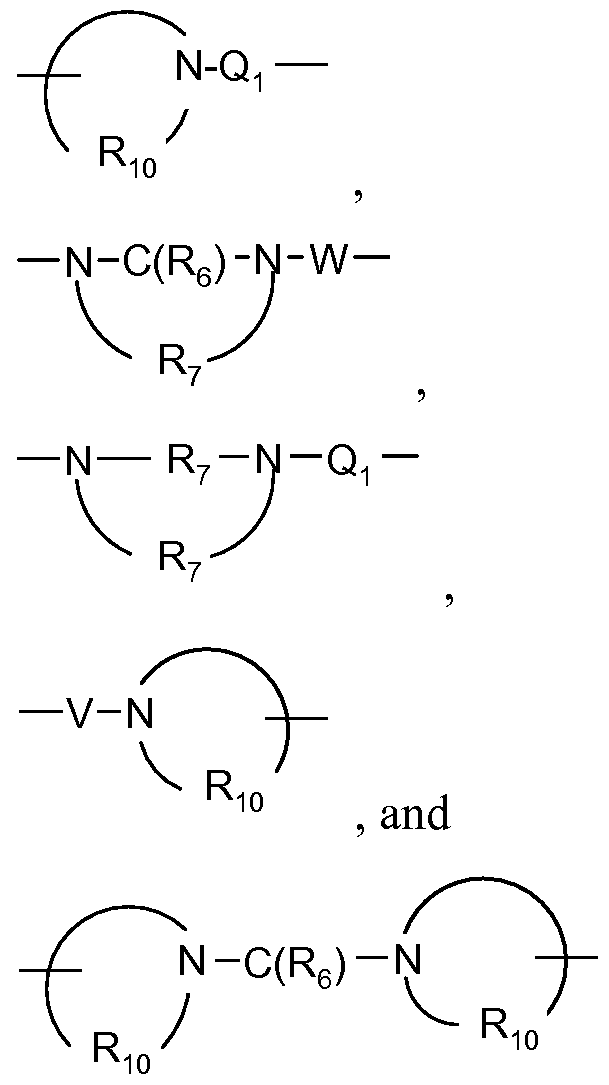WO2007143526A2 - Substituted tetrahydroimidazonaphthyridines and methods - Google Patents
Substituted tetrahydroimidazonaphthyridines and methods Download PDFInfo
- Publication number
- WO2007143526A2 WO2007143526A2 PCT/US2007/070181 US2007070181W WO2007143526A2 WO 2007143526 A2 WO2007143526 A2 WO 2007143526A2 US 2007070181 W US2007070181 W US 2007070181W WO 2007143526 A2 WO2007143526 A2 WO 2007143526A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- compound
- alkyl
- salt
- alkylene
- Prior art date
Links
- 0 CC1(C)Cc2nc(N)c3nc(*)[n](C)c3c2N(*)CC1 Chemical compound CC1(C)Cc2nc(N)c3nc(*)[n](C)c3c2N(*)CC1 0.000 description 15
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/12—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains three hetero rings
- C07D471/14—Ortho-condensed systems
Definitions
- IRMs immune response modifiers
- the present invention provides a new class of compounds that are useful in inducing cytokine biosynthesis in animals.
- examples of such compounds include certain substituted 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amines of the following Formulas I, II, III, and IV:
- R, Ri, R 2 , R 3 , and n are as defined below; and pharmaceutically acceptable salts thereof.
- examples of such compounds also include the following Formulas V, VI, VII, and VIII, which are prodrugs:
- the compounds or salts of Formulas I, II, III, IV, V, VI, VII, and VIII are useful as IRMs due to their ability to modulate cytokine biosynthesis (e.g., induce the biosynthesis or production of one or more cytokines) and otherwise modulate the immune response when administered to animals.
- the ability to modulate cytokine biosynthesis makes the compounds useful in the treatment of a variety of conditions such as viral diseases and neoplastic diseases that are responsive to such changes in the immune response.
- the present invention also provides pharmaceutical compositions containing the compounds of Formulas I, II, III, IV, V, VI, VII, and/or VIII.
- in another aspect of the present invention provides methods of inducing cytokine biosynthesis in animal cells, treating a viral disease in an animal, and/or treating a neoplastic disease in an animal by administering to the animal one or more compounds of the Formulas I, II, III, IV, V, VI, VII, and VIII, and/or pharmaceutically acceptable salts thereof.
- the invention provides methods of synthesizing the compounds of Formulas I, II, III, IV, V, VI, VII, and VIII and intermediate compounds useful in the synthesis of these compounds.
- “a”, “an”, “the”, “at least one”, and “one or more” are used interchangeably.
- the present invention provides compounds of the following Formulas I through
- R, R 1 , R 2 , R3, G, and n are as defined below; and pharmaceutically acceptable salts thereof.
- the present invention provides a compound selected from the group consisting of Formulas I, II, III, and IV:
- R3 is selected from the group consisting of: -Q-R 4 ,
- Q is selected from the group consisting of -C(Re)-, -C(Re)-C(Re)-, -S(O) 2 -, -C(Re)-N(Rg)-W-, -S(O) 2 -N(R 8 )-, -C(Re)-O-, -C(Re)-S-, and -C(Re)-N(OR 9 )-;
- Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups;
- Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino;
- D is CH or N; with the proviso that when D is N, then Q is -C(Re)- or -S(O) 2 -;
- E is selected from the group consisting of -0-, -C(O)-, -S(O) 0-2 -, and -N(Qi-R 4 )-;
- R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl;
- n is O, 1, or 2;
- Ri is selected from the group consisting of:
- D' is selected from the group consisting of -N(Ri')- and -0-;
- R 1 ' is selected from the group consisting of hydrogen, Ci_ 2 o alkyl, hydroxyC 2 _ 2 o alkylenyl, and alkoxyC 2 _ 2 o alkylenyl;
- Xi is C 2 _ 2 o alkylene with the proviso that when Yi is -C(Re)-N(Rg)- or
- Xi Ci_ 2 o alkylene
- Yi is selected from the group consisting of -O-, -S(O) 0-2 -, -S(O) 2 -N(Rg)-,
- R 5a is selected from the group consisting of:
- - R 2 is selected from the group consisting of:
- X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups;
- Y is selected from the group consisting of:
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy; halogen;
- R 8 is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl;
- R 9 is selected from the group consisting of hydrogen and alkyl
- Rio is C3_8 alkylene; A is selected from the group consisting Of -CH 2 -, -O-, -C(O)-, -S(0)o_2-, and
- A' is selected from the group consisting of -0-, -S(0)o-2-, and -CH 2 -;
- Qi is selected from the group consisting of a bond, -C(R 6 )-, -C(Re)-C(R 6 )-, -S(O) 2 -, -C(Rg)-N(Rg)-W-, -S(O) 2 -N(R 8 )-, -C(Rg)-O-, -C(Rg)-S-, and -C(Rg)-N(OR 9 )-;
- Q 2 is selected from the group consisting of a bond, -C(Rg)-, -C(Rg)-C(Rg)-,
- V is selected from the group consisting of a bond, -C(Rg)-, -0-C(Rg)-, -N(Rg)-C(R 6 )-, and -S(O) 2 -;
- W is selected from the group consisting of a bond, -C(O)-, and -S(O) 2 -; and a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7; or a pharmaceutically acceptable salt thereof.
- the present invention provides a compound selected from the group consisting of the Formulas V, VI, VII, and VIII, which are prodrugs:
- G is selected from the group consisting of: -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ -aminoacyl, -C(O)-O-R',
- R and R" are independently selected from the group consisting Of C 1-10 alkyl, C 3 _ 7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_6 alkyl, Ci_4 alkoxy, aryl, heteroaryl, aryl-Ci_4 alkylenyl, heteroaryl-Ci_4 alkylenyl, halo-Ci_4 alkylenyl, halo-Ci_4 alkoxy, -0-C(O)-CH 3 , -C(O)-O-CH 3 , -C(O)-NH 2 , -0-CH 2 -C(O)-NH 2 , -NH 2 , and -S(O) 2 -NH 2 , with the proviso that
- Y' is selected from the group consisting of hydrogen, Ci_6 alkyl, and benzyl;
- Y 0 is selected from the group consisting of Ci_ 6 alkyl, carboxy-Ci_ 6 alkylenyl, amino-Ci_4 alkylenyl, mono-iV-Ci_6 alkylamino-Ci_4 alkylenyl, and alkylamino-Ci_4 alkylenyl;
- Y 2 is selected from the group consisting of mono-iV-Ci_6 alkylamino, di-N,N-C ⁇ - ⁇ alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4-Ci_ 4 alkylpiperazin-l-yl; and R3, R, R 1 , R 2 , and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
- the compound selected from the group consisting of the Formulas I, II, III, and IV is of the Formula II:
- R 3 , R, Ri, R 2 , and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
- the compound selected from the group consisting of the Formulas I, II, III, and IV is of the Formula III:
- R 3 , R, Ri, R 2 , and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
- the -NH 2 group can be replaced by an -NH-G group, as shown in the compounds of Formulas V through VIII , to form prodrugs.
- the compound selected from the group consisting of the Formulas V, VI, VII, and VIII is of the Formula VI:
- R 3 , R, Ri, R 2 , and n are defined as in Formulas I, II, III, and IV above;
- G is defined as in Formulas V, VI, VII, and VIII above; or a pharmaceutically acceptable salt thereof.
- the compound selected from the group consisting of the Formulas V, VI, VII, and VIII is of the Formula VII:
- R 3 , R, R 1 , R 2 , and n are defined as in Formulas I, II, III, and IV above; and G is defined as in Formulas V, VI, VII, and VIII above; or a pharmaceutically acceptable salt thereof.
- each one of the following variables e.g., Ri, R 2 , R 3 , G, R 4 , Q, Qi, Q 2 , Z, X, X 1 , Y, Y 1 , A, and so on
- each one of the following variables e.g., Ri, R 2 , R 3 , G, R 4 , Q, Qi, Q 2 , Z, X, X 1 , Y, Y 1 , A, and so on
- each of the resulting combinations of variables is an embodiment of the present invention.
- R and R" are independently selected from the group consisting of Ci_io alkyl, C3_7 cycloalkyl, phenyl, benzyl, and 2- phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_ 6 alkyl, Ci_ 4 alkoxy, aryl, heteroaryl, aryl-Ci_ 4 alkylenyl, heteroaryl-Ci_ 4 alkylenyl, halo-Ci_ 4 alkylenyl, halo-Ci_ 4 alkoxy, -0-C(O)-CH 3 , -C(O)-O-CH 3 , -C(O)-NH 2 , -0-CH 2 -C(O)-NH 2 , -NH 2 , and -S(O) 2 -NH 2
- Y' is selected from the group consisting of hydrogen, Ci_6 alkyl, and benzyl; Yo is selected from the group consisting of Ci_6 alkyl, carboxy-Ci_6 alkylenyl, amino-Ci_ 4 alkylenyl, mono-iV-Ci_ 6 alkylamino-Ci_ 4 alkylenyl, and alkylamino-Ci_ 4 alkylenyl; and
- Y 2 is selected from the group consisting of mono-JV-Ci_6 alkylamino, alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4-Ci_ 4 alkylpiperazin-1 -yl.
- G is selected from the group consisting of -C(O)-R, ⁇ - aminoacyl, and -C(O)-O-R.
- G is selected from the group consisting of -C(O)-R', ⁇ -amino-C 2 _n acyl, and -C(O)-O-R'.
- ⁇ -amino-C 2 _n acyl includes ⁇ -amino acids containing a total of at least 2 carbon atoms and a total of up to 11 carbon atoms, and may also include one or more heteroatoms selected from the group consisting of O, S, and N.
- R is Ci_io alkyl.
- ⁇ -aminoacyl is an ⁇ -aminoacyl group derived from a naturally occuring ⁇ -amino acid selected from the group consisting of racemic, D-, and L- amino acids.
- ⁇ -aminoacyl is an ⁇ -aminoacyl group derived from an ⁇ - amino acid found in proteins, wherein the the ⁇ -amino acid is selected from the group consisting of racemic, D-, and L-amino acids.
- R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl.
- R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl.
- n is 0, 1, or 2.
- n is 1.
- n is 0.
- Ri is selected from the group consisting of -R 4 , -X-R 4 , -X-Y-R 4 , -X-Y-X-Y-R 4 , -X-R 5 , -N(RO-Qi-R 4 , -D'-Xi-Yi-R 4 , and -D'-Xi-R 5a .
- Ri is selected from the group consisting of -R 4 , -X-R 4 , -X-Y-R 4 , and -X-R 5 .
- Ri is selected from the group consisting of alkyl, arylalkylenyl, aryloxyalkylenyl, hydroxyalkyl, dihydroxyalkyl, -X-Y-R 4 , -X-R 5 , and heterocyclylalkylenyl wherein the heterocyclyl of the heterocyclylalkylenyl group is optionally substituted by one or more alkyl groups; wherein X is alkylene; Y is -N(R 8 )-C(O)-, -N(Rs)-S(O) 2 -, -N(R 8 )-C(R 6 )-N(R 8 )-,
- R 4 is alkyl, aryl, heteroaryl, arylalkylenyl, heteroarylalkylenyl, or arylalkenylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino; and R 5 is
- Ri is selected from the group consisting of 2- hydroxy-2-methylpropyl, 2-methylpropyl, 2,3-dihydroxypropyl, 4- [(methylsulfonyl)amino]butyl, 2-methyl-2-[(methylsulfonyl)amino]propyl, 2- (acetylamino)-2-methylpropyl, 2- ⁇ [(isopropylamino)carbonyl] amino ⁇ -2-methylpropyl,
- Ri is -R 4 , -X-R 4 , -X-Y-R 4 , or -X-R 5
- Ri is selected from the group consisting of -N(Ri')-Qi-R 4 , -D'-Xi-Yi-R 4 , and -D'-Xi-R5 a .
- D' is N(Ri ').
- Ri is Alternatively, for certain of these embodiments, Ri is Alternatively, for certain of these embodiments, Ri is -N(Ri')-Xi-R5 a .
- Ri is -D'-Xi-Yi-R 4 , or -D'-Xi-R 5a
- Ri is -0-Xi-Yi-R 4 or -O-Xi-R 5a .
- R 2 is selected from the group consisting of -R 4 , -X-R 4 , -X-Y-R 4 , -X-R 5 , -OH, -NH 2 , and -NH-Q 2 -R 4 .
- R 2 is selected from the group consisting Of -R 4 , -X-R 4 , -X-Y-R 4 , and -X-R 5 .
- R 2 is -OH, -NH 2 , or -NH-Q 2 -R 4 .
- R 2 is -OH, -NH 2 , or -NH-Q 2 -R 4 .
- R 2 is hydrogen, alkyl, alkoxyalkylenyl, or hy droxy alky lenyl.
- R 2 is selected from the group consisting of hydrogen, methyl, ethyl, propyl, butyl, 2-methoxyethyl, 2-hydroxyethyl, ethoxymethyl, and hydroxymethyl.
- R 2 is -R 4 , -X-R 4 , -X-Y-R 4 , or -X-R 5 , R 2 is -OH.
- Ri is selected from the group consisting of pyridin-3-ylmethyl, isoxazol-5-ylmethyl, isoxazol-3-ylmethyl, [5-(4- fluorophenyl)isoxazol-3-yl]methyl, [3-(4-fluorophenyl)isoxazol-5-yl]methyl, tetrahydro- 2H-pyran-4-ylmethyl, and benzyl, wherein benzyl is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen.
- substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen.
- R 2 is -R 4 , -X-R 4 , -X-Y-R 4 , -X-R 5 , or -OH
- R 2 is -NH-Q 2 -R 4 .
- Q 2 is -C(O)-, -S(O) 2 -, or -C(O)-N(Rg)-.
- -R 4 in -Q 2 -R 4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
- Q 2 is -C(O)-O-.
- R 4 in -Q 2 -R 4 is Ci_ 4 alkyl.
- R 2 is -NH-Q 2 -R 4
- Q 2 is a bond.
- R 4 in -Q 2 -R 4 is hydrogen, Ci_ 4 alkyl, Ci_ 4 alkoxyC 2 _ 4 alkylenyl, or hydroxy C 2 _ 4 alkylenyl.
- R3 is selected from the group consisting of
- R 3 is -Q-R 4 .
- Q is selected from the group consisting of -C(O)-, -S(O) 2 -, -C(O)-N(Rg)-, -C(O)-O-, and -S(O) 2 -N(R 8 )-.
- R 4 in -Q-R 4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
- Q is -C(O)- or -S(O) 2 -.
- R 4 in -Q-R 4 is selected from the group consisting of:
- -R 4 is selected from the group consisting of:
- Q is -C(O)- or -S(O) 2 -.
- Qi in -Qi-R 4 is -C(O)-, -S(O) 2 -, or -C(O)-N(R 8 )-.
- -R 4 in -Qi-R 4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
- D is CH.
- D is N.
- R 3 is -Z-Ar.
- Z is alkylene.
- Z is Ci_ 4 alkylene.
- Ar is pyridinyl, furyl, or phenyl wherein phenyl is optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl.
- R 3 is -X-Y-R 4 or -X-R 5 .
- X in R 3 is alkylenyl.
- X is Ci_ 4 alkylenyl.
- Y in the R 3 group -X-Y-R 4 is selected from the group consisting of -C(O)-, -S(O) 2 -, -C(O)-O-, and -C(O)-N(R 8 )-.
- R 4 in the R 3 group -X-Y-R 4 is selected from the group consisting of alkyl, alkenyl, heterocyclyl, aryl, and arylalkylenyl, wherein aryl and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, alkyl, and amino, and wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of fluoro, alkoxy, and heterocyclyl.
- R 3 is -X-Y-R 4 .
- R 3 is -X-Y-R 4 or -X-R 5 , R 5 in the R 3
- J-H N-alkyl group -X-R 5 is selected from the group consisting of: , ,
- R 3 is -X-R 5 .
- Y in the R 3 group -X-Y-R 4 is -NH-Qi-.
- Qi in -NH-Qi- is -C(O)-, -S(O) 2 -, or -C(O)-N(R 8 )-.
- R 4 in -NH-Qi-R 4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
- R 3 is -X-Y-R 4 .
- R 3 including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R 3 is -Q-R 4 ,
- R 3 is -alkylene-CH(CH 2 -OH)-OH
- R 3 is -CH(CH 2 OH) 2 .
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy;
- R 4 is alkyl, aryl, heteroaryl, arylalkylenyl, heteroarylalkylenyl, or arylalkenylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
- R 4 is alkyl, aryl, heteroaryl, heterocyclyl, or arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
- R 4 is selected from the group consisting of:
- R 4 is selected from the group consisting of:
- R 4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
- R 4 is alkyl, alkenyl, heterocyclyl, aryl, or arylalkylenyl, wherein aryl and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, alkyl, and amino, and wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of fluoro, alkoxy, and heterocyclyl.
- R 4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl wherein alkyl, aryl, and heteroaryl are each unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, and halogen.
- R 4 is selected from the group consisting of alkyl, aryl, arylalkylenyl, and heteroarylalkylenyl wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of alkoxy and hydroxy.
- R 4 is selected from the group consisting of alkyl, arylalkylenyl, aryloxyalkylenyl, and hydroxyalkylenyl.
- R 4 is hydrogen, alkyl, alkoxyalkylenyl, or hydroxyalkylenyl.
- R 4 is hydrogen, Ci_4 alkyl, Ci_4 alkoxyC2-4 alkylenyl, or hydroxy C2-4 alkylenyl.
- R4 is Ci_6 alkyl.
- R 4 is Ci_4 alkyl.
- R 4 is selected from the group consisting of pyridin-3- ylmethyl, isoxazol-5-ylmethyl, isoxazol-3-ylmethyl, tetrahydro-2H-pyran-4-ylmethyl, and benzyl, wherein benzyl is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen.
- R 4 is 4-fluorophenyl.
- R 5 is selected from the group consisting of:
- R5 is selected from the group consisting of: O J. / / —— ⁇ ⁇ O u. / / —— ⁇ ⁇ O u. / / —— ⁇ ⁇ O u. / — ⁇ ⁇ O u. / — ⁇
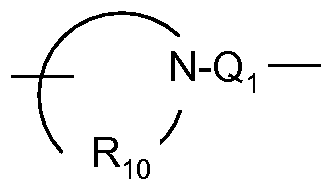
- V-N y J-H N-alkyl V-N N-Q 1 -R 4 VN O
- R5 is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl
- R 5a is selected from the group consisting of:
- R 5a is
- Re S.
- R7 is C2-7 alkylene.
- R7 is C2-4 alkylene.
- R 7 is ethylene.
- Rg a is selected from the group consisting of hydrogen and Ci_4 alkyl.
- Rs a is hydrogen.
- Rs a is Ci_4 alkyl.
- Rs is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl.
- Rs is hydrogen, C 1-10 alkyl, or hydroxy-Ci_io alkylenyl.
- Rs is C 1-10 alkyl or hydroxy-Ci_i 0 alkylenyl.
- Rs is hydrogen.
- Rs is C 1-10 alkyl.
- Rg is selected from the group consisting of hydrogen and alkyl.
- Ri 0 is C3_8 alkylene.
- Ri 0 is C 4-6 alkylene.
- Ri 0 is pentylene
- A is selected from the group consisting Of -CH 2 -, -O-, -C(O)-, -S(OV 2 -, and -NC-Qi-R 4 )-.
- A is selected from the group consisting Of -CH 2 -, -0-, and -N(alkyl)-.
- A is -O- .
- A' is selected from the group consisting of -0-, -S(O) 0-2 -, -NC-Qi-R 4 )-, and -CH 2 -.
- Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino.
- Ar is pyridinyl, furyl, or phenyl wherein phenyl is optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl.
- Ar is phenyl.
- Ar is pyridinyl.
- D is CH or N; with the proviso that when D is N, then Q is -C(R 6 )- or -S(O) 2 -.
- D is CH.
- D is N, and Q is -C(R 6 )- or -S(O) 2 -.
- D' is selected from the group consisting of -N(Ri')- and -0-.
- D' is -N(Ri')-.
- D' is -O- .
- E is selected from the group consisting of -0-, -C(O)-,
- E is -0-.
- E is -N(Qi-R 4 )-.
- Q is selected from the group consisting Of -C(R 6 )-, -C(Re)-C(R 6 )-, -S(O) 2 -, -C(Re)-N(R 8 )- W-, -S(O) 2 -N(R 8 )-, -C(Re)-O-, -C(Re)-S-, and
- Q is selected from the group consisting of -C(O)-, -S(O) 2 -, -C(O)-N(R 8 )-, -C(O)-O-, and -S(O) 2 -N(R 8 )-.
- Q is -C(O)- or -S(O) 2 -.
- Q is -C(O)-O-.
- Qi is selected from the group consisting of a bond, -C(R 6 )-, -C(Re)-C(R 6 )-, -S(O) 2 -, -C(Re)-N(Rg)-W-, -S(O) 2 -N(R 8 )-, -C(Re)-O-, -C(Re)-S-, and -C(Re)-N(OR 9 )-.
- Qi is selected from the group consisting of -C(O)-, -S(O) 2 -, -C(Re)-N(R 8 )-, -S(O) 2 -N(R 8 )-, -C(O)-O-, and -C(O)-S-.
- Qi is -C(O)-, -S(O) 2 -, -C(Re)-N(R 8 )-, or -S(O) 2 -N(R 8 )-.
- Qi is -C(O)-, -S(O) 2 -, -C(Re)-N(R 8 )-, or -C(Re)-O-.
- Qi is -C(O)-, -S(O) 2 -, or -C(O)-N(R 8 )-.
- Qi is -C(R 6 )-.
- Qi is a bond.
- Q 2 is selected from the group consisting of a bond, -C(R 6 )-, -C(Re)-C(R 6 )-, -S(O) 2 -, -C(Re)-N(RSa)-W-, -S(O) 2 -N(R 8a )-, and -C(Re)-O-.
- Q 2 is -C(O)-, -S(O) 2 -, or -C(O)-N(R 8 )-.
- Q 2 is -C(O)-O-.
- Q 2 is a bond
- R 1 ' is selected from the group consisting of hydrogen, Ci_ 2 o alkyl, hydroxyC 2 _ 2 o alkylenyl, and alkoxyC 2 _ 2 o alkylenyl
- R 1 ' is hydrogen
- R 1 ' is C 1-10 alkyl.
- V is selected from the group consisting of a bond, -C(R 6 )-, -0-C(R 6 )-, -N(Rg)-C(R 6 )-, and -S(O) 2 -.
- V is -N(Rg)-C(O)-.
- V is
- W is selected from the group consisting of a bond, -C(O)-, and -S(O) 2 -.
- W is a bond.
- X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups.
- X is alkylene.
- X is C 2 _ 6 alkylene.
- X is Ci_ 4 alkylene.
- X is methylene.
- Xi is C 2 _ 2 o alkylene with the proviso that when Yi is
- Xi is Ci_ 2 o alkylene.
- Xi is C 2 _ 6 alkylene.
- Xi is C 2 _ 4 alkylene.
- Xi is Ci_ 6 alkylene or Ci_ 4 alkylene, and Yi is -C(Re)-N(R 8 )- or
- Y is -N(R 8 )-Qi-.
- Y is -N(R 8 )-C(0)-, -N(Rs)-S(O) 2 -,
- Y is -N(R 8 )-C(0)-, -N(R 8 )-S(O) 2 -,
- Y is -C(O)-, -S(O) 2 -, -C(O)-O-, or -C(O)-N(R 8 )-.
- Y is -NH-Qi-.
- Y is selected from the group consisting of -NH-C(O)-, -NH-S(O) 2 -, -NH-C(Re)-N(R 8 )-, -NH-S(O) 2 -N(R 8 )-, -NH-C(O)-O-, and -NH-C(O)-S-.
- Y is -NH-C(O)-, -NH-S(O) 2 -, or -N(H)-C(O)-N(R 8 )-.
- Yi is selected from the group consisting of -0-, -S(O) 0-2 -, -S(O) 2 -N(R 8 )-, -N(R 8 )-Qi-, -C(Re)-N(R 8 )-, -0-C(Re)-N(R 8 )-, and
- Yi is -0-, -S(O) 2 -N(R 8 )-, -0-C(Re)-N(R 8 )-,
- Yi is -N(Rs)-C(R 6 )-, -N(Rs)-C(Re)-O-, -N(Rs)-S(O) 2 -, -N(Rg)-S(O) 2 -N(R 8 )-, or -N(Rg)-C(Rs)-N(R 8 )-.
- Yi is -N(Rg)-C(O)-, -N(Rg)-S(O) 2 -, or -N(Rg)-C(O)-N(R 8 )-, and R 8 is H or C 1-4 alkyl.
- Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups.
- Z is alkylene
- Z is Ci_ 4 alkylene.
- Z is a bond.
- a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7.
- a and b are each independently 1, 2, or 3.
- a and b are each 2.
- n O.
- n 1.
- n is 2.
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, and a pharmaceutically acceptable carrier.
- the present invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, or a pharmaceutical composition comprising an effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII to the animal.
- the cytokine is selected from the group consisting of IFN- ⁇ , TNF- ⁇ , IL-6, IL-IO, and IL-12.
- the cytokine is IFN- ⁇ or TNF- ⁇ .
- the cytokine is IFN- ⁇ .
- the present invention provides a method of treating a viral disease in an animal comprising administering a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, or a pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII to the animal.
- the present invention provides a method of treating a neoplastic disease in an animal comprising administering a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through
- alkyl As used herein, the terms “alkyl”, “alkenyl”, “alkynyl” and the prefix “alk-” are inclusive of both straight chain and branched chain groups and of cyclic groups, e.g., cycloalkyl and cycloalkenyl. Unless otherwise specified, these groups contain from 1 to 20 carbon atoms, with alkenyl groups containing from 2 to 20 carbon atoms, and alkynyl groups containing from 2 to 20 carbon atoms. In some embodiments, these groups have a total of up to 10 carbon atoms, up to 8 carbon atoms, up to 6 carbon atoms, up to 4 carbon atoms, or up to 2 carbon atoms.
