to 20 atoms not counting hydrogen, or 2 R D groups together are a hydrocarbylene, hydrocarbadiyl or hydrocarbylsilanediyl group of up to 40 atoms not counting hydrogen.
- metal complexes comprising a 1 ,4-butanediyl T 4 group
- the foregoing complexes demonstrate improved catalyst efficiencies, especially at elevated polymerization temperatures.
- Most highly preferred metal complexes according to the invention correspond to the formulas:
- R D independently each occurrence is chloro, methyl or benzyl.
- suitable metal complexes are the following compounds: A) bis ⁇ -oxoyl-S- ⁇ l ⁇ .S ⁇ J ⁇ .g-octahydroanthracen-S-yO-S-CmethyOphenyO ⁇ -phenoxy)-! ⁇ - propanediylzirconium (IV) dimethyl, bis((2-oxoyl-3-( 1 ⁇ SA ⁇ J.S ⁇ -octahydroanthracen-S-y ⁇ -S ⁇ methytyphenyl ⁇ -phenoxy)-!
- the foregoing metal complexes may be conveniently prepared by standard metallation and ligand exchange procedures involving a source of the transition metal and a neutral polyfunctional ligand source.
- the complexes may also be prepared by means of an amide elimination • and hydrocarbylation process starting from the corresponding transition metal tetraamide and a hydrocarbylating agent, such as trimethylaluminum.
- the techniques employed are the same as or analogous to those disclosed in USP's 6,320,005, 6,103,657, WO 02/38628, WO 03/40195, US-A- 2004/0220050, and elsewhere.
- the metal complex is activated to form the active catalyst composition by combination with the cocatalyst. The activation may occur prior to addition of the catalyst composition to the reactor with or without the presence of other components of the reaction mixture, or in situ through separate addition of the metal complex and activating cocatalyst to the reactor.
- Suitable olefin mixtures for use herein include mixtures of ethylene with one or more C 3 .30 aliphatic-, cycloaliphatic- or aromatic- compounds (comonomers) containing one or more ethylenic unsaturations. Examples include aliphatic-, cycloaliphatic- and aromatic olefins or diolefins.
- Preferred comonomers include, but are not limited to, propylene, isobutylene, 1-butene, 1-pentene, 1-hexene, 1-heptene, 1-octene, 1-nonene, 1-decene, and 1-dodecene, 1-tetradecene, 1-hexadecene, 1 -octadecene, 1-eicosene, 3-methyl- ] -butene, 3-methyl-l-pentene, 4-methyl-l-pentene, 4,6- dimethyl-1-heptene, vinylcyclohexane, styrene, cyclopentene, cyclohexene, cyclooctene, 1 ,3- butadiene, 1 ,3-pentadiene, 1 ,4-hexadiene,- 1 ,5-hexadiene, 1 ,7-octadiene, 1 ,9-decadiene,
- novel processes described herein are well suited for the production of olefin polymers comprising monovinylidene aromatic monomers including styrene, o-methyl styrene, p-methyl styrene, t-buty I styrene, and mixtures thereof.
- interpolymers comprising ethylene and styrene can be advantageously prepared by following the teachings herein.
- copolymers comprising ethylene, styrene and/or a C 3 . 2 o alpha olefin, and further optionally comprising a conjugated or non-conjugated C 4 . 20 diene can be prepared.
- Suitable non-conjugated die ⁇ es include straight chain-, branched chain- or cyclic- hydrocarbon dienes having from 6 to 15 carbon atoms.
- suitable non-conjugated dienes include, but are not limited to, straight chain acyclic dienes, such as 1 ,4-hexadiene, 1 ,6-octadiene, 1 ,7-octadiene, 1 ,9-decadiene, branched chain acyclic dienes, such as 5-methyl- 1 ,4-hexadiene; 3,7- dimethyl- 1 ,6-octadiene; 3,7-dimethyl-l ,7-octadiene and mixed isomers of dihydromyricene and dihydroocinene, single ring alicyclic dienes, such as 1 ,3-cyclopentadiene; 1 ,4-cyclohexadiene; 1,5- cyclooctadiene and 1 ,5
- the particularly preferred dienes are 1 ,4-hexadiene (HD), 5-ethylidene-2-norbornene (ENB), 5-vinyIidene-2-norbornene (VNB), 5-methylene-2-norbornene (MNB), and dicyclopentadiene (DCPD).
- the most especially preferred diene is 5-ethylidene-2- norbornene (ENB).
- the polymerization may be accomplished at conditions well known in the prior art for olefin solution polymerization reactions.
- Preferred polymerization temperatures are dependent upon the comonomer content of the resulting polymer.
- the preferred temperatures range from 120-250 0 C, more preferably from 150-220 0 C.
- the preferred temperatures range from 160-250 0 C, more preferably from 180-25O 0 C.
- Preferred polymerization pressures are from atmospheric to 3000 atmospheres (100 kPa to 300 MPa), more preferably from 1 MPa to 10 MPa.
- the molar ratio of catalystrpolymerizable compound employed is from 10 'l2 : l to 10 '1 : 1 , more preferably from 10 " ":l to 10 '5 : l.
- the reaction is conducted under continuous, solution polymerization conditions, that is, conditions wherein the monomer or monomers are continuously added to a reactor operating under solution polymerization conditions, and polymerized product is continuously or semi-con tinuously removed and recovered or forwarded to a second reactor.
- the polymerization mixture comprises an aliphatic or alicyclic liquid diluent.
- aliphatic or alicyclic liquid diluents include straight and branched-chain hydrocarbons such as isobutane, butane, pentane, hexane, heptane, octane, and mixtures thereof; alicyclic hydrocarbons such as cyclohexane, cycloheptane, methylcyclohexane, methylcyclo- heptane, and mixtures thereof; and perfluorinated hydrocarbons such as perfluorinated C 4 . 10 alkanes, and the like.
- a preferred liquid diluent is a hydrogenated oligomeric aliphatic hydrocarbon mixture having a distillation, ASTM D 86, IBP of 1 18 °C, distillation, ASTM D 86, Dry Point of 137 0 C, and Specific Gravity, 15.6 0 C, ASTM D 1250 of 0.72 sold commercially under the trade designation IsoparTM E, available from ExxonMobil Corporation.
- the use of molecular weight control agents or chain transfer agents in the present process is desired.
- molecular weight control agents examples include hydrogen, trialkyl aluminum compounds, or other known chain transfer agents. Hydrogen is a most preferred molecular weight control agent or chain transfer agent.
- a particular benefit of the use of the present invention is the ability (depending on reaction conditions) to produce narrow molecular weight distribution ethylene/ cc-olefin interpolymers. Preferred polymers have Mw/Mn of less than 3.0, more preferably less than 2.6. Such narrow molecular weight distribution polymer products are highly desirable due to improved tensile strength properties as well as reduced levels of extractables and metal values.
- one means for carrying out the present polymerization process is as follows.
- the monomers to be polymerized are introduced continuously together with any solvent or diluent.
- the reactor contains a liquid phase composed substantially of monomers together with any solvent or diluent and dissolved polymer.
- Catalyst along with cocatalyst and optionally chain transfer agent are continuously or intermittently introduced in the reactor liquid phase or any recycled portion thereof.
- the reactor temperature may be controlled by adjusting the solvent/monomer ratio, the catalyst addition rate, as well as by use of cooling or heating coils, jackets or both.
- the polymerization rate is controlled by the rate of catalyst addition.
- Pressure is controlled by the monomer flow rate and partial pressures of volatile components.
- the ethylene content of the polymer product is determined by the ratio of ethylene to comonomer in the reactor, which is controlled by manipulating the respective feed rates of these components to the reactor.
- the polymer product molecular weight is controlled, optionally, by controlling other polymerization variables such as the temperature, monomer concentration, or by the flow rate of he previously mentioned chain transfer agent.
- a catalyst kill agent such as water, steam or an alcohol.
- the polymer solution is optionally heated, and the polymer product is recovered by flashing off gaseous monomers as well as residual solvent or diluent at reduced pressure, and, if necessary, conducting further devolatilization in equipment such as a devolatilizing extruder.
- the mean residence time of the catalyst and polymer in the reactor generally is from 5 minutes to 8 hours, and preferably is from 10 minutes to 6 hours.
- the foregoing polymerization may be carried out in a continuous loop reactor with or without a monomer, comonomer, catalyst or cocatalyst gradient established between differing regions thereof, optionally accompanied by separate addition of catalysts and/or chain transfer agent, and operating under adiabatic or non-adiabatic solution polymerization conditions or combinations of the foregoing reactor conditions.
- suitable loop reactors and a variety of suitable operating conditions for use therewith are found in USP's 5,977,251, 6, 319,989 and 6,683, 149.
- a process for polymerization of ethylene and one or more C 3 ..30 ⁇ -olefins or diolefins under continuous, solution polymerization conditions to prepare a high molecular weight interpolymer having narrow molecular weight distribution and improved processability comprising conducting the polymerization in the presence of a catalyst composition comprising a zirconium complex of a poly valent aryloxyether corresponding to the formula:
- R 20 independently each occurrence is a arylene- or inertly substituted arylene- group of from 6 to 20 atoms not counting hydrogen or any atoms of any substituent, said group being substituted at the position adjacent to the oxyl-metal bond with a cyclic ligand, said cyclic ligand containing from 6 to 30 atoms not counting hydrogen;
- T 3 is a divalent hydrocarbon or s ⁇ lane group having from 1 to 20 atoms not counting hydrogen, or an inertly substituted derivative thereof;
- R D independently each occurrence is a monovalent ligand group of from 1 to 20 atoms, not counting hydrogen, or two R D groups together are a divalent ligand group of from 1 to 20 atoms, not counting hydrogen.
- Ar 2 independently each occurrence is phenylene or an alkyl-, aryl-, alkoxy- or amino- substituted phenylene group of from 6 to 20 atoms not counting hydrogen or any atoms of any substituent, and further substituted at the position adjacent to the oxyl-metal bond with a polycyclic aryl group containing from 6 to 30 atoms not counting hydrogen;
- T 3 is a divalent hydrocarbon bridging group of from 2 to 20 atoms not counting hydrogen, preferably a divalent substituted or unsubstituted C 3 . 6 aliphatic, cycloaliphatic, or bis(alkylene)- substituted cycloaliphatic group; and
- R D independently each occurrence is a monovalent ligand group of from 1 to 20 atoms, not counting hydrogen, or two R D groups together are a divalent ligand group of from 1 to 40 atoms, not counting hydrogen. 22.
- the metal complex corresponds to of the formula:
- Ar 4 independently each occurrence is dibenzo-lH-pyrrole-1-yl, naphthyl, anthracen-5-yI, or l,2,3,4,6,7,8,9-octahydroanthracen-5-yl;
- T 4 independently each occurrence is a propylene- 1,3-diyl group, a cyclohexan-1 ,2-diyl group, a bis(alkylene)cyclohexa ⁇ -l ,2-diyl group, a cyclohexen-4,5-diyl group, or an inertly substituted derivative thereof;
- R 21 independently each occurrence is hydrogen, halo, hydrocarbyl, trihydrocarbylsilyl,
- R D independently each occurrence is halo or a hydrocarbyl or trihydrocarbylsilyl group of up to 20 atoms not counting hydrogen, or 2 R D groups together are a divalent hydrocarbylene, hydrocarbadiyl or trihydrocarbylsilyl group of up to 40 atoms not counting hydrogen.
- R D independently each occurrence is chloro, methyl or benzyl.
- 1,3-propanediylzirconium (IV) dibenzyl bis((2-oxoyl-3-(dibenzo- 1 H-pyrrole- 1 -yl)-5-(methyl)phenyl)-(4-t-butyl-2-phenoxy)- 1 ,3- propanediylzirconium (IV) dimethyl, bis((2-oxoyl-3-(dibenzo-lH-pyrrole-l-yl)-5-(methyl)phenyl)-(4-t-b ⁇ tyl-2-phenoxy)-l ,3- propanediyizirconium (IV) dichloride, bis((2-oxoyI-3-(dibenzo- 1 H-pyrrole- 1 -yl)-5-(methyl)phenyl)-(4-t-butyl-2-phenoxy)- 1 ,3- propanediylzirconium (IV) dibenzyl,
- MI melt index
- a copolymer of ethylene and one or more C 3-8 ⁇ -olef ⁇ ns having a density between 0.855 and 0.885 g/cm 3 , Mw/Mn less than 3.0, a melt index (MI) from 0.1 to 40, and I 10 ZI 2 > 1 1.75(MI)- 0 188 , preferably an I 10 A 2 > 12.72(MI)- 0 168 .
