WO2012032546A2 - Process for the preparation of salmeterol and its intermediates - Google Patents
Process for the preparation of salmeterol and its intermediates Download PDFInfo
- Publication number
- WO2012032546A2 WO2012032546A2 PCT/IN2011/000615 IN2011000615W WO2012032546A2 WO 2012032546 A2 WO2012032546 A2 WO 2012032546A2 IN 2011000615 W IN2011000615 W IN 2011000615W WO 2012032546 A2 WO2012032546 A2 WO 2012032546A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- benzyloxy
- benzoate
- phenylbutoxy
- salmeterol
- Prior art date
Links
- AKWQRVIQUREMOK-UHFFFAOYSA-N COC(c1cc(C(CBr)=O)ccc1OCc1ccccc1)=O Chemical compound COC(c1cc(C(CBr)=O)ccc1OCc1ccccc1)=O AKWQRVIQUREMOK-UHFFFAOYSA-N 0.000 description 2
- MFBFHLSKYGRGIY-UHFFFAOYSA-N OCC1=CC(C(CNCCCCCCOCCCCc2ccccc2)O)=CCC1O Chemical compound OCC1=CC(C(CNCCCCCCOCCCCc2ccccc2)O)=CCC1O MFBFHLSKYGRGIY-UHFFFAOYSA-N 0.000 description 1
- PSHAXLAATQLRBM-UHFFFAOYSA-N OCc(cc(C(CN(CCCCCCOCCCCc1ccccc1)Cc1ccccc1)O)cc1)c1OCc1ccccc1 Chemical compound OCc(cc(C(CN(CCCCCCOCCCCc1ccccc1)Cc1ccccc1)O)cc1)c1OCc1ccccc1 PSHAXLAATQLRBM-UHFFFAOYSA-N 0.000 description 1
- IFMYZHHQPZHOKD-UHFFFAOYSA-N OCc(cc(C(CNCCCCCCOCCCCc(cc1)ccc1-c1ccc(ccc(C(O)=O)c2O)c2c1)O)cc1)c1O Chemical compound OCc(cc(C(CNCCCCCCOCCCCc(cc1)ccc1-c1ccc(ccc(C(O)=O)c2O)c2c1)O)cc1)c1O IFMYZHHQPZHOKD-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/08—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton by reactions not involving the formation of amino groups, hydroxy groups or etherified or esterified hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/02—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton by reactions involving the formation of amino groups from compounds containing hydroxy groups or etherified or esterified hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/10—Separation; Purification; Stabilisation; Use of additives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C229/00—Compounds containing amino and carboxyl groups bound to the same carbon skeleton
- C07C229/38—Compounds containing amino and carboxyl groups bound to the same carbon skeleton having amino groups bound to acyclic carbon atoms and carboxyl groups bound to carbon atoms of six-membered aromatic rings of the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C41/00—Preparation of ethers; Preparation of compounds having groups, groups or groups