- Cyclic groups can be monocyclic or poly cyclic and preferably have from 3 to 10 ring carbon atoms.
- Exemplary cyclic groups include cyclopropyl, cyclopropylmethyl, cyclobutyl, cyclobutylmethyl, cyclopentyl, cyclopentylmethyl, cyclohexyl, cyclohexylmethyl, adamantyl, and substituted and unsubstituted bornyl, norbornyl, and norbornenyl.
- alkylene "-alkylene-"
- alkenylene alkenylene
- alkenylene- "alkynylene”, and “-alkynylene-” are the divalent forms of the "alkyl”, “alkenyl”, and “alkynyl” groups defined above.
- alkylenyl “alkenylenyl”, and “alkynylenyl” are used when “alkylene”, “alkenylene”, and “alkynylene”, respectively, are substituted.
- an arylalkylenyl group comprises an "alkylene” moiety to which an aryl group is attached.
- haloalkyl is inclusive of alkyl groups that are substituted by one or more halogen atoms, including perfluorinated groups. This is also true of other groups that include the prefix "halo-”. Examples of suitable haloalkyl groups are chloromethyl, trifluoromethyl, and the like.
- aryl as used herein includes carbocyclic aromatic rings or ring systems.
- aryl groups include phenyl, naphthyl, biphenyl, fluorenyl and indenyl.
- heteroatom refers to the atoms O, S, or N.
- heteroaryl includes aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N).
- heteroaryl includes a ring or ring system that contains 2 to 12 carbon atoms, 1 to 3 rings, 1 to 4 heteroatoms, and O, S, and/or N as the heteroatoms.
- Suitable heteroaryl groups include furyl, thienyl, pyridyl, quinolinyl, isoquinolinyl, indolyl, isoindolyl, triazolyl, pyrrolyl, tetrazolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, benzofuranyl, benzothiophenyl, carbazolyl, benzoxazolyl, pyrimidinyl, benzimidazolyl, quinoxalinyl, benzothiazolyl, naphthyridinyl, isoxazolyl, isothiazolyl, purinyl, quinazolinyl, pyrazinyl, 1-oxidopyridyl, pyridazinyl, triazinyl, tetrazinyl, oxadiazolyl, thiadiazolyl, and so on.
- heterocyclyl includes non-aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N) and includes all of the fully saturated and partially unsaturated derivatives of the above mentioned heteroaryl groups.
- heterocyclyl includes a ring or ring system that contains 2 to 12 carbon atoms, 1 to 3 rings, 1 to 4 heteroatoms, and O, S, and N as the heteroatoms.
- exemplary heterocyclyl groups include pyrrolidinyl, tetrahydrofuranyl, morpholinyl, thiomorpholinyl,
- 1,1-dioxothiomorpholinyl piperidinyl, piperazinyl, thiazolidinyl, imidazolidinyl, isothiazolidinyl, tetrahydropyranyl, quinuclidinyl, homopiperidinyl (azepanyl), 1 ,A- oxazepanyl, homopiperazinyl (diazepanyl), 1,3-dioxolanyl, aziridinyl, azetidinyl, dihydroisoquinolin-(lH)-yl, octahydroisoquinolin-(lH)-yl, dihydroquinolin-(2H)-yl, octahydroquinolin-(2H)-yl, dihydro-lH-imidazolyl, 3-azabicyclo[3.2.2]non-3-yl, and the like.
- heterocyclyl includes bicylic and tricyclic heterocyclic ring systems. Such ring systems include fused and/or bridged rings and spiro rings. Fused rings can include, in addition to a saturated or partially saturated ring, an aromatic ring, for example, a benzene ring. Spiro rings include two rings joined by one spiro atom and three rings joined by two spiro atoms.
- heterocyclyl contains a nitrogen atom
- the point of attachment of the heterocyclyl group may be the nitrogen atom
- arylene is the divalent forms of the "aryl”, “heteroaryl”, and “heterocyclyl” groups defined above.
- arylenyl is used when “arylene”, “heteroarylene,” and “heterocyclylene”, respectively, are substituted.
- an alkylarylenyl group comprises an arylene moiety to which an alkyl group is attached.
- each group (or substituent or variable) is independently selected, whether
- each R 7 group is independently selected.
- each Y group is independently selected.
- each Y group is independently selected.
- more than one -N(Rg)-Qi-R 4 group is present (e.g., more than one -Y-R 4 group is present, and both contain a -N(Rs)-Qi- group)
- each Rg group is independently selected, each Qi group is independently selected, and each R 4 group is independently selected.
- the invention is inclusive of the compounds described herein (including intermediates) in any of their pharmaceutically acceptable forms, including isomers (e.g., diastereomers and enantiomers), salts, solvates, polymorphs, prodrugs, and the like.
- isomers e.g., diastereomers and enantiomers
- salts e.g., sodium bicarbonate
- solvates e.g., sodium bicarbonate
- polymorphs e.g., sodium bicarbonate
- prodrugs e.g., sodium bicarbonate
- the term “compound” includes any or all of such forms, whether explicitly stated or not (although at times, “salts" are explicitly stated).
- prodrug means a compound that can be transformed in vivo to yield an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above.
- the prodrug itself, may be an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above.
- the transformation may occur by vaious mechanisms, such as through a chemical (e.g., solvolysis or hydrolysis, for example, in the blood) or enzymatic biotransformation.
- a discussion of the use of prodrugs is provided by T. Higuchi and W. Stella, "Pro-drugs as Novel Delivery Systems," Vol. 14 of the A. C. S. Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987.
- tautomer or “tautomeric form” refers to structural isomers of different energies, which are interconvertible via a low energy barrier.
- proton tautomers include interconversions via migration of a proton, such as keto-enol and imine-enamine isomerizations. This is illustrated, for example, when compounds of the present invention have a hydrogen atom on an oxygen or nitrogen atom at the 2-position, allowing proton migration between the oxygen or nitrogen atom at the 2-position and the 3-position.
- Compounds of the invention may be synthesized by synthetic routes that include processes analogous to those well known in the chemical arts, particularly in light of the description contained herein.
- the starting materials are generally available from commercial sources such as Aldrich Chemicals (Milwaukee, Wisconsin, USA) or are readily prepared using methods well known to those skilled in the art (e.g., prepared by methods generally described in Louis F. Fieser and Mary Fieser, Reagents for Organic Synthesis, v. 1-19, Wiley, New York, (1967-1999 ed.); Alan R. Katritsky, Otto Meth- Cohn, Charles W. Rees, Comprehensive Organic Functional Group Transformations, v. 1-
- Suitable amino protecting groups include acetyl, trifluoroacetyl, fert-butoxycarbonyl (Boc), benzyloxycarbonyl, and 9- fluorenylmethoxycarbonyl (Fmoc).
- Suitable hydroxy protecting groups include acetyl and silyl groups such as the tert-butyl dimethylsilyl group.
- Such techniques may include, for example, all types of chromatography (high performance liquid chromatography (HPLC), column chromatography using common absorbents such as silica gel, and thin layer chromatography), recrystallization, and differential (i.e., liquid- liquid) extraction techniques.
- HPLC high performance liquid chromatography
- column chromatography using common absorbents such as silica gel
- thin layer chromatography such as silica gel
- recrystallization i.e., differential (i.e., liquid- liquid) extraction techniques.
- step (1) of Reaction Scheme I a lH-imidazo[4,5-c]naphthyridin-4-amine of Formula X is reduced to provide a compound of Formula XI, or a salt thereof, wherein J 2 contains the necessary atoms to provide the following four 6,7,8,9-tetrahydro-lH- imidazo[4,5-c]naphthyridin-4-amine isomers of Formulas XVII, XVIII, XIX, and XX, wherein R and n are as defined above.
- step (2) of Reaction Scheme I the secondary amine in a 6,7,8,9-tetrahydro-lH- imidazo[4,5-c]naphthyridin-4-amine of Formula XI is functionalized with an R3 group to form a compound of Formula XII, wherein J3 contains the necessary atoms to provide the following compounds of the invention, Formulas I, II, III, and IV, wherein R, n, and R3 are as defined above.
- protection and deprotection steps may be required.
- protection of the 4-amino group may be necessary before installation of the R3 group.
- the 4-amino group can be protected as a cyclic imide such as an JV-phthalimide or an JV-succinimde by reaction of a lH-imidazo[4,5-c]naphthyridine of Formula XIII or XVI with phthalic anhydride or succinic anhydride in a suitable solvent, such as chloroform of ⁇ /, ⁇ /-dimethylformamide (DMF), at elevated temperature.
- a suitable solvent such as chloroform of ⁇ /, ⁇ /-dimethylformamide (DMF)
- the protected lH-imidazo[4,5-c]naphthyridine can then be reduced using the conditions described in step (1) of Reaction Scheme I to yield a 6,7,8,9-tetrahydro-lH-imidazo[4,5- c]naphthyridine where the 4-amino group is protected.
- the protection step can be carried out on a 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridine of Formula XVII or XX.
- the R3 group can be installed using one of the methods described below in step (2) of Reaction Scheme I, and the cyclic imide protecting group can be removed with hydrazine to yield a compound of Formula I or IV.
- a 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amine of Formula XI or a salt thereof can be converted to an amide, sulfonamide, carbamate, sulfamide, or urea of Formula XII, i.e. R3 is -Q-R 4 , using conventional methods.
- R3 is -Q-R 4
- Formula XI or salt thereof can react with an acid chloride of Formula R 4 C(O)Cl to provide a compound of Formula XII wherein R 3 is -C(O)-R 4 .
- a compound of the Formula XI can react with a sulfonyl chloride of Formula R 4 S(O) 2 Cl or a sulfonic anhydride of Formula (R 4 S(O) 2 ) 2 O to provide a compound of Formula XII wherein R3 is - S(O) 2 -R 4 .
- a compound of the Formula XI can also react with a chloroformate of Formula
- R 4 CO(O)Cl to provide a compound of Formula XII in which R 3 is -C(O)-O-R 4 .
- Numerous acid chlorides of Formula R 4 C(O)Cl, sulfonyl chlorides of Formula R 4 S(O) 2 Cl, sulfonic anhydrides of Formula (R 4 S(O) 2 ⁇ O, and chloro formates of Formula R 4 CO(O)Cl are commercially available; others can be readily prepared using known synthetic methods.
- the reaction can be conveniently carried out by combining the acid chloride of Formula
- R 4 C(O)Cl chloroformate of Formula R 4 CO(O)Cl, sulfonyl chloride of Formula R 4 S(O) 2 Cl, or sulfonic anhydride of Formula (R 4 S(O) ⁇ 2 O with a compound of Formula XI and a base such as triethylamine in a suitable solvent such as chloroform, dichloromethane, or acetonitrile.
- a base such as triethylamine
- a suitable solvent such as chloroform, dichloromethane, or acetonitrile.
- the reaction can be carried out at room temperature or at a sub-ambient temperature such as O 0 C.
- the reaction can be conveniently carried out by adding the isocyanate or isothiocyanate to a cooled solution of a compound of Formula XI in a suitable solvent such as dichloromethane or chloroform.
- a suitable solvent such as dichloromethane or chloroform.
- a base such as triethylamine can be added.
- the reaction can be carried out at room temperature or at a sub-ambient temperature such as 0 0 C.
- ureas and sulfamides of Formula XII where R 3 is -C(O)-heterocycle or -S(O) 2 -heterocycle wherein heterocycle is, for example, pyrrolidine or morpholine can be prepared from carbamoyl chlorides of Formula Cl-C(O)-heterocycle or sulfamoyl chlorides of Formula Cl-S(O) 2 -heterocycle.
- Some carbamoyl chlorides and sulfamoyl chlorides are commercially available; others can be prepared using known synthetic methods from commercially available amines of Formula FIN(R 8 )R 4 or appropriate cyclic amines.
- Q is -C(O)- or -S(O) 2 -
- D, Q 1 , and R 4 are as defined above
- D, Q 1 , and R 4 can be prepared by treating a compound of Formula XI with an acid chloride, carbamoyl chloride, or sulfamoyl chloride of Formula
- Some acid chlorides, carbamoyl chlorides, and sulfamoyl chlorides of this formula are commercially available, for example, l-acetylpiperidine-4-carbonyl chloride and A- methylpiperazine-1-carbonyl chloride; others can be prepared using known synthetic methods from piperidine-4-carboxylic acid or piperazine.
- -Qi-R 4 is a tert- butoxycarbonyl (Boc) group in a compound of Formula XII
- the protecting group can be removed under acidic conditions, and the resulting amine can be further elaborated using the methods described above to provide a sulfonamide, amide, carbamate, urea, thiourea, or sulfamide.
- the reductive alkylation is conveniently carried out in two parts by (i) adding an aryl or arylalkylenyl aldehyde or ketone to a solution of a compound of Formula XI or a salt thereof in a suitable solvent such as DMF in the presence of a base such as N,N- diisopropylethylamine.
- a suitable solvent such as DMF
- the reduction is carried out by adding a suitable reducing agent such as the borane-pyridine complex. Both part (i) and part (ii) can be carried out at room temperature.
- Compounds of Formula XII where R3 is -Z-Ar, wherein Z is alkylene, alkenylene, or alkynylene and Ar is as defined above, can also be prepared through an alkylation reaction with an arylalkylenyl, arylalkenylenyl, or arylalkynylenyl halide of Formula Halide-Z-Ar, where Halide is -Cl, -Br, or -I.
- the reaction is carried out by treating a compound of Formula XI with a compound of Formula Halide-Z-Ar in a suitable solvent such as ethanol, DMF, tetrahydrofuran (THF), or acetonitrile in the presence of a base such as triethylamine or potassium carbonate.
- a suitable solvent such as ethanol, DMF, tetrahydrofuran (THF), or acetonitrile
- THF tetrahydrofuran
- acetonitrile such as triethylamine or potassium carbonate.
- Numerous compounds of Formula Halide-Z-Ar are commercially available, for example, substituted benzyl bromides and chlorides.
- Compounds of Formula XII where R 3 is -Z-Ar, wherein Z is a bond and Ar is as defined above, can be prepared using an aromatic nucleophilic substitution reaction.
- a compound of Formula XI can react with an appropriately substituted aryl reagent, for example, l-fluoro-2,4-dinitro-benzene, to yield a compound of Formula XII in which Ar is a 2,4-dinitrobenzene group.
- a compound of Formula XI can undergo a palladium-mediated coupling reaction with an aryl halide to yield a compound of Formula XII.
- Compounds of Formula XII where R 3 is -alkylene-CH(CH 2 -OH)-OH, -alkylene-CH(CH 2 CH 2 -OH)-OH, and -(alkylene) 0 -i-CH(CH 2 OH) 2 can be prepared by a reductive amination reaction between a dihydroxy aldehyde or ketone and a compound of Formula XI.
- a ketone of Formula HO-CH 2 -C(O)-CH 2 -OH can be reacted with a compound of Formula XI under the conditions described above for a reductive amination reaction to provide a compound of Formula XII in which R 3 is
- a dihydroxy aldehyde or ketone wherein the hydroxyl groups are protected with an appropriate protecting group or groups can also be used in the reductive amination reaction; subsequent deprotection provides a compound of Formula XII.
- Many protected dihydroxy aldehydes and ketones are known in the literature and can be readily synthesized; see, for example, Howson, W. et al Eur. J. Med. Chem. Chim. Ther. 25, pp. 595-602 (1990); Hon, Y.-S. et al. Tetrahedron, 57, 6181-6188, (2001); and Rao, A. V. R. et al. Carbohydr. Res. 148, 51-56, (1986).
- reagents are commercially available, for example, tert-butyl 4-(bromomethyl)piperidine-l-carboxylate, 4-(bromomethyl)tetrahydro-2H-pyran, and 4-(bromomethyl)piperidine; other reagents can be prepared using known synthetic methods.
- E is -N(Qi-R 4 )-, wherein Q 1 -R 4 is a protecting group, the protecting group can subsequently be removed, and the resulting amine can be converted into an amide, urea, thiourea, carbamate, sulfonamide, or sulfamide using one of the methods described above.
- a Michael addition reaction can be used to prepare a compound of Formula XII in
- R 3 is -X-Y-R4, -X-R5, or wherein X is alkylene; Y and R 4 are as defined above, R5 is -CN, Z is a bond, E is -C(O)-, and Rio is C 4 _6 alkylene.
- R 3 is -CH 2 CH 2 -Y-R 4 or -CH 2 CH 2 -R 5 , wherein Y is -S(O) 2 -, -C(O)-, -C(O)-O-, -C(O)-N(H)-, and R 5 is -CN.
- Y is -S(O) 2 -, -C(O)-, -C(O)-O-, -C(O)-N(H)-
- R 5 is -CN.
- Many ⁇ , ⁇ -unsaturated sulfones, ketones, esters, amides, and nitriles are commercially available, others can be prepared readily through known methods.
- the reaction can also be carried out with cyclic ⁇ , ⁇ -unsaturated ketones such as 2-cyclohexene-l-one to provide a compound of Formula XII wherein R 3 is
- the reaction can be conveniently carried out by adding an ⁇ , ⁇ -unsaturated sulfone, ketone, ester, amide, or nitrile to a solution of a compound of Formula XI in a suitable solvent such as THF or acetonitrile optionally in the presence of a base such as triethylamine.
- a suitable solvent such as THF or acetonitrile optionally in the presence of a base such as triethylamine.
- the reaction can be carried out at room temperature or at an elevated temperature, for example, the reflux temperature of the solvent.
- Additional compounds of the invention of Formula XII where R 3 is -X-Y-R 4 or -X-R 5 can be prepared through alkylation of a compound of Formula XI with a reagent of Formula HaMe-X-Y-R 4 or Halide-X-R 5 ; or by a reductive amination reaction between an aldehyde of Formula H(O)C-X-Y-R 4 or H(O)C-X-R 5 with a compound of Formula XI using the conditions described above for an alkylation or a reductive amination.
- the amino group in a compound of Formula XII where R3 is -X-CH 2 -NH 2 can be converted to an amide, urea, thiourea, carbamate, sulfonamide, or sulfamide of Formula XII in which R3 is -X-CH 2 -N(Rs)-Qi-R 4 using the methods described above.
- the amino group can also be treated with a an acid chloride of Formula Cl-RyC(O)Cl or a sulfonyl chloride of Formula Cl-RyS(O) 2 Cl using the reaction conditions described above.
- the isolable intermediate chloroalkanesulfonamide or chloroalkanamide can then be treated with a base such as l,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or sodium hydride at room temperature in a suitable solvent such as DMF to effect a cyclization and provide a compound of Formula XII in which R 3 is -X-CH 2 -Rs, wherein R 5 is -N- C(R 6 ) -N- S(O) 2 7 or 7 and R 6 and Ry are as defined above.
- a base such as l,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or sodium hydride
- DBU l,8-diazabicyclo[5.4.0]undec-7-ene
- DMF sodium hydride
- a hydroxyalkyl-substituted compound of Formula XII, in which R3 is -X-OH or -X-CH 2 -OH, can be treated with JV-hydroxyphthalimide under Mitsunobu reaction conditions to provide an JV-phthalimide-protected hydroxylamine.
- the reaction can be conveniently carried out by adding triphenylphosphine and JV-hydroxyphthalimide to a solution of the hydroxyalkyl-substituted compound in a suitable solvent such as THF or
- the reaction can be carried out at room temperature or at an elevated temperature, such as 60 0 C.
- the phthalimide group can then be removed from the resulting JV-phthalimide-protected hydroxylamine by treatment with hydrazine at room temperature in a suitable solvent such as ethanol.
- the hydroxylamine prepared after the hydrazine deprotection may be treated with one of numerous acid chlorides, sulfonyl chlorides, isocyanates, carbamoyl chlorides, or sulfamoyl chlorides as described above to provide a compound of the invention wherein R3 is -X-Y-R 4 where Y is -O-NH-Q1-, and Qi and R 4 are as defined above.
- the alcohol in a compound of Formula XII wherein R 3 is -X-OH or -X-CH 2 -OH, can be converted into a chloride group that can be displaced by a nucleophile, such as a cyclic amine of Formula
- the chlorination reaction can be conveniently carried out by combining a hydroxyl-substituted compound of Formula XII with thionyl chloride in a suitable solvent such as dichloromethane at room temperature. The resulting chloro-substituted compound is then combined with a cyclic amine in the presence of a base such as potassium carbonate and in a suitable solvent such as DMF. Catalytic sodium iodide can optionally be added. The reaction can be carried out at an elevated temperature such as 50 0 C or 90 0 C-IOO 0 C.
- reaction conditions also can be used employing a variety of phenols to provide compounds of Formula XII where R 3 is -X-Y-R 4 where Y is -O- and R 4 is an unsubstituted or substituted phenyl group.
- the reaction can be carried out by combining an aqueous solution of a hydroxylamine salt of Formula NH 2 ORs ⁇ HCl and a solution of the ketone in a suitable solvent such as methanol or ethanol and then adding a base such as sodium hydroxide and heating at an elevated temperature.
- a suitable solvent such as methanol or ethanol
- the oxime so prepared may be reduced with sodium cyanoborohydride in a mixture of ethanol or methanol in acetic acid to provide a hydroxylamine, which may be treated with one of numerous acid chlorides, sulfonyl chlorides, isocyanates, carbamoyl chlorides, or sulfamoyl chlorides as described above to provide a compound of the invention wherein
- R 3 is -X-Y-R 4 wherein Y is and Qi and Rs are as defined above.
- lH-Imidazo[4,5-c]naphthyridin-4-amine compounds of Formula XII can also be prepared according to Reaction Scheme II wherein R 1 , R 2 , J 1 , and J3 are as defined above, Hal is -Br or -I, M is -B(O-alkyl) 2 , -B(OH) 2 , -Sn(alkyl) 3 , or -Zn-Halide, Di is hydrogen, -C(O)C(CH 3 ) 3 (tert-butylcarbonyl), or Boc, and J 4 and J 5 are defined below.
- step (1) of Reaction Scheme II aminomalonitrile (Formula XXI), which is available commercially as the /?-toluenesulfonic acid salt, is reacted with an orthoester to generate an imidate intermediate, which is treated with a primary amine.
- the orthoester and amine are selected such that they will provide the desired R 2 and Ri substituents in a compound of Formula XXII.
- the reaction can be conveniently carried out by heating a solution of aminomalonitrile /?-toluenesulfonate and the orthoester in a suitable solvent such as THF in the presence of a base such as triethylamine. The solution is allowed to cool to ambient temperature and the primary amine is added.
- amines of Formula Ri-NH 2 provide a compound of Formula XXII that contain a functional group or protected functional group that can be transformed in a subsequent step in Reaction Scheme II to provide compounds of Formula XII with a variety of different Ri groups.
- amines of formula RiNH 2 may contain a protected functional group, such as a Boc-protected amino group.
- Amines of formula RiNH 2 can also contain other protected functional groups, such as ketal-protected ketones.
- ketal-protected ketones For example, 2,2-dimethyl-3-(2-methyl-l,3-dioxolan-2- yl)propylamine, prepared in Example 22 of International Patent Application Publication Nos. WO2005/051317 (Krepski et al.), can be used, and the ketal protecting group can later be removed by conventional methods to provide a compound of the invention in which Ri is 2,2-dimethyl-4-oxopentyl.
- WO2005/066169 (Bonk and Dellaria), WO2005/018551 (Kshirsagar et al.), WO2005/018556 (Kshirsagar et al.), and WO2005/051324 (Krepski et al.), respectively.
- the amine of formula RiNH 2 may be tert-butyl carbazate, and the resulting Ri group can be manipulated in subsequent steps, for example, after step (4), using the methods of U.S. Patent Application Publication No. 2006/026760 to provide compounds of the invention wherein Ri is or -N(Ri')-Xi-R 5a . Other transformations at the Ri position can be made. See, for example, U.S. Patent Nos.
- step (2) of Reaction Scheme II a compound of Formula XXII is added to a solution of bromoform or diiodomethane and an alkyl nitrite such as isoamyl nitrite or tert-butyi nitrite to yield a compound of Formula XXIII where Hal is Br or I, respectively.
- the reaction can be carried out at an elevated temperature such as 90 0 C, and a co-solvent such as chloroform can be employed.
- step (3) of Reaction Scheme II an iodo- or bromo-substituted compound of Formula XXIII undergoes a transition-metal catalyzed cross coupling reaction with a reagent of Formula XXIV, in which J 4 contains the necessary atoms to provide a compound of Formula XXIV as the following four pyridine isomers of Formula XXVI, XXVII, XXVIII, and XXIX, wherein R and n are as defined above:
- Reagents of Formula XXIV are known to undergo coupling reactions, such as Suzuki couplings, Stille couplings, and Negishi couplings, and are either commercially available or can be prepared using known synthetic methods.
- a compound of Formula XXVI, 3-amino-2-tri-/?-butylstannylpyridine, can be prepared by a halogen-lithium exchange of a 2-bromopyridine that has a protected amino group in the 3-position followed by reaction with tributyltin chloride and deprotection of the amino group.
- a reagent of Formula XXVII is commercially available (2,2-dimethyl-iV-[3-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)pyridin-4-yl]propanamide, CB Research and Development, Inc. in New Castle, DE).
- Compounds of Formula XXVII, XXVIII, and XXIX can be prepared starting from te/t-butylcarbonyl or Boc-protected 4-, 3-, and 2- aminopyridines, which undergo directed ortho metalation in the presence of butyllithium reagents.
- the resulting organo lithium intermediate can react with electrophiles such as B(O-alkyl) 3 and ClSn(alkyl) 3 to provide compounds of Formula XXIV, where M is -B(O-alkyl) 2 or -B(OH) 2 and -Sn(alkyl) 3 , respectively.
- the Boc protecting group can be removed with acid to provide a compound of Formula XXIV where Di is hydrogen.
- a Suzuki coupling reaction can be conveniently carried out by heating a mixture of the compound of Formula XXIII, palladium (II) acetate, triphenylphosphine, and a boron reagent of Formula XXIV, where M is -B(OH) 2 or -B(O-alkyl) 2 , in the presence of a base such as sodium carbonate.
- the reaction can be carried out in a suitable solvent or solvent mixture such as n-propanol: water and can be heated at an elevated temperature such as 100 0 C.
- the product of the Suzuki coupling is a compound of Formula XXV where J5 contains the necessary atoms to provide the following four isomers of Formula XXX, XXXI, XXXII, and XXXIII, wherein R and n are as defined above.
- a compound of Formula XXV cyclizes to form a lH-imidazo[4,5-c]naphthyridin-4-amine of Formula X.
- the Suzuki reaction in step (3) is carried out with a compound of Formula XXIV wherein Di is hydrogen or Boc
- the cyclization can be carried out under acidic conditions.
- the intramolecular cyclization can be conveniently carried out by treating a compound of Formula XXV with a solution of hydrochloric acid in ethanol. The reaction is then heated at reflux to provide a IH- imidazo[4,5-c]naphthyridin-4-amine of Formula X.
- a compound of Formula XXV wherein Di is hydrogen may undergo cyclization to the compound of Formula X under the Suzuki coupling reaction conditions in step (3).
- the cyclization in step (4) can be carried out under basic conditions.
- the reaction can be conveniently carried out by heating a compound of Formula XXV with potassium tert-butoxide in a suitable solvent such as ethanol at an elevated temperature such as the reflux temperature of the solvent.
- compounds of the invention can be prepared according to Reaction Scheme III wherein R, R 1 , and n are as defined above; Hal, M, and Di are defined as in Scheme II, and P is a protecting group, for example, an alkyl or benzyl group.