- A3 bis((2-oxoyl-3-(3,5-bis-(l ,1 -dimethylethyl)
- A4 bis((2-oxoyl-3-(dibenzo- 1 H-pyrrole- 1 -yl)-5- phenyl)-5-(methyl)phenyl)-2- phenoxy)- (msthyl)phenyl)-2-phenoxy)- 1 ,3-propanediyl zirconium (IV) dimethyl 1 ,3-propanediyl zirconium (IV) dimethyl
- A5 bis((2-oxoyl-3-(dibenzo-lH-pyrrole- l-yl)-5-
- A6 bis((2-oxoyl-3-(diben2D- lH-pyrrole-l-yl)-5- (rr B thyl)phenyl)-2-phenoxy)-cis- (rnethyl)phenyl)-2-phenoxymethyl)- cis-
- A7 bis((2-oxoyl-3-(dIbenzo- lH-pyrrole- l-yI)-5-
- A8 bis((2-oxoyl-3-(dibenzo-lH-pymote-l-yl)-5- (rrEtl ⁇ l)pheny ⁇ (4-n ⁇ ethyl-2-phenoxyniethyl))- (methyl)phenyI)-2-phenoxy)- trans l,2-cyctohexanezirconiurn(IV) dimethyl 1,4-butanedJyJzirconium (TV) dimethyl
- A9 bis((2-oxoy ⁇ 3-(dibenzo- lH-pyrrole-l-yO-5-
- A10 bis((2-oxoy ⁇ 3-(dibenzo- lH-pyrrole- l-yl>5- (m 2 thyl)phenyD-(5-(2-irethyl)propane-2-yl)-2- (nnethyDphenyI)-(5-(2-methyl)propane-2-yl)-2- phenoxy> 1 ,3-propanediyl zirconium (IV) dirrerthyl phenoxy)- 1 ,3-propanediyl zirconium (IV) dichloride
- Continuous solution polymerizations are carried out in a computer controlled autoclave reactor equipped with an internal stirrer.
- Purified mixed alkanes solvent IsoparTM E available from ExxonMobil, Inc.
- ethylene, 1-octene, and hydrogen are supplied to a 3.8 L reactor equipped with a jacket for temperature control and an internal thermocouple.
- the solvent feed to the reactor is measured by a mass-flow controller.
- a variable speed diaphragm pump controls the solvent flow rate and pressure to the reactor.
- a side stream is taken to provide flush flows for the catalyst and cocatalyst injection lines and the reactor agitator. These flows are measured by mass flow meters and controlled by control valves or by the manual adjustment of needle valves.
- the remaining solvent is combined with l-octene, ethylene, and hydrogen and fed to the reactor.
- a mass flow controller is used to deliver hydrogen to the reactor as needed.
- the temperature of the solvent/monomer solution is controlled by use of a heat exchanger before entering the reactor. This stream enters the bottom of the reactor.
- the cocatalyst used is a long- chain alkyl ammonium borate of approximate stoichiometry equal to methyldi(octadecyl)- ammonium tetrakis(pentafl ⁇ orophenyl)borate (MDB) combined with a tertiary component, tri(isobutyl)aluminum modified methalumoxane (MMAO) containing a molar ratio of i- butyl/methyl groups of about 1/3.
- MDB methyldi(octadecyl)- ammonium tetrakis(pentafl ⁇ orophenyl)borate
- MMAO tri(isobutyl)aluminum modified methalumoxane
- the catalyst component solutions are metered using pumps and mass flow meters and are combined with the catalyst flush solvent and introduced into the bottom of the reactor.
- the reactor is run liquid-full at 500 psig (3.45 MPa)
- Product is removed through exit lines at the top of the reactor. All exit lines from the reactor are steam traced and insulated. Polymerization is stopped by the addition of a small amount of water into the exit line along with any stabilizers or other additives and passing the mixture through a static mixer. The product stream is then heated by passing through a heat exchanger before de volatilization. The polymer product is recovered by extrusion using a devolatilizing extruder and water cooled pelletizer.
- Tables 1 and 2 More process details and results are contained in Tables 1 and 2, while Table 3 contains polymer data from runs in Table 2.
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BRPI0711018A BRPI0711018A8 (en) | 2006-05-17 | 2007-04-24 | PROCESS FOR THE POLYMERIZATION OF ETHYLENE AND ONE OR MORE C-3-30 ALPHA-OLEFINS OR DIOLEFINS ETHYLENE AND ONE OR MORE C3-8 ALPHA-OLEFINS |
| CA002652551A CA2652551A1 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| EP07776212.8A EP2024403B1 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| ES07776212.8T ES2524779T3 (en) | 2006-05-17 | 2007-04-24 | Solution polymerization process of polyolefins and polymer |
| KR1020087030627A KR101396058B1 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| CN2007800256425A CN101484475B (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| JP2009510956A JP5666129B2 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization method and polymer |
| MX2008014669A MX2008014669A (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer. |
| US12/300,861 US8101696B2 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| US13/329,486 US8349984B2 (en) | 2006-05-17 | 2011-12-19 | Polyolefin solution polymerization process and polymer |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US80118206P | 2006-05-17 | 2006-05-17 | |
| US60/801,182 | 2006-05-17 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/300,861 A-371-Of-International US8101696B2 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| US13/329,486 Division US8349984B2 (en) | 2006-05-17 | 2011-12-19 | Polyolefin solution polymerization process and polymer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007136506A2 true WO2007136506A2 (en) | 2007-11-29 |
| WO2007136506A3 WO2007136506A3 (en) | 2008-01-24 |
Family
ID=38572813
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/009844 WO2007136496A2 (en) | 2006-05-17 | 2007-04-24 | High temperature polyethylene solution polymerization process |
| PCT/US2007/009842 WO2007136494A2 (en) | 2006-05-17 | 2007-04-24 | Ethylene/ alpha-olefin/ diene solution polymerization process |
| PCT/US2007/009843 WO2007136495A2 (en) | 2006-05-17 | 2007-04-24 | High efficiency solution polymerization process |
| PCT/US2007/010070 WO2007136506A2 (en) | 2006-05-17 | 2007-04-24 | Polyolefin solution polymerization process and polymer |
| PCT/US2007/009841 WO2007136493A2 (en) | 2006-05-17 | 2007-04-24 | Polypropylene solution polymerization process |
| PCT/US2007/009845 WO2007136497A2 (en) | 2006-05-17 | 2007-04-24 | High temperature solution polymerization process |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/009844 WO2007136496A2 (en) | 2006-05-17 | 2007-04-24 | High temperature polyethylene solution polymerization process |
| PCT/US2007/009842 WO2007136494A2 (en) | 2006-05-17 | 2007-04-24 | Ethylene/ alpha-olefin/ diene solution polymerization process |
| PCT/US2007/009843 WO2007136495A2 (en) | 2006-05-17 | 2007-04-24 | High efficiency solution polymerization process |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/009841 WO2007136493A2 (en) | 2006-05-17 | 2007-04-24 | Polypropylene solution polymerization process |
| PCT/US2007/009845 WO2007136497A2 (en) | 2006-05-17 | 2007-04-24 | High temperature solution polymerization process |
Country Status (14)
| Country | Link |
|---|---|
| US (7) | US8058373B2 (en) |
| EP (8) | EP2024401B1 (en) |
| JP (6) | JP5666129B2 (en) |
| KR (6) | KR101397337B1 (en) |
| CN (8) | CN101490096B (en) |
| AR (6) | AR060639A1 (en) |
| AU (1) | AU2007254428A1 (en) |
| BR (3) | BRPI0711018A8 (en) |
| CA (5) | CA2653534A1 (en) |
| ES (7) | ES2534469T3 (en) |
| MX (4) | MX2008014669A (en) |
| RU (4) | RU2463311C2 (en) |
| SG (1) | SG158102A1 (en) |
| WO (6) | WO2007136496A2 (en) |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010141557A1 (en) | 2009-06-05 | 2010-12-09 | Dow Global Technologies Inc. | Process to make long chain branched (lcb), block, or interconnected copolymers of ethylene |
| WO2010144784A1 (en) | 2009-06-11 | 2010-12-16 | Dow Global Technologies Inc. | Novel ldpe enabling high output and good optics when blended with other polymers |
| US20110015346A1 (en) * | 2009-07-01 | 2011-01-20 | Dow Global Technologies Inc. | Ethylene-based polymer compositions |
| WO2011019563A1 (en) | 2009-08-10 | 2011-02-17 | Dow Global Technologies Inc. | Ldpe for use as a blend component in shrinkage film applications |
| WO2011032172A1 (en) | 2009-09-14 | 2011-03-17 | Dow Global Technologies Inc. | Polymers comprising units derived from ethylene and siloxane |
| US20110130533A1 (en) * | 2009-06-05 | 2011-06-02 | Dow Global Technologies Inc. | Process to make long chain branched (lcb), block, or interconnected copolymers of ethylene |
| WO2011066469A1 (en) | 2009-11-24 | 2011-06-03 | Dow Global Technologies Inc. | Extrusion coating composition |
| WO2011109563A3 (en) * | 2010-03-02 | 2011-11-10 | Dow Global Technologies Llc | Ethylene-based polymer compositions |
| US20120101241A1 (en) * | 2006-05-17 | 2012-04-26 | Harold W Boone | ETHYLENE/a-OLEFIN/DIENE SOLUTION POLYMERIZATION PROCESS AND POLYMER |
| WO2012064630A2 (en) | 2010-11-08 | 2012-05-18 | Dow Global Technologies Llc | Solution polymerization process and procatalyst carrier systems useful therein |
| JP2012532231A (en) * | 2009-07-01 | 2012-12-13 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene polymer and use thereof |
| WO2013096573A1 (en) | 2011-12-20 | 2013-06-27 | Dow Global Technologies Llc | Ethylene/alpha-olefin/nonconjugated polyene interpolymers and processes to form the same |
| JP2013528689A (en) * | 2010-06-14 | 2013-07-11 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene-based polymer composition for use as a blend component in shrink film applications |
| US20130281643A1 (en) * | 2010-12-21 | 2013-10-24 | Dow Global Technologies Llc | Olefin-based polymers and dispersion polymerizations |
| US8598276B2 (en) | 2009-09-14 | 2013-12-03 | Dow Global Technologies Llc | Polymers comprising units derived from ethylene and poly(alkoxide) |
| US20140163186A1 (en) * | 2010-05-17 | 2014-06-12 | Dow Global Technologies Llc | Process for selectively polymerizing ethylene and catalyst therefor |
| US8784996B2 (en) | 2009-11-24 | 2014-07-22 | Dow Global Technologies Llc | Extrusion coating composition |
| WO2014113046A1 (en) | 2013-01-18 | 2014-07-24 | Dow Global Technologies Llc | Polymerization processes for high molecular weight polyolefins |
| US20140242304A1 (en) * | 2011-10-24 | 2014-08-28 | Peter Sandkuehler | Artificial turf yarn |
| US20140248816A1 (en) * | 2011-10-05 | 2014-09-04 | Dow Global Technologies Llc | Bi-component fiber and fabrics made therefrom |
| US8987385B2 (en) | 2009-09-14 | 2015-03-24 | Dow Global Technologies Llc | Interconnected copolymers of ethylene in combination with one other polyalkene |
| KR20150037832A (en) * | 2012-07-20 | 2015-04-08 | 다우 글로벌 테크놀로지스 엘엘씨 | A linear low density polyethylene composition suitable for cast film |
| US10336846B2 (en) | 2015-05-28 | 2019-07-02 | Dow Global Technologies Llc | Process to form ethylene/α-olefin interpolymers |
Families Citing this family (195)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8420760B2 (en) | 2007-11-19 | 2013-04-16 | Dow Global Technologies Llc | Long chain branched propylene-alpha-olefin copolymers |
| KR101149755B1 (en) | 2009-01-06 | 2012-06-01 | 에스케이종합화학 주식회사 | The Manufacture of Ethylene-Propylene-Diene Copolymer |
| US8629214B2 (en) | 2009-07-01 | 2014-01-14 | Dow Global Technologies Llc. | Ethylene-based polymer compositions for use as a blend component in shrinkage film applications |
| KR20120107066A (en) | 2009-07-01 | 2012-09-28 | 다우 글로벌 테크놀로지스 엘엘씨 | Ethylenic polymer and its use |