- C07C41/01—Preparation of ethers
- C07C41/16—Preparation of ethers by reaction of esters of mineral or organic acids with hydroxy or O-metal groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/30—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group
- C07C67/307—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group by introduction of halogen; by substitution of halogen atoms by other halogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/30—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group
- C07C67/31—Preparation of carboxylic acid esters by modifying the acid moiety of the ester, such modification not being an introduction of an ester group by introduction of functional groups containing oxygen only in singly bound form
Definitions
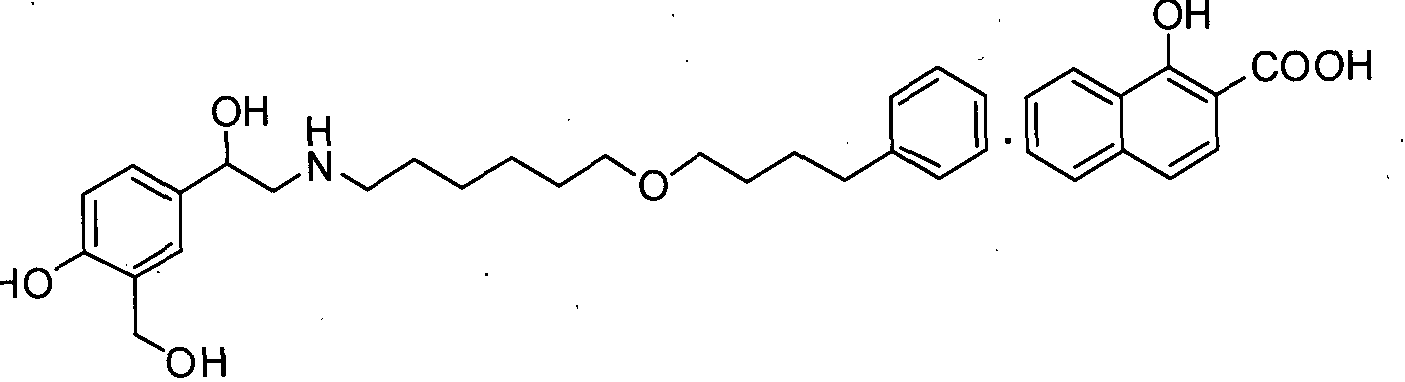
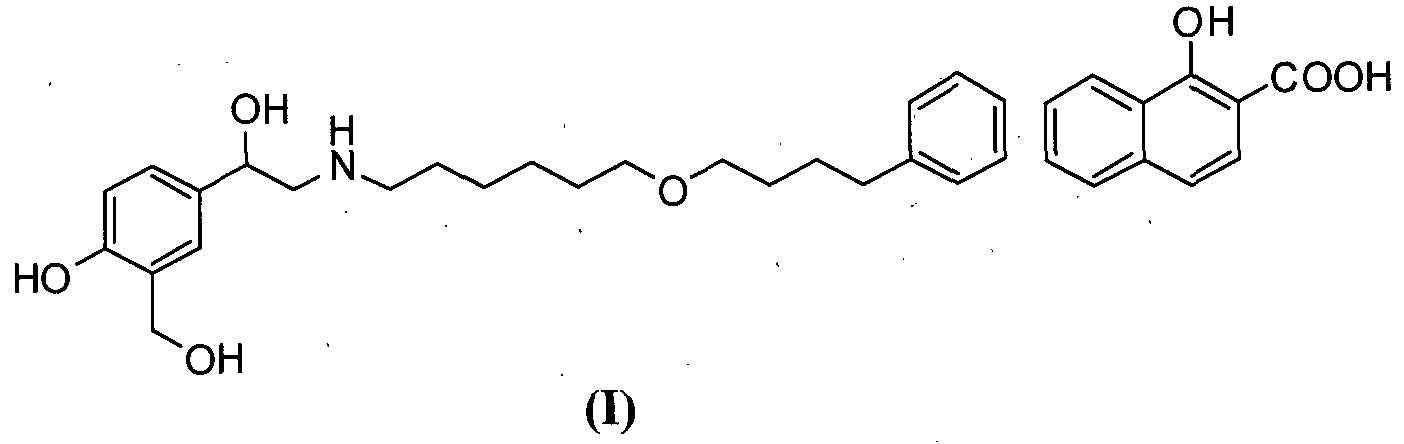
- the present invention relates to an improved process for the preparation of salmeterol and intermediates thereof.
- the present invention particularly relates to processes for the preparation of 4-hydroxy-a'-[[[6-(4-phenylbutoxy)hexyl)amino]methyl]- 1,3-benzenedimethanol 1 -hydroxy-2-naphthonate (salmeterol Xinafoate) [I], the preparation of (2-(benzyl(6-(4-phenylbutoxy)hexyl) amino)- l-(4-benzyloxy)-3 - (hydroxymethyl)phenyl)ethanol [III] and the preparation of 5-(2-(benzyl- (6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] as shown below.
- Salmeterol is the international common term of 4-hydroxy-a'-[[[6-(4- phenylbutoxy)hexyl)amino] methyl] -1, 3 -benzenedimethanol used in the treatment of asthma and of the chronic bronchitis. It is commercialized like Salmeterol Xinafoate i.e. racemate salt of 1 -hydroxy-2-naphthanoic acid and salmeterol.
- Salmeterol Xinafoate is a selective 2-adrenore.ceptor agonist. It is clinically used as long-active inhaled bronchodilator for maintenance treatment of asthama and to control nocturnal asthma. Unlike other bronchodilator drugs, salmeterol is more lipophilic and has many unusual pharmacological properties. The dosage strength is very small (0.021 mg as a metered dose and 0.046 mg as a dry powder inhaler).