- Compounds of Formula XXIIa can be prepared by reacting aminomalonitrile or a salt thereof with triphosgene and a primary amine of Formula Ri-NH 2 . The reaction can be conveniently carried out in a suitable solvent such as THF in the presence of triethylamine or JV,jV-diisopropylethylamine. See, for example, the method described by Hirota, K. et al, Heterocycles, 55, pp. 2279-2282 (2001).
- a 2-hydroxy imidazole of Formula XXIIa is protected as an alkyl or benzyl ether to provide an imidazole of Formula XXIIb.
- the reaction can be carried out by treating a compound of Formula XXIIa with an alkyl or benzyl halide in the presence of a base such as potassium carbonate in a suitable solvent such as acetone, methanol, or ethanol.
- the reaction can be carried out at room temperature or at an elevated temperature such as the reflux temperature of the solvent.
- steps (2) through (4) of Reaction Scheme III a compound of Formula XXIIb is converted into a lH-imidazo[4,5-c]- 1 ,6-naphthyridin-4-amine of Formula XIVa using the methods described in steps (2) through (4) of Reaction Scheme II.
- step (5) of Reaction Scheme III a lH-imidazo[4,5-c]-l,6-naphthyridin-4-amine of Formula XIVa is converted to a 4-amino-lH-imidazo[4,5-c]-l,6-naphthyridin-2-ol of Formula XIVb using conventional dealkylation methods.
- a dealkylation reaction can be carried out by treating a compound of Formula XIVa wherein P is a
- a compound of Formula XIVa wherein P is benzyl can be deprotected by hetereogeneous hydrogenation or by treatment with trifluoroacetic acid using conditions known to one of skill in the art.
- steps (6) and (7), a compound of Formula XIVb can be converted into a compound of the invention of Formula lib using the methods described in steps (1) and (2) of Reaction Scheme I.
- naphthyridine isomers can be formed if pyridine isomers of Formula XXVI, XXVIII, or XXIX are used instead of the pyridine of Formula XXVII in step (3) of Reaction Scheme III.
- compounds of the invention can be prepared according to Reaction Scheme IV, wherein R 1 , R 2 , G, and J3 are as defined above, and J 6 contains the necessary atoms to provide a compound of Formula XXV as the compounds of the invention of Formula V, VI, VII, and VIII.
- Compounds of Formula XII can be prepared according to the methods described above in Reaction Schemes I, II, or III.
- Particularly useful compounds of Formula XXXIV are amides derived from carboxylic acids containing one to ten carbon atoms, amides derived from amino acids, and carbamates containing one to ten carbon atoms.
- the reaction can be carried out, for example, by combining a compound of Formula XII with a chloro formate or acid chloride, such as ethyl chloroformate or acetyl chloride, in the presence of a base such as triethylamine in a suitable solvent such as dichloromethane at room temperature.
- prodrugs can be prepared in a variety of ways.
- a compound wherein Ri or R 2 is hydroxyalkyl can be converted into a prodrug wherein Ri or R 2 is, for example, -alkylenyl-O-C(R ⁇ )-R4, -alkylenyl-O-C(R ⁇ )-O-R 4 , or -alkylenyl-O-C(R6)-N(Rg)-R4, wherein R 4 , R 6 , and Rg are as defined above, using methods known to one skilled in the art.
- a compound wherein R is hydroxy or a compound of Formula lib or its positional isomers may also be converted to an ester, an ether, a carbonate, or a carbamate.
- a prodrug can be formed by the replacement of the hydrogen atom of the alcohol group with a group such as Ci_6 alkanoyloxymethyl, 1-(C 1-6 alkanoyloxy)ethyl,
- esters can be prepared by heating a compound of Formula lib, for example, with a carboxylic acid optionally in the presence of a base.
- the esterification reaction can be carried out in a suitable solvent such as methanol or ethanol.
- the reaction can be carried out by treating the compound with a carboxylic acid or amino acid under Mitsunobu reaction conditions in the presence of triphenylphosphine and diisopropyl azodicarboxylate in a suitable solvent such as THF.
- the reaction can be run at a sub-ambient temperature such as 0 0 C.
- Compounds of the invention can also be prepared using variations of the synthetic route shown in Reaction Schemes I through IV that would be apparent to one of skill in the art, including variations described in the EXAMPLES below.
- compositions of the invention contain a therapeutically effective amount of a compound or salt described above in combination with a pharmaceutically acceptable carrier.
- a therapeutically effective amount and “effective amount” mean an amount of the compound or salt sufficient to induce a therapeutic or prophylactic effect, such as cytokine induction, immunomodulation, antitumor activity, and/or antiviral activity.
- cytokine induction cytokine induction
- immunomodulation antitumor activity
- antiviral activity cytokine induction
- amount of compound or salt used in a pharmaceutical composition of the invention will vary according to factors known to those of skill in the art, such as the physical and chemical nature of the compound or salt, the nature of the carrier, and the intended dosing regimen.
- compositions of the invention will contain sufficient active ingredient or prodrug to provide a dose of about 100 nanograms per kilogram (ng/kg) to about 50 milligrams per kilogram (mg/kg), preferably about 10 micrograms per kilogram ( ⁇ g/kg) to about 5 mg/kg, of the compound or salt to the subject.
- the method includes administering sufficient compound to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- dosage forms may be used, such as tablets, lozenges, capsules, parenteral formulations, syrups, creams, ointments, aerosol formulations, transdermal patches, transmucosal patches and the like.
- These dosage forms can be prepared with conventional pharmaceutically acceptable carriers and additives using conventional methods, which generally include the step of bringing the active ingredient into association with the carrier.
- the compounds or salts of the invention can be administered as the single therapeutic agent in the treatment regimen, or the compounds or salts described herein may be administered in combination with one another or with other active agents, including additional immune response modifiers, antivirals, antibiotics, antibodies, proteins, peptides, oligonucleotides, etc.
- Cytokines whose production may be induced by the administration of compounds or salts of the invention generally include interferon- ⁇ (IFN- ⁇ ) and tumor necrosis factor- ⁇ (TNF- ⁇ ) as well as certain interleukins (IL). Cytokines whose biosynthesis may be induced by compounds or salts of the invention include IFN- ⁇ , TNF- ⁇ , IL-I, IL-6, IL-10 and IL-12, and a variety of other cytokines. Among other effects, these and other cytokines can inhibit virus production and tumor cell growth, making the compounds or salts useful in the treatment of viral diseases and neoplastic diseases.
- IFN- ⁇ interferon- ⁇
- TNF- ⁇ tumor necrosis factor- ⁇
- IL-6 tumor necrosis factor- ⁇
- IL-12 interleukins
- the invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of the invention to the animal.
- the animal to which the compound or salt is administered for induction of cytokine biosynthesis may have a disease as described infra, for example a viral disease or a neoplastic disease, and administration of the compound or salt may provide therapeutic treatment.
- the compound or salt may be administered to the animal prior to the animal acquiring the disease so that administration of the compound or salt may provide a prophylactic treatment.
- compounds or salts described herein can affect other aspects of the innate immune response. For example, natural killer cell activity may be stimulated, an effect that may be due to cytokine induction.
- the compounds or salts may also activate macrophages, which in turn stimulate secretion of nitric oxide and the production of additional cytokines. Further, the compounds or salts may cause proliferation and differentiation of B-lymphocytes. Compounds or salts described herein can also have an effect on the acquired immune response.
- T H 1 T helper type 1
- T R 2 T helper type 2
- the compound or salt or composition may be administered alone or in combination with one or more active components as in, for example, a vaccine adjuvant.
- the compound or salt or composition and other component or components may be administered separately; together but independently such as in a solution; or together and associated with one another such as (a) covalently linked or (b) non-covalently associated, e.g., in a colloidal suspension.
- Conditions for which compounds or salts or compositions identified herein may be used as treatments include, but are not limited to:
- viral diseases such as, for example, diseases resulting from infection by an adenovirus, a herpesvirus (e.g., HSV-I, HSV-II, CMV, or VZV), a poxvirus (e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum), a picornavirus (e.g., rhinovirus or enterovirus), an orthomyxovirus (e.g., influenzavirus), a paramyxovirus (e.g., parainfluenzavirus, mumps virus, measles virus, and respiratory syncytial virus (RSV)), a coronavirus (e.g., SARS), a papovavirus (e.g., papillomaviruses, such as those that cause genital warts, common warts, or plantar warts), a hepadnavirus (e.g., hepatitis B virus),
- bacterial diseases such as, for example, diseases resulting from infection by bacteria of, for example, the genus Escherichia, Enterobacter, Salmonella, Staphylococcus, Shigella, Listeria, Aerobacter, Helicobacter, Klebsiella, Proteus, Pseudomonas, Streptococcus, Chlamydia, Mycoplasma, Pneumococcus, Neisseria, Clostridium, Bacillus, Corynebacterium, Mycobacterium, Campylobacter, Vibrio, Serratia, Providencia, Chromobacterium, Brucella, Yersinia, Haemophilus, or Bordetella;
- infectious diseases such as chlamydia, fungal diseases including but not limited to candidiasis, aspergillosis, histoplasmosis, cryptococcal meningitis, or parasitic diseases including but not limited to malaria, Pneumocystis carnii pneumonia, leishmaniasis, cryptosporidiosis, toxoplasmosis, and trypanosome infection;
- neoplastic diseases such as intraepithelial neoplasias, cervical dysplasia, actinic keratosis, basal cell carcinoma, squamous cell carcinoma, renal cell carcinoma, Kaposi's sarcoma, melanoma, leukemias including but not limited to acute myeloid leukemia, acute lymphocytic leukemia, chronic myeloid leukemia, chronic lymphocytic leukemia, multiple myeloma, Hodgkin's lymphoma, non-Hodgkin's lymphoma, cutaneous T-cell lymphoma, B-cell lymphoma, and hairy cell leukemia, and other cancers;
- atopic diseases such as atopic dermatitis or eczema, eosinophilia, asthma, allergy, allergic rhinitis, and Ommen's syndrome;
- diseases associated with wound repair such as, for example, inhibition of keloid formation and other types of scarring (e.g., enhancing wound healing, including chronic wounds).
- a compound or salt identified herein may be useful as a vaccine adjuvant for use in conjunction with any material that raises either humoral and/or cell mediated immune response, such as, for example, live viral, bacterial, or parasitic immunogens; inactivated viral, tumor-derived, protozoal, organism-derived, fungal, or bacterial immunogens; toxoids; toxins; self-antigens; polysaccharides; proteins; glycoproteins; peptides; cellular vaccines; DNA vaccines; autologous vaccines; recombinant proteins; and the like, for use in connection with, for example, BCG, cholera, plague, typhoid, hepatitis A, hepatitis B, hepatitis C, influenza A, influenza B, parainfluenza, polio, rabies, measles, mumps, rubella, yellow fever, tetanus, diphtheria, hemophilus influenza b, tuberculosis, meningo
- Compounds or salts identified herein may be particularly helpful in individuals having compromised immune function.
- compounds or salts may be used for treating the opportunistic infections and tumors that occur after suppression of cell mediated immunity in, for example, transplant patients, cancer patients and HIV patients.
- one or more of the above diseases or types of diseases for example, a viral disease or a neoplastic disease may be treated in an animal in need thereof (having the disease) by administering a therapeutically effective amount of a compound or salt of the invention to the animal.
- An animal may also be vaccinated by administering an effective amount of a compound or salt described herein, as a vaccine adjuvant.
- a method of vaccinating an animal comprising administering an effective amount of a compound or salt described herein to the animal as a vaccine adjuvant.
- An amount of a compound or salt effective to induce cytokine biosynthesis is an amount sufficient to cause one or more cell types, such as monocytes, macrophages, dendritic cells and B-cells to produce an amount of one or more cytokines such as, for example, IFN- ⁇ , TNF- ⁇ , IL-I, IL-6, IL-IO and IL- 12 that is increased (induced) over a background level of such cytokines.
- the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- the amount is expected to be a dose of, for example, from about 0.01 mg/m 2 to about 5.0 mg/m 2 , (computed according to the Dubois method as described above) although in some embodiments the induction or inhibition of cytokine biosynthesis may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt or composition to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- the invention also provides a method of treating a viral infection in an animal and a method of treating a neoplastic disease in an animal comprising administering an effective amount of a compound or salt of the invention to the animal.
- An amount effective to treat or inhibit a viral infection is an amount that will cause a reduction in one or more of the manifestations of viral infection, such as viral lesions, viral load, rate of virus production, and mortality as compared to untreated control animals.
- the precise amount that is effective for such treatment will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- An amount of a compound or salt effective to treat a neoplastic condition is an amount that will cause a reduction in tumor size or in the number of tumor foci. Again, the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg. In other embodiments, the amount is expected to be a dose of, for example, from about 0.01 mg/m 2 to about 5.0 mg/m 2 , (computed according to the Dubois method as described above) although in some embodiments either of these methods may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- the slurry was then cooled again to -78 0 C, and triisopropylborate (17.5 mL, 76.0 mmol) was added dropwise via syringe over 10 min.
- the reaction mixture was then warmed to ambient temperature and allowed to stir for 20 min.
- the reaction mixture initially became clear, and then a white precipitate formed.
- the resultant slurry was then poured into saturated aqueous ammonium choride (100 mL) and allowed to stir vigorously for an additional 20 min.
- Part D A mixture of l-(2-methylpropyl)-2-propyl-lH-imidazo[4,5-c]-l,6-naphthyridin-4- amine (1.50 g, 5.29 mmol), platinum oxide (1.0 g), and trifluoroacetic acid (20 mL) was placed under 50 psi (3.5 x 10 5 Pa) of hydrogen pressure on a Parr apparatus. After 14 hours, the mixture was diluted with dichloromethane (200 mL) and filtered through a pad of CELITE filter agent.
- Example 3 Ethyl 3-[4-amino- 1 -(2-methylpropyl)-2-propyl- 1 ,6,7,9-tetrahydro-8H-imidazo[4,5-c]- 1 ,6- naphthyridin-8-yl]propanoate l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (650 mg, 2.26 mmol, prepared as described in Part D of Example 1) was dissolved in T ⁇ F (20 mL).
- Triethylamine (0.95 rnL, 6.78 mmol) and ethyl acrylate (0.27 mL, 2.49 mmol) were added via syringe.
- the resultant solution was allowed to stir overnight at ambient temperature.
- the solution was then partitioned between dichloromethane and saturated aqueous sodium bicarbonate, and the aqueous layer was extracted with additional portions of dichloromethane.
- the combined organic layers were washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure.
- the residue was purified via flash chromatography on silica gel (gradient elution with 25-50% ethyl acetate in hexanes).
- Part B A 250 mL round bottom flask equipped with magnetic stirring bar was charged with the material from Part A (4.60 g, 12.6 mmol) and ethanol (18 mL, reagent grade). A solution of potassium carbonate (2.6O g, 19.0 mmol) in water (7 mL) was added, and the resultant reaction mixture was heated at reflux for 3 hours. The reaction mixture was then concentrated under reduced pressure, and the residue was partitioned between dichloromethane (50 mL) and water (30 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to afford the crude product.
- dichloromethane 50 mL
- water 30 mL
- the solid was suspended in methanol (24 mL) and 1 M aqueous hydrochloric acid (24 mL) was added. The resultant solution was heated at reflux for 3 hours, then concentrated under reduced pressure. A 50% aqueous sodium hydroxide solution was then added until a basic p ⁇ was reached.
- Triethylamine (0.40 mL, 2.6 mmol) was added, followed by methanesulfonyl chloride (0.14 mL, 1.8 mmol). The resultant solution was allowed to stir at ambient temperature for 1 hour. The reaction mixture was then concentrated under reduced pressure to afford a white solid that was purified by flash chromatography on silica gel (elution with 5% methanol in dichloromethane). The product was then slurried in water (20 mL), and potassium carbonate was added until p ⁇ 10 was reached.
- Examples 32-38 l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (862 mg, 3.0 mmol, prepared as described in Part D of Example 1) was diluted with methanol to provide 30 mL of solution. The solution (1 mL) was added to a test tube containing an aldehyde or ketone (1.25 equivalents, 0.125 mmol) from the table below. The test tubes were capped and vortexed for 15 minutes.
- Examples 39-41 l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (260 mg, 0.90 mmol, prepared as described in Part D of Example 1) was diluted with T ⁇ F to provide 9 mL of solution. The solution (1 mL) was added to a test tube containing a reagent (1 equivalent, 0.1 mmol) from the table below. The test tube was capped and stirred for 116 hours at 70 0 C. The solvent was removed by vacuum centrifugation. The compounds were purified as described for Examples 7-31. The table below shows the reagent used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt. Examples 39-41
- Certain exemplary compounds including some of those described above in the Examples, have the following Formula (Ha or Ilia) and one R 1 , one R 2 , and one R3 substituent shown in the following table, wherein each line of the table is matched with the Formula (Ha or Ilia) to represent a specific embodiment of the invention.
- Compounds of the invention have been found to modulate cytokine biosynthesis by inducing the production of interferon ⁇ and/or tumor necrosis factor ⁇ in human cells when tested using one of the methods described below.
- cytokine induction An in vitro human blood cell system is used to assess cytokine induction. Activity is based on the measurement of interferon ( ⁇ ) and tumor necrosis factor ( ⁇ ) (IFN- ⁇ and TNF- ⁇ , respectively) secreted into culture media as described by Testerman et. al. in “Cytokine Induction by the Immunomodulators Imiquimod and S-27609", Journal of Leukocyte Biology, 58, 365-372 (September, 1995).
- ⁇ interferon
- ⁇ tumor necrosis factor
- PBMC Peripheral blood mononuclear cells
- HISTOPAQUE- 1077 Sigma, St. Louis, MO
- Ficoll-Paque Plus Amersham Biosciences Piscataway, NJ
- Blood is diluted 1 : 1 with Dulbecco's Phosphate Buffered Saline (DPBS) or Hank's Balanced Salts Solution (HBSS).
- DPBS Dulbecco's Phosphate Buffered Saline
- HBSS Hank's Balanced Salts Solution
- PBMC whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium.
- the PBMC layer is collected and washed twice with DPBS or HBSS and re-suspended at 4 x 10 6 cells/mL in RPMI complete.
- the PBMC suspension is added to 96 well flat bottom sterile tissue culture plates containing an equal volume of RPMI complete media containing test compound.
- the compounds are solubilized in dimethyl sulfoxide (DMSO).
- DMSO concentration should not exceed a final concentration of 1% for addition to the culture wells.
- the compounds are generally tested at concentrations ranging from 30-0.014 ⁇ M.
- Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with reference compound. Incubation
- test compound is added at 60 ⁇ M to the first well containing RPMI complete and serial 3 fold dilutions are made in the wells.
- the PBMC suspension is then added to the wells in an equal volume, bringing the test compound concentrations to the desired range (usually 30-0.014 ⁇ M).
- the final concentration of PBMC suspension is 2 x 10 6 cells/mL.
- the plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere. Separation Following incubation the plates are centrifuged for 10 minutes at 1000 rpm
- IFN- ⁇ concentration is determined with a human multi-subtype colorimetric sandwich ELISA (Catalog Number 41105) from PBL Biomedical Laboratories,
- the TNF- ⁇ concentration is determined by ORIGEN M-Series Immunoassay and read on an IGEN M-8 analyzer from BioVeris Corporation, formerly known as IGEN International, Gaithersburg, MD.
- the immunoassay uses a human TNF- ⁇ capture and detection antibody pair (Catalog Numbers AHC3419 and AHC3712) from Biosource International, Camarillo, CA. Results are expressed in pg/mL. Assay Data and Analysis
- the data output of the assay consists of concentration values of TNF- ⁇ and IFN- ⁇ (y-axis) as a function of compound concentration (x-axis).
- Analysis of the data has two steps. First, the greater of the mean DMSO (DMSO control wells) or the experimental background (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ) is subtracted from each reading. If any negative values result from background subtraction, the reading is reported as " * ", and is noted as not reliably detectable. In subsequent calculations and statistics, " * ", is treated as a zero. Second, all background subtracted values are multiplied by a single adjustment ratio to decrease experiment to experiment variability.
- the adjustment ratio is the area of the reference compound in the new experiment divided by the expected area of the reference compound based on the past 61 experiments (unadjusted readings). This results in the scaling of the reading (y-axis) for the new data without changing the shape of the dose-response curve.
- the reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ - dimethyl-lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from the past 61 experiments.
- the minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound.
- the minimum effective concentration ( ⁇ molar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ).
- the maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
- the CYTOKINE INDUCTION IN HUMAN CELLS test method described above was modified as follows for high throughput screening.
- PBMC Peripheral blood mononuclear cells
- HISTOPAQUE- 1077 Sigma, St. Louis, MO
- Ficoll-Paque Plus Amersham Biosciences Piscataway, NJ
- Whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium.
- the PBMC layer is collected and washed twice with DPBS or HBSS and re- suspended at 4 x 10 6 cells/mL in RPMI complete (2-fold the final cell density).
- the PBMC suspension is added to 96-well flat bottom sterile tissue culture plates.
- Compound Preparation The compounds are solubilized in dimethyl sulfoxide (DMSO). The compounds are generally tested at concentrations ranging from 30 - 0.014 ⁇ M.
- Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with a reference compound 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ -dimethyl- lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) on each plate.
- the solution of test compound is added at 7.5 mM to the first well of a dosing plate and serial 3 fold dilutions are made for the 7 subsequent concentrations in DMSO.
- RPMI Complete media is then added to the test compound dilutions in order to reach a final compound concentration of 2-fold higher (60 - 0.028 ⁇ M) than the final tested concentration range.
- test compound solution is then added to the wells containing the PBMC suspension bringing the test compound concentrations to the desired range (usually 30 - 0.014 ⁇ M) and the DMSO concentration to 0.4 %.
- the final concentration of PBMC suspension is 2x10 6 cells/mL.
- the plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere. Separation Following incubation the plates are centrifuged for 10 minutes at 1000 rpm
- MSD MULTI-SPOT plates contain within each well capture antibodies for human TNF- ⁇ and human IFN- ⁇ that have been pre-coated on specific spots.
- Each well contains four spots: one human TNF- ⁇ capture antibody (MSD) spot, one human IFN- ⁇ capture antibody (PBL Biomedical Laboratories, Piscataway, NJ) spot, and two inactive bovine serum albumin spots.
- the human TNF- ⁇ capture and detection antibody pair is from MesoScale Discovery.
- the human IFN- ⁇ multi-subtype antibody (PBL Biomedical Laboratories) captures all IFN- ⁇ subtypes except IFN- ⁇ F (IFNA21).
- the data output of the assay consists of concentration values of TNF- ⁇ or IFN- ⁇ (y-axis) as a function of compound concentration (x-axis).
- a plate-wise scaling is performed within a given experiment aimed at reducing plate-to-plate variability associated within the same experiment.
- the greater of the median DMSO (DMSO control wells) or the experimental background (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ) is subtracted from each reading. Negative values that may result from background subtraction are set to zero.
- Each plate within a given experiment has a reference compound that serves as a control. This control is used to calculate a median expected area under the curve across all plates in the assay.
- a plate- wise scaling factor is calculated for each plate as a ratio of the area of the reference compound on the particular plate to the median expected area for the entire experiment.
- the data from each plate are then multiplied by the plate-wise scaling factor for all plates. Only data from plates bearing a scaling factor of between 0.5 and 2.0 (for both cytokines IFN- ⁇ , TNF- ⁇ ) are reported. Data from plates with scaling factors outside the above mentioned interval are retested until they bear scaling factors inside the above mentioned interval. The above method produces a scaling of the y- values without altering the shape of the curve.
- the reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9- tetrahydro- ⁇ , ⁇ -dimethyl-lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91).
- the median expected area is the median area across all plates that are part of a given experiment.
- a second scaling may also be performed to reduce inter-experiment variability (across multiple experiments). All background- subtracted values are multiplied by a single adjustment ratio to decrease experiment-to-experiment variability.
- the adjustment ratio is the area of the reference compound in the new experiment divided by the expected area of the reference compound based on an average of previous experiments (unadjusted readings). This results in the scaling of the reading (y-axis) for the new data without changing the shape of the dose-response curve.
- the reference compound used is 2-[4- amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ -dimethyl- lH-imidazo[4,5-c]quinolin- 1 - yljethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from an average of previous experiments.
- the minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound.
- the minimum effective concentration ( ⁇ molar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ).
- the maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
Abstract
6,7,8,9-Tetrahydro-1H-imidazo[4,5-c]naphthyridines with a substituent at the 6-, 7-, 8-, or 9-position nitrogen atom, pharmaceutical compositions containing these compounds, methods of making the compounds, intermediates, and methods of use of these compounds as immunomodulators, for inducing cytokine biosynthesis in animals and in the treatment of diseases including viral and neoplastic diseases, are disclosed.
Description
SUBSTITUTED TETRAHYDROIMIDAZONAPHTHYRIDINES AND METHODS
CROSS REFERENCE TO RELATED APPLICATIONS
The present invention claims priority to U.S. Provisional Application Serial No. 60/803927, filed June 05, 2006, which is incorporated herein by reference.
BACKGROUND Certain compounds have been found to be useful as immune response modifiers
(IRMs), rendering them useful in the treatment of a variety of disorders. However, there continues to be interest in and a need for compounds that have the ability to modulate the immune response, by induction of cytokine biosynthesis or other means.
SUMMARY OF THE INVENTION
The present invention provides a new class of compounds that are useful in inducing cytokine biosynthesis in animals. Examples of such compounds include certain substituted 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amines of the following Formulas I, II, III, and IV:
IV wherein R, Ri, R2, R3, and n are as defined below; and pharmaceutically acceptable salts thereof.
Examples of such compounds also include the following Formulas V, VI, VII, and VIII, which are prodrugs:
VIII wherein R, Ri, R2, R3, G, and n are as defined below; and pharmaceutically acceptable salts thereof.
The compounds or salts of Formulas I, II, III, IV, V, VI, VII, and VIII are useful as IRMs due to their ability to modulate cytokine biosynthesis (e.g., induce the biosynthesis or production of one or more cytokines) and otherwise modulate the immune response when administered to animals. The ability to modulate cytokine biosynthesis makes the compounds useful in the treatment of a variety of conditions such as viral diseases and neoplastic diseases that are responsive to such changes in the immune response. In another aspect, the present invention also provides pharmaceutical compositions containing the compounds of Formulas I, II, III, IV, V, VI, VII, and/or VIII.
In another aspect of the present invention provides methods of inducing cytokine biosynthesis in animal cells, treating a viral disease in an animal, and/or treating a neoplastic disease in an animal by administering to the animal one or more compounds of the Formulas I, II, III, IV, V, VI, VII, and VIII, and/or pharmaceutically acceptable salts thereof.
In another aspect, the invention provides methods of synthesizing the compounds of Formulas I, II, III, IV, V, VI, VII, and VIII and intermediate compounds useful in the synthesis of these compounds.
As used herein, "a", "an", "the", "at least one", and "one or more" are used interchangeably.
The terms "comprising" and variations thereof do not have a limiting meaning where these terms appear in the description and claims.
The above summary of the present invention is not intended to describe each disclosed embodiment or every implementation of the present invention. The description that follows more particularly exemplifies illustrative embodiments. Guidance is also provided herein through lists of examples, which can be used in various combinations. In each instance, the recited list serves only as a representative group and should not be interpreted as an exclusive list.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS OF THE
INVENTION
The present invention provides compounds of the following Formulas I through
VIII:
II III
VIII wherein R, R1, R2, R3, G, and n are as defined below; and pharmaceutically acceptable salts thereof.