| ES2638913T3 (en) | 2009-08-31 | 2017-10-24 | Dow Global Technologies Llc | Catalyst and process for polymerizing an olefin and polyolefin prepared by it |
| JP5623043B2 (en) * | 2009-09-04 | 2014-11-12 | 出光興産株式会社 | Polyolefin production method, production apparatus thereof, and polymerization apparatus |
| WO2011079207A1 (en) | 2009-12-24 | 2011-06-30 | Dow Global Technologies Llc | Polymer compositions, methods of making the same, and articles prepared from the same |
| ES2672893T3 (en) * | 2010-01-14 | 2018-06-18 | Exxonmobil Chemical Patents Inc. | Processes and apparatus for continuous polymerization in solution |
| EP2576685A1 (en) * | 2010-05-26 | 2013-04-10 | Dow Global Technologies LLC | Electronic device module comprising polyolefin copolymer with low unsaturation and optional vinyl silane |
| BR112012030649A2 (en) | 2010-06-04 | 2016-08-16 | Dow Global Technologies Llc | electronic device module comprising homogeneous polyolefin copolymer film and adhesive enhancing graft polymer |
| US20130233383A1 (en) | 2010-06-04 | 2013-09-12 | John A. Naumovitz | Electronic Device Module Comprising Film of Homogeneous Polyolefin Copolymer and Grafted Silane |
| JP5937071B2 (en) * | 2010-07-06 | 2016-06-22 | ティコナ ゲゼルシャフト ミット ベシュレンクテル ハフツング | Method for producing high molecular weight polyethylene |
| WO2012061168A1 (en) | 2010-11-02 | 2012-05-10 | Dow Global Technologies Llc | A sealant composition, method of producing the same |
| WO2012074812A1 (en) | 2010-12-03 | 2012-06-07 | Dow Global Technologies Llc | Processes to prepare ethylene-based polymer compositions |
| KR101856719B1 (en) * | 2010-12-21 | 2018-05-11 | 다우 글로벌 테크놀로지스 엘엘씨 | Polymerization process and raman analysis for olefin-based polymers |
| KR101871533B1 (en) | 2010-12-30 | 2018-06-26 | 다우 글로벌 테크놀로지스 엘엘씨 | Compositions, methods of making the same, and articles prepared from the same |
| WO2014209927A1 (en) * | 2013-06-28 | 2014-12-31 | Dow Global Technologies Llc | Hyperbranched ethylene-based oligomers |
| KR101916986B1 (en) | 2011-09-07 | 2018-11-08 | 다우 글로벌 테크놀로지스 엘엘씨 | Polymer compositions and articles prepared from the same |
| EP2751191B1 (en) * | 2011-09-12 | 2020-07-22 | Dow Global Technologies LLC | Compositions and articles formed from the same |
| WO2013043796A2 (en) * | 2011-09-23 | 2013-03-28 | Exxonmobil Chemical Patents Inc. | Modified polyethylene compositions |
| US20160272798A1 (en) | 2011-09-23 | 2016-09-22 | Exxonmobil Chemical Patents Inc. | Modified Polyethylene Compositions with Enhanced Melt Strength |
| BR112014008455B1 (en) | 2011-10-10 | 2021-01-05 | Dow Global Technologies Llc | composition, lattice composition and article |
| JP6170056B2 (en) | 2011-10-20 | 2017-07-26 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene-based polymer composition and articles made therefrom |
| WO2013056466A1 (en) * | 2011-10-21 | 2013-04-25 | Dow Global Technologies Llc | Multi-layered shrink films |
| CA2857290C (en) | 2011-12-13 | 2020-02-11 | Brian W. Walther | Ethylene-propylene-diene interpolymer composition |
| KR20140107260A (en) | 2011-12-19 | 2014-09-04 | 다우 글로벌 테크놀로지스 엘엘씨 | Ethylene-based polymers prepared by dispersion polymerization |
| KR101989208B1 (en) * | 2011-12-29 | 2019-06-13 | 다우 글로벌 테크놀로지스 엘엘씨 | Process for producing low molecular weight ethylene- and alpha-olefin-based materials |
| CA2861516C (en) * | 2011-12-29 | 2021-01-05 | Dow Global Technologies Llc | Hyperbranched olefin oil based dielectric fluid |
| EP2617741B1 (en) * | 2012-01-18 | 2016-01-13 | Borealis AG | Process for polymerizing olefin polymers in the presence of a catalyst system and a method of controlling the process |
| US9362436B2 (en) | 2012-02-03 | 2016-06-07 | Dow Global Technologies Llc | Silane-containing ethylene interpolymer formulation including films and electronic device module comprising same |
| US9334349B2 (en) | 2012-05-09 | 2016-05-10 | Dow Global Technologies Llc | Polyolefin polymerization process, semi-crystalline ethylene-based polymer made therefrom, and articles made from the polymer |
| WO2014003857A1 (en) * | 2012-06-29 | 2014-01-03 | Dow Global Technologies Llc | Ethylene/alpha-olefin/nonconjugated polyene based compositions for thermoplastic vulcanizates |
| EP2880006B1 (en) | 2012-08-03 | 2017-12-20 | ExxonMobil Chemical Patents Inc. | Non-symmetric catalysts comprising salan ligands |
| US9382349B2 (en) | 2012-08-03 | 2016-07-05 | Exxonmobil Chemical Patents Inc. | Polyalphaolefins prepared using modified Salan catalyst compounds |
| US9045568B2 (en) | 2012-08-03 | 2015-06-02 | Exxonmobil Chemical Patents Inc. | Vinyl terminated polyethylene with long chain branching |
| EP2880096B1 (en) | 2012-08-03 | 2018-01-03 | ExxonMobil Chemical Patents Inc. | Process for preparing polyalphaolefins using modified salan catalyst compounds and polyalphaolefins prepared therewith |
| WO2014022008A1 (en) | 2012-08-03 | 2014-02-06 | Exxonmobil Chemical Patents Inc. | Catalysts comprising salan ligands |
| US8952114B2 (en) | 2012-08-03 | 2015-02-10 | Exxonmobil Chemical Patents Inc. | Halogenated catalysts comprising Salan ligands |
| US10822479B2 (en) | 2012-09-20 | 2020-11-03 | Exxonmobil Chemical Patents Inc. | Foamed polyethylene compositions |
| US10836853B2 (en) | 2012-09-20 | 2020-11-17 | Exxonmobil Chemical Patents Inc. | Crack-resistant polyethylene compositions |
| US9120879B2 (en) | 2012-11-02 | 2015-09-01 | Exxonmobil Chemical Patents Inc. | Supported Salan catalysts |
| JP6207336B2 (en) * | 2012-11-12 | 2017-10-04 | 日本ポリプロ株式会社 | Diol compound, catalyst for olefin polymerization, and method for producing olefin polymer |
| KR102215306B1 (en) | 2012-11-30 | 2021-02-16 | 다우 글로벌 테크놀로지스 엘엘씨 | Ethylene/alpha-olefin/polyene based compositions |
| WO2014105415A1 (en) | 2012-12-27 | 2014-07-03 | Dow Global Technologies Llc | A polymerization process for producing ethylene based polymers |
| US20150337062A1 (en) | 2012-12-27 | 2015-11-26 | Dow Global Technologies Llc | Ethylene Based Polymer |
| BR112015015548B1 (en) | 2012-12-27 | 2021-05-18 | Dow Global Technologies Llc | polymerization process for the production of ethylene-based polymers |
| KR102161865B1 (en) * | 2012-12-27 | 2020-10-05 | 다우 글로벌 테크놀로지스 엘엘씨 | An ethylene based polymer |
| ES2665586T3 (en) * | 2012-12-27 | 2018-04-26 | Dow Global Technologies Llc | Catalyst systems for olefin polymerization |
| CN104968668B (en) | 2013-02-08 | 2017-11-17 | 三井化学株式会社 | Solid-like poly-aluminium silicone compositions, catalyst for olefines polymerizing, the manufacture method of the manufacture method of olefin polymer and solid-like poly-aluminium silicone compositions |
| US8937137B2 (en) | 2013-03-13 | 2015-01-20 | Exxonmobil Chemical Patents Inc. | Diphenylamine salan catalyst |
| KR101657262B1 (en) * | 2013-04-05 | 2016-09-13 | 한화케미칼 주식회사 | Catalyst for olefin polymerization and method for preparing polyolefin using the same |
| US9200099B2 (en) | 2013-06-20 | 2015-12-01 | Exxonmobil Chemical Patents Inc. | Salenol catalyst |
| US9150676B2 (en) | 2013-06-20 | 2015-10-06 | Exxonmobil Chemical Patents Inc. | Thio-salalen catalyst |
| US9200100B2 (en) | 2013-06-20 | 2015-12-01 | Exxonmobil Chemical Patents Inc. | Long-bridged salen catalyst |
| EP2883890A4 (en) | 2013-06-28 | 2016-07-20 | Lg Chemical Ltd | Trinary elastic copolymer comprising diene and method for preparing same |
| KR101585204B1 (en) | 2013-06-28 | 2016-01-13 | 주식회사 엘지화학 | Elastic diene terpolymer and preparation method thereof |
| KR102218415B1 (en) | 2013-06-28 | 2021-02-22 | 다우 글로벌 테크놀로지스 엘엘씨 | Molecular weight control of polyolefins using halogenated bis-phenylphenoxy catalysts |
| JP2015536368A (en) | 2013-06-28 | 2015-12-21 | エルジー・ケム・リミテッド | Ternary elastic copolymer containing diene and method for producing the same |
| US9493593B2 (en) | 2013-06-28 | 2016-11-15 | Lg Chem, Ltd. | Elastic diene terpolymer and preparation method thereof |
| US10155831B2 (en) * | 2013-09-05 | 2018-12-18 | Univation Technologies, Llc | Process control for long chain branching control in polyethylene production |
| CN104628920B (en) * | 2013-11-08 | 2017-02-01 | 中国石油天然气股份有限公司 | Preparation method of solution polymerized ethylene propylene (EP) rubber |
| US9290589B2 (en) | 2013-12-13 | 2016-03-22 | Exxonmobil Chemical Patents Inc. | Cyclopentadienyl-substituted salan catalysts |
| WO2015116382A1 (en) * | 2014-01-30 | 2015-08-06 | Exxonmobil Chemical Patents Inc. | Foamed polyethylene compositions |
| WO2015116381A1 (en) * | 2014-01-30 | 2015-08-06 | Exxonmobil Chemical Patents Inc. | Crack-resistant polyethylene composiitions |
| US9382361B2 (en) | 2014-03-21 | 2016-07-05 | Exxonmobil Chemical Patents Inc. | Process to produce ethylene propylene copolymers |
| US9193813B2 (en) | 2014-03-31 | 2015-11-24 | Exxonmobil Chemical Patents Inc. | Phenylene-bridged salalen catalysts |
| EP3131934B1 (en) * | 2014-04-17 | 2020-01-22 | Borealis AG | Improved catalyst system for producing polyethylene copolymers in a high temperature solution polymerization process |
| US10526431B2 (en) * | 2014-06-30 | 2020-01-07 | Dow Global Technologies Llc | Catalyst systems for olefin polymerization |
| BR112016029439B1 (en) | 2014-06-30 | 2022-01-04 | Dow Global Technologies Llc | PROCESS TO FORM AN OLEFIN-BASED POLYMER |
| EP3578578A1 (en) | 2014-07-24 | 2019-12-11 | Dow Global Technologies Llc | Bis-biphenylphenoxy catalysts for polymerization of low molecular weight ethylene-based polymers |
| EP3227304B1 (en) | 2014-12-04 | 2020-01-29 | Dow Global Technologies LLC | Five-coordinate bis-phenylphenoxy catalysts for the preparation of ethylene-based polymers |
| US10351646B2 (en) * | 2014-12-31 | 2019-07-16 | Dow Global Technologies Llc | Polyolefin composition and method of producing the same |
| BR112017020525B1 (en) * | 2015-03-31 | 2022-07-19 | Dow Global Technologies Llc | PROCESS FOR FORMING A POLYMER COMPOSITION |
| CN107771199B (en) * | 2015-06-17 | 2021-05-25 | 陶氏环球技术有限责任公司 | Process for making crosslinked cable insulation using high melt strength ethylene-based polymers made in a tubular reactor and optionally modified with a branching agent |
| CN107735414A (en) * | 2015-06-22 | 2018-02-23 | 陶氏环球技术有限责任公司 | The method that the polymer based on ethene is prepared using carbon carbon radicals initiator |
| WO2017004462A1 (en) | 2015-06-30 | 2017-01-05 | Dow Global Technologies Llc | A polymerization process for producing ethylene based polymers |
| KR102588244B1 (en) | 2015-06-30 | 2023-10-12 | 다우 글로벌 테크놀로지스 엘엘씨 | Polymerization method for producing ethylene-based polymer |
| CN108137855B (en) | 2015-09-02 | 2021-09-28 | 陶氏环球技术有限责任公司 | Flexible crosslinked cable insulation and method of making same |
| CA2996616C (en) | 2015-09-02 | 2023-09-26 | Dow Global Technologies Llc | Flexible crosslinked cable insulation and methods for making flexible crosslinked cable insulation |
| US10800865B2 (en) | 2015-09-14 | 2020-10-13 | Exxonmobil Chemical Patents Inc. | Process for making branched EPDM and the EPDM therefrom |