- Scheme-1 ES 2065269 describes a novel process for the synthesis of salmeterol by reacting 6- (4-phenylbutoxy)]hexyl]benzylamine (Ila) with salicil aldehyde derivative 4 followed by catalytic hydrogenation of the formed compound 5 (process in which the reduction of groups takes simultaneously to end carbonyls of aldehyde and ketone, as well as the deprotection of amino group) to obtain Salmeterol as shown in scheme-2.
- GB 2140800 and Spanish patent ES 531722, ES 539625 and ES 2065269 discloses the process for the preparation of compound [6-(4-phenylbutoxy)hexyl]benzylamine (Ila) from (4-(6-bromohexyl- oxy)butyl)benzene by displacement of bromine with benzylamine.
- R Methyl, trifluromethyl, 2,2,2,-trifluoroethyl, monoflourobutyl, tolyl, p-bromophenyl, p-nitro phenyl etc.
- ES 539625 and ES 531722 discloses various processes for the preparation of Salmeterol.
- the condensation of compound (II) and (III) provides compound of formula (V) which on reduction with LiAlH4 followed by hydrogenation provides Salmeterol.
- compound of formula (II) on condensation with (IV). provides compound of formula (VI), which on hydrogenation results in Salmeterol as shown in Scheme-5.
- the starting material compound of formula (IV) is prepared by hydrolysis of acetoxy-5 -(2 -bromoacetyl)benzyl acetate (X), which is prepared from hydroxyacetophenone as per the process reported in ES345365 and shown in scheme-6.
- Scheme-6 ES 2065269 Bl discloses an alternative approach for the synthesis of 4-hydroxy-a'- [[[6-(4-phenylbutoxy)hexyl)amino]methyl]-l,3-benzenedimethanol i.e. Salmeterol and new intermediates.
- Such procedure consists in the hydrogenation of the new intermediate 5-[(6-(4- (phenylbutoxy)hexyl)benzylaminoacetyl]salicylaldehyde of formula (XIV), which in turn is obtained from the condensation between 5-bromoacetylsalicylaldehyde and 6-[4-(phenylbutoxy)] hexylbenzylamine.
- the transformation (XIV) in (I) is carried out in a single step and without the detection of lateral reactions, thus substantially shortening the synthetic procedures.
- N-(6-(4- phenylbutoxy)hexyl)benzenemethamine (II) serves as the key intermediate in the synthesis of Salmeterol (GB Patent 2,176,476; US Patent 4,992,474; Tetrahedron Letters, Vol. 35(50), pages 9375-9378, 1994; Synthetic Communication, Vol. 29 (12a), pages 2155- 2162, 1999; and Indian Journal of Chemistry, Vol. 34B, 629-632, 1995).
- WO2007045857 Al discloses a chemical method for purification of intermediate (II) via formation of an acid salt (8). This method affords intermediate (II) of very high purity of more than 99.5%.
- US 71 12701 B2 uses KOH as a base and phase transfer (tetrabutyl ammonium hydrogen sulfate) as a catalyst in toluene as a solvent.
- the product is isolated after high vacuum distillation.
- WO 2007045857 Al discloses use of NaH as a base, and tetrabutyl ammonium bromide as a catalyst.
- the present invention uses Nal, which helps to minimize side reactions and to get a cleaner product.
- the product need not be distilled, but can be taken up as such for the preparation of the key intermediate (II) for salmeterol.
- US 6756508 B2 claims cinnamic acid salt of Salmeterol.
- US 6680345 B2 claims Salicylic acid salt of Salmterol.
- US 5795594 claims Salmeterol Xinafoate in crystalline from having dynamic bulk density less than 0.1 gm/cm .
- EP 1073429 Bl discloses two polymorphic forms of Salmeterol Xinafoate characterized by XRD and DSC.
- the inventors of the present invention has found that the use of novel intermediates in the synthesis of Salmeterol would alleviates the hitherto problems associated with the prior art for preparing Salmeterol of high purity.
- the present inventors also provides novel intermediate that it allows to prepare product to industrial scale, with good yield and high purity, and therefore to obtain salmeterol with an appropriate purity for employed being like active principle in the preparation of pharmaceutical formulations.
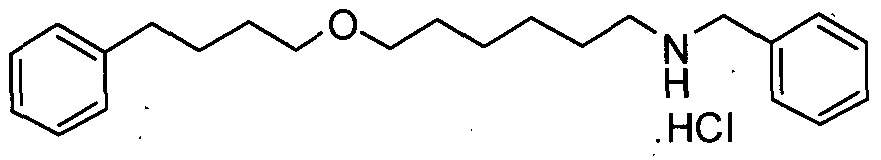
- Salmeterol (I) can be prepared in high purity and yield by preparing the intermediates, methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI).