In one embodiment, the present invention provides a compound selected from the group consisting of Formulas I, II, III, and IV:
IV wherein:
R3 is selected from the group consisting of: -Q-R4,
-Q -D ^N-Q1 -R4
Z-Ar,
X-Y-R4,
X-R5, alkylene-CH(CH 2-OH)-OH,
-alkylene-CH(CH2CH2-OH)-OH, and -(alkylene)o-i-CH(CH2OH)2;
Q is selected from the group consisting of -C(Re)-, -C(Re)-C(Re)-, -S(O)2-, -C(Re)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Re)-O-, -C(Re)-S-, and -C(Re)-N(OR9)-; Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups;
Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino; D is CH or N; with the proviso that when D is N, then Q is -C(Re)- or -S(O)2-; E is selected from the group consisting of -0-, -C(O)-, -S(O)0-2-, and -N(Qi-R4)-; R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl; n is O, 1, or 2;
Ri is selected from the group consisting of:
-R4, -X-R4, -X-Y-R4,
-X-Y-X-Y-R4, -X-R5,
D' is selected from the group consisting of -N(Ri')- and -0-; R1' is selected from the group consisting of hydrogen, Ci_2o alkyl, hydroxyC2_2o alkylenyl, and alkoxyC2_2o alkylenyl;
Xi is C2_2o alkylene with the proviso that when Yi is -C(Re)-N(Rg)- or
-C(R6) -N /--x y Ri° , or R5a is
, then Xi is Ci_2o alkylene;
Yi is selected from the group consisting of -O-, -S(O)0-2-, -S(O)2-N(Rg)-,
R5a is selected from the group consisting of:
-R4,
-X-R4,
-X-Y-R4,
-X-R5, -OH,
-NH2, and
-NH-Q2-R4;
X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups; Y is selected from the group consisting of:
-0-,
-S(O)0-2-, -S(O)2-N(R8)-,
-C(R6)-,
-C(Re)-O-,
-0-C(R6)-,
-C(Re)-N(R8)-,
-0-C(Re)-N(R8)-,
-CC=N-O-R8)-,
-CHC-NC-O-Re)-Qi-R4)-,
R4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy; halogen; nitro; hydroxy; mercapto; cyano; aryl; aryloxy; arylalkyleneoxy; heteroaryl; heteroaryloxy; heteroarylalkyleneoxy; heterocyclyl; amino; alkylamino; dialkylamino; (dialkylamino)alkyleneoxy; and, in the case of alkyl, alkenyl, alkynyl, and heterocyclyl, oxo; R5 is selected from the group consisting of:
Re is selected from the group consisting of =0 and =S;
R7 is C2-7 alkylene; Rga is selected from the group consisting of hydrogen and Ci_4 alkyl;
R8 is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl;
R9 is selected from the group consisting of hydrogen and alkyl;
Qi is selected from the group consisting of a bond, -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Rg)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Rg)-O-, -C(Rg)-S-, and -C(Rg)-N(OR9)-; Q2 is selected from the group consisting of a bond, -C(Rg)-, -C(Rg)-C(Rg)-,
-S(O)2-, -C(Rg)-N(R8a)-W-, -S(O)2-N(R8a)-, and -C(Rg)-O-;
V is selected from the group consisting of a bond, -C(Rg)-, -0-C(Rg)-, -N(Rg)-C(R6)-, and -S(O)2-;
W is selected from the group consisting of a bond, -C(O)-, and -S(O)2-; and a and b are independently integers from 1 to 6 with the proviso that a + b is < 7; or a pharmaceutically acceptable salt thereof.
In another embodiment, the present invention provides a compound selected from the group consisting of the Formulas V, VI, VII, and VIII, which are prodrugs:
V VI VII
VIII wherein:
G is selected from the group consisting of: -C(O)-R', α-aminoacyl, α-aminoacyl-α-aminoacyl, -C(O)-O-R',
-C(0)-N(R")R, -C(=NY')-R', -CH(OH)-C(O)-OY', -CH(OCi-4 alkyl)Yo, -CH2Y2, and
-CH(CH3)Y2;
R and R" are independently selected from the group consisting Of C1-10 alkyl, C3_7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_6 alkyl, Ci_4 alkoxy, aryl, heteroaryl, aryl-Ci_4 alkylenyl, heteroaryl-Ci_4 alkylenyl, halo-Ci_4 alkylenyl, halo-Ci_4 alkoxy, -0-C(O)-CH3, -C(O)-O-CH3, -C(O)-NH2, -0-CH2-C(O)-NH2, -NH2, and -S(O)2-NH2, with the proviso that R" can also be hydrogen;
α-aminoacyl is an α-aminoacyl group derived from an α-amino acid selected from the group consisting of racemic, D-, and L-amino acids;
Y' is selected from the group consisting of hydrogen, Ci_6 alkyl, and benzyl;
Y0 is selected from the group consisting of Ci_6 alkyl, carboxy-Ci_6 alkylenyl, amino-Ci_4 alkylenyl, mono-iV-Ci_6 alkylamino-Ci_4 alkylenyl, and alkylamino-Ci_4 alkylenyl;
Y2 is selected from the group consisting of mono-iV-Ci_6 alkylamino, di-N,N-Cι-β alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4-Ci_4 alkylpiperazin-l-yl; and R3, R, R1, R2, and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
For certain embodiments, the compound selected from the group consisting of the Formulas I, II, III, and IV is of the Formula II:
II wherein R3, R, Ri, R2, and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
For certain embodiments, the compound selected from the group consisting of the Formulas I, II, III, and IV is of the Formula III:
III wherein R3, R, Ri, R2, and n are defined as in Formulas I, II, III, and IV above; or a pharmaceutically acceptable salt thereof.
For certain embodiments of the compounds of Formulas I through IV, the
-NH2 group can be replaced by an -NH-G group, as shown in the compounds of Formulas V through VIII , to form prodrugs.
For certain embodiments, the compound selected from the group consisting of the Formulas V, VI, VII, and VIII is of the Formula VI:
VI wherein R3, R, Ri, R2, and n are defined as in Formulas I, II, III, and IV above; and
G is defined as in Formulas V, VI, VII, and VIII above; or a pharmaceutically acceptable salt thereof.
For certain embodiments, the compound selected from the group consisting of the Formulas V, VI, VII, and VIII is of the Formula VII:
VII wherein R3, R, R1, R2, and n are defined as in Formulas I, II, III, and IV above; and G is defined as in Formulas V, VI, VII, and VIII above; or a pharmaceutically acceptable salt thereof.
For any of the compounds presented herein, each one of the following variables (e.g., Ri, R2, R3, G, R4, Q, Qi, Q2, Z, X, X1, Y, Y1, A, and so on) in any of its embodiments can be combined with any one or more of the other variables in any of their embodiments and associated with any one of the formulas described herein, as would be understood by one of skill in the art. Each of the resulting combinations of variables is an embodiment of the present invention.
For certain embodiments, e.g., of Formulas V, VI, VII, and VIII, G is selected from the group consisting of-C(O)-R', α-aminoacyl, α-aminoacyl-α-aminoacyl, -C(O)-O-R', -C(O)-N(R")R', -C(=NY')-R, -CH(OH)-C(O)-OY', -CH(OCL4 alkyl) Y0, -CH2Y2, and -CH(CH3)Y2. For certain of these embodiments, R and R" are independently selected from the group consisting of Ci_io alkyl, C3_7 cycloalkyl, phenyl, benzyl, and 2- phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_6 alkyl, Ci_4 alkoxy, aryl, heteroaryl, aryl-Ci_4 alkylenyl, heteroaryl-Ci_4 alkylenyl, halo-Ci_4 alkylenyl, halo-Ci_4 alkoxy, -0-C(O)-CH3, -C(O)-O-CH3, -C(O)-NH2, -0-CH2-C(O)-NH2, -NH2, and -S(O)2-NH2, with the proviso that R" can also be hydrogen; α-aminoacyl is an α-aminoacyl group derived from an α-amino acid selected from the group consisting of racemic, D-, and L-amino acids;
Y' is selected from the group consisting of hydrogen, Ci_6 alkyl, and benzyl; Yo is selected from the group consisting of Ci_6 alkyl, carboxy-Ci_6 alkylenyl, amino-Ci_4 alkylenyl, mono-iV-Ci_6 alkylamino-Ci_4 alkylenyl, and alkylamino-Ci_4 alkylenyl; and
Y2 is selected from the group consisting of mono-JV-Ci_6 alkylamino, alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4-Ci_4 alkylpiperazin-1 -yl.
For certain embodiments, including any one of the above embodiments of Formulas V, VI, VII, and VIII, G is selected from the group consisting of -C(O)-R, α- aminoacyl, and -C(O)-O-R.
For certain embodiments, including any one of the above embodiments of Formula V, VI, VII, and VIII, G is selected from the group consisting of -C(O)-R', α-amino-C2_n acyl, and -C(O)-O-R'. For certain of these embodiments, α-amino-C2_n acyl includes α-amino acids containing a total of at least 2 carbon atoms and a total of up to 11 carbon atoms, and may also include one or more heteroatoms selected from the group consisting of O, S, and N. For certain of these embodiments, R is Ci_io alkyl. For certain embodiments, including any one of the above embodiments which include an α-aminoacyl group, α-aminoacyl is an α-aminoacyl group derived from a
naturally occuring α-amino acid selected from the group consisting of racemic, D-, and L- amino acids.
For certain embodiments, including any one of the above embodiments which include an α-aminoacyl group, α-aminoacyl is an α-aminoacyl group derived from an α- amino acid found in proteins, wherein the the α-amino acid is selected from the group consisting of racemic, D-, and L-amino acids.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl. For certain embodiments, including any one of the above embodiments of
Formulas I, II, III, IV, V, VI, VII, and VIII, n is 0, 1, or 2. For certain of these embodiments, n is 1. Alternatively, for certain of these embodiments, n is 0.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, Ri is selected from the group consisting of -R4, -X-R4, -X-Y-R4, -X-Y-X-Y-R4, -X-R5, -N(RO-Qi-R4, -D'-Xi-Yi-R4, and -D'-Xi-R5a.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, Ri is selected from the group consisting of -R4, -X-R4, -X-Y-R4, and -X-R5. For certain of these embodiments, Ri is selected from the group consisting of alkyl, arylalkylenyl, aryloxyalkylenyl, hydroxyalkyl, dihydroxyalkyl, -X-Y-R4, -X-R5, and heterocyclylalkylenyl wherein the heterocyclyl of the heterocyclylalkylenyl group is optionally substituted by one or more alkyl groups; wherein X is alkylene; Y is -N(R8)-C(O)-, -N(Rs)-S(O)2-, -N(R8)-C(R6)-N(R8)-,
-N(Rg)-C(Re)-O-, -C(O)-, -S(O)2-, or
; R4 is alkyl, aryl, heteroaryl, arylalkylenyl, heteroarylalkylenyl, or arylalkenylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino; and R5 is
-
. For certain of these embodiments, X is C2-6 alkylene.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, Ri is selected from the group consisting of 2- hydroxy-2-methylpropyl, 2-methylpropyl, 2,3-dihydroxypropyl, 4- [(methylsulfonyl)amino]butyl, 2-methyl-2-[(methylsulfonyl)amino]propyl, 2- (acetylamino)-2-methylpropyl, 2- { [(isopropylamino)carbonyl] amino } -2-methylpropyl,
4- { [(isopropylamino)carbonyl] amino } butyl, 4-( 1 , 1 -dioxidoisothiazolidin-2-yl)butyl, tetrahydro-2H-pyran-4-ylmethyl, (2,2-dimethyl- 1 ,3-dioxolan-4-yl)methyl, 2,2-dimethyl-4- oxopentyl, (1 -hydroxy cyclobutyl)methyl, (1 -hydroxy cyclopentyl)methyl, and (1- hy droxy cy clohexy l)methy 1. For certain embodiments, including any one of the above embodiments of
Formulas I, II, III, IV, V, VI, VII, and VIII, except where Ri is -R4, -X-R4, -X-Y-R4, or -X-R5, Ri is selected from the group consisting of -N(Ri')-Qi-R4, -D'-Xi-Yi-R4, and -D'-Xi-R5a. For certain of these embodiments, D' is N(Ri '). For certain of these embodiments, Ri is
Alternatively, for certain of these embodiments, Ri is
Alternatively, for certain of these embodiments, Ri is -N(Ri')-Xi-R5a.
Alternatively, for certain embodiments where Ri is
-D'-Xi-Yi-R4, or -D'-Xi-R5a, Ri is -0-Xi-Yi-R4 or -O-Xi-R5a.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, R2 is selected from the group consisting of -R4, -X-R4, -X-Y-R4, -X-R5, -OH, -NH2, and -NH-Q2-R4. For certain of these embodiments, R2 is selected from the group consisting Of -R4, -X-R4, -X-Y-R4, and -X-R5. Alternatively, for certain of these embodiments, R2 is -OH, -NH2, or -NH-Q2-R4. For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R2 is -OH, -NH2, or -NH-Q2-R4, R2 is -R4. For certain of these embodiments, R2 is hydrogen, alkyl, alkoxyalkylenyl, or hy droxy alky lenyl. For certain of these embodiments, R2 is selected from the group consisting of hydrogen, methyl, ethyl, propyl, butyl, 2-methoxyethyl, 2-hydroxyethyl, ethoxymethyl, and hydroxymethyl.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R2 is -R4, -X-R4, -X-Y-R4, or -X-R5, R2 is -OH. For certain of these embodiments, Ri is selected from the group consisting of pyridin-3-ylmethyl, isoxazol-5-ylmethyl, isoxazol-3-ylmethyl, [5-(4-
fluorophenyl)isoxazol-3-yl]methyl, [3-(4-fluorophenyl)isoxazol-5-yl]methyl, tetrahydro- 2H-pyran-4-ylmethyl, and benzyl, wherein benzyl is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen. For certain embodiments, including any one of the above embodiments of
Formulas I, II, III, IV, V, VI, VII, and VIII, except where R2 is -R4, -X-R4, -X-Y-R4, -X-R5, or -OH, R2 is -NH-Q2-R4. For certain of these embodiments, Q2 is -C(O)-, -S(O)2-, or -C(O)-N(Rg)-. For certain of these embodiments, -R4 in -Q2-R4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino. Alternatively, for certain of these embodiments, Q2 is -C(O)-O-. For certain of these embodiments, R4 in -Q2-R4 is Ci_4 alkyl. Alternatively, for certain embodiments where R2 is -NH-Q2-R4, Q2 is a bond. For certain of these embodiments, R4 in -Q2-R4 is hydrogen, Ci_4 alkyl, Ci_4 alkoxyC2_4 alkylenyl, or hydroxy C2_4 alkylenyl.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, R3 is selected from the group consisting of
-Q-R4,
, -X-Y-R4, -X-R5, -alkylene-CH(CH2-OH)-OH, -alkylene-CH(CH2CH2-OH)-OH, and -(alkylene)0-i-CH(CH2OH)2.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, R3 is -Q-R4. For certain of these embodiments, Q is selected from the group consisting of -C(O)-, -S(O)2-, -C(O)-N(Rg)-, -C(O)-O-, and -S(O)2-N(R8)-.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, R4 in -Q-R4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and
wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, Q is -C(O)- or -S(O)2-. For certain of these embodiments, R4 in -Q-R4 is selected from the group consisting of:
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where Q is -C(O)- or -S(O)2-, Q is -C(O)-O-. For certain of these embodiments, -R4 is selected from the group consisting of:
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R3 is -Q-R4, R3 is
-Q -D N-Q1 -R4
. For certain of these embodiments, Q is -C(O)- or -S(O)2-. For certain of these embodiments, Qi in -Qi-R4 is -C(O)-, -S(O)2-, or -C(O)-N(R8)-. For certain of these embodiments, -R4 in -Qi-R4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino. For certain of these embodiments, D is CH. Alternatively, for certain of these embodiments, D is N.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R3 is -Q-R4 or
-Q -D N-Q1 -R4
, R3 is -Z-Ar. For certain of these embodiments, Z is alkylene. For certain of these embodiments, Z is Ci_4 alkylene. For certain of these embodiments, Ar is pyridinyl, furyl, or phenyl wherein phenyl is optionally substituted by one or more
substituents independently selected from the group consisting of halogen, alkoxy, and alkyl.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R3 is -Q-R4,
-Q -D N-Q1 -R4 , or -Z-Ar, R3 is -X-Y-R4 or -X-R5. For certain of these embodiments, X in R3 is alkylenyl. For certain of these embodiments, X is Ci_4 alkylenyl. For certain of these embodiments, Y in the R3 group -X-Y-R4 is selected from the group consisting of -C(O)-, -S(O)2-, -C(O)-O-, and -C(O)-N(R8)-. For certain of these embodiments, R4 in the R3 group -X-Y-R4 is selected from the group consisting of alkyl, alkenyl, heterocyclyl, aryl, and arylalkylenyl, wherein aryl and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, alkyl, and amino, and wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of fluoro, alkoxy, and heterocyclyl. For certain of these embodiments, R3 is -X-Y-R4. For certain embodiments, including any one of the above embodiments of
Formulas I, II, III, IV, V, VI, VII, and VIII where R3 is -X-Y-R4 or -X-R5, R5 in the R3
VN ) J-H N-alkyl group -X-R5 is selected from the group consisting of: , ,
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII where R3 is -X-Y-R4 or -X-R5, except where Y is -C(O)-, -
S(O)2-, -C(O)-O-, or -C(O)-N(R8)-, Y in the R3 group -X-Y-R4 is -NH-Qi-. For certain of these embodiments, Qi in -NH-Qi- is -C(O)-, -S(O)2-, or -C(O)-N(R8)-. For certain of these embodiments, R4 in -NH-Qi-R4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo. For certain of these embodiments, R3 is -X-Y-R4.
For certain embodiments, including any one of the above embodiments of Formulas I, II, III, IV, V, VI, VII, and VIII, except where R3 is -Q-R4,
-Q -D N-Q1 -R4
— , -Z-Ar, -X-Y-R4, or -X-R5, R3 is -alkylene-CH(CH2-OH)-OH,
-alkylene-CH(CH2CH2-OH)-OH, or -(alkylene)0-i-CH(CH2OH)2. For certain of these embodiments, R3 is -CH(CH2OH)2.
For certain embodiments, R4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy; halogen; nitro; hydroxy; mercapto; cyano; aryl; aryloxy; arylalkyleneoxy; heteroaryl; heteroaryloxy; heteroarylalkyleneoxy; heterocyclyl; amino; alkylamino; dialkylamino; (dialkylamino)alkyleneoxy; and, in the case of alkyl, alkenyl, alkynyl, and heterocyclyl, oxo.
For certain embodiments, R4 is alkyl, aryl, heteroaryl, arylalkylenyl, heteroarylalkylenyl, or arylalkenylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
For certain embodiments, R4 is alkyl, aryl, heteroaryl, heterocyclyl, or arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo. For certain embodiments, R4 is selected from the group consisting of:
For certain embodiments, R4 is selected from the group consisting of:
For certain embodiments, R4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
For certain embodiments, R4 is alkyl, alkenyl, heterocyclyl, aryl, or arylalkylenyl, wherein aryl and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, alkyl, and amino, and wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of fluoro, alkoxy, and heterocyclyl.
For certain embodiments, R4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl wherein alkyl, aryl, and heteroaryl are each unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, and halogen.
For certain embodiments, R4 is selected from the group consisting of alkyl, aryl, arylalkylenyl, and heteroarylalkylenyl wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of alkoxy and hydroxy.
For certain embodiments, R4 is selected from the group consisting of alkyl, arylalkylenyl, aryloxyalkylenyl, and hydroxyalkylenyl.
For certain embodiments, R4 is hydrogen, alkyl, alkoxyalkylenyl, or hydroxyalkylenyl.
For certain embodiments, R4 is hydrogen, Ci_4 alkyl, Ci_4 alkoxyC2-4 alkylenyl, or hydroxy C2-4 alkylenyl.
For certain embodiments, R4 is Ci_6 alkyl.
For certain embodiments, R4 is Ci_4 alkyl.
For certain embodiments, R4 is selected from the group consisting of pyridin-3- ylmethyl, isoxazol-5-ylmethyl, isoxazol-3-ylmethyl, tetrahydro-2H-pyran-4-ylmethyl, and benzyl, wherein benzyl is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen.
For certain embodiments, R4 is 4-fluorophenyl.
For certain embodiments, R5 is selected from the group consisting of:
For certain embodiments, R5 is selected from the group consisting of: O J. / / —— \ \ O u. / / —— \ \ O u. / / —— \ \ O u. / — \
V-N y J-H N-alkyl V-N N-Q1-R4 VN O
For certain embodiments, R5 is
-
For certain embodiments, Re is selected from the group consisting of =0 and =S. For certain embodiments, Re is =0. For certain embodiments, Re is =S.
For certain embodiments, R7 is C2-7 alkylene. For certain embodiments, R7 is C2-4 alkylene.
For certain embodiments, R7 is ethylene.
For certain embodiments, Rga is selected from the group consisting of hydrogen and Ci_4 alkyl.
For certain embodiments, Rsa is hydrogen. For certain embodiments, Rsa is Ci_4 alkyl.
For certain embodiments, Rs is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl.
For certain embodiments, Rs is hydrogen, C1-10 alkyl, or hydroxy-Ci_io alkylenyl.
For certain embodiments, Rs is C1-10 alkyl or hydroxy-Ci_i0 alkylenyl. For certain embodiments, Rs is hydrogen.
For certain embodiments, Rs is C1-10 alkyl.
For certain embodiments, Rg is selected from the group consisting of hydrogen and alkyl.
For certain embodiments, Ri0 is C3_8 alkylene. For certain embodiments, Ri0 is C4-6 alkylene.
For certain embodiments, Ri0 is pentylene.
For certain embodiments, A is selected from the group consisting Of -CH2-, -O-, -C(O)-, -S(OV2-, and -NC-Qi-R4)-.
For certain embodiments, A is selected from the group consisting Of -CH2-, -0-, and -N(alkyl)-.
For certain embodiments, A is -O- .
For certain embodiments, A' is selected from the group consisting of -0-, -S(O)0-2-, -NC-Qi-R4)-, and -CH2-.
For certain embodiments, Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino.
For certain embodiments, Ar is pyridinyl, furyl, or phenyl wherein phenyl is optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl.
For certain embodiments, Ar is phenyl.
For certain embodiments, Ar is pyridinyl.
For certain embodiments, D is CH or N; with the proviso that when D is N, then Q is -C(R6)- or -S(O)2-.
For certain embodiments, D is CH. For certain embodiments, D is N, and Q is -C(R6)- or -S(O)2-.
For certain embodiments, D' is selected from the group consisting of -N(Ri')- and -0-.
For certain embodiments, D' is -N(Ri')-.
For certain embodiments, D' is -O- . For certain embodiments, E is selected from the group consisting of -0-, -C(O)-,
-S(O)0-2-, and -N(Qi-R4)-.
For certain embodiments, E is -0-.
For certain embodiments, E is -N(Qi-R4)-.
For certain embodiments, Q is selected from the group consisting Of -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Re)-N(R8)- W-, -S(O)2-N(R8)-, -C(Re)-O-, -C(Re)-S-, and
-C(Re)-N(OR9)-.
For certain embodiments, Q is selected from the group consisting of -C(O)-, -S(O)2-, -C(O)-N(R8)-, -C(O)-O-, and -S(O)2-N(R8)-.
For certain embodiments, Q is -C(O)- or -S(O)2-. For certain embodiments, Q is -C(O)-O-.
For certain embodiments, Qi is selected from the group consisting of a bond, -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Re)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Re)-O-, -C(Re)-S-, and -C(Re)-N(OR9)-.
For certain embodiments, Qi is selected from the group consisting of -C(O)-, -S(O)2-, -C(Re)-N(R8)-, -S(O)2-N(R8)-, -C(O)-O-, and -C(O)-S-.
For certain embodiments, Qi is -C(O)-, -S(O)2-, -C(Re)-N(R8)-, or -S(O)2-N(R8)-.
For certain embodiments, Qi is -C(O)-, -S(O)2-, -C(Re)-N(R8)-, or -C(Re)-O-.
For certain embodiments, Qi is -C(O)-, -S(O)2-, or -C(O)-N(R8)-.
For certain embodiments, Qi is -C(R6)-. For certain embodiments, Qi is a bond.
For certain embodiments, Q2 is selected from the group consisting of a bond, -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Re)-N(RSa)-W-, -S(O)2-N(R8a)-, and -C(Re)-O-.
For certain embodiments, Q2 is -C(O)-, -S(O)2-, or -C(O)-N(R8)-.
For certain embodiments, Q2 is -C(O)-O-.
For certain embodiments, Q2 is a bond.
For certain embodiments, R1' is selected from the group consisting of hydrogen, Ci_2o alkyl, hydroxyC2_2o alkylenyl, and alkoxyC2_2o alkylenyl
For certain embodiments, R1' is hydrogen.
For certain embodiments, R1' is C1-10 alkyl.
For certain embodiments, V is selected from the group consisting of a bond, -C(R6)-, -0-C(R6)-, -N(Rg)-C(R6)-, and -S(O)2-. For certain embodiments, V is -N(Rg)-C(O)-. For certain embodiments, V is
-N(H)-C(O)-.
For certain embodiments, W is selected from the group consisting of a bond, -C(O)-, and -S(O)2-.
For certain embodiments, W is a bond. For certain embodiments, X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups.
For certain embodiments, X is alkylene. For certain embodiments, X is C2_6 alkylene.
For certain embodiments, X is Ci_4 alkylene. For certain embodiments, X is methylene.
For certain embodiments, Xi is C2_2o alkylene with the proviso that when Yi is
For certain embodiments, Xi is C2_6 alkylene. For certain embodiments, Xi is C2_4 alkylene. For certain embodiments, Xi is Ci_6 alkylene or Ci_4 alkylene, and Yi is
-C(Re)-N(R8)- or
For certain embodiments, Y is selected from the group consisting of -O-, -S(O)0-2-, -S(O)2-N(R8)-, -C(R6)-, -C(Re)-O-, -0-C(R6)-, -0-C(O)-O-, -N(Rg)-Qi-, -C(Re)-N(R8)-, -0-C(Re)-N(R8)-, -C(Re)-N(OR9)-, -0-N(Rg)-Qi-, -0-N=C(R4)-,
-C(=N-O-R8)-, -CH(-N(-O-R8)-Qi-R4)-,
For certain embodiments, Y is -N(R8)-Qi-.
For certain embodiments, Y is -N(R8)-C(0)-, -N(Rs)-S(O)2-,
-N(Rg)-C(Re)-N(R8)-, -N(Rs)-C(Re)-O-, -C(O)-, -S(O)2-, or
For certain embodiments, Y is -N(R8)-C(0)-, -N(R8)-S(O)2-,
-N(R8)-C(Re)-N(R8)-, -N(Rs)-S(O)2-N(R8)-, -N(Rg)-C(Re)-O-, or -N(Rg)-C(Re)-S-. For certain embodiments, Y is -C(O)-, -S(O)2-, -C(O)-O-, or -C(O)-N(R8)-. For certain embodiments, Y is -NH-Qi-.
For certain embodiments, Y is selected from the group consisting of -NH-C(O)-, -NH-S(O)2-, -NH-C(Re)-N(R8)-, -NH-S(O)2-N(R8)-, -NH-C(O)-O-, and -NH-C(O)-S-.
For certain embodiments, Y is -NH-C(O)-, -NH-S(O)2-, or -N(H)-C(O)-N(R8)-. For certain embodiments, Yi is selected from the group consisting of -0-, -S(O)0-2-, -S(O)2-N(R8)-, -N(R8)-Qi-, -C(Re)-N(R8)-, -0-C(Re)-N(R8)-, and
-C(Re)-N(R8)-, -N(Rg)-C(R6)-, -N(Rg)-C(Re)-O-, -N(Rg)-S(O)2-, -N(Rg)-S(O)2-N(R8)-, or -N(R8)-C(Re)-N(R8)-.
For certain embodiments, Yi is -N(Rs)-C(R6)-, -N(Rs)-C(Re)-O-, -N(Rs)-S(O)2-,
-N(Rg)-S(O)2-N(R8)-, or -N(Rg)-C(Rs)-N(R8)-.
For certain embodiments, Yi is -N(Rg)-C(O)-, -N(Rg)-S(O)2-, or -N(Rg)-C(O)-N(R8)-, and R8 is H or C1-4 alkyl.