| EP3356374A1 (en) | 2015-09-30 | 2018-08-08 | Dow Global Technologies LLC | Multi- or dual-headed compositions useful for chain shuttling and process to prepare the same |
| BR112018004696B1 (en) | 2015-09-30 | 2022-06-28 | Dow Global Technologies Llc | PROCESS FOR THE PREPARATION OF A FUNCTIONAL POLYMER |
| ES2804554T3 (en) | 2015-09-30 | 2021-02-08 | Dow Global Technologies Llc | Procatalyst and polymerization process using the same |
| ES2925720T3 (en) | 2015-09-30 | 2022-10-19 | Dow Global Technologies Llc | A polymerization process to produce ethylene-based polymers |
| BR112018006725B1 (en) | 2015-10-29 | 2022-04-19 | Dow Global Technologies Llc | Crosslinkable polymer composition and coated conductor |
| AU2016383056B2 (en) | 2015-12-29 | 2020-11-19 | Dow Global Technologies Llc | Highly grafted ethylene-based polymers, highly grafted ethylene-based polymer compositions, and processes for forming the same |
| EP3214115B1 (en) | 2016-03-03 | 2018-10-03 | Dow Global Technologies LLC | Polyethylene composition, method of making the same, and films made therefrom |
| WO2017206008A1 (en) | 2016-05-30 | 2017-12-07 | Dow Global Technologies Llc | Ethylene/alpha-olefin/diene interpolymer |
| WO2017206009A1 (en) | 2016-05-30 | 2017-12-07 | Dow Global Technologies Llc | Ethylene/alpha-olefin/diene interpolymers compositions |
| WO2018005789A1 (en) | 2016-06-30 | 2018-01-04 | Dow Global Technologies Llc | Procatalyst compositions useful for low comonomer incorporation and process for preparing the same |
| US11098144B2 (en) | 2016-06-30 | 2021-08-24 | Dow Global Technologies Llc | Ethylene/alpha-olefin/polyene interpolymers and compositions containing the same |
| WO2018005852A1 (en) | 2016-06-30 | 2018-01-04 | Dow Global Technologies Llc | Ethylene/alpha-olefin/polyene based compositions |
| WO2018013283A2 (en) | 2016-07-13 | 2018-01-18 | Exxonmobil Chemical Patents Inc. | Dual metallocene catalyst copolymer compositions |
| CN109641990B (en) | 2016-07-13 | 2022-08-19 | 埃克森美孚化学专利公司 | Dual metallocene catalyst copolymer compositions |
| EP3484984B1 (en) | 2016-07-14 | 2022-08-03 | ExxonMobil Chemical Patents Inc. | Lubricating oil compositions comprising dual metallocene-catalyzed bimodal copolymer compositions useful as viscosity modifiers |
| BR112018077058A2 (en) | 2016-07-21 | 2019-04-02 | Dow Global Technologies Llc | composite padding structures and manufacturing methods thereof |
| US11111318B2 (en) | 2016-07-29 | 2021-09-07 | Dow Global Technologies Llc | Silyl-bridged bis-biphenyl-phenoxy catalysts for olefin polymerization |
| BR112019001249B1 (en) | 2016-07-29 | 2022-10-18 | Dow Global Technologies Llc | MULTIMODAL ELASTOMER, THERMOPLATIC OLEFIN AND METHOD TO PRODUCE A MULTIMODAL ELASTOMER |
| JP7050055B2 (en) | 2016-09-29 | 2022-04-07 | ダウ グローバル テクノロジーズ エルエルシー | Modified Ziegler-Natta (Pro) catalysts and systems |
| CN109790246A (en) | 2016-09-29 | 2019-05-21 | 陶氏环球技术有限责任公司 | The method of olefin polymerization |
| WO2018063899A1 (en) | 2016-09-29 | 2018-04-05 | Dow Global Technologies Llc | Magnesium halide-supported titanium (pro) catalysts |
| JP7123040B2 (en) | 2016-09-30 | 2022-08-22 | ダウ グローバル テクノロジーズ エルエルシー | Method for preparing multi-headed or double-headed compositions useful for chain shuttling |
| KR102444560B1 (en) | 2016-09-30 | 2022-09-20 | 다우 글로벌 테크놀로지스 엘엘씨 | Multi- or dual-head components useful for chain shuttling and the process of preparing them |
| CN109952324B (en) | 2016-09-30 | 2023-06-09 | 陶氏环球技术有限责任公司 | Blocked multi-or double-headed compositions suitable for chain shuttling and methods of making the same |
| SG11201903065VA (en) | 2016-10-27 | 2019-05-30 | Univation Tech Llc | Method of preparing a molecular catalyst |
| US10280234B2 (en) | 2016-11-11 | 2019-05-07 | Exxonmobil Chemical Patents Inc. | Catalyst compositions and use thereof |
| WO2018089165A1 (en) * | 2016-11-11 | 2018-05-17 | Exxonmobil Chemical Patents Inc. | Catalyst compositions and use thereof |
| TW201829574A (en) | 2016-11-16 | 2018-08-16 | 美商陶氏全球科技有限責任公司 | Tie layer compositions and multilayer films incorporating same |
| EP4273180A3 (en) | 2017-02-28 | 2024-02-07 | Dow Global Technologies LLC | Phosphoramidate catalysts for ethylene-based interpolymers |
| KR102631009B1 (en) | 2017-03-15 | 2024-01-30 | 다우 글로벌 테크놀로지스 엘엘씨 | Catalyst system for forming multi-block copolymers |
| WO2018170056A1 (en) | 2017-03-15 | 2018-09-20 | Dow Global Technologies Llc | Catalyst system for multi-block copolymer formation |
| US11208502B2 (en) | 2017-03-15 | 2021-12-28 | Dow Global Technologies Llc | Catalyst system for multi-block coploymer formation |
| SG11201908414YA (en) | 2017-03-15 | 2019-10-30 | Dow Global Technologies Llc | Catalyst system for multi-block copolymer formation |
| ES2954446T3 (en) | 2017-03-15 | 2023-11-22 | Dow Global Technologies Llc | Catalyst system for multiblock copolymer formation |
| JP2018162231A (en) * | 2017-03-27 | 2018-10-18 | 三井化学株式会社 | Transition metal compound, catalyst for olefin polymerization and method for producing olefin polymer |
| EP3409697A1 (en) | 2017-05-28 | 2018-12-05 | SABIC Global Technologies B.V. | Preparation of polymer dispersions |
| EP3638730A1 (en) | 2017-06-14 | 2020-04-22 | ExxonMobil Chemical Patents Inc. | Ethylene copolymer blends for cross-linking applications |
| JP7381449B2 (en) | 2017-08-24 | 2023-11-15 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene/C5-C10 alpha-olefin/polyethylene interpolymer |
| JP7221948B2 (en) | 2017-09-29 | 2023-02-14 | ダウ グローバル テクノロジーズ エルエルシー | Bisphenylphenoxypolyolefin catalysts with methylenetrialkylsilicon ligands on metals to improve solubility |
| WO2019067274A1 (en) | 2017-09-29 | 2019-04-04 | Dow Global Technologies Llc | Bis-phenyl-phenoxy polyolefin catalysts having two methylenetrialkylsilicon ligands on the metal for improved solubility |
| KR102590973B1 (en) | 2017-09-29 | 2023-10-19 | 다우 글로벌 테크놀로지스 엘엘씨 | Bis-phenyl-phenoxy polyolefin catalysts with alkoxy or amido ligands on metal for improved solubility |
| US10995166B2 (en) | 2017-11-07 | 2021-05-04 | Nova Chemicals (International) S.A. | Ethylene interpolymer products and films |
| US10683376B2 (en) | 2017-11-07 | 2020-06-16 | Nova Chemicals (International) S.A. | Manufacturing ethylene interpolymer products at higher production rate |
| KR102647144B1 (en) | 2017-12-26 | 2024-03-15 | 다우 글로벌 테크놀로지스 엘엘씨 | Composition comprising multimodal ethylene-based polymer and low-density polyethylene (LDPE) |
| KR20200103723A (en) | 2017-12-26 | 2020-09-02 | 다우 글로벌 테크놀로지스 엘엘씨 | Multimode ethylene-based polymer treatment system and method |
| WO2019133368A1 (en) | 2017-12-26 | 2019-07-04 | Dow Global Technologies Llc | Dual reactor solution process for the production of multimodal ethylene-based polymer |
| JP7278286B2 (en) | 2017-12-26 | 2023-05-19 | ダウ グローバル テクノロジーズ エルエルシー | Multimodal ethylene-based polymer composition with improved toughness |
| EP3732216B1 (en) | 2017-12-26 | 2023-04-19 | Dow Global Technologies LLC | Process for the production of multimodal ethylene-based polymers |
| WO2019133400A1 (en) | 2017-12-26 | 2019-07-04 | Dow Global Technologies Llc | Compositions with multimodal ethylene-based polymers having improved toughness at low temperatures |
| JP7381464B2 (en) | 2017-12-29 | 2023-11-15 | ダウ グローバル テクノロジーズ エルエルシー | End-capped double-headed organoaluminum composition |
| JP7344872B2 (en) | 2017-12-29 | 2023-09-14 | ダウ グローバル テクノロジーズ エルエルシー | Double-headed organoaluminum composition |
| US20190248934A1 (en) | 2018-02-09 | 2019-08-15 | Exxonmobil Chemical Patents Inc. | Ethylene-a-olefin-diene Elastomers and Methods of Making Them |
| EP3752560A4 (en) | 2018-02-14 | 2021-08-25 | Dow Global Technologies LLC | Ethylene/alpha-olefin interpolymer compositions with improved long term heat aging performance |
| CN111741997B (en) | 2018-03-19 | 2022-08-09 | 美国陶氏有机硅公司 | Polyolefin-polydiorganosiloxane block copolymers and hydrosilylation reaction methods for their synthesis |
| SG11202009138QA (en) | 2018-03-19 | 2020-10-29 | Dow Global Technologies Llc | Silicon-terminated organo-metal compounds and processes for preparing the same |
| JP7378416B2 (en) | 2018-03-19 | 2023-11-13 | ダウ シリコーンズ コーポレーション | Polyolefin-polydiorganosiloxane block copolymers and methods for their synthesis |
| CN111886314B (en) | 2018-03-19 | 2022-06-17 | 美国陶氏有机硅公司 | Hot melt adhesive compositions containing polyolefin-polydiorganosiloxane block copolymers and methods of making and using the same |
| JP2021518406A (en) | 2018-03-19 | 2021-08-02 | ダウ グローバル テクノロジーズ エルエルシー | A process for functionalizing an organometallic compound with a silyl-based functionalizing agent and a silyl-functionalizing compound prepared thereby. |
| BR112020019174A2 (en) | 2018-03-19 | 2021-01-05 | Dow Global Technologies Llc | TELEQUELIC POLYOLEFINE COMPOSITIONS FINISHED WITH SILICON AND PROCESSES FOR THE PREPARATION OF THE SAME |
| KR20200133230A (en) | 2018-03-19 | 2020-11-26 | 다우 글로벌 테크놀로지스 엘엘씨 | Method for functionalization of organo-zinc compounds having halosilanes using basic nitrogen containing heterocycles and silyl-functionalized compounds prepared thereby |
| CN111868196B (en) | 2018-03-19 | 2022-08-30 | 美国陶氏有机硅公司 | Polyorganosiloxane hot melt adhesive compositions containing polyolefin-polydiorganosiloxane copolymers and methods of making and using the same |
| KR20200133354A (en) | 2018-03-19 | 2020-11-27 | 다우 글로벌 테크놀로지스 엘엘씨 | Silicon-terminated organo-metal compound and preparation method thereof |
| CN111918917A (en) | 2018-03-29 | 2020-11-10 | 陶氏环球技术有限责任公司 | Resin for use as tie layer in multilayer structure and multilayer structure comprising same |
| US11542350B2 (en) | 2018-03-30 | 2023-01-03 | Dow Global Technologies Llc | Binuclear olefin polymerization activators |
| BR112020019520A2 (en) | 2018-03-30 | 2020-12-29 | Dow Global Technologies Llc | CATALYST SYSTEM AND POLYMERIZATION PROCESS. |
| KR20200138272A (en) | 2018-03-30 | 2020-12-09 | 다우 글로벌 테크놀로지스 엘엘씨 | Olefin polymerization activator |
| US11447586B2 (en) | 2018-03-30 | 2022-09-20 | Dow Global Technologies Llc | Olefin polymerization activators |
| EP3802045B1 (en) | 2018-05-31 | 2023-07-19 | Dow Global Technologies LLC | Molded articles, and methods thereof |
| EP3807332A1 (en) | 2018-06-15 | 2021-04-21 | Dow Global Technologies LLC | Process for the production of bimodal ethylene-based polymers having high molecular weight high density fractions |
| CA3103015A1 (en) | 2018-06-15 | 2019-12-19 | Dow Global Technologies Llc | Cast films comprising bimodal ethylene-based polymers having high molecular weight high density fractions |
| WO2019241475A1 (en) | 2018-06-15 | 2019-12-19 | Dow Global Technologies Llc | Bimodal ethylene-based polymers having high molecular weight high density fractions |
| JP7419269B2 (en) | 2018-06-15 | 2024-01-22 | ダウ グローバル テクノロジーズ エルエルシー | Blown films containing bimodal ethylene-based polymers with high molecular weight high-density fractions |