- the prior art discloses the process for the preparation of N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) with desired purity and converting back to N-(6-(4-phenylbutoxy)hexyl)benzenemethamine by treating with base.
- the present inventors have found that there is no need to convert N-(6-(4- phenylbutoxy)hexyl) benzenemethamine hydrochloride to N-(6-(4- phenylbutoxy)hexyl)benzenemethamine.
- the hydrochloride salt with purity greater than 98% can be reacted as such with methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) with purity , greater than 99% to obtain 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV]. This significantly improves the process economics and commercial viability.
- the isolation may include filtration, filtration under vacuum, centrifugation, and decantation.
- the product obtain may be further or additionally dried to achieve the desired moisture values.
- the product may be dried in a tray dryer, dried under vacuum and/or in a fluid bed drier.
- process for the preparation of Salmeterol includes reacting methyl 2-(benzyloxy)-5-(2- bromoacetyl)benzoate (V) and - N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) in a suitable polar organic solvent in presence of base to obtain novel intermediate 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)- acetyl)-2-
- the process further includes reacting 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] with suitable reducing agent in a suitable organic solvent followed by treating with suitable base under heating to get 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l -(4-(benzyloxy)-3-
- the process further includes hydrogenating 2- (benzyl-(6-(4-phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3- (hydroxymethyl)phenyl)ethanol (III) with suitable catalyst in polar solvent under 5-6 Kg/cm 2 pressure and temperature of about 30°C to 80°C to obtain Salmeterol base (II).
- suitable catalyst in polar solvent under 5-6 Kg/cm 2 pressure and temperature of about 30°C to 80°C to obtain Salmeterol base (II).
- the process further includes conversion of salmeterol base (II) into its pharmaceutically acceptable salts preferably xinafoate salt by reaction with xinafoic acid in polar organic solvent with optionally purification in polar organic solvent to get salmeterol xinafoate (I) with high yield and purity.
- pharmaceutically acceptable salts preferably xinafoate salt by reaction with xinafoic acid in polar organic solvent with optionally purification in polar organic solvent to get salmeterol xinafoate (I) with high yield and purity.
- the process includes reacting methyl 2- (benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) in a suitable polar organic solvent in presence of base to obtain 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV]; reducing 5-(2-(benzyl-(6- (4-phenylbutoxy)hexyl)amino)- acetyl)-2-(benzyloxy)benzoate [IV] with suitable reducing agent in a suitable organic solvent followed by treating with suitable base under heating to get 2-(benzyl-(6-(4-phenylbutoxy)hexyl)
- Salmeterol Xinafoate can be done by any process known in the art, which may optionally include crystallization in one or more suitable polar solvents like C]-C 4 alcohols, C 2 -C 8 ketones, amides, nitriles etc.
- the process includes reacting benzylating methyl-5-acetyl-2-hydroxybenzoate (VIII) with benzyl chloride in presence of base and catalyst in polar solvent followed by bromination of methyl 5-acetyl-2-(benzyloxy)benzoate (VII) with suitable brominating agent in suitable solvent in presence of acid catalyst and optionally purifying thus obtained methyl 2-
- the process includes reacting 4-phenyl-l-butanol (XII) with 1 ,6-dibromobutane (XI) in presence of base and phase transfer catalyst in a hydrocarbon solvent to obtain (4-(6- bromohexyloxy)butyl)benzene (X); reacting with benzylamine in presence of base and catalyst in polar solvent and optionally purifying the obtained N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI).
- Salmeterol obtained by the process for the present invention can be converted into its pharmaceutically acceptable salts like Xinafoate.
- the Salmeterol Xinafoate obtained by the process of the present invention is crystalline form.
- FIG.l X-ray diffraction of crystalline Salmeterol Xinafoate (I).
- FIG.2 Differential Scanning Calorimetry of Salmeterol Xinafoate (I).
- the process includes:
- base includes inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc., preferably potassium carbonate.
- any catalyst which promotes the reaction, can be used as catalyst for example potassium iodide.
- Benzylation reaction can be carried out in one or more suitable polar solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-Q alcohols, C 3 -C 6 ketones or C 3 -C 6 esters, preferably dimethylformamide.