For certain embodiments, Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups.
For certain embodiments, Z is alkylene.
For certain embodiments, Z is Ci_4 alkylene.
For certain embodiments, Z is a bond. For certain embodiments, a and b are independently integers from 1 to 6 with the proviso that a + b is < 7.
For certain embodiments, a and b are each independently 1, 2, or 3.
For certain embodiments, a and b are each 2.
For certain embodiments, n is O. For certain embodiments, n is 1.
For certain embodiments, n is 2.
For certain embodiments, the present invention provides a pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, and a pharmaceutically acceptable carrier.
For certain embodiments, the present invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, or a pharmaceutical composition comprising an effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII to the animal. For certain of these embodiments, the cytokine is selected from the group consisting of IFN-α, TNF-α, IL-6, IL-IO, and IL-12. For certain of these embodiments, the cytokine is IFN-α or TNF- α. For certain of these embodiments, the cytokine is IFN-α.
For certain embodiments, the present invention provides a method of treating a viral disease in an animal comprising administering a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII, or a
pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII to the animal. For certain embodiments, the present invention provides a method of treating a neoplastic disease in an animal comprising administering a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through
VIII, or a pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of the above embodiments of Formulas I through VIII to the animal.
As used herein, the terms "alkyl", "alkenyl", "alkynyl" and the prefix "alk-" are inclusive of both straight chain and branched chain groups and of cyclic groups, e.g., cycloalkyl and cycloalkenyl. Unless otherwise specified, these groups contain from 1 to 20 carbon atoms, with alkenyl groups containing from 2 to 20 carbon atoms, and alkynyl groups containing from 2 to 20 carbon atoms. In some embodiments, these groups have a total of up to 10 carbon atoms, up to 8 carbon atoms, up to 6 carbon atoms, up to 4 carbon atoms, or up to 2 carbon atoms. Cyclic groups can be monocyclic or poly cyclic and preferably have from 3 to 10 ring carbon atoms. Exemplary cyclic groups include cyclopropyl, cyclopropylmethyl, cyclobutyl, cyclobutylmethyl, cyclopentyl, cyclopentylmethyl, cyclohexyl, cyclohexylmethyl, adamantyl, and substituted and unsubstituted bornyl, norbornyl, and norbornenyl. Unless otherwise specified, "alkylene", "-alkylene-", "alkenylene",
"-alkenylene-", "alkynylene", and "-alkynylene-" are the divalent forms of the "alkyl", "alkenyl", and "alkynyl" groups defined above. The terms "alkylenyl", "alkenylenyl", and "alkynylenyl" are used when "alkylene", "alkenylene", and "alkynylene", respectively, are substituted. For example, an arylalkylenyl group comprises an "alkylene" moiety to which an aryl group is attached.
The term "haloalkyl" is inclusive of alkyl groups that are substituted by one or more halogen atoms, including perfluorinated groups. This is also true of other groups that include the prefix "halo-". Examples of suitable haloalkyl groups are chloromethyl, trifluoromethyl, and the like. The term "aryl" as used herein includes carbocyclic aromatic rings or ring systems.
Examples of aryl groups include phenyl, naphthyl, biphenyl, fluorenyl and indenyl.
Unless otherwise indicated, the term "heteroatom" refers to the atoms O, S, or N.
The term "heteroaryl" includes aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N). In some embodiments, the term "heteroaryl" includes a ring or ring system that contains 2 to 12 carbon atoms, 1 to 3 rings, 1 to 4 heteroatoms, and O, S, and/or N as the heteroatoms. Suitable heteroaryl groups include furyl, thienyl, pyridyl, quinolinyl, isoquinolinyl, indolyl, isoindolyl, triazolyl, pyrrolyl, tetrazolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, benzofuranyl, benzothiophenyl, carbazolyl, benzoxazolyl, pyrimidinyl, benzimidazolyl, quinoxalinyl, benzothiazolyl, naphthyridinyl, isoxazolyl, isothiazolyl, purinyl, quinazolinyl, pyrazinyl, 1-oxidopyridyl, pyridazinyl, triazinyl, tetrazinyl, oxadiazolyl, thiadiazolyl, and so on. The term "heterocyclyl" includes non-aromatic rings or ring systems that contain at least one ring heteroatom (e.g., O, S, N) and includes all of the fully saturated and partially unsaturated derivatives of the above mentioned heteroaryl groups. In some embodiments, the term "heterocyclyl" includes a ring or ring system that contains 2 to 12 carbon atoms, 1 to 3 rings, 1 to 4 heteroatoms, and O, S, and N as the heteroatoms. Exemplary heterocyclyl groups include pyrrolidinyl, tetrahydrofuranyl, morpholinyl, thiomorpholinyl,
1,1-dioxothiomorpholinyl, piperidinyl, piperazinyl, thiazolidinyl, imidazolidinyl, isothiazolidinyl, tetrahydropyranyl, quinuclidinyl, homopiperidinyl (azepanyl), 1 ,A- oxazepanyl, homopiperazinyl (diazepanyl), 1,3-dioxolanyl, aziridinyl, azetidinyl, dihydroisoquinolin-(lH)-yl, octahydroisoquinolin-(lH)-yl, dihydroquinolin-(2H)-yl, octahydroquinolin-(2H)-yl, dihydro-lH-imidazolyl, 3-azabicyclo[3.2.2]non-3-yl, and the like.
The term "heterocyclyl" includes bicylic and tricyclic heterocyclic ring systems. Such ring systems include fused and/or bridged rings and spiro rings. Fused rings can include, in addition to a saturated or partially saturated ring, an aromatic ring, for example, a benzene ring. Spiro rings include two rings joined by one spiro atom and three rings joined by two spiro atoms.
When "heterocyclyl" contains a nitrogen atom, the point of attachment of the heterocyclyl group may be the nitrogen atom.
The terms "arylene", "heteroarylene", and "heterocyclylene" are the divalent forms of the "aryl", "heteroaryl", and "heterocyclyl" groups defined above. The terms, "arylenyl", "heteroarylenyl", and "heterocyclylenyl" are used when "arylene",
"heteroarylene," and "heterocyclylene", respectively, are substituted. For example, an alkylarylenyl group comprises an arylene moiety to which an alkyl group is attached.
When a group (or substituent or variable) is present more than once in any Formula described herein, each group (or substituent or variable) is independently selected, whether
explicitly stated or not. For example, for the formula
each R7 group is independently selected. In another example, when more than one Y group is present, each Y group is independently selected. In a further example, when more than one -N(Rg)-Qi-R4 group is present (e.g., more than one -Y-R4 group is present, and both contain a -N(Rs)-Qi- group) each Rg group is independently selected, each Qi group is independently selected, and each R4 group is independently selected.
The invention is inclusive of the compounds described herein (including intermediates) in any of their pharmaceutically acceptable forms, including isomers (e.g., diastereomers and enantiomers), salts, solvates, polymorphs, prodrugs, and the like. In particular, if a compound is optically active, the invention specifically includes each of the compound's enantiomers as well as racemic and scalemic mixtures of the enantiomers. It should be understood that the term "compound" includes any or all of such forms, whether explicitly stated or not (although at times, "salts" are explicitly stated).
The term "prodrug" means a compound that can be transformed in vivo to yield an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above. The prodrug, itself, may be an immune response modifying compound, including any of the salt, solvated, polymorphic, or isomeric forms described above. The transformation may occur by vaious mechanisms, such as through a chemical (e.g., solvolysis or hydrolysis, for example, in the blood) or enzymatic biotransformation. A discussion of the use of prodrugs is provided by T. Higuchi and W. Stella, "Pro-drugs as Novel Delivery Systems," Vol. 14 of the A. C. S. Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987.
Compounds (including intermediates) of the present invention may exist in different tautomeric forms, and all such forms are embraced within the scope of the invention. The term "tautomer" or "tautomeric form" refers to structural isomers of different energies, which are interconvertible via a low energy barrier. For example,
proton tautomers (prototropic tautomers) include interconversions via migration of a proton, such as keto-enol and imine-enamine isomerizations. This is illustrated, for example, when compounds of the present invention have a hydrogen atom on an oxygen or nitrogen atom at the 2-position, allowing proton migration between the oxygen or nitrogen atom at the 2-position and the 3-position.
Preparation of the Compounds
Compounds of the invention may be synthesized by synthetic routes that include processes analogous to those well known in the chemical arts, particularly in light of the description contained herein. The starting materials are generally available from commercial sources such as Aldrich Chemicals (Milwaukee, Wisconsin, USA) or are readily prepared using methods well known to those skilled in the art (e.g., prepared by methods generally described in Louis F. Fieser and Mary Fieser, Reagents for Organic Synthesis, v. 1-19, Wiley, New York, (1967-1999 ed.); Alan R. Katritsky, Otto Meth- Cohn, Charles W. Rees, Comprehensive Organic Functional Group Transformations, v. 1-
6, Pergamon Press, Oxford, England, (1995); Barry M. Trost and Ian Fleming, Comprehensive Organic Synthesis, v. 1-8, Pergamon Press, Oxford, England, (1991); or Beilsteins Handbuch der organischen Chemie, 4, Aufl. Ed. Springer-Verlag, Berlin, Germany, including supplements (also available via the Beilstein online database)). For illustrative purposes, the reaction schemes depicted below provide potential routes for synthesizing the compounds of the present invention as well as key intermediates. For more detailed description of the individual reaction steps, see the EXAMPLES section below. Those skilled in the art will appreciate that other synthetic routes may be used to synthesize the compounds of the invention. Although specific starting materials and reagents are depicted in the reaction schemes and discussed below, other starting materials and reagents can be easily substituted to provide a variety of derivatives and/or reaction conditions. In addition, many of the compounds prepared by the methods described below can be further modified in light of this disclosure using conventional methods well known to those skilled in the art. In the preparation of compounds of the invention it may sometimes be necessary to protect a particular functionality while reacting other functional groups on an intermediate. The need for such protection will vary depending on the nature of the particular functional
group and the conditions of the reaction step. Suitable amino protecting groups include acetyl, trifluoroacetyl, fert-butoxycarbonyl (Boc), benzyloxycarbonyl, and 9- fluorenylmethoxycarbonyl (Fmoc). Suitable hydroxy protecting groups include acetyl and silyl groups such as the tert-butyl dimethylsilyl group. For a general description of protecting groups and their use, see T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, 3rd edition, John Wiley & Sons, New York, USA, 1999.
Conventional methods and techniques of separation and purification can be used to isolate compounds of the invention, as well as various intermediates related thereto. Such techniques may include, for example, all types of chromatography (high performance liquid chromatography (HPLC), column chromatography using common absorbents such as silica gel, and thin layer chromatography), recrystallization, and differential (i.e., liquid- liquid) extraction techniques.
Compounds of the invention can be prepared according to Reaction Scheme I, starting from a compound of Formula X in which Ri and R2 are as defined above and Ji contains the necessary atoms to provide the following four lH-imidazo[4,5- c]naphthyridin-4-amine isomers of Formulas XIII, XIV, XV, and XVI, wherein R and n are as defined above.
Many lH-imidazo[4,5-c]naphthyridin-4-amine compounds of Formula X are known or can be prepared by known methods. See, for example, U. S. Patent No. 6,194,425 (Gerster et al), International Publication Nos. WO 2005/018551 (Kshirsagar et al), WO 2005/018556 (Kshirsagar et al.), WO 2005/048945 (Kshirsagar et al.), WO 2005/048933 (Kshirsagar et al.), WO 2005/051324 (Rrepski et al.), WO 2005/123079 (Kshirsagar et al.), WO 2006/009832 (Dellaria et al.), WO 2006/029115 (Kshirsagar et al.), WO 2005/066169 (Bonk and Dellaria), WO 2006/028545 (Stoermer et al.), WO 2006/026760 (Stoermer et al.), WO 2006/028962 (Rrepski et al.), WO 2005/076783 (Radmer et al.), and WO 2005/094531 (Rrepski et al.), and International Application No. PCT/US2005/021570 (Moser et al.).
In step (1) of Reaction Scheme I, a lH-imidazo[4,5-c]naphthyridin-4-amine of Formula X is reduced to provide a compound of Formula XI, or a salt thereof, wherein J2 contains the necessary atoms to provide the following four 6,7,8,9-tetrahydro-lH- imidazo[4,5-c]naphthyridin-4-amine isomers of Formulas XVII, XVIII, XIX, and XX, wherein R and n are as defined above.
XVIl XVIII XIX XX
Several 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amines of Formula XVII, XIX, and XX are known; see for example, U. S. Patent No. 6,194,425 (Gerster et al), International Publication Nos. WO 2006/028545 (Stoermer et al.) and WO 2006/026760 (Stoermer et al.), and International Application No. PCT/US2005/021570 (Moser et al.). The reduction can be carried out under hydrogen pressure using a hydrogenation catalyst such as platinum oxide in a solvent such as trifluoroacetic acid. The reaction can be conveniently carried out on a Parr apparatus at room temperature.
In step (2) of Reaction Scheme I, the secondary amine in a 6,7,8,9-tetrahydro-lH- imidazo[4,5-c]naphthyridin-4-amine of Formula XI is functionalized with an R3 group to form a compound of Formula XII, wherein J3 contains the necessary atoms to provide the following compounds of the invention, Formulas I, II, III, and IV, wherein R, n, and R3 are as defined above.
IV
For some of the chemistry described below in the synthesis of compounds of Formula XII, protection and deprotection steps may be required. For example, in the synthesis of a substituted 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,5-naphthyridin-4-amine of Formula I and a substituted 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,8-naphthyridin-4-
amine of Formula IV, protection of the 4-amino group may be necessary before installation of the R3 group. The 4-amino group can be protected as a cyclic imide such as an JV-phthalimide or an JV-succinimde by reaction of a lH-imidazo[4,5-c]naphthyridine of Formula XIII or XVI with phthalic anhydride or succinic anhydride in a suitable solvent, such as chloroform of Λ/,Λ/-dimethylformamide (DMF), at elevated temperature. The protected lH-imidazo[4,5-c]naphthyridine can then be reduced using the conditions described in step (1) of Reaction Scheme I to yield a 6,7,8,9-tetrahydro-lH-imidazo[4,5- c]naphthyridine where the 4-amino group is protected. Alternatively, the protection step can be carried out on a 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridine of Formula XVII or XX. The R3 group can be installed using one of the methods described below in step (2) of Reaction Scheme I, and the cyclic imide protecting group can be removed with hydrazine to yield a compound of Formula I or IV.
A 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amine of Formula XI or a salt thereof can be converted to an amide, sulfonamide, carbamate, sulfamide, or urea of Formula XII, i.e. R3 is -Q-R4, using conventional methods. For example, a compound of
Formula XI or salt thereof can react with an acid chloride of Formula R4C(O)Cl to provide a compound of Formula XII wherein R3 is -C(O)-R4. In addition, a compound of the Formula XI can react with a sulfonyl chloride of Formula R4S(O)2Cl or a sulfonic anhydride of Formula (R4S(O)2)2O to provide a compound of Formula XII wherein R3 is - S(O)2-R4. A compound of the Formula XI can also react with a chloroformate of Formula
R4CO(O)Cl to provide a compound of Formula XII in which R3 is -C(O)-O-R4. Numerous acid chlorides of Formula R4C(O)Cl, sulfonyl chlorides of Formula R4S(O)2Cl, sulfonic anhydrides of Formula (R4S(O)2^O, and chloro formates of Formula R4CO(O)Cl are commercially available; others can be readily prepared using known synthetic methods. The reaction can be conveniently carried out by combining the acid chloride of Formula
R4C(O)Cl, chloroformate of Formula R4CO(O)Cl, sulfonyl chloride of Formula R4S(O)2Cl, or sulfonic anhydride of Formula (R4S(O)^2O with a compound of Formula XI and a base such as triethylamine in a suitable solvent such as chloroform, dichloromethane, or acetonitrile. The reaction can be carried out at room temperature or at a sub-ambient temperature such as O 0C.
Ureas and thioureas of Formula XII where R3 is -C(RO)-N(RS)-W-R4, wherein W is a bond, Rs is Η, and R4 and R6 are as defined above, can be prepared by reacting a
compound of Formula XI or a salt thereof with isocyanates of Formula R4N=C=O or isothiocyanates of Formula R4N=C=S. Numerous isocyanates and isothiocyanates are commercially available; others can be readily prepared using known synthetic methods. The reaction can be conveniently carried out by adding the isocyanate or isothiocyanate to a cooled solution of a compound of Formula XI in a suitable solvent such as dichloromethane or chloroform. Optionally, a base such as triethylamine can be added. The reaction can be carried out at room temperature or at a sub-ambient temperature such as 0 0C.
Ureas and sulfamides of Formula XII where R3 is -C(R^)-N(Rs)-W-R4 or -S(O)2-N(Rg)-R4, wherein R6 is =0, W is a bond, and Rg and R4 are as defined above, can be synthesized by treating a compound of Formula XI with a carbamoyl chloride of Formula R4(R8)N-C(O)Cl or a sulfamoyl chloride of Formula R4(R8)N-S(O)2Cl under the reaction conditions described above for reaction of compounds of Formula XI with acid chlorides. In addition, ureas and sulfamides of Formula XII where R3 is -C(O)-heterocycle or -S(O)2-heterocycle wherein heterocycle is, for example, pyrrolidine or morpholine, can be prepared from carbamoyl chlorides of Formula Cl-C(O)-heterocycle or sulfamoyl chlorides of Formula Cl-S(O)2-heterocycle. Some carbamoyl chlorides and sulfamoyl chlorides are commercially available; others can be prepared using known synthetic methods from commercially available amines of Formula FIN(R8)R4 or appropriate cyclic amines.
An amide, urea, or sulfamide of Formula XII where R3 is
-Q-D N-Q1 -R4
wherein Q is -C(O)- or -S(O)2-, and D, Q1, and R4 are as defined above can be prepared by treating a compound of Formula XI with an acid chloride, carbamoyl chloride, or sulfamoyl chloride of Formula
Cl-Q-D N-Q1 -R4
Some acid chlorides, carbamoyl chlorides, and sulfamoyl chlorides of this formula are commercially available, for example, l-acetylpiperidine-4-carbonyl chloride and A- methylpiperazine-1-carbonyl chloride; others can be prepared using known synthetic methods from piperidine-4-carboxylic acid or piperazine. When -Qi-R4 is a tert-
butoxycarbonyl (Boc) group in a compound of Formula XII, the protecting group can be removed under acidic conditions, and the resulting amine can be further elaborated using the methods described above to provide a sulfonamide, amide, carbamate, urea, thiourea, or sulfamide. Compounds of Formula XII where R3 is -Z-Ar, wherein Z is alkylene and Ar is as defined above, can be prepared by a reductive amination reaction between an aryl or arylalkylenyl aldehyde or ketone and a compound of Formula XI. Numerous aldehydes and ketones of this type are commercially available, especially substituted benzaldehydes. The reductive alkylation is conveniently carried out in two parts by (i) adding an aryl or arylalkylenyl aldehyde or ketone to a solution of a compound of Formula XI or a salt thereof in a suitable solvent such as DMF in the presence of a base such as N,N- diisopropylethylamine. In part (ii) the reduction is carried out by adding a suitable reducing agent such as the borane-pyridine complex. Both part (i) and part (ii) can be carried out at room temperature. Compounds of Formula XII where R3 is -Z-Ar, wherein Z is alkylene, alkenylene, or alkynylene and Ar is as defined above, can also be prepared through an alkylation reaction with an arylalkylenyl, arylalkenylenyl, or arylalkynylenyl halide of Formula Halide-Z-Ar, where Halide is -Cl, -Br, or -I. The reaction is carried out by treating a compound of Formula XI with a compound of Formula Halide-Z-Ar in a suitable solvent such as ethanol, DMF, tetrahydrofuran (THF), or acetonitrile in the presence of a base such as triethylamine or potassium carbonate. The reaction can be carried out at room temperature or at elevated temperature. Numerous compounds of Formula Halide-Z-Ar are commercially available, for example, substituted benzyl bromides and chlorides.
Compounds of Formula XII where R3 is -Z-Ar, wherein Z is a bond and Ar is as defined above, can be prepared using an aromatic nucleophilic substitution reaction. A compound of Formula XI can react with an appropriately substituted aryl reagent, for example, l-fluoro-2,4-dinitro-benzene, to yield a compound of Formula XII in which Ar is a 2,4-dinitrobenzene group. Alternatively, a compound of Formula XI can undergo a palladium-mediated coupling reaction with an aryl halide to yield a compound of Formula XII.
Compounds of Formula XII where R3 is -alkylene-CH(CH2-OH)-OH,
-alkylene-CH(CH2CH2-OH)-OH, and -(alkylene)0-i-CH(CH2OH)2 can be prepared by a reductive amination reaction between a dihydroxy aldehyde or ketone and a compound of Formula XI. For example, a ketone of Formula HO-CH2-C(O)-CH2-OH can be reacted with a compound of Formula XI under the conditions described above for a reductive amination reaction to provide a compound of Formula XII in which R3 is
-CH(CH2OH)2. A dihydroxy aldehyde or ketone wherein the hydroxyl groups are protected with an appropriate protecting group or groups can also be used in the reductive amination reaction; subsequent deprotection provides a compound of Formula XII. Many protected dihydroxy aldehydes and ketones are known in the literature and can be readily synthesized; see, for example, Howson, W. et al Eur. J. Med. Chem. Chim. Ther. 25, pp. 595-602 (1990); Hon, Y.-S. et al. Tetrahedron, 57, 6181-6188, (2001); and Rao, A. V. R. et al. Carbohydr. Res. 148, 51-56, (1986).
A compound of Formula XII where R3 is
; wherein Z is a bond, alkylene, or -CH2-alkylene, E is -O-, -S(0)o-2-, or -N(Qi-R4)-, and Rio is as defined above; can be prepared by alkylation of a compound of
Formula XI with a reagent of Formula
, or through a reductive amination between a compound of Formula XI and a compound of Formula
10 using the conditions described above. Some useful reagents are commercially available, for example, tert-butyl 4-(bromomethyl)piperidine-l-carboxylate, 4-(bromomethyl)tetrahydro-2H-pyran, and 4-(bromomethyl)piperidine; other reagents can be prepared using known synthetic methods. IfE is -N(Qi-R4)-, wherein Q1-R4 is a protecting group, the protecting group can subsequently be removed, and the resulting amine can be converted into an amide, urea, thiourea, carbamate, sulfonamide, or sulfamide using one of the methods described above.
A Michael addition reaction can be used to prepare a compound of Formula XII in
which R3 is -X-Y-R4, -X-R5, or
wherein X is alkylene; Y and R4 are as defined above, R5 is -CN, Z is a bond, E is -C(O)-, and Rio is C4_6 alkylene. For example, a compound of Formula XI can be treated with an α,β-unsaturated sulfone, ketone, ester, amide, or nitrile of Formula H2C=C(H)-Y-R4 or H2C=C(H)-R5 to provide a compound of
Formula XII where R3 is -CH2CH2-Y-R4 or -CH2CH2-R5, wherein Y is -S(O)2-, -C(O)-, -C(O)-O-, -C(O)-N(H)-, and R5 is -CN. Many α,β-unsaturated sulfones, ketones, esters, amides, and nitriles are commercially available, others can be prepared readily through known methods. The reaction can also be carried out with cyclic α,β-unsaturated ketones such as 2-cyclohexene-l-one to provide a compound of Formula XII wherein R3 is
The reaction can be conveniently carried out by adding an α,β-unsaturated sulfone, ketone, ester, amide, or nitrile to a solution of a compound of Formula XI in a suitable solvent such as THF or acetonitrile optionally in the presence of a base such as triethylamine. The reaction can be carried out at room temperature or at an elevated temperature, for example, the reflux temperature of the solvent. An alkyne of Formula HC=C-Y-R4 or HC=C-R5 can be used in place of an alkene of Formula H2C=C(H)-Y-R4 or H2C=C(H)-R5 in the Michael reaction to yield compound of Formula XII in which R3 is -X-Y-R4 or -X-R5 wherein X is alkenylene. Additional compounds of the invention of Formula XII where R3 is -X-Y-R4 or -X-R5 can be prepared through alkylation of a compound of Formula XI with a reagent of Formula HaMe-X-Y-R4 or Halide-X-R5; or by a reductive amination reaction between an aldehyde of Formula H(O)C-X-Y-R4 or H(O)C-X-R5 with a compound of Formula XI using the conditions described above for an alkylation or a reductive amination. A compound of Formula XII in which R3 is -X-Y-R4 or -X-R5 obtained from a
Michael reaction or by other means, can be elaborated to provide additional compounds of the invention. For example, compounds of Formula XII in which R3 is -X-CN or -X-CO2R4 can be treated with a reducing agent such as lithium aluminum hydride to provide a compound of Formula XII in which R3 is -X-CH2-NH2 or -X-CH2-OH,
respectively. The amino group in a compound of Formula XII where R3 is -X-CH2-NH2 can be converted to an amide, urea, thiourea, carbamate, sulfonamide, or sulfamide of Formula XII in which R3 is -X-CH2-N(Rs)-Qi-R4 using the methods described above. The amino group can also be treated with a an acid chloride of Formula Cl-RyC(O)Cl or a sulfonyl chloride of Formula Cl-RyS(O)2Cl using the reaction conditions described above.
The isolable intermediate chloroalkanesulfonamide or chloroalkanamide can then be treated with a base such as l,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or sodium hydride at room temperature in a suitable solvent such as DMF to effect a cyclization and provide a compound of Formula XII in which R3 is -X-CH2-Rs, wherein R5 is -N- C(R6) -N- S(O)2 7 or 7 and R6 and Ry are as defined above.
A hydroxyalkyl-substituted compound of Formula XII, in which R3 is -X-OH or -X-CH2-OH, can be treated with JV-hydroxyphthalimide under Mitsunobu reaction conditions to provide an JV-phthalimide-protected hydroxylamine. The reaction can be conveniently carried out by adding triphenylphosphine and JV-hydroxyphthalimide to a solution of the hydroxyalkyl-substituted compound in a suitable solvent such as THF or
DMF and then slowly adding diisopropyl azodicarboxylate. The reaction can be carried out at room temperature or at an elevated temperature, such as 60 0C. The phthalimide group can then be removed from the resulting JV-phthalimide-protected hydroxylamine by treatment with hydrazine at room temperature in a suitable solvent such as ethanol. The resulting hydroxylamine can then be treated with one of numerous commercially available aldehydes or ketones in a suitable solvent such as methanol to provide an oxime in a compound of Formula XII where R3 is -X-Y-R4 or -X-R5, wherein Y is -0-N=C(R4)-, R5 is
^(CH2)a -. -C-N= A.
(CH2 )[J ^ an(j R4^ a^ j^ an(j A' are as defined above. Alternatively, the hydroxylamine prepared after the hydrazine deprotection may be treated with one of numerous acid chlorides, sulfonyl chlorides, isocyanates, carbamoyl chlorides, or sulfamoyl chlorides as described above to provide a compound of the invention wherein R3 is -X-Y-R4 where Y is -O-NH-Q1-, and Qi and R4 are as defined above.