| US11702512B2 (en) | 2018-07-17 | 2023-07-18 | Dow Silicones Corporation | Polysiloxane resin-polyolefin copolymer and methods for the preparation and use thereof |
| US20210179827A1 (en) | 2018-08-29 | 2021-06-17 | Exxonmobil Chemical Patents Inc. | Methods of Making Polymer Compositions with Enhanced Elasticity by Employing VTP and HMP Catalyst Systems in Parallel Processes |
| TW202031692A (en) | 2018-11-30 | 2020-09-01 | 美商陶氏全球科技有限責任公司 | Polymer-based film with balanced properties |
| BR112021012786A2 (en) | 2018-12-28 | 2021-09-14 | Dow Global Technologies Llc | CURABLE FORMULATION |
| KR20210121028A (en) | 2018-12-28 | 2021-10-07 | 다우 글로벌 테크놀로지스 엘엘씨 | organometallic chain transfer agent |
| CN113454130A (en) | 2018-12-28 | 2021-09-28 | 陶氏环球技术有限责任公司 | Telechelic polyolefins and process for preparing telechelic polyolefins |
| CN113498413A (en) | 2018-12-28 | 2021-10-12 | 陶氏环球技术有限责任公司 | Curable compositions comprising unsaturated polyolefins |
| SG11202107057WA (en) | 2018-12-28 | 2021-07-29 | Dow Global Technologies Llc | Curable compositions comprising unsaturated polyolefins |
| US11028805B2 (en) | 2019-01-09 | 2021-06-08 | Saudi Arabian Oil Company | System and method for on-board catalytic upgrading of hydrocarbon fuels |
| KR101995951B1 (en) * | 2019-03-12 | 2019-07-03 | 주식회사 라이온켐텍 | A continuous method for manufacturing polyolefin copolymer |
| ES2944469T3 (en) | 2019-03-28 | 2023-06-21 | Dow Global Technologies Llc | Group III Anion Complexes as Weakly Coordinating Anions for Olefin Polymerization Catalyst Activators |
| US20230183401A1 (en) | 2019-04-30 | 2023-06-15 | Dow Global Technologies Llc | Alkene functionalized activators and their use in electrical applications |
| JP7288977B2 (en) | 2019-04-30 | 2023-06-08 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene/propylene/non-conjugated diene interpolymer composition |
| US20220251360A1 (en) | 2019-06-26 | 2022-08-11 | Dow Global Technologies Llc | EPDM Blends with Long Chain Branching |
| KR20220025824A (en) | 2019-06-26 | 2022-03-03 | 다우 글로벌 테크놀로지스 엘엘씨 | Controlled Long Chain Branching of EPDM by Post-Reactor Modification |
| EP3763745A1 (en) | 2019-07-10 | 2021-01-13 | Borealis AG | High temperature solution process for the copolymerization of alpha-olefins |
| EP4004071A1 (en) | 2019-07-31 | 2022-06-01 | Dow Global Technologies LLC | Polymerization of ethylene in solution processes using a ziegler-natta catalyst and a hydrogenation procatalyst |
| WO2021035709A1 (en) | 2019-08-30 | 2021-03-04 | Dow Global Technologies Llc | Polyolefin compositions having improved electrical properties |
| CN114514280A (en) | 2019-09-24 | 2022-05-17 | 陶氏环球技术有限责任公司 | Polymer composition for extrusion profiles |
| US20220298344A1 (en) | 2019-09-24 | 2022-09-22 | Dow Global Technologies Llc | Ethylene/alpha-olefin/polyene interpolymer compositions |
| JP2023508013A (en) | 2019-12-30 | 2023-02-28 | ダウ グローバル テクノロジーズ エルエルシー | Process for preparing alpha-substituted acrylates |
| US20220402856A1 (en) | 2019-12-30 | 2022-12-22 | Dow Global Technologies Llc | Process for preparing an alpha-substituted acrylate |
| CN115298236A (en) | 2020-03-27 | 2022-11-04 | 陶氏环球技术有限责任公司 | Long chain branched ethylene-based polymers |
| US20230128870A1 (en) | 2020-03-27 | 2023-04-27 | Dow Global Technologies Llc | A process for producing long-chain branched ethylene-based polymers |
| EP4126990A1 (en) | 2020-03-27 | 2023-02-08 | Dow Global Technologies LLC | Long-chain branched ethylene-based polymers |
| KR20220158763A (en) | 2020-03-27 | 2022-12-01 | 다우 글로벌 테크놀로지스 엘엘씨 | long-chain branched ethylenic polymers |
| EP4127061A1 (en) | 2020-03-30 | 2023-02-08 | ExxonMobil Chemical Patents Inc. | Comb-block copolymers and methods thereof |
| JP2023524452A (en) | 2020-04-29 | 2023-06-12 | ダウ グローバル テクノロジーズ エルエルシー | High-flow propylene-based interpolymer composition |
| CN111662403B (en) * | 2020-07-14 | 2022-08-05 | 万华化学集团股份有限公司 | Cascade catalytic system and method for preparing LLDPE (Linear Low Density polyethylene) by using same |
| KR20230039687A (en) | 2020-07-17 | 2023-03-21 | 다우 글로벌 테크놀로지스 엘엘씨 | Hydrocarbyl-modified methylaluminoxane cocatalyst for bis-phenylphenoxy metal-ligand complexes |
| BR112023000737A2 (en) | 2020-07-17 | 2023-02-07 | Dow Global Technologies Llc | POLYMERIZATION PROCESS OF OLEFIN MONOMERS |
| BR112023000804A2 (en) | 2020-07-17 | 2023-02-07 | Dow Global Technologies Llc | PROCESSES FOR POLYMERIZING OLEFIN AND POLYMERIZATION MONOMERS |
| CN111909196B (en) * | 2020-08-10 | 2023-05-26 | 万华化学集团股份有限公司 | IVB-group-containing bimetallic complex catalyst, and preparation method and application thereof |
| WO2022073805A1 (en) | 2020-10-05 | 2022-04-14 | Borealis Ag | High temperature solution process for the copolymerization of ethylene with one ore more α-Olefin comonomer(s) |
| WO2022103800A1 (en) | 2020-11-10 | 2022-05-19 | Dow Global Technologies Llc | Preparation of polyolefin-polyacrylate block copolymers additives for increasing surface energy of polyethylene |
| WO2022103803A1 (en) | 2020-11-10 | 2022-05-19 | Dow Global Technologies Llc | Preparation of non-polar–polar block copolymers via vinyl-terminated polyolefins |
| EP4251664A2 (en) | 2020-11-24 | 2023-10-04 | Dow Global Technologies LLC | Process to produce long chain branching in epdm and product |
| CN116897168A (en) * | 2021-02-26 | 2023-10-17 | 陶氏环球技术有限责任公司 | Bis-phenoxy-ether ligands for Group IV polyolefin catalysis |
| CA3228273A1 (en) | 2021-08-04 | 2023-02-09 | China Petroleum & Chemical Corporation | Modified flexible polypropylene insulating material and preparation method and use thereof |
| WO2023039515A2 (en) | 2021-09-10 | 2023-03-16 | Dow Global Technologies Llc | Borate cocatalysts for polyolefin production |
| WO2023039514A1 (en) | 2021-09-10 | 2023-03-16 | Dow Global Technologies Llc | Hydrocarbon soluble borate cocatalysts for olefin polymerization |
| CN114957530B (en) * | 2022-06-28 | 2023-09-29 | 杭州双安科技有限公司 | Solution polymerization method of ethylene and alpha-olefin |
| CN115160472B (en) * | 2022-08-09 | 2023-07-11 | 万华化学集团股份有限公司 | High insertion rate ethylene copolymer solution polymerization method |
Citations (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0277003A1 (en) | 1987-01-30 | 1988-08-03 | Exxon Chemical Patents Inc. | Catalysts, method of preparing these catalysts, and polymerization processes wherein these catalysts are used |
| EP0277004A1 (en) | 1987-01-30 | 1988-08-03 | Exxon Chemical Patents Inc. | Catalysts, method of preparing these catalysts and method of using said catalysts |
| EP0426637A2 (en) | 1989-10-30 | 1991-05-08 | Fina Technology, Inc. | Preparation of metallocene catalysts for polymerization of olefins |
| US5066741A (en) | 1990-03-22 | 1991-11-19 | The Dow Chemical Company | Process for preparation of syndiotactic vinyl aromatic polymers |
| EP0495375A2 (en) | 1991-01-16 | 1992-07-22 | The Dow Chemical Company | Process for preparing addition polymerization catalysts via metal center oxidation |
| EP0500944A1 (en) | 1990-07-24 | 1992-09-02 | MITSUI TOATSU CHEMICALS, Inc. | CATALYST FOR $g(a)-OLEFIN POLYMERIZATION AND PRODUCTION OF POLY-$g(a)-OLEFIN THEREWITH |
| US5153157A (en) | 1987-01-30 | 1992-10-06 | Exxon Chemical Patents Inc. | Catalyst system of enhanced productivity |
| EP0520732A1 (en) | 1991-06-24 | 1992-12-30 | The Dow Chemical Company | Homogeneous olefin polymerization catalyst by ligand abstraction with lewis acids |
| US5198401A (en) | 1987-01-30 | 1993-03-30 | Exxon Chemical Patents Inc. | Ionic metallocene catalyst compositions |
| US5206197A (en) | 1991-03-04 | 1993-04-27 | The Dow Chemical Company | Catalyst composition for preparation of syndiotactic vinyl aromatic polymers |
| US5241025A (en) | 1987-01-30 | 1993-08-31 | Exxon Chemical Patents Inc. | Catalyst system of enhanced productivity |
| EP0570982A1 (en) | 1992-05-22 | 1993-11-24 | Tosoh Corporation | Catalysts and process for producing olefin polymers |
| EP0573403A2 (en) | 1992-06-04 | 1993-12-08 | Fina Technology, Inc. | Cationic metallocene catalysts based on triphenylcarbenium aluminum |
| US5272236A (en) | 1991-10-15 | 1993-12-21 | The Dow Chemical Company | Elastic substantially linear olefin polymers |
| EP0608369A1 (en) | 1991-10-15 | 1994-08-03 | Dow Chemical Co | Elastic substantially linear olefin polymers. |
| US5384299A (en) | 1987-01-30 | 1995-01-24 | Exxon Chemical Patents Inc. | Ionic metallocene catalyst compositions |
| US5387568A (en) | 1989-10-30 | 1995-02-07 | Fina Technology, Inc. | Preparation of metallocene catalysts for polymerization of olefins |
| US5502124A (en) | 1992-07-01 | 1996-03-26 | Exxon Chemical Patents Inc. | Transition metal olefin polymerization processes |
| US6395671B2 (en) | 1998-02-20 | 2002-05-28 | The Dow Chemical Company | Catalyst activators comprising expanded anions |
| US6897276B2 (en) | 2002-04-24 | 2005-05-24 | Symyx Technologies, Inc. | Bridged bi-aromatic ligands, catalysts, processes for polymerizing and polymers therefrom |
| WO2006020624A1 (en) | 2004-08-09 | 2006-02-23 | Dow Global Technologies Inc. | Supported bis(hydroxyarylaryloxy) catalysts for manufacture of polymers |
Family Cites Families (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3485706A (en) | 1968-01-18 | 1969-12-23 | Du Pont | Textile-like patterned nonwoven fabrics and their production |
| US3914342A (en) | 1971-07-13 | 1975-10-21 | Dow Chemical Co | Ethylene polymer blend and polymerization process for preparation thereof |
| US3953413A (en) | 1974-06-13 | 1976-04-27 | Chemplex Company | Supported chromium-containing catalyst and process of polymerizing 1-olefins |
| US4173548A (en) | 1977-02-02 | 1979-11-06 | Chemplex Company | Ethylene polymerization catalyst and method |
| US4330646A (en) | 1979-08-13 | 1982-05-18 | Asahi Kasei Kogyo Kabushiki Kaisha | Polymerization of an α-olefin |
| US4322027A (en) | 1980-10-02 | 1982-03-30 | Crown Zellerbach Corporation | Filament draw nozzle |
| US4413110A (en) | 1981-04-30 | 1983-11-01 | Allied Corporation | High tenacity, high modulus polyethylene and polypropylene fibers and intermediates therefore |
| US4588790A (en) | 1982-03-24 | 1986-05-13 | Union Carbide Corporation | Method for fluidized bed polymerization |
| US4543399A (en) | 1982-03-24 | 1985-09-24 | Union Carbide Corporation | Fluidized bed reaction systems |
| DZ520A1 (en) | 1982-03-24 | 2004-09-13 | Union Carbide Corp | Improved process for increasing the space-time yield of an exothermic polymerization reaction in a fluidized bed. |
| US4430563A (en) | 1982-04-30 | 1984-02-07 | Minnesota Mining And Manufacturing Company | Data processing form |
| US4564660A (en) | 1983-06-30 | 1986-01-14 | Union Carbide Corporation | Use of alkylaluminum compounds and hydroxyl-containing compounds to initiate polymerization of ethylene with chromium oxide catalysts |
| US4612300A (en) * | 1985-06-06 | 1986-09-16 | The Dow Chemical Company | Novel catalyst for producing relatively narrow molecular weight distribution olefin polymers |