- suitable polar solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-Q alcohols, C 3 -C 6 ketones or C 3 -C 6 esters, preferably dimethylformamide.
- the bromination of intermediate methyl 5-acetyl-2-(benzyloxy)benzoate (VII) can be carried out in with suitable brominating agent selected from N-bromosuccinimide, Hydrobromic acid, dibromodimethylhydatoin (DBDMH), Liquid bromine etc, preferably N-bromosuccinimide in presence of acid catalyst.
- suitable brominating agent selected from N-bromosuccinimide, Hydrobromic acid, dibromodimethylhydatoin (DBDMH), Liquid bromine etc, preferably N-bromosuccinimide in presence of acid catalyst.
- any acid catalyst which promotes the reaction, can be used as a catalyst.
- mineral acids like hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid or lewis acids like aluminum chloride, zinc chloride, ferric chloride, copper chloride or magnesium chloride can be used as catalyst.
- the acid catalyst is sulfuric acid or aluminum chloride.
- Embodiments of the process may include purification of methyl 2-(benzyloxy)-5- (2-bromoacetyl)benzoate (V) by using suitable solvent selected from Ci -6 alcohols, C 3- 6 ketones, C 3-6 esters, acetonitrile, dimethylformamide etc.
- suitable solvent selected from Ci -6 alcohols, C 3- 6 ketones, C 3-6 esters, acetonitrile, dimethylformamide etc.
- the solvents for purification may include methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
- the compound methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) can be isolated by filtration, centrifugation, decantation etc, preferably filtration followed drying.
- the process comprises:
- Embodiments of the process may include condensation of methyl 2-(benzyloxy)-5-(2- bromoacetyl)benzoate (V) and N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) in suitable polar solvent can be selected from one or more solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-C 6 alcohols, C 3 -C 6 ketones or C 3 -C 6 esters, preferably dimethylformamide.
- suitable polar solvent can be selected from one or more solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-C 6 alcohols
- base may include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium carbonate.
- suitable reducing agent can be selected from lithium aluminum hydride, vitride, sodium borohydride etc, preferably lithium aluminum hydride in suitable organic solvent which includes C]-C 5 alcohols, C3-C6 ethers, tetrahydrofuran, 2-methyl tetrahydrofuran etc, preferably tetrahydrofuran.
- Embodiments of the process further provides quenching of reaction mixture with suitable base under heating at about 35°C to get 2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)-l-(4-(benzyloxy)-3-(hydroxymethyl)- phenyl)ethanol (III).
- Base is selected from inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably sodium hydroxide.
- catalyst which includes Pd/C, Pt/C, mixture of Pd/C and ZnCl 2 , Raney Nickel etc., preferably Pd/C in polar solvents including Ci- alcohols, C 3-6 ketones, C 3- esters, acetonitrile, dimethylformamide etc.
- the polar solvent can be selected from methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethyl formamide etc., preferably acetone or methanol.
- the hydrogenation reaction is carried out under 5-6 Kg/cm pressure and temperature of about 30°C to 80°C, preferably at about 40°C to obtain Salmeterol base (II) in suitable polar organic solvent which includes methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone.
- suitable polar organic solvent which includes methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone.
- Salmeterol Xinafoate (I) after hydrogenation reaction is completed is achieved by addition of suitable antisolvent before purification which includes ethyl acetate, n-butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n-hexane, n-heptane etc, preferably methyl tertbutyl ether.
- the Salmeterol Xinafoate (I) prepared by the process as described above is required to be purified to obtain desired purity.
- the process of purification thus includes treatment in suitable solvent selected from Ci -6 alcohols, C 3- ketones, C 3-6 esters, acetonitrile, dimethylformamide etc.
- the solvents for purification includes but not limited to methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n- butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
- base may include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium hydroxide.
- phase transfer catalyst may include tetrabutyl ammonium bromide (TBAB), benzyltriethyl ammonium chloride (TEBAC), polyethylene Glycol (PEG-200, 400, 600, 800, 1000 etc.), tetrabutylammonium hydrogen sulphate (TBAHS), preferably TBAHS in a hydrocarbon solvent selected from toluene, xylene, ethyl benzene, cyclohexane, pentane, hexane, heptane etc., preferably toluene.