The alcohol in a compound of Formula XII wherein R3 is -X-OH or -X-CH2-OH, can be converted into a chloride group that can be displaced by a nucleophile, such as a cyclic amine of Formula
s wherein a, b, and A are as defined above. Several cyclic amines of this formula are commercially available; others can be prepared by conventional methods. The chlorination reaction can be conveniently carried out by combining a hydroxyl-substituted compound of Formula XII with thionyl chloride in a suitable solvent such as dichloromethane at room temperature. The resulting chloro-substituted compound is then combined with a cyclic amine in the presence of a base such as potassium carbonate and in a suitable solvent such as DMF. Catalytic sodium iodide can optionally be added. The reaction can be carried out at an elevated temperature such as 50 0C or 90 0C-IOO 0C. These reaction conditions also can be used employing a variety of phenols to provide compounds of Formula XII where R3 is -X-Y-R4 where Y is -O- and R4 is an unsubstituted or substituted phenyl group. In another example, a ketone of Formula XII, which is a compound of the invention wherein R3 is -X-Y-R4 where Y is -C(O)-, X is alkylene, and R4 is as defined above, which may be obtained through a Michael reaction, can be converted to an oxime, wherein Y is -Q=N-ORs)-, and Rs is as defined above. The reaction can be carried out by combining an aqueous solution of a hydroxylamine salt of Formula NH2ORs^HCl and a solution of the ketone in a suitable solvent such as methanol or ethanol and then adding a base such as sodium hydroxide and heating at an elevated temperature. The oxime so prepared may be reduced with sodium cyanoborohydride in a mixture of ethanol or methanol in acetic acid to provide a hydroxylamine, which may be treated with one of numerous acid chlorides, sulfonyl chlorides, isocyanates, carbamoyl chlorides, or sulfamoyl chlorides as described above to provide a compound of the invention wherein
X Xl XIl
lH-Imidazo[4,5-c]naphthyridin-4-amine compounds of Formula XII can also be prepared according to Reaction Scheme II wherein R1, R2, J1, and J3 are as defined above, Hal is -Br or -I, M is -B(O-alkyl)2, -B(OH)2, -Sn(alkyl)3, or -Zn-Halide, Di is hydrogen, -C(O)C(CH3 )3 (tert-butylcarbonyl), or Boc, and J4 and J5 are defined below. In step (1) of Reaction Scheme II, aminomalonitrile (Formula XXI), which is available commercially as the /?-toluenesulfonic acid salt, is reacted with an orthoester to generate an imidate intermediate, which is treated with a primary amine. The orthoester and amine are selected such that they will provide the desired R2 and Ri substituents in a compound of Formula XXII. The reaction can be conveniently carried out by heating a solution of aminomalonitrile /?-toluenesulfonate and the orthoester in a suitable solvent such as THF in the presence of a base such as triethylamine. The solution is allowed to cool to ambient temperature and the primary amine is added. Numerous primary amines suitable for this reaction are commercially available; others can be prepared by known methods. See, for example, the methods in U.S. Patent Nos. 6,451,810 (Coleman et al), 6,660,747 (Crooks et al.), 6,683,088 (Crooks et al.), and 6,656,938 (Crooks et al.); U.S. Patent Application Publication No. 2004/0147543 (Hays et al.); International Patent Application Publication Nos. WO 2005/066169 (Bonk and Dellaria), WO 2006/028545 (Stoermer et al.), WO 2005/051317 (Krepski et al.), WO 2005/076783 (Radmer et al.), and WO 2005/094531 (Krepski et al.); and International Application No. PCT/US2005/021570 (Moser et al.). Certain amines of Formula Ri-NH2 provide a compound of Formula XXII that contain a functional group or protected functional group that can be transformed in a subsequent step in Reaction Scheme II to provide compounds of Formula XII with a variety of different Ri groups. For example, amines of formula RiNH2 may contain a protected functional group, such as a Boc-protected amino group. For example, protected
diamines of Formula BoC-N(Rg)-X-NH2,
, or
are commercially available or can be prepared by known methods; see, for example, U.S. Patent Nos. 6,660,747 (Crooks et al), 6,683,088 (Crooks et al.), and 6,656,938 (Crooks et al.) and Carceller, E. et al., J. Med. Chem., 39, pp.487- 493 (1996). The protecting group may be removed, for example, after step (4) of Reaction Scheme II, and the resultant amino group can be functionalized using one of the methods described in step (2) of Reaction Scheme I to provide a compound of the invention in which Ri is -X-N(Rs)-Qi-R4,
Amines of formula RiNH2 can also contain other protected functional groups, such as ketal-protected ketones. For example, 2,2-dimethyl-3-(2-methyl-l,3-dioxolan-2- yl)propylamine, prepared in Example 22 of International Patent Application Publication Nos. WO2005/051317 (Krepski et al.), can be used, and the ketal protecting group can later be removed by conventional methods to provide a compound of the invention in which Ri is 2,2-dimethyl-4-oxopentyl.
Amino alcohols of formula H2N-X-OH can be used, and the hydroxy functional group can be converted in subsequent steps, for example, after step (4), to a compound of the invention having an -X-S(O)0-2-R* -X-S(O)2-N(Rs)-R4, -X-O-N(Rs)-Qi-R4, -X-O-N=C(R4)-R4, -X-CH(-N(-O-R8)-Qi-R4)-R4 group at the Ri position using methods described in U. S. Patent No. 6,664,264 (Dellaria et al.) and International Patent Application Publication Nos. WO2005/066169 (Bonk and Dellaria), WO2005/018551 (Kshirsagar et al.), WO2005/018556 (Kshirsagar et al.), and WO2005/051324 (Krepski et al.), respectively. The amine of formula RiNH2 may be tert-butyl carbazate, and the resulting Ri group can be manipulated in subsequent steps, for example, after step (4), using the methods of U.S. Patent Application Publication No. 2006/026760 to provide compounds of the invention wherein Ri is
or -N(Ri')-Xi-R5a.
Other transformations at the Ri position can be made. See, for example, U.S. Patent Nos. 5,389,640 (Gerster et al), 6,331,539 (Crooks et al), 6,451,810 (Coleman et al), 6,541,485 (Crooks et al.), 6,660,747 (Crooks et al.), 6,670,372 (Charles et al.), 6,683,088 (Crooks et al.), 6,656,938 (Crooks et al.), 6,664,264 (Dellaria et al.), 6,677,349 (Griesgraber), and 6,664,260 (Charles et al.).
Functional group transformations can also be made at the R2 position in a number of compounds shown in Reaction Scheme II if the orthoester used in step (1) contains a protected hydroxy group, for example. See the methods in, for example, U.S. Patent No. 5,389,640 (Gerster et al.) and International Publication Nos. WO 2005/018551 (Kshirsagar et al.), WO 2005/048945 (Kshirsagar et al.) and WO 2005/048933 (Kshirsagar et al.).
In step (2) of Reaction Scheme II, a compound of Formula XXII is added to a solution of bromoform or diiodomethane and an alkyl nitrite such as isoamyl nitrite or tert-butyi nitrite to yield a compound of Formula XXIII where Hal is Br or I, respectively. The reaction can be carried out at an elevated temperature such as 90 0C, and a co-solvent such as chloroform can be employed.
In step (3) of Reaction Scheme II, an iodo- or bromo-substituted compound of Formula XXIII undergoes a transition-metal catalyzed cross coupling reaction with a reagent of Formula XXIV, in which J4 contains the necessary atoms to provide a compound of Formula XXIV as the following four pyridine isomers of Formula XXVI, XXVII, XXVIII, and XXIX, wherein R and n are as defined above:
XXVI XXVII XXVIII XXlX
Reagents of Formula XXIV are known to undergo coupling reactions, such as Suzuki couplings, Stille couplings, and Negishi couplings, and are either commercially available or can be prepared using known synthetic methods. A compound of Formula XXVI, 3-amino-2-tri-/?-butylstannylpyridine, can be prepared by a halogen-lithium exchange of a 2-bromopyridine that has a protected amino group in the 3-position
followed by reaction with tributyltin chloride and deprotection of the amino group. A reagent of Formula XXVII is commercially available (2,2-dimethyl-iV-[3-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)pyridin-4-yl]propanamide, CB Research and Development, Inc. in New Castle, DE). Compounds of Formula XXVII, XXVIII, and XXIX can be prepared starting from te/t-butylcarbonyl or Boc-protected 4-, 3-, and 2- aminopyridines, which undergo directed ortho metalation in the presence of butyllithium reagents. The resulting organo lithium intermediate can react with electrophiles such as B(O-alkyl)3 and ClSn(alkyl)3 to provide compounds of Formula XXIV, where M is -B(O-alkyl)2 or -B(OH)2 and -Sn(alkyl)3, respectively. The Boc protecting group can be removed with acid to provide a compound of Formula XXIV where Di is hydrogen.
In step (3), a Suzuki coupling reaction can be conveniently carried out by heating a mixture of the compound of Formula XXIII, palladium (II) acetate, triphenylphosphine, and a boron reagent of Formula XXIV, where M is -B(OH)2 or -B(O-alkyl)2, in the presence of a base such as sodium carbonate. The reaction can be carried out in a suitable solvent or solvent mixture such as n-propanol: water and can be heated at an elevated temperature such as 100 0C. The product of the Suzuki coupling is a compound of Formula XXV where J5 contains the necessary atoms to provide the following four isomers of Formula XXX, XXXI, XXXII, and XXXIII, wherein R and n are as defined above.
In step (4) of Reaction Scheme II, a compound of Formula XXV cyclizes to form a lH-imidazo[4,5-c]naphthyridin-4-amine of Formula X. When the Suzuki reaction in step (3) is carried out with a compound of Formula XXIV wherein Di is hydrogen or Boc, the cyclization can be carried out under acidic conditions. The intramolecular cyclization can be conveniently carried out by treating a compound of Formula XXV with a solution of hydrochloric acid in ethanol. The reaction is then heated at reflux to provide a IH-
imidazo[4,5-c]naphthyridin-4-amine of Formula X. Alternatively, a compound of Formula XXV wherein Di is hydrogen may undergo cyclization to the compound of Formula X under the Suzuki coupling reaction conditions in step (3).
When the Suzuki reaction in step (3) is carried out with a compound of Formula XXIV wherein Di is tert-butycarbonyl, the cyclization in step (4) can be carried out under basic conditions. The reaction can be conveniently carried out by heating a compound of Formula XXV with potassium tert-butoxide in a suitable solvent such as ethanol at an elevated temperature such as the reflux temperature of the solvent.
In steps (5) and (6) of Reaction Scheme II, a lH-imidazo[4,5-c]naphthyridin-4- amine of Formula X is transformed into a compound of Formula XII using the methods discussed in steps (1) and (2), respectively, of Reaction Scheme I.
Reaction Scheme II
For some embodiments, compounds of the invention can be prepared according to Reaction Scheme III wherein R, R1, and n are as defined above; Hal, M, and Di are defined as in Scheme II, and P is a protecting group, for example, an alkyl or benzyl group. Compounds of Formula XXIIa can be prepared by reacting aminomalonitrile or a salt thereof with triphosgene and a primary amine of Formula Ri-NH2. The reaction can be conveniently carried out in a suitable solvent such as THF in the presence of
triethylamine or JV,jV-diisopropylethylamine. See, for example, the method described by Hirota, K. et al, Heterocycles, 55, pp. 2279-2282 (2001).
In step (1) of Reaction Scheme III, a 2-hydroxy imidazole of Formula XXIIa is protected as an alkyl or benzyl ether to provide an imidazole of Formula XXIIb. The reaction can be carried out by treating a compound of Formula XXIIa with an alkyl or benzyl halide in the presence of a base such as potassium carbonate in a suitable solvent such as acetone, methanol, or ethanol. The reaction can be carried out at room temperature or at an elevated temperature such as the reflux temperature of the solvent. In steps (2) through (4) of Reaction Scheme III, a compound of Formula XXIIb is converted into a lH-imidazo[4,5-c]- 1 ,6-naphthyridin-4-amine of Formula XIVa using the methods described in steps (2) through (4) of Reaction Scheme II.
In step (5) of Reaction Scheme III, a lH-imidazo[4,5-c]-l,6-naphthyridin-4-amine of Formula XIVa is converted to a 4-amino-lH-imidazo[4,5-c]-l,6-naphthyridin-2-ol of Formula XIVb using conventional dealkylation methods. For example, a dealkylation reaction can be carried out by treating a compound of Formula XIVa wherein P is a
Ci_4 alkyl group with boron tribromide in a suitable solvent such as dichloromethane at room temperature or a sub-ambient temperature such as -78 0C. A compound of Formula XIVa wherein P is benzyl can be deprotected by hetereogeneous hydrogenation or by treatment with trifluoroacetic acid using conditions known to one of skill in the art. In steps (6) and (7), a compound of Formula XIVb can be converted into a compound of the invention of Formula lib using the methods described in steps (1) and (2) of Reaction Scheme I. Other naphthyridine isomers can be formed if pyridine isomers of Formula XXVI, XXVIII, or XXIX are used instead of the pyridine of Formula XXVII in step (3) of Reaction Scheme III.
Reaction Scheme III
XVlIa Nb
For certain embodiments, compounds of the invention can be prepared according to Reaction Scheme IV, wherein R1, R2, G, and J3 are as defined above, and J6 contains the necessary atoms to provide a compound of Formula XXV as the compounds of the invention of Formula V, VI, VII, and VIII. Compounds of Formula XII can be prepared according to the methods described above in Reaction Schemes I, II, or III. The 4-amino group of a compound of a 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]naphthyridin-4-amine of Formula XII can be converted using conventional methods to a functional group such as an amide, carbamate, urea, amidine, or another hydrolyzable group by the replacement of a hydrogen atom in an amino group with a group such as -C(O)-R, α-aminoacyl, α-aminoacyl-α-aminoacyl, -C(O)-O-R, -C(0)-N(R")-R, -C(=NY')-R, -CH(OH)-C(O)-OY', -CH(OCL4 alkyl)Y0, -CH2Y2, or -CH(CH3)Y2; wherein R and R" are each independently C1-10 alkyl, C3_7 cycloalkyl, phenyl, benzyl, or 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_6 alkyl, Ci_4 alkoxy, aryl, heteroaryl, arylCi_4 alkylenyl, heteroarylCi_4 alkylenyl, haloCi.4 alkylenyl, haloCM alkoxy, -0-C(O)-CH3, -C(O)-O-CH3, -C(O)-NH2,
-0-CH2-C(O)-NH2, -NH2, and -S(O)2-NH2; with the proviso that R" may also be hydrogen; each α-aminoacyl group is independently selected from racemic, D, or L-amino acids; Y' is hydrogen, Ci_6 alkyl, or benzyl; Y0 is Ci_6 alkyl, carboxyCi_6 alkylenyl, aminoCi_4 alkylenyl, mono-N-Ci_6 alkylaminoCi_4 alkylenyl, or alkylaminoCi_4 alkylenyl; and Y2 is mono-N-Ci_6 alkylamino,
alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, or 4-Ci_4 alkylpiperazin-1-yl. Particularly useful compounds of Formula XXXIV are amides derived from carboxylic acids containing one to ten carbon atoms, amides derived from amino acids, and carbamates containing one to ten carbon atoms. The reaction can be carried out, for example, by combining a compound of Formula XII with a chloro formate or acid chloride, such as ethyl chloroformate or acetyl chloride, in the presence of a base such as triethylamine in a suitable solvent such as dichloromethane at room temperature.
Reaction Scheme IV
XIl XXXIV
Other prodrugs can be prepared in a variety of ways. For example, a compound wherein Ri or R2 is hydroxyalkyl can be converted into a prodrug wherein Ri or R2 is, for example, -alkylenyl-O-C(Rδ)-R4, -alkylenyl-O-C(Rδ)-O-R4, or -alkylenyl-O-C(R6)-N(Rg)-R4, wherein R4, R6, and Rg are as defined above, using methods known to one skilled in the art. In addition, a compound wherein R is hydroxy or a compound of Formula lib or its positional isomers may also be converted to an ester, an ether, a carbonate, or a carbamate. For any of these compounds containing an alcohol functional group, a prodrug can be formed by the replacement of the hydrogen atom of the alcohol group with a group such as Ci_6 alkanoyloxymethyl, 1-(C1-6 alkanoyloxy)ethyl,
1 -methyl- 1 -(C i_6 alkanoyloxy)ethyl, Ci_6 alkoxycarbonyloxymethyl, N-(C1-6 alkoxycarbonyl)aminomethyl, succinoyl, Ci_6 alkanoyl, α-aminoCi_4 alkanoyl, arylacyl, -P(O)(OH)2, -P(O)(O-C i_6 alkyl)2, C1-6 alkoxycarbonyl, Ci_6 alkylcarbamoyl, and
α-aminoacyl or α-aminoacyl-α-aminoacyl, where each α-aminoacyl group is independently selected from the naturally occurring racemic, D-, and L-amino acids. For compounds containing an alcohol functional group, particularly useful prodrugs are esters made from carboxylic acids containing one to six carbon atoms, unsubstituted or substituted benzoic acid esters, or esters made from naturally occurring L-amino acids.
These esters can be prepared by heating a compound of Formula lib, for example, with a carboxylic acid optionally in the presence of a base. The esterification reaction can be carried out in a suitable solvent such as methanol or ethanol. Alternatively, for compounds wherein Ri or R2 is hydroxyalkyl, the reaction can be carried out by treating the compound with a carboxylic acid or amino acid under Mitsunobu reaction conditions in the presence of triphenylphosphine and diisopropyl azodicarboxylate in a suitable solvent such as THF. The reaction can be run at a sub-ambient temperature such as 0 0C. Compounds of the invention can also be prepared using variations of the synthetic route shown in Reaction Schemes I through IV that would be apparent to one of skill in the art, including variations described in the EXAMPLES below.
Pharmaceutical Compositions and Biological Activity
Pharmaceutical compositions of the invention contain a therapeutically effective amount of a compound or salt described above in combination with a pharmaceutically acceptable carrier.
The terms "a therapeutically effective amount" and "effective amount" mean an amount of the compound or salt sufficient to induce a therapeutic or prophylactic effect, such as cytokine induction, immunomodulation, antitumor activity, and/or antiviral activity. The exact amount of compound or salt used in a pharmaceutical composition of the invention will vary according to factors known to those of skill in the art, such as the physical and chemical nature of the compound or salt, the nature of the carrier, and the intended dosing regimen.
In some embodiments, the compositions of the invention will contain sufficient active ingredient or prodrug to provide a dose of about 100 nanograms per kilogram (ng/kg) to about 50 milligrams per kilogram (mg/kg), preferably about 10 micrograms per kilogram (μg/kg) to about 5 mg/kg, of the compound or salt to the subject.
In other embodiments, the compositions of the invention will contain sufficient active ingredient or prodrug to provide a dose of, for example, from about 0.01 mg/m2 to about 5.0 mg/m2, computed according to the Dubois method, in which the body surface area of a subject (m2) is computed using the subject's body weight: m2 = (wt kg0 425 x height cm0'725) x 0.007184, although in some embodiments the methods may be performed by administering a compound or salt or composition in a dose outside this range. In some of these embodiments, the method includes administering sufficient compound to provide a dose of from about 0.1 mg/m2 to about 2.0 mg/ m2 to the subject, for example, a dose of from about 0.4 mg/m2 to about 1.2 mg/m2. A variety of dosage forms may be used, such as tablets, lozenges, capsules, parenteral formulations, syrups, creams, ointments, aerosol formulations, transdermal patches, transmucosal patches and the like. These dosage forms can be prepared with conventional pharmaceutically acceptable carriers and additives using conventional methods, which generally include the step of bringing the active ingredient into association with the carrier.
The compounds or salts of the invention can be administered as the single therapeutic agent in the treatment regimen, or the compounds or salts described herein may be administered in combination with one another or with other active agents, including additional immune response modifiers, antivirals, antibiotics, antibodies, proteins, peptides, oligonucleotides, etc.
Compounds or salts of the invention have been shown to induce the production of certain cytokines in experiments performed according to the tests set forth below. These results indicate that the compounds or salts are useful for modulating the immune response in a number of different ways, rendering them useful in the treatment of a variety of disorders.
Cytokines whose production may be induced by the administration of compounds or salts of the invention generally include interferon-α (IFN-α) and tumor necrosis factor-α (TNF-α) as well as certain interleukins (IL). Cytokines whose biosynthesis may be induced by compounds or salts of the invention include IFN-α, TNF-α, IL-I, IL-6, IL-10 and IL-12, and a variety of other cytokines. Among other effects, these and other cytokines can inhibit virus production and tumor cell growth, making the compounds or salts useful in the treatment of viral diseases and neoplastic diseases. Accordingly, the
invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of the invention to the animal. The animal to which the compound or salt is administered for induction of cytokine biosynthesis may have a disease as described infra, for example a viral disease or a neoplastic disease, and administration of the compound or salt may provide therapeutic treatment. Alternatively, the compound or salt may be administered to the animal prior to the animal acquiring the disease so that administration of the compound or salt may provide a prophylactic treatment.
In addition to the ability to induce the production of cytokines, compounds or salts described herein can affect other aspects of the innate immune response. For example, natural killer cell activity may be stimulated, an effect that may be due to cytokine induction. The compounds or salts may also activate macrophages, which in turn stimulate secretion of nitric oxide and the production of additional cytokines. Further, the compounds or salts may cause proliferation and differentiation of B-lymphocytes. Compounds or salts described herein can also have an effect on the acquired immune response. For example, the production of the T helper type 1 (TH1) cytokine IFN- γ may be induced indirectly and the production of the T helper type 2 (TR2) cytokines IL- 4, IL-5 and IL-13 may be inhibited upon administration of the compounds or salts.
Whether for prophylaxis or therapeutic treatment of a disease, and whether for effecting innate or acquired immunity, the compound or salt or composition may be administered alone or in combination with one or more active components as in, for example, a vaccine adjuvant. When administered with other components, the compound or salt or composition and other component or components may be administered separately; together but independently such as in a solution; or together and associated with one another such as (a) covalently linked or (b) non-covalently associated, e.g., in a colloidal suspension.
Conditions for which compounds or salts or compositions identified herein may be used as treatments include, but are not limited to:
(a) viral diseases such as, for example, diseases resulting from infection by an adenovirus, a herpesvirus (e.g., HSV-I, HSV-II, CMV, or VZV), a poxvirus (e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum), a picornavirus (e.g., rhinovirus or enterovirus), an orthomyxovirus (e.g., influenzavirus), a paramyxovirus
(e.g., parainfluenzavirus, mumps virus, measles virus, and respiratory syncytial virus (RSV)), a coronavirus (e.g., SARS), a papovavirus (e.g., papillomaviruses, such as those that cause genital warts, common warts, or plantar warts), a hepadnavirus (e.g., hepatitis B virus), a flavivirus (e.g., hepatitis C virus or Dengue virus), or a retrovirus (e.g., a lenti virus such as HIV);
(b) bacterial diseases such as, for example, diseases resulting from infection by bacteria of, for example, the genus Escherichia, Enterobacter, Salmonella, Staphylococcus, Shigella, Listeria, Aerobacter, Helicobacter, Klebsiella, Proteus, Pseudomonas, Streptococcus, Chlamydia, Mycoplasma, Pneumococcus, Neisseria, Clostridium, Bacillus, Corynebacterium, Mycobacterium, Campylobacter, Vibrio, Serratia, Providencia, Chromobacterium, Brucella, Yersinia, Haemophilus, or Bordetella;
(c) other infectious diseases, such as chlamydia, fungal diseases including but not limited to candidiasis, aspergillosis, histoplasmosis, cryptococcal meningitis, or parasitic diseases including but not limited to malaria, Pneumocystis carnii pneumonia, leishmaniasis, cryptosporidiosis, toxoplasmosis, and trypanosome infection;
(d) neoplastic diseases, such as intraepithelial neoplasias, cervical dysplasia, actinic keratosis, basal cell carcinoma, squamous cell carcinoma, renal cell carcinoma, Kaposi's sarcoma, melanoma, leukemias including but not limited to acute myeloid leukemia, acute lymphocytic leukemia, chronic myeloid leukemia, chronic lymphocytic leukemia, multiple myeloma, Hodgkin's lymphoma, non-Hodgkin's lymphoma, cutaneous T-cell lymphoma, B-cell lymphoma, and hairy cell leukemia, and other cancers;
(e) TH2 -mediated, atopic diseases, such as atopic dermatitis or eczema, eosinophilia, asthma, allergy, allergic rhinitis, and Ommen's syndrome;
(f) certain autoimmune diseases such as systemic lupus erythematosus, essential thrombocythaemia, multiple sclerosis, discoid lupus, alopecia areata; and
(g) diseases associated with wound repair such as, for example, inhibition of keloid formation and other types of scarring (e.g., enhancing wound healing, including chronic wounds).
Additionally, a compound or salt identified herein may be useful as a vaccine adjuvant for use in conjunction with any material that raises either humoral and/or cell mediated immune response, such as, for example, live viral, bacterial, or parasitic immunogens; inactivated viral, tumor-derived, protozoal, organism-derived, fungal, or
bacterial immunogens; toxoids; toxins; self-antigens; polysaccharides; proteins; glycoproteins; peptides; cellular vaccines; DNA vaccines; autologous vaccines; recombinant proteins; and the like, for use in connection with, for example, BCG, cholera, plague, typhoid, hepatitis A, hepatitis B, hepatitis C, influenza A, influenza B, parainfluenza, polio, rabies, measles, mumps, rubella, yellow fever, tetanus, diphtheria, hemophilus influenza b, tuberculosis, meningococcal and pneumococcal vaccines, adenovirus, HIV, chicken pox, cytomegalovirus, dengue, feline leukemia, fowl plague, HSV-I and HSV-2, hog cholera, Japanese encephalitis, respiratory syncytial virus, rotavirus, papilloma virus, yellow fever, and Alzheimer's Disease. Compounds or salts identified herein may be particularly helpful in individuals having compromised immune function. For example, compounds or salts may be used for treating the opportunistic infections and tumors that occur after suppression of cell mediated immunity in, for example, transplant patients, cancer patients and HIV patients. Thus, one or more of the above diseases or types of diseases, for example, a viral disease or a neoplastic disease may be treated in an animal in need thereof (having the disease) by administering a therapeutically effective amount of a compound or salt of the invention to the animal.
An animal may also be vaccinated by administering an effective amount of a compound or salt described herein, as a vaccine adjuvant. In one embodiment, there is provided a method of vaccinating an animal comprising administering an effective amount of a compound or salt described herein to the animal as a vaccine adjuvant.
An amount of a compound or salt effective to induce cytokine biosynthesis is an amount sufficient to cause one or more cell types, such as monocytes, macrophages, dendritic cells and B-cells to produce an amount of one or more cytokines such as, for example, IFN-α, TNF-α, IL-I, IL-6, IL-IO and IL- 12 that is increased (induced) over a background level of such cytokines. The precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 μg/kg to about 5 mg/kg. In other embodiments, the amount is expected to be a dose of, for example, from about 0.01 mg/m2 to about 5.0 mg/m2, (computed according to the Dubois method as described above) although in some embodiments the induction or inhibition of cytokine biosynthesis may be performed by administering a compound or salt in a dose outside this range. In some of these
embodiments, the method includes administering sufficient compound or salt or composition to provide a dose of from about 0.1 mg/m2 to about 2.0 mg/ m2 to the subject, for example, a dose of from about 0.4 mg/m2 to about 1.2 mg/m2.
The invention also provides a method of treating a viral infection in an animal and a method of treating a neoplastic disease in an animal comprising administering an effective amount of a compound or salt of the invention to the animal. An amount effective to treat or inhibit a viral infection is an amount that will cause a reduction in one or more of the manifestations of viral infection, such as viral lesions, viral load, rate of virus production, and mortality as compared to untreated control animals. The precise amount that is effective for such treatment will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 μg/kg to about 5 mg/kg. An amount of a compound or salt effective to treat a neoplastic condition is an amount that will cause a reduction in tumor size or in the number of tumor foci. Again, the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 μg/kg to about 5 mg/kg. In other embodiments, the amount is expected to be a dose of, for example, from about 0.01 mg/m2 to about 5.0 mg/m2, (computed according to the Dubois method as described above) although in some embodiments either of these methods may be performed by administering a compound or salt in a dose outside this range. In some of these embodiments, the method includes administering sufficient compound or salt to provide a dose of from about 0.1 mg/m2 to about 2.0 mg/ m2 to the subject, for example, a dose of from about 0.4 mg/m2 to about 1.2 mg/m2.