| US4665208A (en) | 1985-07-11 | 1987-05-12 | Exxon Chemical Patents Inc. | Process for the preparation of alumoxanes |
| US4663220A (en) | 1985-07-30 | 1987-05-05 | Kimberly-Clark Corporation | Polyolefin-containing extrudable compositions and methods for their formation into elastomeric products including microfibers |
| US4668566A (en) | 1985-10-07 | 1987-05-26 | Kimberly-Clark Corporation | Multilayer nonwoven fabric made with poly-propylene and polyethylene |
| ATE114678T1 (en) | 1986-09-24 | 1994-12-15 | Mitsui Petrochemical Ind | PROCESSES FOR POLYMERIZATION OF POLYOLEFINS. |
| JPH0780933B2 (en) | 1986-11-20 | 1995-08-30 | 三井石油化学工業株式会社 | Olefin Polymerization Method |
| JPS63154753A (en) | 1986-12-18 | 1988-06-28 | Nippon Oil Co Ltd | Polyethylene composition |
| JPH0742301B2 (en) | 1987-02-14 | 1995-05-10 | 三井石油化学工業株式会社 | Particulate aluminoxane, its manufacturing method and its use |
| JP2538588B2 (en) | 1987-04-03 | 1996-09-25 | 三井石油化学工業株式会社 | Method for producing solid catalyst for olefin polymerization |
| US5206199A (en) | 1987-04-20 | 1993-04-27 | Mitsui Petrochemical Industries, Ltd. | Catalyst for polymerizing an olefin and process for polymerizing an olefin |
| US4981927A (en) * | 1987-05-20 | 1991-01-01 | National Distillers And Chemical Corporation | Chromium catalyst compositions and polymerization utilizing same |
| FR2634212B1 (en) | 1988-07-15 | 1991-04-19 | Bp Chimie Sa | APPARATUS AND METHOD FOR POLYMERIZATION OF GASEOUS OLEFINS IN A FLUIDIZED BED REACTOR |
| US5091352A (en) | 1988-09-14 | 1992-02-25 | Mitsui Petrochemical Industries, Ltd. | Olefin polymerization catalyst component, olefin polymerization catalyst and process for the polymerization of olefins |
| US4908463A (en) | 1988-12-05 | 1990-03-13 | Ethyl Corporation | Aluminoxane process |
| US5103031A (en) | 1989-02-21 | 1992-04-07 | Ethyl Corporation | Falling film aluminoxane process |
| US4968827A (en) | 1989-06-06 | 1990-11-06 | Ethyl Corporation | Alkylaluminoxane process |
| US4924018A (en) * | 1989-06-26 | 1990-05-08 | Ethyl Corporation | Alkylaluminoxane process |
| US5064802A (en) * | 1989-09-14 | 1991-11-12 | The Dow Chemical Company | Metal complex compounds |
| FR2656314B1 (en) * | 1989-12-22 | 1992-04-17 | Bp Chemicals Snc | ZIRCONIUM CATALYST SUPPORTED ON MAGNESIUM CHLORIDE, PROCESS FOR THE PREPARATION AND USE OF THE CATALYST IN OLEFIN POLYMERIZATION. |
| US5032562A (en) | 1989-12-27 | 1991-07-16 | Mobil Oil Corporation | Catalyst composition and process for polymerizing polymers having multimodal molecular weight distribution |
| JP2545006B2 (en) | 1990-07-03 | 1996-10-16 | ザ ダウ ケミカル カンパニー | Addition polymerization catalyst |
| US5296443A (en) * | 1991-03-01 | 1994-03-22 | Dai Nippon Printing Co., Ltd. | Thermal transfer image receiving sheet |
| US5308815A (en) | 1991-07-26 | 1994-05-03 | Ethyl Corporation | Heterogeneous methylaluminoxane catalyst system |
| US5235081A (en) | 1992-03-18 | 1993-08-10 | Ethyl Corporation | Method of removing gel forming materials from methylaluminoxanes |
| US5157137A (en) | 1991-07-26 | 1992-10-20 | Ethyl Corporation | Method of making gel free alkylaluminoxane solutions |
| US5674342A (en) * | 1991-10-15 | 1997-10-07 | The Dow Chemical Company | High drawdown extrusion composition and process |
| US5525695A (en) | 1991-10-15 | 1996-06-11 | The Dow Chemical Company | Elastic linear interpolymers |
| US6448355B1 (en) * | 1991-10-15 | 2002-09-10 | The Dow Chemical Company | Elastic fibers, fabrics and articles fabricated therefrom |
| KR100262023B1 (en) * | 1992-01-06 | 2000-07-15 | 그레이스 스티븐 에스. | Improved catalyst composition |
| US5329032A (en) | 1992-03-18 | 1994-07-12 | Akzo Chemicals Inc. | Polymethylaluminoxane of enhanced solution stability |
| US5436304A (en) | 1992-03-19 | 1995-07-25 | Exxon Chemical Patents Inc. | Process for polymerizing monomers in fluidized beds |
| US5352749A (en) | 1992-03-19 | 1994-10-04 | Exxon Chemical Patents, Inc. | Process for polymerizing monomers in fluidized beds |
| US5296433A (en) | 1992-04-14 | 1994-03-22 | Minnesota Mining And Manufacturing Company | Tris(pentafluorophenyl)borane complexes and catalysts derived therefrom |
| US5288933A (en) * | 1992-04-16 | 1994-02-22 | Union Carbide Chemicals & Plastics Technology Corporation | Process for the production of polyethylene |
| US5350723A (en) * | 1992-05-15 | 1994-09-27 | The Dow Chemical Company | Process for preparation of monocyclopentadienyl metal complex compounds and method of use |
| BE1005957A5 (en) | 1992-06-05 | 1994-04-05 | Solvay | Preparation method of catalyst system, process (co) polymerization of olefins and (co) polymer at least one olefine. |
| US5248801A (en) | 1992-08-27 | 1993-09-28 | Ethyl Corporation | Preparation of methylaluminoxanes |
| KR950703595A (en) | 1992-09-29 | 1995-09-20 | 벤셔 카펜헤르 | LONG CHAIN BRANCHED POLYMERS AND A PROCESS TO MAKE LONG CHAIN BRANCHED POLYMERS |
| CA2146012A1 (en) | 1992-10-02 | 1994-04-14 | Brian W. S. Kolthammer | Supported homogenous catalyst complexes for olefin polymerization |
| DE4322884A1 (en) | 1992-10-09 | 1994-04-14 | Bayer Ag | Biologically active polymers |
| US5939346A (en) | 1992-11-02 | 1999-08-17 | Akzo Nobel N.V. | Catalyst system comprising an aryloxyaluminoxane containing an electron withdrawing group |
| US5391793A (en) | 1992-11-02 | 1995-02-21 | Akzo Nobel N.V. | Aryloxyaluminoxanes |
| US5608019A (en) | 1992-12-28 | 1997-03-04 | Mobil Oil Corporation | Temperature control of MW in olefin polymerization using supported metallocene catalyst |
| US5420220A (en) * | 1993-03-25 | 1995-05-30 | Mobil Oil Corporation | LLDPE films |
| US5332706A (en) | 1992-12-28 | 1994-07-26 | Mobil Oil Corporation | Process and a catalyst for preventing reactor fouling |
| US5391529A (en) | 1993-02-01 | 1995-02-21 | Albemarle Corporation | Siloxy-aluminoxane compositions, and catalysts which include such compositions with a metallocene |
| TW298593B (en) | 1993-02-12 | 1997-02-21 | Hoechst Ag | |
| KR100311244B1 (en) * | 1993-02-22 | 2001-12-15 | 가지와라 야스시 | Process for producing ethylene / α-olefin copolymer |
| US6313240B1 (en) * | 1993-02-22 | 2001-11-06 | Tosoh Corporation | Process for producing ethylene/α-olefin copolymer |
| US5462999A (en) * | 1993-04-26 | 1995-10-31 | Exxon Chemical Patents Inc. | Process for polymerizing monomers in fluidized beds |
| WO1994025495A1 (en) | 1993-05-20 | 1994-11-10 | Exxon Chemical Patents Inc. | Process for polymerizing monomers in fluidized beds |
| HU214694B (en) * | 1993-04-28 | 1998-04-28 | The Dow Chemical Co. | Ethylene polymer compositions and fabricated articles made therefrom |
| BE1007148A3 (en) | 1993-05-17 | 1995-04-11 | Solvay | Support for catalyst, method for producing gel precursor media for catalyst, method for preparing a catalyst support, catalyst for olefin polymerization and method for olefin polymerization using the catalyst . |
| FR2705252B1 (en) | 1993-05-19 | 1995-07-21 | Bp Chemicals Snc | Process for introducing a solid into a reactor and apparatus. |
| ZA943399B (en) * | 1993-05-20 | 1995-11-17 | Bp Chem Int Ltd | Polymerisation process |
| JP2736938B2 (en) | 1993-06-24 | 1998-04-08 | ザ ダウ ケミカル カンパニー | Titanium (II) or zirconium (II) complex and addition polymerization catalyst comprising the same |
| US5372682A (en) * | 1993-06-24 | 1994-12-13 | The Dow Chemical Company | Electrochemical preparation of addition polymerization catalysts |
| JPH09505340A (en) | 1993-11-19 | 1997-05-27 | エクソン・ケミカル・パテンツ・インク | Polymerization catalyst system, production method and use thereof |
| JPH07144455A (en) | 1993-11-25 | 1995-06-06 | Canon Inc | Ink jet recording apparatus |
| US5674795A (en) | 1993-12-23 | 1997-10-07 | Union Carbide Chemicals & Plastics Technology Corporation | Spray dried, filled metallocene catalyst composition for use in polyolefin manufacture |
| US5648310A (en) | 1993-12-23 | 1997-07-15 | Union Carbide Chemicals & Plastics Technology Corporation | Spray dried, filled metallocene catalyst composition for use in polyolefin manufacture |
| US5461123A (en) | 1994-07-14 | 1995-10-24 | Union Carbide Chemicals & Plastics Technology Corporation | Gas phase fluidized bed polyolefin polymerization process using sound waves |
| US5453471B1 (en) * | 1994-08-02 | 1999-02-09 | Carbide Chemicals & Plastics T | Gas phase polymerization process |
| US5625087A (en) | 1994-09-12 | 1997-04-29 | The Dow Chemical Company | Silylium cationic polymerization activators for metallocene complexes |
| DE4436392C2 (en) | 1994-10-12 | 2002-10-31 | Fraunhofer Ges Forschung | Metal niobates and / or tantalates, processes for their preparation and their further processing into perovskites |
| US5616661A (en) | 1995-03-31 | 1997-04-01 | Union Carbide Chemicals & Plastics Technology Corporation | Process for controlling particle growth during production of sticky polymers |
| UA47394C2 (en) * | 1995-05-16 | 2002-07-15 | Юнівейшн Текнолоджіз, Ллс | Ethylene polymer with improved processability and an article containing the ethylene polymer |
| US5869723A (en) | 1995-06-08 | 1999-02-09 | Showa Denko K.K. | Ionic compound and olefin polymerization catalyst containing the same |
| US5731253A (en) | 1995-07-27 | 1998-03-24 | Albemarle Corporation | Hydrocarbylsilloxy - aluminoxane compositions |
| US5869575A (en) | 1995-08-02 | 1999-02-09 | The Dow Chemical Company | Ethylene interpolymerizations |
| US5767208A (en) | 1995-10-20 | 1998-06-16 | Exxon Chemical Patents Inc. | High temperature olefin polymerization process |
| US5693838A (en) | 1995-11-13 | 1997-12-02 | Albemarle Corporation | Aluminoxane process and product |
| US5814714A (en) * | 1995-11-30 | 1998-09-29 | The Dow Chemical Company | Mono-olefin/polyene interpolymers, method of preparation, compositions containing the same, and articles made thereof |
| EP0786466B1 (en) | 1996-01-25 | 2003-04-16 | Tosoh Corporation | Olefin polymerisation process which comprises a transition metal catalyst. |
| ATE206445T1 (en) * | 1996-03-05 | 2001-10-15 | Dow Chemical Co | RHEOLOGICALLY MODIFIED POLYOLEFINS |
| ATE234870T1 (en) * | 1996-03-27 | 2003-04-15 | Dow Global Technologies Inc | SOLUTION POLYMERIZATION PROCESS WITH DISPERSED CATALYST ACTIVATORS |
| KR100437238B1 (en) | 1996-03-27 | 2004-08-16 | 다우 글로벌 테크놀로지스 인크. | Highly soluble olefin polymerization catalyst activator |
| US5977251A (en) | 1996-04-01 | 1999-11-02 | The Dow Chemical Company | Non-adiabatic olefin solution polymerization |
| US5731451A (en) | 1996-07-12 | 1998-03-24 | Akzo Nobel Nv | Modified polyalkylauminoxane composition formed using reagent containing aluminum trialkyl siloxide |
| US5854166A (en) | 1996-08-19 | 1998-12-29 | Northwestern University | Synthesis and use of (perfluoroaryl) fluoro-aluminate anion |
| DE69728677T2 (en) | 1996-09-06 | 2005-03-31 | Hyundai Pertrochemical Co., Ltd. | CATALYST SYSTEM FOR THE (CO) POLYMERIZATION OF OLEFINES AND METHOD FOR THE PRODUCTION OF OLEFIN (CO) POLYMERS USING THE CATALYST SYSTEM |