- TBAB tetrabutyl ammonium bromide
- TEBAC benzyltriethyl ammonium chloride
- PEG-200 polyethylene Glycol
- TAAHS tetrabutylammonium hydrogen sulphate
- TBAHS tetrabutylammonium hydrogen sulphate
- the compound of formula (X) is reacted with benzylamine in presence of base, which include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium carbonate and in presence of catalyst.
- base include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium carbonate and in presence of catalyst.
- any catalyst which promotes the reaction, can be used as catalyst for example potassium iodide.
- the reaction with benzylamine can be performed in suitable polar solvent may include one or more solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N-methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-C 6 alcohols, C 3 -C 6 ketones or C 3 -C 6 esters preferably dimethylformamide.
- the N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) prepared by the process as described above is required to be purified to obtain desired purity.
- the process of purification thus includes treatment in suitable solvent selected from Ci-6 alcohols, C 3-6 ketones, C 3-6 esters, acetonitrile, dimethylformamide etc. preferably ethyl acetate.
- Embodiments of the process may include one or more of the following features.
- the solution of suspension may be obtained by dissolving or suspending
- Salmeterol and pharmaceutically acceptable acid in suitable solvent may be obtained directly from a reaction mixture in a process in which Salmeterol is formed.
- suitable solvent may include methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone and heating the reaction mixture at about 45°C
- compositions of the process includes, isolation of Salmeterol and pharmaceutically acceptable salt (I) by addition of suitable antisolvent which may include ethyl acetate, n- butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n- hexane, n-heptane etc, preferably methyl tertbutyl ether.
- suitable antisolvent may include ethyl acetate, n- butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n- hexane, n-heptane etc, preferably methyl tertbutyl ether.
- the Salmeterol and pharmaceutically acceptable salt (I) prepared by the process as described above is required to be purified to obtain desired purity.
- the process of purification thus includes treatment in suitable solvent selected from Ci -6 alcohols, C 3-6 ketones, C 3-6 esters, acetonitrile, dimethylformamide etc.
- the solvents for purification includes but not limited to methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
- Salmeterol Xinafoate (I) according to the process provided herein above is obtained in crystalline form characterized by X-ray diffraction pattern as shown in Figure- 1 having characteristic XRD peaks 4.0, 17.1 , 17.6, 19.4, 21.1 and 22.0 ⁇ 0.2° ⁇
- Salmeterol Xinafoate (I) according to the process provided herein above is obtained in crystalline form characterized by characterized by DSC as shown in Figure-2 having endotherm peak at about 126°C.
- Embodiments of the present invention provides salmeterol Xinafoate (I) in crystalline Form I characterized by XRD as shown in Figure-I and DSC as shown in Figure-2 and according to European Patent 1073429 Bl which is incorporated herein as reference.
- Fraction-I was separated around 40°C-45°C vapor temperature at 90°C to 100°C
- Fraction-II (1,6-dibromohexane) was separated around 95°C-1 15°C vapor temperature at 145°C to 200°C
- Fraction-Ill (4-(6-bromohexyloxy)butyl)benzene (X) was separated around 150°C-210°C vapor temperature at 220°C to250°C.
- Fraction-Ill was again subjected to fractional distillation to remove Fraction-I and Fraction-II to get 160 g (77%) (4-(6-bromohexyloxy)butyl)benzene (X).
- the separated organic layer was dried over 20 g of anhydrous sodium sulfate. Ethyl acetate was distilled under vacuum at 60°C. The residue and 700 mL dichloromethane were taken in another round bottom flask at 30°C and treated with 16% hydrochloric acid to adjust the pH of about 1.0 to 1.5. The reaction mixture was allowed to settle and the organic layer was separated. The organic layer was washed with water and distilled to remove dichloromethane under vacuum at 45°C. 100 mL ethyl acetate was added to the residue and cooled to 25°C followed by addition of 1.5 L of methyltertbutyl ether and further cooled to 10°C.
- the present invention provides a novel process for the preparation of Salmeterol Xinafoate (I).
- the present invention provides a novel process for preparation of methyl 2- (benzyloxy)-5-(2-bromoacetyl)benzoate (V).
- the main advantage of the present invention is to provide Salmeterol Xinafoate with high yield and high purity by HPLC greater than 99% with single individual impurity ⁇ 0.1%.