In addition to the formulations and uses described specifically herein, other formulations, uses, and administration devices suitable for compounds of the present invention are described in, for example, International Publication Nos. WO 03/077944 and
WO 02/036592, U.S. Patent No. 6,245,776, and U.S. Publication Nos. 2003/0139364, 2003/185835, 2004/0258698, 2004/0265351, 2004/076633, and 2005/0009858.
Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention.
EXAMPLES
Preparation of 5-Iodo-l -(2 -methylpropyl)-2-propyl-lH-imidazole-4-carbonitrile Part A
Aminomalononitrile/?-toluenesulfonate (744.65 g, 2.94 mol), triethylamine (297.5 g, 2.94 mol), and trimethyl orthobutyrate (436 g, 2.94 mol) were combined in toluene
(7.45 L) and the solution was heated at 60 0C for 2.5 hours. Additional trimethyl orthobutyrate (21 g) was added, and the reaction was allowed to cool over a period of 30 minutes, and triethylamine (297.5 g, 2.94 mol) was added. The solution was further cooled to about 3 0C over 30 minutes, and isobutylamine (215.03 g, 2.94 mol) was added over a period of 90 minutes. During the addition, the reaction temperature rose to 10 0C. The reaction was warmed to room temperature slowly and stirred overnight. Deionized water (7.35 L) and sodium carbonate (735 g) were added over a period of 15 minutes while maintaining the temperature of the mixture at 20 0C with the addition of ice. The mixture was stirred for 15 minutes, and then the precipitate was isolated by filtration, washed sequentially with toluene (300 mL) and water (3 L), air-dried, and further dried under vacuum at 70 0C for 24 hours to provide 394.4 g (65%) of 5 -amino- 1 -(2- methylpropyl)-2-propyl-lH-imidazole-4-carbonitrile as a beige solid, mp 134.5-136.5 0C. Part B
A solution of isoamylnitrite (106.3 g, 0.908 mol) and diiodomethane (1.296 kg, 4.84 mol) in chloroform (500 mL) was heated at 70 0C under a nitrogen atmosphere. A solution of 5-amino-l-(2-methylpropyl)-2-propyl-lH-imidazole-4-carbonitrile (50.0 g, 0.242 mol) in chloroform (500 mL) was heated to 70 0C and added over a period of 30 minutes while maintaining the reaction temperature at 68 0C to 70 0C during the addition. The reaction was heated for an additional 10 minutes, allowed to cool to room temperature, and concentrated under reduced pressure at 75 0C. The residue was purified by column chromatography on silica gel (eluting sequentially with dichloromethane, 1% methanol in dichloromethane, 2% methanol in dichloromethane, and 3% methanol in dichloromethane) to provide 39.4 g (51.3%) of 5-iodo-l-(2-methylpropyl)-2-propyl-lH- imidazole-4-carbonitrile as a tan oil that solidified upon standing.
Example 1
l-(2-Methylpropyl)-8-[2-(methylsulfonyl)ethyl]-2-propyl-6,7,8,9-tetrahydro-lH- imidazo [4 , 5 -c] - 1 , 6-naphthyridin-4-amine
Part A A 500 rnL two-necked round bottom flask equipped with nitrogen inlet adaptor and magnetic stirring bar was charged with tert-butyi pyridin-4-ylcarbamate (3.88 g, 20.0 mmol, prepared as described in Spivey, A. C. et al. J. Org. Chem. 64, pp. 9430-9443 (1999)), ΛWΛ^-tetramethylethylenediamine (TMEDA) (8.15 mL, 54.0 mmol), and anhydrous tetrahydrofuran (THF) (10 mL). The resultant clear, colorless solution was degassed with several cycles of evacuation under reduced pressure followed by a nitrogen purge, then cooled to -78 0C with a dry ice/acetone bath. A solution of n-butyllithium in hexanes (20.0 mL of a 2.5 M solution, 50.0 mmol) was added via syringe under nitrogen atmosphere over 15 minutes (min), resulting in the formation of a thick tan slurry. After 10 min, the cooling bath was replaced with a 5 0C ice bath, and the slurry was allowed to stir for 2 hours. The slurry was then cooled again to -78 0C, and triisopropylborate (17.5 mL, 76.0 mmol) was added dropwise via syringe over 10 min. The reaction mixture was then warmed to ambient temperature and allowed to stir for 20 min. The reaction mixture initially became clear, and then a white precipitate formed. The resultant slurry was then poured into saturated aqueous ammonium choride (100 mL) and allowed to stir vigorously for an additional 20 min. The white precipitate was isolated by filtration, washed sequentially with water (100 mL) and diethyl ether (100 mL), and then dried overnight under reduced pressure, providing 3.10 g of 4-[(tert-butoxycarbonyl)amino]pyridin-3- ylboronic acid as a white solid. Part B A 100 mL round bottom flask was charged with 4-[(tert- butoxycarbonyl)amino]pyridin-3-ylboronic acid (2.38 g, 10.0 mmol) and ethanol (10 mL). To the clear, colorless solution was added a solution of hydrochloric acid in 1,4-dioxane
(10 niL of a 4.0 M solution, 40.0 mmol). Upon complete addition, the reaction mixture became warm, and a white precipitate formed. Additional ethanol (about 5 mL) was added until the reaction mixture became homogeneous. The flask was then fitted with a reflux condenser, and the solution was heated in a 90 0C oil bath. After 1 hour, the flask was allowed to cool to ambient temperature, and most of the solvent was removed under reduced pressure. The remaining viscous oil was poured into diethyl ether (100 mL), using a small amount of methanol to assist in the transfer. A white precipitate formed immediately, and the resultant slurry was allowed to stir vigorously for 10 min. The precipitate was isolated by filtration and washed with an additional portion of diethyl ether (50 mL). The collected solid was then dried under reduced pressure overnight, providing 1.74 g of 4-aminopyridin-3-ylboronic acid hydrochloride as a white solid. Part C
A 500 mL round bottom flask was charged with 5-iodo-l-(2-methylpropyl)-2- propyl-lH-imidazole-4-carbonitrile (7.26 g, 22.9 mmol), 4-aminopyridin-3-ylboronic acid hydrochloride (4.40 g, 25.2 mmol), and n-propanol (150 mL). A solution of sodium carbonate (6.55 g, 3.44 mmol) in water (60 mL) was added, and nitrogen was bubbled through the resultant solution for 15 min. Palladium (II) acetate (258 mg, 1.15 mmol) and triphenylphosphine (902 mg, 3.44 mmol) were added, and the solution was further degassed using several cycles of evacuation under reduced pressure followed by a nitrogen purge. The solution was then heated at reflux under nitrogen atmosphere for 18 hours. After cooling to room temperature, the reaction mixture was partitioned between ethyl acetate and saturated aqueous sodium bicarbonate, and the aqueous layer was extracted with additional portions of ethyl acetate. The combined organic layers were then washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via flash chromatography on silica gel (eluting with 7% methanol in dichloromethane) to afford 1.50 g of l-(2-methylpropyl)-2-propyl-lH- imidazo[4,5-c]-l,6-naphthyridin-4-amine as a tan solid. Recrystallization from acetonitrile provided an analytically pure sample as white needles, mp 253-255 0C. MS (ESI) m/z 284 (M + H)+; Anal. Calcd for Ci6H2iN5: C, 66.76; H, 7.53; N, 24.33. Found: C, 66.96; H, 7.44; N, 23.98. Part D
A mixture of l-(2-methylpropyl)-2-propyl-lH-imidazo[4,5-c]-l,6-naphthyridin-4- amine (1.50 g, 5.29 mmol), platinum oxide (1.0 g), and trifluoroacetic acid (20 mL) was placed under 50 psi (3.5 x 105 Pa) of hydrogen pressure on a Parr apparatus. After 14 hours, the mixture was diluted with dichloromethane (200 mL) and filtered through a pad of CELITE filter agent. The filtrate was concentrated under reduced pressure, and the residue was then dissolved in dichloromethane (100 mL) and water (100 mL). An aqueous 1 M sodium hydroxide solution was then added until a pΗ of 11-12 was reached. The organic layer was drawn off, and the aqueous layer was extracted with additional portions of dichloromethane (3 x 100 mL). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to provide a tan solid. Crystallization from a mixture of acetonitrile and dichloromethane afforded 1.40 g of l-(2-methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine as white needles. Part E To a solution of l-(2-methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5- c]-l,6-naphthyridin-4-amine (300 mg, 0.75 mmol) in TΗF (15 mL) was added triethylamine (0.37 mL, 2.6 mmol) and methyl vinyl sulfone (70 μL, 0.82 mmol). The resultant slurry was allowed to stir for several hours at ambient temperature, after which time analysis by high pressure liquid chromatography (ΗPLC) indicated only partial conversion to the desired product. Additional triethylamine (0.37 mL, 2.6 mmol) was added, and the reaction mixture was heated at reflux for 14 hours. The reaction mixture was then partitioned between saturated aqueous sodium bicarbonate and dichloromethane, and the aqueous layer was extracted with additional portions of dichloromethane. The combined organic layers were dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via flash chromatography on silica gel (gradient elution with 5-10% methanol in dichloromethane) to afford 200 mg of l-(2- methylpropyl)-8-[2-(methylsulfonyl)ethyl]-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5- c]-l,6-naphthyridin-4-amine as a yellow solid, mp 82-84 0C. 1U NMR (300 MHz, CDCl3) δ 5.21 (br s, 2H), 3.96 (s, 2H), 3.91 (d, J= 7.5 Hz, 2H), 3.25 (t, J= 6.0 Hz, 2H), 3.12 (t, J = 6.0 Hz, 2H), 3.04 (s, 3H), 2.95 (t, J= 5.4 Hz, 2H), 2.87 (t, J= 5.4 Hz, 2H), 2.77 (t, J = 7.5 Hz, 2H), 2.02 (m, IH), 1.87 (m, 2H), 1.04 (t, J= 7.4 Hz, 3H), 0.94 (d, J= 6.7 Hz, 6H);
MS (APCI) m/z 394 (M + H)+; Anal, calcd for C19H31N5O2S-O-S H2O: C, 56.69; H, 8.01; N, 17.40. Found: C, 56.61; H, 8.34; N, 17.38.
Example 2 l-(2-Methylpropyl)-8-(methylsulfonyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-
1 ,6-naphthyridin-4-amine
l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (940 mg, 2.34 mmol, prepared as described in Part D of Example 1) was dissolved in dichloromethane (20 mL). Triethylamine (1.14 mL, 8.19 mmol) was added via syringe. The resultant solution was cooled in an ice bath, and methanesulfonyl chloride (0.18 mL, 2.34 mmol) was added via syringe. After 3 hours, the reaction solution was washed with saturated aqueous sodium bicarbonate (20 mL), dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via flash chromatography on silica gel (gradient elution with 3-8% methanol in dichloromethane) to afford 500 mg of l-(2-methylpropyl)-8-(methylsulfonyl)-2-propyl- 6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6-naphthyridin-4-amine as a white solid, mp 271- 272 0C (dec). 1H NMR (300 MHz, CDCl3) δ 5.03 (br s, 2H), 4.73 (s, 2H), 3.92 (d, J= 7.5 Hz, 2H), 3.67 (t, J= 6.1 Hz, 2H), 3.03 (t, J= 6.1 Hz, 2H), 2.87 (s, 3H), 2.78 (t, J= 7.6 Hz, 2H), 2.02 (pent, J= 6.8 Hz, IH), 1.87 (m, 2H), 1.05 (t, J= 7.4 Hz, 3H), 0.97 (d, J= 6.8
Hz, 6H); MS (APCI) m/z 366 (M + H)+; Anal, calcd for Ci7H27N5O2S: C, 55.87; H, 7.45; N, 19.16. Found: C, 55.65; H, 7.65; N, 19.09.
Example 3 Ethyl 3-[4-amino- 1 -(2-methylpropyl)-2-propyl- 1 ,6,7,9-tetrahydro-8H-imidazo[4,5-c]- 1 ,6- naphthyridin-8-yl]propanoate
l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (650 mg, 2.26 mmol, prepared as described in Part D of Example 1) was dissolved in TΗF (20 mL). Triethylamine (0.95 rnL, 6.78 mmol) and ethyl acrylate (0.27 mL, 2.49 mmol) were added via syringe. The resultant solution was allowed to stir overnight at ambient temperature. The solution was then partitioned between dichloromethane and saturated aqueous sodium bicarbonate, and the aqueous layer was extracted with additional portions of dichloromethane. The combined organic layers were washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via flash chromatography on silica gel (gradient elution with 25-50% ethyl acetate in hexanes). Further purification was accomplished by trituration with diethyl ether to afford 300 mg of ethyl 3-(4-amino-l-(2-methylpropyl)-2- propyl-l,6,7,9-tetrahydro-8H-imidazo[4,5-c]-l,6-naphthyridin-8-yl)propanoate as a yellow solid, mp 109-110 0C. 1H NMR (300 MHz, CDCl3) δ 5.97 (br s, 2H), 4.08 (q, J = 7.1 Hz, 2H), 3.85 (d, J= 7.6 Hz, 2H), 3.81 (s, 2H), 2.94 (m, 2H), 2.86 (t, J= 7.2 Hz, 2H),
2.78-2.68 (m, 4H), 2.53 (t, J= 7.0 Hz, 2H), 1.98 (hextet, J= 7.0 Hz, IH), 1.81 (hextet, J = 7.6 Hz, 2H), 1.19 (t, J= 7.1 Hz, 3H), 0.98 (t, J= 7.4 Hz, 3H), 0.89 (d, J= 7.0 Hz, 6H); MS (APCI) m/z 388 (M + H)+; Anal, calcd for C2iH33N5O2'0.3 H2O: C, 64.19; H, 8.62; N, 17.82. Found: C, 63.92; H, 8.38; N, 18.19.
Example 4
4-Amino-l-(2-methylpropyl)-Λ/-phenyl-2-propyl-l,6,7,9-tetrahydro-8H-imidazo[4,5-c]-
1 ,6-naphthyridine-8-carboxamide
l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (500 mg, 1.74 mmol, prepared as described in Part D of Example 1) was dissolved in dichloromethane (15 rnL). Triethylamine (0.73 rnL, 5.22 mmol) and phenyl isocyanate (0.21 mL, 1.91 mmol) were added. The resultant solution was allowed to stir overnight at ambient temperature. The reaction solution was then partitioned between dichloromethane and saturated aqueous sodium bicarbonate, and the aqueous layer was extracted with additional portions of dichloromethane. The combined organic layers were washed with brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was crystallized from acetonitrile to afford 500 mg of 4-amino-l-(2-methylpropyl)-N-phenyl-2-propyl-l,6,7,9-tetrahydro-8H-imidazo[4,5-c]- l,6-naphthyridine-8-carboxamide as a white solid, mp 206-207 0C. 1H NMR (300 MHz, CDCl3) δ 7.19-7.07 (m, 4H), 6.85 (t, J= 7.3 Hz, IH), 6.39 (br s, IH), 4.92 (br s, 2H), 4.75 (s, 2H), 3.79 (d, J= 7.5 Hz, 2H), 3.55 (t, J= 6.1 Hz, 2H), 2.84 (t, J= 6.1 Hz, 2H), 2.58 (t, J= 7.5 Hz, 2H), 1.93-1.84 (m, 3H), 1.67 (hextet, J= 7.6 Hz, 2H), 0.85 (t, J= 7.4 Hz, 3H), 0.77 (d, J= 7.0 Hz, 6H); MS (APCI) m/z 407 (M + H)+; Anal, calcd for C23H30N6OO^ H2O: C, 67.06; H, 7.49; N, 20.40. Found: C, 66.72; H, 7.48; N, 20.24.
Example 5 l-(2-Methylpropyl)-7-(methylsulfonyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-
1 ,7-naphthyridin-4-amine
A 250 rnL round bottom flask equipped with magnetic stirring bar was charged with Λ/5-(2-methylpropyl)tetraazolo[l,5-α]-l,7-naphthyridine-4,5-diamine (4.10 g, 16.0 mmol, Example 20 in U. S. Patent No. 6,194,425) and acetonitrile (80 mL). Butyryl chloride (1.80 mL, 17.6 mmol) was added via syringe, and the reaction solution was allowed to stir for 22 hours at ambient temperature. The solids were collected by filtration and washed with acetonitrile. The orange solid was utilized in the following step without further purification. Part B A 250 mL round bottom flask equipped with magnetic stirring bar was charged with the material from Part A (4.60 g, 12.6 mmol) and ethanol (18 mL, reagent grade). A solution of potassium carbonate (2.6O g, 19.0 mmol) in water (7 mL) was added, and the resultant reaction mixture was heated at reflux for 3 hours. The reaction mixture was then concentrated under reduced pressure, and the residue was partitioned between dichloromethane (50 mL) and water (30 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to afford the crude product. Purification by flash chromatography on silica gel (elution with 5% methanol in dichloromethane) provided 1.70 g of 6-(2-methylpropyl)-5-propyl-6H-imidazo[4,5- c]tetraazolo[l,5-α]-l,7-naphthyridine as an off-white solid. Part C
A 500 mL round bottom flask equipped with magnetic stirring bar was charged with 6-(2-methylpropyl)-5-propyl-6H-imidazo[4,5-c]tetraazolo[l,5-α]-l,7-naphthyridine (1.50 g, 4.85 mmol) and 1 ,2-dichlorobenzene (24 mL). Triphenylphosphine (3.10 g, 11.6 mmol) was added all at once, and the resultant reaction mixture was heated at reflux for 3 hours. After cooling to ambient temperature, the mixture was diluted with hexanes (50 mL). The resultant suspension was allowed to stir overnight, and the solid was then collected by filtration and was washed with hexanes. The solid was suspended in methanol (24 mL) and 1 M aqueous hydrochloric acid (24 mL) was added. The resultant solution was heated at reflux for 3 hours, then concentrated under reduced pressure. A 50% aqueous sodium hydroxide solution was then added until a basic pΗ was reached.
The solid that precipitated was collected by filtration and washing with water. Purification of this material by flash chromatography on silica gel (elution with 5% methanol in
dichloromethane) provided 1.0 g of l-(2-methylpropyl)-2-propyl-lH-imidazo[4,5-c]-l,7- naphthyridin-4-amine as a white solid. Part D
A mixture of l-(2-methylpropyl)-2-propyl-lH-imidazo[4,5-c]-l,7-naphthyridin-4- amine (1.00 g, 3.53 mmol), platinum oxide (0.5 g), and trifluoroacetic acid (18 mL) was placed under 50 psi (3.5 x 105 Pa) of hydrogen pressure on a Parr apparatus. After 44 hours, the mixture was filtered through a pad of CELITE filter agent, washing with dichloromethane (100 mL). The filtrate was concentrated under reduced pressure, and the residue was dissolved in water (20 mL). The solution was adjusted to pΗ 12-13 with 50% w/v aqueous sodium hydroxide solution. The product, l-(2-methylpropyl)-2-propyl-
6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,7-naphthyridin-4-amine, was isolated by filtration, washed with water, dried, and used in the following step without further purification. Part E l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,7- naphthyridin-4-amine (500 mg, 1.74 mmol) was dissolved in dichloromethane (7 mL). Triethylamine (0.40 mL, 2.6 mmol) was added, followed by methanesulfonyl chloride (0.14 mL, 1.8 mmol). The resultant solution was allowed to stir at ambient temperature for 1 hour. The reaction mixture was then concentrated under reduced pressure to afford a white solid that was purified by flash chromatography on silica gel (elution with 5% methanol in dichloromethane). The product was then slurried in water (20 mL), and potassium carbonate was added until pΗ 10 was reached. The solid was isolated by filtration and dried under house vacuum at 65 0C for 18 hours to provide 0.40 g of l-(2- methylpropyl)-7-(methylsulfonyl)-2-propyl-6,7,8,9-tetrahydro- lH-imidazo[4,5-c]- 1 ,7- naphthyridin-4-amine as a white solid, mp 205-209 0C. 1H NMR (300 MHz, DMSO-J6) δ 5.74 (bs, 2H), 4.18 (s, 2H), 4.13 (d, J= 7.5 Hz, 2H ), 3.45 (t, J= 5.6 Hz, 2H), 2.94 (s, 3H),
2.92-2.91 (m, 2H), 2.79 (t, J= 7.5 Hz, 2H), 2.06 (pentet, J= 6.9 Hz, IH), 1.79 (sextet, J = 7.5 Hz, 2H), 0.98 (t, J= 7.5 Hz, 3H), 0.80 (t, J= 6.9 Hz, 6H); MS (APCI) m/z 366 (M + H)+; Anal, calcd for Ci7H27N5O2S: C, 55.87; H, 7.45: N, 19.16. Found: C, 55.65; H, 7.68; N, 19.14.
Example 6 l-(2-Methylpropyl)-7-[2-(methylsulfonyl)ethyl]-2-propyl-6,7,8,9-tetrahydro-lH- imidazo [4 , 5 -c] - 1 , 7-naphthyridin-4-amine
l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,7- naphthyridin-4-amine (450 mg, 1.57 mmol, prepared in step F of Example 5) was dissolved in TΗF (6 mL). Methyl vinyl sulfone (180 mg, 1.72 mmol) was added via syringe to the solution. The reaction mixture was heated at reflux for 1.5 hours, and then was concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (eluted with 10% methanol in dichloromethane) to afford a white solid that was crystallized from ethyl acetate to give 350 mg of l-(2-methylpropyl)- 7-[2-(methylsulfonyl)ethyl]-2-propyl-6,7,8,9-tetrahydro- lH-imidazo[4,5-c]- 1 ,7- naphthyridin-4-amine as white crystals, mp 169-170 0C. 1H NMR (300 MHz, DMSO-J6) δ 5.57 (bs, 2H), 4.11 (d, J= 7.5 Hz, 2H), 3.49 (s, 2H), 3.39 (t, J= 6.9 Hz, 2H), 3.02 (s, 3H), 2.90 (t, J= 6.9 Hz, 2H), 2.82-2.74 (m, 6H), 2.07 (pentet, J= 6.9 Hz, IH), 1.78
(sextet, J= 7.5 Hz, 2H), 0.98 (t, J= 7.5 Hz, 3H), 0.80 (t, J= 6.9 Hz, 6H); MS (APCI) m/z 394 (M + H)+; Anal, calcd for C19H31N5O2S: C, 57.99; H, 7.94: N, 17.80. Found: C, 58.18; H, 8.17; N, 18.11.
Examples 7-31
A mixture of l-(2-methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]- l,6-naphthyridin-4-amine (1.3 g, 4.5 mmol, prepared as described in Part D of Example 1) and JV,jV-diisopropylethylamine (1.6 mL, 9.0 mmol) was diluted with chloroform to yield 45 mL of a solution. The solution (1 mL) was added to a test tube containing a reagent from the table below (1.1 equivalents, 0.11 mmol). The test tube was capped and placed on a shaker at ambient temperature overnight (approximately 18 hours). Water (50 μL) was added to each tube. The solvent was removed by vacuum centrifugation. The compounds were purified by reversed phase preparative high-performance liquid
chromatography (prep HPLC) using a Waters FractionLynx automated purification system. The prep HPLC fractions were analyzed using a Waters LC/TOF-MS, and the appropriate fractions were centrifuge evaporated to provide the trifluoroacetate salt of the desired compound. Reversed phase preparative liquid chromatography was performed with non- linear gradient elution from 5-95% B where A is 0.05% trifluoroacetic acid/water and B is 0.05% trifluoroacetic acid/acetonitrile. Fractions were collected by mass-selective triggering. The table below shows the acylating agent used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
Examples 7-31
Examples 32-38 l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (862 mg, 3.0 mmol, prepared as described in Part D of Example 1) was diluted with methanol to provide 30 mL of solution. The solution (1 mL) was added to a test tube containing an aldehyde or ketone (1.25 equivalents, 0.125 mmol) from the table below. The test tubes were capped and vortexed for 15 minutes. Borane-pyridine complex (16 μL, 0.13 mmol, 1.3 eq) was added to each tube, which was then vortexed overnight (approximately 18 hours). Water (50 μL) was added to each tube. The solvent was removed by vacuum centrifugation. The compounds were purified as described for Examples 7-31. The table below shows the aldehyde or ketone used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
Examples 32-38
35 4-Fluorobenzaldehyde 396.2549
36 3 -Pheny lpropionaldehy de 406.2998
37 o-Anisaldehyde 408.2786
Examples 39-41 l-(2-Methylpropyl)-2-propyl-6,7,8,9-tetrahydro-lH-imidazo[4,5-c]-l,6- naphthyridin-4-amine (260 mg, 0.90 mmol, prepared as described in Part D of Example 1) was diluted with TΗF to provide 9 mL of solution. The solution (1 mL) was added to a test tube containing a reagent (1 equivalent, 0.1 mmol) from the table below. The test tube was capped and stirred for 116 hours at 70 0C. The solvent was removed by vacuum centrifugation. The compounds were purified as described for Examples 7-31. The table below shows the reagent used for each example, the structure of the resulting compound, and the observed accurate mass for the isolated trifluoroacetate salt.
Examples 39-41
Exemplary Compounds
Certain exemplary compounds, including some of those described above in the Examples, have the following Formula (Ha or Ilia) and one R1, one R2, and one R3 substituent shown in the following table, wherein each line of the table is matched with the Formula (Ha or Ilia) to represent a specific embodiment of the invention.
Compounds of the invention have been found to modulate cytokine biosynthesis by inducing the production of interferon α and/or tumor necrosis factor α in human cells when tested using one of the methods described below.
CYTOKINE INDUCTION IN HUMAN CELLS
An in vitro human blood cell system is used to assess cytokine induction. Activity is based on the measurement of interferon (α) and tumor necrosis factor (α) (IFN-α and TNF-α, respectively) secreted into culture media as described by Testerman et. al. in
"Cytokine Induction by the Immunomodulators Imiquimod and S-27609", Journal of Leukocyte Biology, 58, 365-372 (September, 1995).
Blood Cell Preparation for Culture Whole blood from healthy human donors is collected by venipuncture into vacutainer tubes or syringes containing EDTA. Peripheral blood mononuclear cells (PBMC) are separated from whole blood by density gradient centrifugation using HISTOPAQUE- 1077 (Sigma, St. Louis, MO) or Ficoll-Paque Plus (Amersham Biosciences Piscataway, NJ). Blood is diluted 1 : 1 with Dulbecco's Phosphate Buffered Saline (DPBS) or Hank's Balanced Salts Solution (HBSS). Alternately, whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium. The PBMC layer is collected and washed twice with DPBS or HBSS and re-suspended at 4 x 106 cells/mL in RPMI complete. The PBMC suspension is added to 96 well flat bottom sterile tissue culture plates containing an equal volume of RPMI complete media containing test compound. Compound Preparation
The compounds are solubilized in dimethyl sulfoxide (DMSO). The DMSO concentration should not exceed a final concentration of 1% for addition to the culture wells. The compounds are generally tested at concentrations ranging from 30-0.014 μM. Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with reference compound. Incubation
The solution of test compound is added at 60 μM to the first well containing RPMI complete and serial 3 fold dilutions are made in the wells. The PBMC suspension is then added to the wells in an equal volume, bringing the test compound concentrations to the desired range (usually 30-0.014 μM). The final concentration of PBMC suspension is 2 x 106 cells/mL. The plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere. Separation Following incubation the plates are centrifuged for 10 minutes at 1000 rpm
(approximately 200 x g) at 40C. The cell-free culture supernatant is removed and transferred to sterile polypropylene tubes. Samples are maintained at -30 to -700C until
analysis. The samples are analyzed for IFN-α by ELISA and for TNF-α by
IGEN/BioVeris Assay.
Interferon (α) and Tumor Necrosis Factor (α) Analysis
IFN-α concentration is determined with a human multi-subtype colorimetric sandwich ELISA (Catalog Number 41105) from PBL Biomedical Laboratories,
Piscataway, NJ. Results are expressed in pg/mL.