| US5744656A (en) | 1996-10-25 | 1998-04-28 | Boulder Scientific Company | Conversion of hexafluorobenzene to bromopentafluorobenzene |
| US5783512A (en) * | 1996-12-18 | 1998-07-21 | The Dow Chemical Company | Catalyst component dispersion comprising an ionic compound and solid addition polymerization catalysts containing the same |
| FI970349A (en) | 1997-01-28 | 1998-07-29 | Borealis As | New activator systems for metallocene compounds |
| IL132189A (en) | 1997-04-03 | 2003-10-31 | Univ Colorado State Res Found | Polyhalogenated monoheteroborane anion compositions |
| US6420507B1 (en) | 1997-05-01 | 2002-07-16 | The Dow Chemical Company | Olefin polymers prepared with substituted indenyl containing metal complexes |
| US6103657A (en) | 1997-07-02 | 2000-08-15 | Union Carbide Chemicals & Plastics Technology Corporation | Catalyst for the production of olefin polymers |
| US6319989B1 (en) * | 1997-07-21 | 2001-11-20 | The Dow Chemical Company | Broad MWD, compositionally uniform ethylene interpolymer compositions, process for making the same and article made therefrom |
| JP2001517714A (en) | 1997-09-19 | 2001-10-09 | ザ ダウ ケミカル カンパニー | Modified aluminoxane catalyst activator |
| US6696379B1 (en) * | 1997-09-19 | 2004-02-24 | The Dow Chemical Company | Supported modified alumoxane catalyst activator |
| ZA988572B (en) | 1997-09-19 | 2000-03-22 | Dow Chemical Co | Narrow MWD, compositionally optimized ethylene interpolymer composition, process for making the same and article made therefrom. |
| DE19744102A1 (en) | 1997-10-06 | 1999-04-15 | Targor Gmbh | Metallocene catalyst system useful in (co)polyolefin production |
| DE69925114T2 (en) | 1998-03-04 | 2005-10-27 | Exxonmobil Chemical Patents Inc., Baytown | METHOD FOR POLYMERIZING OLEFINES AT HIGH TEMPERATURES |
| US6827976B2 (en) | 1998-04-29 | 2004-12-07 | Unaxis Trading Ag | Method to increase wear resistance of a tool or other machine component |
| DE69902548T2 (en) | 1998-08-11 | 2003-04-10 | Dow Chemical Co | catalyst activator |
| DE69901451T2 (en) * | 1998-08-11 | 2002-12-12 | Dow Chemical Co | ANSA TO (.MU.-ALUMINUM) -SUBSTITUED GROUP-4-METAL COMPLEXES |
| DE69922548T2 (en) * | 1998-10-23 | 2006-01-05 | Exxonmobil Chemical Patents Inc., Baytown | BROKEN METAL OENCENE FOR OLEFIN COPOLYMERIZATION |
| JP2000129045A (en) * | 1998-10-27 | 2000-05-09 | Asahi Chem Ind Co Ltd | Clean vessel made of polyethylene |
| BR9915199B1 (en) * | 1998-11-02 | 2010-09-08 | fine-shear ethylene / alpha-olefin interpolymer, ethylene / alpha-olefin interpolymer preparation process, manufactured article, polymer blend composition and thermoplastic vulcanized composition. | |
| AU2716700A (en) * | 1998-12-31 | 2000-07-31 | Homedics, Inc. | Percussive massager |
| AU1919900A (en) | 1999-02-19 | 2000-09-04 | Dow Chemical Company, The | Process for preparing trifluoroarylaluminum etherates |
| US6420298B1 (en) * | 1999-08-31 | 2002-07-16 | Exxonmobil Oil Corporation | Metallocene catalyst compositions, processes for making polyolefin resins using such catalyst compositions, and products produced thereby |
| JP2001098028A (en) * | 1999-09-29 | 2001-04-10 | Japan Polychem Corp | Polyethylene resin for medical and medical container |
| US6750302B1 (en) * | 1999-12-16 | 2004-06-15 | Phillips Petroleum Company | Organometal catalyst compositions |
| US6686490B1 (en) * | 2000-11-06 | 2004-02-03 | Ramot University Authority For Applied Research & Industrial Development Ltd. | Active non-metallocene pre-catalyst and method for tactic catalytic polymerization of alpha-olefin monomers |
| US6713577B2 (en) | 2000-11-07 | 2004-03-30 | Symyx Technologies, Inc. | Substituted pyridyl amine catalysts and processes for polymerizing and polymers |
| WO2002046246A2 (en) * | 2000-12-04 | 2002-06-13 | Univaton Technologies, Llc | Polimerization process |
| JP3549868B2 (en) * | 2001-02-08 | 2004-08-04 | 三井化学株式会社 | Ethylene polymer, method for producing the same, and molded article using the same |
| MY131000A (en) | 2001-03-16 | 2007-07-31 | Dow Global Technologies Inc | High melt strength polymers and method of making same |
| US6727329B2 (en) | 2001-07-23 | 2004-04-27 | Dow Global Technology Inc. | Salts of lewis acid/acid adducts and catalyst activators therefrom |
| US6960635B2 (en) | 2001-11-06 | 2005-11-01 | Dow Global Technologies Inc. | Isotactic propylene copolymers, their preparation and use |
| US6908972B2 (en) * | 2002-04-16 | 2005-06-21 | Equistar Chemicals, Lp | Method for making polyolefins |
| US6794908B2 (en) | 2002-05-31 | 2004-09-21 | Honeywell International Inc. | Radiation-hard circuit |
| US6756455B2 (en) | 2002-05-31 | 2004-06-29 | Equistar Chemicals, Lp | High-temperature solution process for polyolefin manufacture |
| US6953764B2 (en) | 2003-05-02 | 2005-10-11 | Dow Global Technologies Inc. | High activity olefin polymerization catalyst and process |
| JP4879882B2 (en) * | 2004-03-17 | 2012-02-22 | ダウ グローバル テクノロジーズ エルエルシー | Catalyst composition comprising a shuttling agent for forming higher order olefin multi-block copolymers |
| JP5255833B2 (en) | 2004-03-24 | 2013-08-07 | エクソンモービル・ケミカル・パテンツ・インク | Method for producing ethylene interpolymer, interpolymer produced by the method, composition, and electrical device comprising the interpolymer |
| US20050288461A1 (en) | 2004-06-25 | 2005-12-29 | Jensen Michael D | Polymerization catalysts for producing polymers with low levels of long chain branching |
| CA2479704C (en) * | 2004-08-31 | 2013-08-13 | Nova Chemicals Corporation | High density homopolymer blends |
| BRPI0516675A (en) * | 2004-11-05 | 2008-09-16 | Dow Global Technologies Inc | substituted ferrocene compound, process for the polymerization by addition of one or more olefinic monomers and process for the synthesis of a group 3-10 organometallic compound |
| KR100639696B1 (en) * | 2005-07-01 | 2006-10-30 | 에스케이 주식회사 | ARYLPHENOXY CATALYST SYSTEM FOR PRODUCING ETHYLENE HOMOPOLYMERS OR ETHYLENE COPOLYMERS WITH alpha;-OLEFINS |
| KR101397337B1 (en) * | 2006-05-17 | 2014-05-19 | 다우 글로벌 테크놀로지스 엘엘씨 | High Efficiency Solution Polymerization Process |
-
2007
- 2007-04-24 KR KR1020087030632A patent/KR101397337B1/en active IP Right Grant
- 2007-04-24 BR BRPI0711018A patent/BRPI0711018A8/en not_active IP Right Cessation
- 2007-04-24 ES ES07776021.3T patent/ES2534469T3/en active Active
- 2007-04-24 RU RU2008149711/04A patent/RU2463311C2/en not_active IP Right Cessation
- 2007-04-24 US US12/300,515 patent/US8058373B2/en active Active
- 2007-04-24 ES ES12169643.9T patent/ES2634440T3/en active Active
- 2007-04-24 ES ES07755919T patent/ES2394225T3/en active Active
- 2007-04-24 RU RU2008149714/04A patent/RU2008149714A/en not_active Application Discontinuation
- 2007-04-24 CN CN2007800266677A patent/CN101490096B/en active Active
- 2007-04-24 WO PCT/US2007/009844 patent/WO2007136496A2/en active Application Filing
- 2007-04-24 RU RU2008149709/04A patent/RU2450026C2/en not_active IP Right Cessation
- 2007-04-24 BR BRPI0711819-8A patent/BRPI0711819A2/en not_active IP Right Cessation
- 2007-04-24 CN CN2007800233160A patent/CN101472952B/en active Active
- 2007-04-24 KR KR1020087030629A patent/KR101401785B1/en active IP Right Grant
- 2007-04-24 JP JP2009510956A patent/JP5666129B2/en active Active
- 2007-04-24 CA CA002653534A patent/CA2653534A1/en not_active Abandoned
- 2007-04-24 AR ARP070101767A patent/AR060639A1/en not_active Application Discontinuation
- 2007-04-24 CA CA002652465A patent/CA2652465A1/en not_active Abandoned
- 2007-04-24 EP EP07776021.3A patent/EP2024401B1/en active Active
- 2007-04-24 JP JP2009510950A patent/JP5603595B2/en active Active
- 2007-04-24 ES ES07776212.8T patent/ES2524779T3/en active Active
- 2007-04-24 AR ARP070101770A patent/AR060642A1/en not_active Application Discontinuation
- 2007-04-24 EP EP07755918.5A patent/EP2024399B1/en active Active
- 2007-04-24 KR KR1020087030639A patent/KR101395970B1/en active IP Right Grant
- 2007-04-24 WO PCT/US2007/009842 patent/WO2007136494A2/en active Application Filing
- 2007-04-24 CN CN2007800256425A patent/CN101484475B/en active Active
- 2007-04-24 EP EP07776022.1A patent/EP2024402B2/en active Active
- 2007-04-24 ES ES07776022T patent/ES2651590T5/en active Active
- 2007-04-24 KR KR1020087030593A patent/KR101384380B1/en active IP Right Grant
- 2007-04-24 MX MX2008014669A patent/MX2008014669A/en unknown
- 2007-04-24 KR KR1020087030627A patent/KR101396058B1/en active IP Right Grant
- 2007-04-24 WO PCT/US2007/009843 patent/WO2007136495A2/en active Application Filing
- 2007-04-24 EP EP20140174865 patent/EP2787013A1/en not_active Withdrawn
- 2007-04-24 CN CN201210266123.0A patent/CN102786619B/en active Active
- 2007-04-24 AR ARP070101769A patent/AR060641A1/en not_active Application Discontinuation
- 2007-04-24 CN CNA2007800259531A patent/CN101490095A/en active Pending
- 2007-04-24 AR ARP070101771A patent/AR060643A1/en unknown
- 2007-04-24 MX MX2008014667A patent/MX2008014667A/en unknown
- 2007-04-24 US US12/302,050 patent/US8354484B2/en active Active
- 2007-04-24 RU RU2008149712/04A patent/RU2008149712A/en not_active Application Discontinuation
- 2007-04-24 CA CA002652460A patent/CA2652460A1/en not_active Abandoned
- 2007-04-24 CN CN200780023101.9A patent/CN101472951B/en active Active
- 2007-04-24 WO PCT/US2007/010070 patent/WO2007136506A2/en active Application Filing
- 2007-04-24 MX MX2008014668A patent/MX2008014668A/en unknown
- 2007-04-24 WO PCT/US2007/009841 patent/WO2007136493A2/en active Application Filing
- 2007-04-24 JP JP2009510952A patent/JP5623738B2/en active Active
- 2007-04-24 CN CN2007800259052A patent/CN101490094B/en active Active
- 2007-04-24 AR ARP070101766A patent/AR060638A1/en not_active Application Discontinuation
- 2007-04-24 CA CA002652456A patent/CA2652456A1/en not_active Abandoned
- 2007-04-24 CN CN201510094535.4A patent/CN104725535A/en active Pending
- 2007-04-24 JP JP2009510949A patent/JP5645400B2/en active Active
- 2007-04-24 JP JP2009510951A patent/JP5583402B2/en active Active
- 2007-04-24 EP EP12169643.9A patent/EP2492287B1/en active Active
- 2007-04-24 WO PCT/US2007/009845 patent/WO2007136497A2/en active Application Filing
- 2007-04-24 US US12/300,857 patent/US8450438B2/en active Active
- 2007-04-24 MX MX2008014671A patent/MX2008014671A/en unknown
- 2007-04-24 EP EP07755919A patent/EP2024400B1/en active Active
- 2007-04-24 SG SG200908023-5A patent/SG158102A1/en unknown
- 2007-04-24 ES ES07755917.7T patent/ES2567140T3/en active Active
- 2007-04-24 US US12/300,863 patent/US8299189B2/en active Active
- 2007-04-24 EP EP07755917.7A patent/EP2021378B1/en active Active
- 2007-04-24 ES ES07755918.5T patent/ES2475159T3/en active Active
- 2007-04-24 US US12/300,861 patent/US8101696B2/en active Active
- 2007-04-24 KR KR1020087030640A patent/KR101352674B1/en active IP Right Grant
- 2007-04-24 EP EP07776212.8A patent/EP2024403B1/en active Active
- 2007-04-24 BR BRPI0711029-4A patent/BRPI0711029A2/en not_active IP Right Cessation