Abstract
The present invention discloses a process for the preparation of methyl 2-(benzyloxy)- 5-(2-bromoacetyl)benzoate (V), comprising: (d) benzylating methyl-5-acetyl-2-hydroxybenzoate (VIII) with benzyl chloride in the presence of a base and a catalyst in a suitable polar solvent to obtain 5-acetyl-2- benzyloxy benzoate (VII); (e) brominating methyl 5-acetyl-2-(benzyloxy)benzoate (VII) with a suitable brominating agent in one or more suitable' solvents in the presence of an acid catalyst to obtain methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate V; (c) optionally, purifying the methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) in a suitable solvent; and (f) isolating the methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V).
Description
PROCESS FOR THE PREPARATION OF SALMETEROL AND ITS INTERMEDIATES FIELD OF THE INVENTION
The present invention relates to an improved process for the preparation of salmeterol and intermediates thereof. The present invention particularly relates to processes for the preparation of 4-hydroxy-a'-[[[6-(4-phenylbutoxy)hexyl)amino]methyl]- 1,3-benzenedimethanol 1 -hydroxy-2-naphthonate (salmeterol Xinafoate) [I], the preparation of (2-(benzyl(6-(4-phenylbutoxy)hexyl) amino)- l-(4-benzyloxy)-3 - (hydroxymethyl)phenyl)ethanol [III] and the preparation of 5-(2-(benzyl- (6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] as shown below.
(III)
(IV)
These intermediates (III) and (IV) are useful compounds in the synthesis of Salmeterol (II) and Salmeterol Xinafoate (I).
BACKGROUND OF THE INVENTION
The following discussion of the prior art is intended to present the invention in an appropriate technical context and allow its significance to be properly appreciated. Unless clearly indicated to the contrary, however, reference to any prior art in this specification should be construed as an admission that such art is widely known or forms part of common general knowledge in the field.
Salmeterol is the international common term of 4-hydroxy-a'-[[[6-(4- phenylbutoxy)hexyl)amino] methyl] -1, 3 -benzenedimethanol used in the treatment of asthma and of the chronic bronchitis. It is commercialized like Salmeterol Xinafoate i.e. racemate salt of 1 -hydroxy-2-naphthanoic acid and salmeterol.
Salmeterol Xinafoate, represented above, is a selective 2-adrenore.ceptor agonist. It is clinically used as long-active inhaled bronchodilator for maintenance treatment of asthama and to control nocturnal asthma. Unlike other bronchodilator drugs, salmeterol is more lipophilic and has many unusual pharmacological properties. The dosage strength is very small (0.021 mg as a metered dose and 0.046 mg as a dry powder inhaler).
The salmeterol was described for the first time in GB 2140800. In apparent GB 2140800, ES 531722 and ES 539625 describe various processes for the preparation of Salmeterol. The synthetic strategy of these synthesis procedures is based mainly on the disconnection of the bond C-N that provides intermediate V and VI, and that therefore requires the 6-(4-phenylbutoxy)]N-substitUted hexylamines like synthetic precursors as shown in scheme 1. .
Scheme-1
ES 2065269 describes a novel process for the synthesis of salmeterol by reacting 6- (4-phenylbutoxy)]hexyl]benzylamine (Ila) with salicil aldehyde derivative 4 followed by catalytic hydrogenation of the formed compound 5 (process in which the reduction of groups takes simultaneously to end carbonyls of aldehyde and ketone, as well as the deprotection of amino group) to obtain Salmeterol as shown in scheme-2.
Scheme-2
GB 2140800 and Spanish patent ES 531722, ES 539625 and ES 2065269 discloses the process for the preparation of compound [6-(4-phenylbutoxy)hexyl]benzylamine (Ila) from (4-(6-bromohexyl- oxy)butyl)benzene by displacement of bromine with benzylamine.
Reference article Synth. Commun.1999, 29 (12), pages 2155-2162 discloses above reaction takes to end with an excess of benzylamine (2.0 5 eq.) and triethylamine like base in DMSO using Nal like catalyst to accelerate the reaction. In these conditions, after the
necessary purification by colum chromatography, the excess of benzylamine and the formed by products are removed, the intermediate Ila with an yield of 70% is obtained.
Reference article. J. Label. Compd. Radiopharm. 2002, 45 (9), pages 755-762 discloses alternative reaction conditions in DMF at 60-80°C and in the presence of a slight excess of benzylamine (1.1 eq.) using Cs2C03 base.