The TNF-α concentration is determined by ORIGEN M-Series Immunoassay and read on an IGEN M-8 analyzer from BioVeris Corporation, formerly known as IGEN International, Gaithersburg, MD. The immunoassay uses a human TNF-α capture and detection antibody pair (Catalog Numbers AHC3419 and AHC3712) from Biosource International, Camarillo, CA. Results are expressed in pg/mL. Assay Data and Analysis
In total, the data output of the assay consists of concentration values of TNF-α and IFN-α (y-axis) as a function of compound concentration (x-axis). Analysis of the data has two steps. First, the greater of the mean DMSO (DMSO control wells) or the experimental background (usually 20 pg/mL for IFN-α and 40 pg/mL for TNF-α) is subtracted from each reading. If any negative values result from background subtraction, the reading is reported as " * ", and is noted as not reliably detectable. In subsequent calculations and statistics, " * ", is treated as a zero. Second, all background subtracted values are multiplied by a single adjustment ratio to decrease experiment to experiment variability. The adjustment ratio is the area of the reference compound in the new experiment divided by the expected area of the reference compound based on the past 61 experiments (unadjusted readings). This results in the scaling of the reading (y-axis) for the new data without changing the shape of the dose-response curve. The reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro-α,α- dimethyl-lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from the past 61 experiments.
The minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound. The minimum effective concentration (μmolar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested
cytokine (usually 20 pg/mL for IFN-α and 40 pg/mL for TNF-α). The maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
CYTOKINE INDUCTION IN HUMAN CELLS (High Throughput Screen)
The CYTOKINE INDUCTION IN HUMAN CELLS test method described above was modified as follows for high throughput screening.
Blood Cell Preparation for Culture Whole blood from healthy human donors is collected by venipuncture into vacutainer tubes or syringes containing EDTA. Peripheral blood mononuclear cells (PBMC) are separated from whole blood by density gradient centrifugation using HISTOPAQUE- 1077 (Sigma, St. Louis, MO) or Ficoll-Paque Plus (Amersham Biosciences Piscataway, NJ). Whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium. The PBMC layer is collected and washed twice with DPBS or HBSS and re- suspended at 4 x 106 cells/mL in RPMI complete (2-fold the final cell density). The PBMC suspension is added to 96-well flat bottom sterile tissue culture plates. Compound Preparation The compounds are solubilized in dimethyl sulfoxide (DMSO). The compounds are generally tested at concentrations ranging from 30 - 0.014 μM. Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with a reference compound 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro-α,α-dimethyl- lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) on each plate. The solution of test compound is added at 7.5 mM to the first well of a dosing plate and serial 3 fold dilutions are made for the 7 subsequent concentrations in DMSO. RPMI Complete media is then added to the test compound dilutions in order to reach a final compound concentration of 2-fold higher (60 - 0.028 μM) than the final tested concentration range. Incubation
Compound solution is then added to the wells containing the PBMC suspension bringing the test compound concentrations to the desired range (usually 30 - 0.014 μM)
and the DMSO concentration to 0.4 %. The final concentration of PBMC suspension is 2x106 cells/mL. The plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere. Separation Following incubation the plates are centrifuged for 10 minutes at 1000 rpm
(approximately 200 g) at 40C. 4-plex Human Panel MSD MULTI-SPOT 96-well plates are pre-coated with the appropriate capture antibodies by MesoScale Discovery, Inc. (MSD, Gaithersburg, MD). The cell-free culture supernatants are removed and transferred to the MSD plates. Fresh samples are typically tested, although they may be maintained at -30 to -700C until analysis.
Interferon-α and Tumor Necrosis Factor-α Analysis
MSD MULTI-SPOT plates contain within each well capture antibodies for human TNF-α and human IFN-α that have been pre-coated on specific spots. Each well contains four spots: one human TNF-α capture antibody (MSD) spot, one human IFN- α capture antibody (PBL Biomedical Laboratories, Piscataway, NJ) spot, and two inactive bovine serum albumin spots. The human TNF-α capture and detection antibody pair is from MesoScale Discovery. The human IFN-α multi-subtype antibody (PBL Biomedical Laboratories) captures all IFN-α subtypes except IFN-α F (IFNA21). Standards consist of recombinant human TNF-α (R&D Systems, Minneapolis, MN) and IFN-α (PBL Biomedical Laboratories). Samples and separate standards are added at the time of analysis to each MSD plate. Two human IFN-α detection antibodies (Cat. Nos. 21112 & 21100, PBL) are used in a two to one ratio (weight: weight) to each other to determine the IFN-α concentrations. The cytokine-specific detection antibodies are labeled with the SULFO-TAG reagent (MSD). After adding the SULFO-TAG labeled detection antibodies to the wells, each well's electrochemoluminescent levels are read using MSD's SECTOR HTS READER. Results are expressed in pg/mL upon calculation with known cytokine standards. Assay Data and Analysis
In total, the data output of the assay consists of concentration values of TNF-α or IFN-α (y-axis) as a function of compound concentration (x-axis).
A plate-wise scaling is performed within a given experiment aimed at reducing plate-to-plate variability associated within the same experiment. First, the greater of the
median DMSO (DMSO control wells) or the experimental background (usually 20 pg/mL for IFN-α and 40 pg/mL for TNF-α) is subtracted from each reading. Negative values that may result from background subtraction are set to zero. Each plate within a given experiment has a reference compound that serves as a control. This control is used to calculate a median expected area under the curve across all plates in the assay. A plate- wise scaling factor is calculated for each plate as a ratio of the area of the reference compound on the particular plate to the median expected area for the entire experiment. The data from each plate are then multiplied by the plate-wise scaling factor for all plates. Only data from plates bearing a scaling factor of between 0.5 and 2.0 (for both cytokines IFN-α, TNF-α) are reported. Data from plates with scaling factors outside the above mentioned interval are retested until they bear scaling factors inside the above mentioned interval. The above method produces a scaling of the y- values without altering the shape of the curve. The reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9- tetrahydro-α,α-dimethyl-lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91). The median expected area is the median area across all plates that are part of a given experiment.
A second scaling may also be performed to reduce inter-experiment variability (across multiple experiments). All background- subtracted values are multiplied by a single adjustment ratio to decrease experiment-to-experiment variability. The adjustment ratio is the area of the reference compound in the new experiment divided by the expected area of the reference compound based on an average of previous experiments (unadjusted readings). This results in the scaling of the reading (y-axis) for the new data without changing the shape of the dose-response curve. The reference compound used is 2-[4- amino-2-ethoxymethyl-6,7,8,9-tetrahydro-α,α-dimethyl- lH-imidazo[4,5-c]quinolin- 1 - yljethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from an average of previous experiments.
The minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound. The minimum effective concentration (μmolar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN-α and 40 pg/mL for TNF-α). The maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
The complete disclosures of the patents, patent documents, and publications cited herein are incorporated by reference in their entirety as if each were individually incorporated. Various modifications and alterations to this invention will become apparent to those skilled in the art without departing from the scope and spirit of this invention. It should be understood that this invention is not intended to be unduly limited by the illustrative embodiments and examples set forth herein and that such examples and embodiments are presented by way of example only with the scope of the invention intended to be limited only by the claims set forth herein as follows.
Claims
1. A compound selected from the group consisting of Formulas I, II, III, and IV:
IV wherein:
R3 is selected from the group consisting of: -Q-R4,
-Q -D N-Q1 -R4
-Z-Ar,
-X-Y-R4,
-X-R5,
-alkylene-CH(CH2-OH)-OH, -alkylene-CH(CH2CH2-OH)-OH, and -(alkylene)o-i-CH(CH2OH)2;
Q is selected from the group consisting Of -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Rs)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Rs)-O-, -C(Re)-S-, and -C(Re)-N(OR9)-; Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups;
Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino; D is CH or N; with the proviso that when D is N, then Q is -C(R6)- or -S(O)2-; E is selected from the group consisting of -0-, -C(O)-, -S(O)0-2-, and -N(Qi-R4)-; R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl; n is O, 1, or 2;
Ri is selected from the group consisting of:
-R4, -X-R4,
-D'-Xi-R5a;
D' is selected from the group consisting of -N(Ri')- and -O-; R1' is selected from the group consisting of hydrogen, Ci_2o alkyl, hydroxyC2-2o alkylenyl, and alkoxyC2-2o alkylenyl; Xi is C2-20 alkylene with the proviso that when Yi is or
R2 is selected from the group consisting of: -R4, -X-R4, -X-Y-R4,
-X-R5, -OH,
-NH2, and -NH-Q2-R4; X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups;
Y is selected from the group consisting of: -O-,
-S(O)0-2-, -S(O)2-N(R8)-, -C(R6)-, -C(Re)-O-, -0-C(R6)-,
-0-C(O)-O-,
R4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy; halogen; nitro; hydroxy; mercapto; cyano; aryl; aryloxy; arylalkyleneoxy; heteroaryl; heteroaryloxy; heteroarylalkyleneoxy; heterocyclyl; amino; alkylamino; dialkylamino; (dialkylamino)alkyleneoxy; and, in the case of alkyl, alkenyl, alkynyl, and heterocyclyl, oxo;
R5 is selected from the group consisting of:
Rga is selected from the group consisting of hydrogen and Ci_4 alkyl; Rg is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl; R9 is selected from the group consisting of hydrogen and alkyl;
A' is selected from the group consisting of -0-, -S(O)0-2-, -NC-Q1-R4)-, and -CH2-; Qi is selected from the group consisting of a bond, -C(R6)-, -C(Re)-C(R6)-,
-S(O)2-, -C(Rs)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Rs)-O-, -C(Rs)-S-, and -C(Rs)-N(OR9)-;
Q2 is selected from the group consisting of a bond, -C(R6)-, -C(Rs)-C(R6)-, -S(O)2-, -C(Rs)-N(R8a)-W-, -S(O)2-NCRSa)-, and -C(Ke)-O-;
V is selected from the group consisting of a bond, -C(R6)-, -0-C(R6)-, -N(Rg)-C(R6)-, and -S(O)2-;
W is selected from the group consisting of a bond, -C(O)-, and -S(O)2-; and a and b are independently integers from 1 to 6 with the proviso that a + b is < 7; or a pharmaceutically acceptable salt thereof.
2. The compound or salt of claim 1 wherein the compound is of the Formula II:
R3 11 or a pharmaceutically acceptable salt thereof.
III or a pharmaceutically acceptable salt thereof.
4. A compound selected from the group consisting of the Formulas V, VI, VII, and VIII:
V VI VII
G is selected from the group consisting of:
-C(O)-R', α-aminoacyl, α-aminoacyl-α-aminoacyl,
-C(O)-O-R',
-C(0)-N(R")R,
-C(=NY')-R',
-CH(OH)-C(O)-OY', -CH(OCL4 alkyl)Y0,
-CH2Y2, and -CH(CH3)Y2;
R' and R" are independently selected from the group consisting Of C1-10 alkyl, C3-7 cycloalkyl, phenyl, benzyl, and 2-phenylethyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from the group consisting of halogen, hydroxy, nitro, cyano, carboxy, Ci_6 alkyl, Ci_4 alkoxy, aryl, heteroaryl, aryl-Ci_4 alkylenyl, heteroaryl-Ci_4 alkylenyl, halo-Ci_4 alkylenyl, halo-Ci_4 alkoxy, -0-C(O)-CH3, -C(O)-O-CH3, -C(O)-NH2, -0-CH2-C(O)-NH2, -NH2, and -S(O)2-NH2, with the proviso that R" can also be hydrogen; α-aminoacyl is an α-aminoacyl group derived from an α-amino acid selected from the group consisting of racemic, D-, and L-amino acids;
Y' is selected from the group consisting of hydrogen, Ci_6 alkyl, and benzyl; Y0 is selected from the group consisting of Ci_6 alkyl, carboxy-Ci_6 alkylenyl, amino-Ci_4 alkylenyl, mono-iV-Ci_6 alkylamino-Ci_4 alkylenyl, and alkylamino-Ci_4 alkylenyl; Y2 is selected from the group consisting of mono-iV-Ci_6 alkylamino, di-N,N-C\-6 alkylamino, morpholin-4-yl, piperidin-1-yl, pyrrolidin-1-yl, and 4-Ci_4 alkylpiperazin-1-yl;
R3 is selected from the group consisting of: -Q-R4,
-Q -D N-Q1 -R4 ^^
-Z-Ar,
-X-Y-R4, -X-R5, -alkylene-CH(CH2-OH)-OH,
-alkylene-CH(CH2CH2-OH)-OH, and -(alkylene)0-i-CH(CH2OH)2;
Q is selected from the group consisting Of -C(R6)-, -C(Re)-C(R6)-, -S(O)2-, -C(Rs)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Rs)-O-, -C(Re)-S-, and -C(Re)-N(OR9)-; Z is selected from the group consisting of a bond, alkylene, alkenylene, and alkynylene, wherein the alkylene, alkenylene, and alkynylene groups are optionally interrupted by one or more -O- groups;
Ar is selected from the group consisting of aryl and heteroaryl each of which is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, cyano, amino, alkylamino, and dialkylamino; D is CH or N; with the proviso that when D is N, then Q is -C(R6)- or -S(O)2-; E is selected from the group consisting of -0-, -C(O)-, -S(O)0-2-, and -N(Qi-R4)-; R is selected from the group consisting of alkyl, alkoxy, fluoro, hydroxy, and trifluoromethyl; n is O, 1, or 2;
Ri is selected from the group consisting of:
-R4, -X-R4,
-D'-Xi-R5a;
D' is selected from the group consisting of -N(Ri')- and -O-; R1' is selected from the group consisting of hydrogen, Ci_2o alkyl, hydroxyC2-2o alkylenyl, and alkoxyC2-2o alkylenyl; Xi is C2-20 alkylene with the proviso that when Yi is or
R2 is selected from the group consisting of: -R4, -X-R4, -X-Y-R4,
-X-R5, -OH,
-NH2, and -NH-Q2-R4; X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated by arylene, heteroarylene or heterocyclylene and optionally interrupted by one or more -O- groups;
Y is selected from the group consisting of: -O-,
-S(O)0-2-, -S(O)2-N(R8)-, -C(R6)-, -C(Re)-O-, -0-C(R6)-,
-0-C(O)-O-,
R4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl; alkoxy; hydroxyalkyl; haloalkyl; haloalkoxy; halogen; nitro; hydroxy; mercapto; cyano; aryl; aryloxy; arylalkyleneoxy; heteroaryl; heteroaryloxy; heteroarylalkyleneoxy; heterocyclyl; amino; alkylamino; dialkylamino; (dialkylamino)alkyleneoxy; and, in the case of alkyl, alkenyl, alkynyl, and heterocyclyl, oxo;
R5 is selected from the group consisting of:
Rga is selected from the group consisting of hydrogen and Ci_4 alkyl; Rg is selected from the group consisting of hydrogen, alkyl, alkenyl, hydroxyalkylenyl, alkoxyalkylenyl, arylalkylenyl, and heteroarylalkylenyl; R9 is selected from the group consisting of hydrogen and alkyl;
A' is selected from the group consisting of -0-, -S(O)0-2-, -NC-Q1-R4)-, and -CH2-; Qi is selected from the group consisting of a bond, -C(R6)-, -C(Re)-C(R6)-,
-S(O)2-, -C(Rs)-N(Rg)-W-, -S(O)2-N(R8)-, -C(Rs)-O-, -C(Rs)-S-, and -C(Rs)-N(OR9)-;
Q2 is selected from the group consisting of a bond, -C(R6)-, -C(Rs)-C(R6)-, -S(O)2-, -C(Rs)-N(R8a)-W-, -S(O)2-NCRSa)-, and -C(Ke)-O-;
V is selected from the group consisting of a bond, -C(R6)-, -0-C(R6)-, -N(Rg)-C(R6)-, and -S(O)2-;
W is selected from the group consisting of a bond, -C(O)-, and -S(O)2-; and a and b are independently integers from 1 to 6 with the proviso that a + b is < 7; or a pharmaceutically acceptable salt thereof.
5. The compound or salt of claim 4 wherein the compound is of the Formula VI:
VI or a pharmaceutically acceptable salt thereof.
VII or a pharmaceutically acceptable salt thereof.
7. The compound or salt of any one of claims 1 through 6 wherein n is 0.
8. The compound or salt of any one of claims 1 through 7 wherein Ri is selected from the group consisting Of -R4, -X-R4, -X-Y-R4, and -X-R5.
9. The compound or salt of any one of claims 1 through 8 wherein Ri is selected from the group consisting of alkyl, arylalkylenyl, aryloxyalkylenyl, hydroxyalkyl, dihydroxyalkyl, -X-Y-R4, -X-R5, and heterocyclylalkylenyl wherein the heterocyclyl of the heterocyclylalkylenyl group is optionally substituted by one or more alkyl groups; wherein X is alkylene; Y is -N(R8)-C(O)-, -N(Rs)-S(O)2-, -N(Rs)-C(Re)-N(R8)-,
-N(R8)-C(R6)-O-, -C(O)-, -S(O)2-, or ; R4 is alkyl, aryl, heteroaryl, arylalkylenyl, heteroarylalkylenyl, or arylalkenylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino; and R5 is
/-(CH2)a ■. -N- C(R6) -N- S(O)2 -N(R8) -C(O) -N A V , V , or -^
10. The compound or salt of the claim 9 wherein X is C2-6 alkylene.
11. The compound or salt of any one of claims 1 through 10 wherein Ri is selected from the group consisting of 2-hydroxy-2-methylpropyl, 2-methylpropyl, 2,3- dihydroxypropyl, 4-[(methylsulfonyl)amino]butyl, 2-methyl-2- [(methylsulfonyl)amino]propyl, 2-(acetylamino)-2-methylpropyl, 2- { [(isopropylamino)carbonyl] amino } -2-methylpropyl,
4- { [(isopropylamino)carbonyl] amino } butyl, 4-( 1 , 1 -dioxidoisothiazolidin-2-yl)butyl, tetrahydro-2H-pyran-4-ylmethyl, (2,2-dimethyl-l,3-dioxolan-4-yl)methyl, 2,2-dimethyl-4- oxopentyl, (1 -hydroxy cyclobutyl)methyl, (1 -hydroxy cyclopentyl)methyl, and (1- hy droxy cy clohexy l)methy 1.
12. The compound or salt of any one of claims 1 through 7 wherein Ri is selected from the group consisting of -N(Ri')-Qi-R4, -0'-Xi-Yi-R4, and -D'-Xi-R5a.
13. The compound or salt of any one of claims 1 through 12 wherein R2 is -R4.
14. The compound or salt of claim 13 wherein R2 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, and hy droxy alky lenyl.
15. The compound or salt of claim 14 wherein R2 is selected from the group consisting of hydrogen, methyl, ethyl, propyl, butyl, 2-methoxyethyl, 2-hydroxyethyl, ethoxymethyl, and hydroxymethyl.
16. The compound or salt of any one of claims 1 through 8 wherein R2 is -OH, and Ri is selected from the group consisting of pyridin-3-ylmethyl, isoxazol-5-ylmethyl, isoxazol- 3-ylmethyl, [5-(4-fluorophenyl)isoxazol-3-yl]methyl, [3-(4-fluorophenyl)isoxazol-5- yljmethyl, tetrahydro-2H-pyran-4-ylmethyl, and benzyl, wherein benzyl is unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, haloalkyl, haloalkoxy, and halogen.
17. The compound or salt of any one of claims 1 through 12 wherein R2 is -NH-Q2-R4.
18. The compound or salt of any one of claims 1 through 17 wherein R3 is -Q-R4.
19. The compound or salt of claim 18 wherein Q is selected from the group consisting of -C(O)-, -S(O)2-, -C(O)-N(R8)-, -C(O)-O-, and -S(O)2-N(R8)-.
20. The compound or salt of claim 18 or claim 19 wherein R4 in -Q-R4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
21. The compound or salt of any one of claims 18, 19, and 20 wherein Q is -C(O)- or -S(O)2-, and -R4 is selected from the group consisting of:
22. The compound or salt of any one of claims 18, 19, and 20 wherein Q is -C(O)-O-, and -R4 is selected from the group consisting of:
23. The compound or salt of any one of claims 1 through 17 wherein R3 is
-Q -D N-Q1 -R4
24. The compound or salt of claim 23 wherein Q is -C(O)- or -S(O)2-, Qi in -Qi-R4 is -C(O)-, -S(O)2-, or -C(O)-N(R8)-, and -R4 in -Qi-R4 is alkyl, aryl, heteroaryl, arylalkylenyl, or heteroarylalkylenyl, wherein alkyl, aryl, heteroaryl, or arylalkylenyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl, halogen, haloalkyl, haloalkoxy, heterocyclyl, cyano, alkoxy, and dialkylamino.
25. The compound or salt of any one of claims 1 through 17 wherein R3 is -Z-Ar.
26. The compound or salt of claim 25 wherein Z is alkylene.
27. The compound or salt of claim 26 wherein Z is Ci_4 alkylene.
28. The compound or salt of any one of claims 25, 26, and 27 wherein Ar is pyridinyl, furyl, or phenyl wherein phenyl is optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl.
29. The compound or salt of any one of claims 1 through 17 wherein R3 is -X-Y-R4 or -X-R5.
30. The compound or salt of claim 29 wherein X in R3 is alkylene.
31. The compound or salt of claim 30 wherein X is Ci_4 alkylene.
32. The compound or salt of any one of claims 29, 30, and 31 wherein R3 is -X-Y-R4.
33. The compound or salt of any one of claims 29, 30, 31, and 32 wherein Y in the R3 group -X-Y-R4 is selected from the group consisting of -C(O)-, -S(O)2-, -C(O)-O-, and -C(O)-N(R8)-.
34. The compound or salt of any one of claims 29, 30, 31, 32, and 33 wherein R4 in the R3 group -X-Y-R4 is selected from the group consisting of alkyl, alkenyl, heterocyclyl, aryl, and arylalkylenyl, wherein aryl and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, alkyl, and amino, and wherein alkyl is optionally substituted by one or more substituents independently selected from the group consisting of fluoro, alkoxy, and heterocyclyl.
35. The compound or salt of any one of claims 29, 30, 31, and 32 wherein Y in R3 is
36. The compound or salt of claim 35 wherein Qi in -NH-Qi- is -C(O)-, -S(O)2-, or -C(O)-N(Rg)-, and R4 in -NH-Qi-R4 is selected from the group consisting of alkyl, aryl, heteroaryl, heterocyclyl, and arylalkylenyl, wherein aryl, alkyl, and aryl in arylalkylenyl are optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkoxy, and alkyl, and wherein heterocyclyl is optionally substituted by one or more substituents independently selected from the group consisting of alkyl and oxo.
37. The compound or salt of any one of claims 29, 30, and 31 wherein R3 is -X-R5.
38. The compound or salt of any one of claims 29, 30, 31 , and 37 wherein R5 in the R3 group -X-R5 is selected from the group consisting of:
O ^^ O ^^ O ^^ O ^^
/ / / ~1 4 /
, and '
39. The compound or salt of any one of claims 1 through 17 wherein R3 is -alkylene-CH(CH2-OH)-OH, -alkylene-CH(CH2CH2-OH)-OH, or -(alkylene)o-i-CH(CH2OH)2.
40. The compound or salt of claim 39 wherein R3 is -CH(CH2OH)2.
41. A pharmaceutical composition comprising a therapeutically effective amount of a compound or salt of any one of claims 1 through 40 and a pharmaceutically acceptable carrier.
42. A method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of any one of claims 1 through 40 or the pharmaceutical composition of claim 41 to the animal.
43. A method of treating a viral disease in an animal in need thereof comprising administering a therapeutically effective amount of a compound or salt of any one of claims 1 through 40 or the pharmaceutical composition of claim 41 to the animal.
44. A method of treating a neoplastic disease in an animal in need thereof comprising administering a therapeutically effective amount of a compound or salt of any one of claims 1 through 40 or the pharmaceutical composition of claim 41 to the animal.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US80392706P | 2006-06-05 | 2006-06-05 | |
| US60/803,927 | 2006-06-05 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007143526A2 true WO2007143526A2 (en) | 2007-12-13 |
| WO2007143526A3 WO2007143526A3 (en) | 2008-02-07 |
Family
ID=38802237
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2007/070181 WO2007143526A2 (en) | 2006-06-05 | 2007-06-01 | Substituted tetrahydroimidazonaphthyridines and methods |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007143526A2 (en) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8697873B2 (en) | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| JP2018526422A (en) * | 2015-09-14 | 2018-09-13 | ファイザー・インク | Novel imidazo [4,5-c] quinoline and imidazo [4,5-c] [1,5] naphthyridine derivatives as LRRK2 inhibitors |
| WO2022204112A1 (en) * | 2021-03-22 | 2022-09-29 | Incyte Corporation | Imidazole and triazole kras inhibitors |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6194425B1 (en) * | 1997-12-11 | 2001-02-27 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US6518280B2 (en) * | 1998-12-11 | 2003-02-11 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US20040147543A1 (en) * | 2002-12-20 | 2004-07-29 | 3M Innovative Properties Company | Aryl substituted imidazoquinolines |
-
2007
- 2007-06-01 WO PCT/US2007/070181 patent/WO2007143526A2/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6194425B1 (en) * | 1997-12-11 | 2001-02-27 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US6518280B2 (en) * | 1998-12-11 | 2003-02-11 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US20040147543A1 (en) * | 2002-12-20 | 2004-07-29 | 3M Innovative Properties Company | Aryl substituted imidazoquinolines |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| US8697873B2 (en) | 2004-03-24 | 2014-04-15 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US10071156B2 (en) | 2005-02-04 | 2018-09-11 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| JP2018526422A (en) * | 2015-09-14 | 2018-09-13 | ファイザー・インク | Novel imidazo [4,5-c] quinoline and imidazo [4,5-c] [1,5] naphthyridine derivatives as LRRK2 inhibitors |
| WO2022204112A1 (en) * | 2021-03-22 | 2022-09-29 | Incyte Corporation | Imidazole and triazole kras inhibitors |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007143526A3 (en) | 2008-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007143526A2 (en) | Substituted tetrahydroimidazonaphthyridines and methods | |
| JP5247458B2 (en) | Hydroxy and alkoxy substituted 1H-imidazoquinolines and methods | |
| US7943610B2 (en) | Pyrazolopyridine-1,4-diamines and analogs thereof | |
| US7943636B2 (en) | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases | |
| US7897609B2 (en) | Aryl substituted imidazonaphthyridines | |
| US8329721B2 (en) | Hydroxy and alkoxy substituted 1H-imidazonaphthyridines and methods | |
| US20090030030A1 (en) | Arylalkenyl and arylalkynyl substituted imidazoquinolines | |
| WO2007079086A1 (en) | Pyrazoloalkyl substituted imidazo ring compounds and methods | |
| EP1877056A2 (en) | Oxime and hydroxylamine substituted thiazoloý4,5-c¨ring compounds and methods | |
| EP1846405A2 (en) | Oxime and hydroxylamine substituted imidazo 4,5-c ring compounds and methods | |
| WO2006009826A1 (en) | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines | |
| EP1845988A2 (en) | Substituted imidazoquinolines and imidazonaphthyridines | |
| EP1784180A2 (en) | 2-amino 1h imidazo ring systems and methods | |
| WO2007028129A1 (en) | Hydroxy substituted 1h-imidazopyridines and methods | |
| JP2008530112A (en) | Substituted condensed [1,2] imidazo “4,5-c] ring compounds and methods | |
| WO2006091567A2 (en) | Hydroxyalkyl substituted imidazoquinoline compounds and methods | |
| WO2008045543A1 (en) | Substituted 4h-imidazo [4, 5, 1-ij] [1, 6] naphthyridine-9-amines and their pharmaceutical use | |
| EP1851220A2 (en) | Hydroxyalkyl substituted imidazonaphthyridines | |
| WO2006028451A1 (en) | 1-amino 1-h-imidazoquinolines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07797985 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: RU |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07797985 Country of ref document: EP Kind code of ref document: A2 |