- 2007-04-24 AR ARP070101768A patent/AR060640A1/en not_active Application Discontinuation
- 2007-04-24 US US12/300,859 patent/US8202953B2/en active Active
- 2007-04-24 CA CA002652551A patent/CA2652551A1/en not_active Abandoned
- 2007-04-24 AU AU2007254428A patent/AU2007254428A1/en not_active Abandoned
-
2011
- 2011-12-19 US US13/329,486 patent/US8349984B2/en active Active
-
2014
- 2014-12-10 JP JP2014249797A patent/JP6001042B2/en active Active
Patent Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5384299A (en) | 1987-01-30 | 1995-01-24 | Exxon Chemical Patents Inc. | Ionic metallocene catalyst compositions |
| US5153157A (en) | 1987-01-30 | 1992-10-06 | Exxon Chemical Patents Inc. | Catalyst system of enhanced productivity |
| US5241025A (en) | 1987-01-30 | 1993-08-31 | Exxon Chemical Patents Inc. | Catalyst system of enhanced productivity |
| EP0277003A1 (en) | 1987-01-30 | 1988-08-03 | Exxon Chemical Patents Inc. | Catalysts, method of preparing these catalysts, and polymerization processes wherein these catalysts are used |
| US5198401A (en) | 1987-01-30 | 1993-03-30 | Exxon Chemical Patents Inc. | Ionic metallocene catalyst compositions |
| EP0277004A1 (en) | 1987-01-30 | 1988-08-03 | Exxon Chemical Patents Inc. | Catalysts, method of preparing these catalysts and method of using said catalysts |
| EP0426637A2 (en) | 1989-10-30 | 1991-05-08 | Fina Technology, Inc. | Preparation of metallocene catalysts for polymerization of olefins |
| US5387568A (en) | 1989-10-30 | 1995-02-07 | Fina Technology, Inc. | Preparation of metallocene catalysts for polymerization of olefins |
| US5066741A (en) | 1990-03-22 | 1991-11-19 | The Dow Chemical Company | Process for preparation of syndiotactic vinyl aromatic polymers |
| EP0500944A1 (en) | 1990-07-24 | 1992-09-02 | MITSUI TOATSU CHEMICALS, Inc. | CATALYST FOR $g(a)-OLEFIN POLYMERIZATION AND PRODUCTION OF POLY-$g(a)-OLEFIN THEREWITH |
| EP0495375A2 (en) | 1991-01-16 | 1992-07-22 | The Dow Chemical Company | Process for preparing addition polymerization catalysts via metal center oxidation |
| US5206197A (en) | 1991-03-04 | 1993-04-27 | The Dow Chemical Company | Catalyst composition for preparation of syndiotactic vinyl aromatic polymers |
| EP0520732A1 (en) | 1991-06-24 | 1992-12-30 | The Dow Chemical Company | Homogeneous olefin polymerization catalyst by ligand abstraction with lewis acids |
| US5272236A (en) | 1991-10-15 | 1993-12-21 | The Dow Chemical Company | Elastic substantially linear olefin polymers |
| EP0608369A1 (en) | 1991-10-15 | 1994-08-03 | Dow Chemical Co | Elastic substantially linear olefin polymers. |
| EP0570982A1 (en) | 1992-05-22 | 1993-11-24 | Tosoh Corporation | Catalysts and process for producing olefin polymers |
| EP0573403A2 (en) | 1992-06-04 | 1993-12-08 | Fina Technology, Inc. | Cationic metallocene catalysts based on triphenylcarbenium aluminum |
| US5502124A (en) | 1992-07-01 | 1996-03-26 | Exxon Chemical Patents Inc. | Transition metal olefin polymerization processes |
| US6395671B2 (en) | 1998-02-20 | 2002-05-28 | The Dow Chemical Company | Catalyst activators comprising expanded anions |
| US6897276B2 (en) | 2002-04-24 | 2005-05-24 | Symyx Technologies, Inc. | Bridged bi-aromatic ligands, catalysts, processes for polymerizing and polymers therefrom |
| US20050164872A1 (en) | 2002-04-24 | 2005-07-28 | Symyx Technologies, Inc. | Bridged bi-aromatic ligands, catalysts, processes for polymerizing and polymers therefrom |
| WO2006020624A1 (en) | 2004-08-09 | 2006-02-23 | Dow Global Technologies Inc. | Supported bis(hydroxyarylaryloxy) catalysts for manufacture of polymers |
Non-Patent Citations (1)
| Title |
|---|
| "Periodic Table of the Elements", 2003, CRC PRESS, INC. |
Cited By (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120101241A1 (en) * | 2006-05-17 | 2012-04-26 | Harold W Boone | ETHYLENE/a-OLEFIN/DIENE SOLUTION POLYMERIZATION PROCESS AND POLYMER |
| US8299189B2 (en) * | 2006-05-17 | 2012-10-30 | Dow Global Technologies, Llc | Ethylene/α-olefin/diene solution polymerization process and polymer |
| US20100311927A1 (en) * | 2009-06-05 | 2010-12-09 | Dow Global Technologies Inc. | Process to make long chain branched (lcb), block, or interconnected copolymers of ethylene |
| WO2010141557A1 (en) | 2009-06-05 | 2010-12-09 | Dow Global Technologies Inc. | Process to make long chain branched (lcb), block, or interconnected copolymers of ethylene |
| US8722817B2 (en) | 2009-06-05 | 2014-05-13 | Dow Global Technologies Llc | Process to make long chain branched (LCB), block, or interconnected copolymers of ethylene |
| US20110130533A1 (en) * | 2009-06-05 | 2011-06-02 | Dow Global Technologies Inc. | Process to make long chain branched (lcb), block, or interconnected copolymers of ethylene |
| WO2010144784A1 (en) | 2009-06-11 | 2010-12-16 | Dow Global Technologies Inc. | Novel ldpe enabling high output and good optics when blended with other polymers |
| EP2700661A1 (en) | 2009-06-11 | 2014-02-26 | Dow Global Technologies LLC | Novel ldpe enabling high output and good optics when blended with other polymers |
| US9243087B2 (en) | 2009-06-11 | 2016-01-26 | Dow Global Technologies Llc | LDPE enabling high output and good optics when blended with other polymers |
| US20130046061A1 (en) * | 2009-07-01 | 2013-02-21 | Dow GBlobal Technologies LLC | Ethylene-based polymer composition |
| EP2449025B1 (en) * | 2009-07-01 | 2017-04-05 | Dow Global Technologies LLC | Ethylene-based polymer compositions |
| JP2012532218A (en) * | 2009-07-01 | 2012-12-13 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene polymer composition |
| JP2012532231A (en) * | 2009-07-01 | 2012-12-13 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene polymer and use thereof |
| US8372931B2 (en) | 2009-07-01 | 2013-02-12 | Dow Global Technologies Llc | Ethylene-based polymer compositions |
| US20110015346A1 (en) * | 2009-07-01 | 2011-01-20 | Dow Global Technologies Inc. | Ethylene-based polymer compositions |
| JP2015110782A (en) * | 2009-07-01 | 2015-06-18 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene-based polymer composition |
| US8829115B2 (en) * | 2009-07-01 | 2014-09-09 | Dow Global Technologies Llc | Ethylene-based polymer composition |
| US8729200B2 (en) | 2009-07-01 | 2014-05-20 | Dow Global Technologies Llc | Ethylene-based polymer compositions |
| WO2011019563A1 (en) | 2009-08-10 | 2011-02-17 | Dow Global Technologies Inc. | Ldpe for use as a blend component in shrinkage film applications |
| US8987385B2 (en) | 2009-09-14 | 2015-03-24 | Dow Global Technologies Llc | Interconnected copolymers of ethylene in combination with one other polyalkene |
| US8598276B2 (en) | 2009-09-14 | 2013-12-03 | Dow Global Technologies Llc | Polymers comprising units derived from ethylene and poly(alkoxide) |
| WO2011032172A1 (en) | 2009-09-14 | 2011-03-17 | Dow Global Technologies Inc. | Polymers comprising units derived from ethylene and siloxane |
| US8691923B2 (en) | 2009-09-14 | 2014-04-08 | Dow Global Technologies Llc | Interconnected copolymers of ethylene in combination with at least one polysiloxane |
| US8679639B2 (en) | 2009-11-24 | 2014-03-25 | Dow Global Technologies Llc | Extrusion coating composition |
| WO2011066469A1 (en) | 2009-11-24 | 2011-06-03 | Dow Global Technologies Inc. | Extrusion coating composition |
| US8784996B2 (en) | 2009-11-24 | 2014-07-22 | Dow Global Technologies Llc | Extrusion coating composition |
| JP2013521382A (en) * | 2010-03-02 | 2013-06-10 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene polymer composition |
| WO2011109563A3 (en) * | 2010-03-02 | 2011-11-10 | Dow Global Technologies Llc | Ethylene-based polymer compositions |
| US9000108B2 (en) * | 2010-05-17 | 2015-04-07 | Dow Global Technologies Llc | Process for selectively polymerizing ethylene and catalyst therefor |
| US20140163186A1 (en) * | 2010-05-17 | 2014-06-12 | Dow Global Technologies Llc | Process for selectively polymerizing ethylene and catalyst therefor |
| EP2580279B1 (en) | 2010-06-14 | 2019-01-09 | Dow Global Technologies LLC | Ethylene-based polymer compositions for use as a blend component in shrinkage film applications |
| JP2013528689A (en) * | 2010-06-14 | 2013-07-11 | ダウ グローバル テクノロジーズ エルエルシー | Ethylene-based polymer composition for use as a blend component in shrink film applications |
| WO2012064630A2 (en) | 2010-11-08 | 2012-05-18 | Dow Global Technologies Llc | Solution polymerization process and procatalyst carrier systems useful therein |
| US20130281643A1 (en) * | 2010-12-21 | 2013-10-24 | Dow Global Technologies Llc | Olefin-based polymers and dispersion polymerizations |
| EP3091038B1 (en) | 2010-12-21 | 2019-09-18 | Dow Global Technologies LLC | Olefin-based polymers and dispersion polymerizations |
| US9388254B2 (en) * | 2010-12-21 | 2016-07-12 | Dow Global Technologies Llc | Olefin-based polymers and dispersion polymerizations |
| US20140248816A1 (en) * | 2011-10-05 | 2014-09-04 | Dow Global Technologies Llc | Bi-component fiber and fabrics made therefrom |
| US10391739B2 (en) * | 2011-10-05 | 2019-08-27 | Dow Global Technologies Llc | Bi-component fiber and fabrics made therefrom |
| US20140242304A1 (en) * | 2011-10-24 | 2014-08-28 | Peter Sandkuehler | Artificial turf yarn |
| WO2013096573A1 (en) | 2011-12-20 | 2013-06-27 | Dow Global Technologies Llc | Ethylene/alpha-olefin/nonconjugated polyene interpolymers and processes to form the same |
| KR20150037832A (en) * | 2012-07-20 | 2015-04-08 | 다우 글로벌 테크놀로지스 엘엘씨 | A linear low density polyethylene composition suitable for cast film |
| US9534070B2 (en) | 2013-01-18 | 2017-01-03 | Dow Global Technologies Llc | Polymerization processes for high molecular weight polymers |
| WO2014113046A1 (en) | 2013-01-18 | 2014-07-24 | Dow Global Technologies Llc | Polymerization processes for high molecular weight polyolefins |
| US10336846B2 (en) | 2015-05-28 | 2019-07-02 | Dow Global Technologies Llc | Process to form ethylene/α-olefin interpolymers |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8349984B2 (en) | Polyolefin solution polymerization process and polymer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200780025642.5 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07776212 Country of ref document: EP Kind code of ref document: A2 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2008/014669 Country of ref document: MX Ref document number: 2007776212 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2652551 Country of ref document: CA Ref document number: 2009510956 Country of ref document: JP Ref document number: 6263/CHENP/2008 Country of ref document: IN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020087030627 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008149709 Country of ref document: RU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12300861 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: PI0711018 Country of ref document: BR Kind code of ref document: A2 Effective date: 20081117 |