In both articles, intermediate [4(6-bromohexyloxy)butyl]benzene 6 obtained by means of reaction of 4-phenyl-l -butanol with 1 ,6-dibromohexane in the presence of a base, generally sodium hydride, at room temperature as to reflux as much for 7 hours to 8 days, and with ields in the range of 48 to 81 %.
In the article Tetrahedron Lett.1994, 35, 9375-9378, describes the process for the preparation of intermediate Ila useful for the preparation of salmeterol as shown in scheme
Scheme-3
ES2288114 Bl discloses the process for the preparation of intermediate II as shown in
scheme-4.
R = Methyl, trifluromethyl, 2,2,2,-trifluoroethyl, monoflourobutyl, tolyl, p-bromophenyl, p-nitro phenyl etc.
Scheme-4
ES 539625 and ES 531722 discloses various processes for the preparation of Salmeterol. The condensation of compound (II) and (III) provides compound of formula (V) which on reduction with LiAlH4 followed by hydrogenation provides Salmeterol. Alternatively, compound of formula (II) on condensation with (IV). provides compound of formula (VI), which on hydrogenation results in Salmeterol as shown in Scheme-5.
Scheme-5
The starting material compound of formula (IV) is prepared by hydrolysis of acetoxy-5 -(2 -bromoacetyl)benzyl acetate (X), which is prepared from hydroxyacetophenone as per the process reported in ES345365 and shown in scheme-6.
Scheme-6
ES 2065269 Bl discloses an alternative approach for the synthesis of 4-hydroxy-a'- [[[6-(4-phenylbutoxy)hexyl)amino]methyl]-l,3-benzenedimethanol i.e. Salmeterol and new intermediates. Such procedure consists in the hydrogenation of the new intermediate 5-[(6-(4- (phenylbutoxy)hexyl)benzylaminoacetyl]salicylaldehyde of formula (XIV), which in turn is obtained from the condensation between 5-bromoacetylsalicylaldehyde and 6-[4-(phenylbutoxy)] hexylbenzylamine. The transformation (XIV) in (I) is carried out in a single step and without the detection of lateral reactions, thus substantially shortening the synthetic procedures.
In all the reported synthetic schemes, N-(6-(4- phenylbutoxy)hexyl)benzenemethamine (II) serves as the key intermediate in the synthesis of Salmeterol (GB Patent 2,176,476; US Patent 4,992,474; Tetrahedron Letters, Vol. 35(50), pages 9375-9378, 1994; Synthetic Communication, Vol. 29 (12a), pages 2155- 2162, 1999; and Indian Journal of Chemistry, Vol. 34B, 629-632, 1995).
WO2007045857 Al discloses a chemical method for purification of intermediate (II) via formation of an acid salt (8). This method affords intermediate (II) of very high purity of more than 99.5%.
US 71 12701 B2 uses KOH as a base and phase transfer (tetrabutyl ammonium hydrogen sulfate) as a catalyst in toluene as a solvent. The product is isolated after high vacuum distillation. WO 2007045857 Al discloses use of NaH as a base, and tetrabutyl ammonium bromide as a catalyst. In addition to this catalyst the present invention uses Nal, which helps to minimize side reactions and to get a cleaner product. The product need not be distilled, but can be taken up as such for the preparation of the key intermediate (II) for salmeterol.
US 6756508 B2 claims cinnamic acid salt of Salmeterol. US 6680345 B2 claims Salicylic acid salt of Salmterol. US 5795594 claims Salmeterol Xinafoate in crystalline from having dynamic bulk density less than 0.1 gm/cm . EP 1073429 Bl discloses two polymorphic forms of Salmeterol Xinafoate characterized by XRD and DSC.
The processes described in the above patents/publications are lengthy, results in low yield and purity and are cumbersome. They are difficult to be adopted for commercial scale production. As described above, that the dosage strength of Salmeterol is very small and due to this it is of utmost importance to have the highest purity of the API.
The inventors of the present invention has found that the use of novel intermediates in the synthesis of Salmeterol would alleviates the hitherto problems associated with the prior art for preparing Salmeterol of high purity. The present inventors also provides novel intermediate that it allows to prepare product to industrial scale, with good yield and high
purity, and therefore to obtain salmeterol with an appropriate purity for employed being like active principle in the preparation of pharmaceutical formulations.
SUMMARY OF THE INVENTION
The present inventors have found that Salmeterol (I) can be prepared in high purity and yield by preparing the intermediates, methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI). The prior art discloses the process for the preparation of N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) with desired purity and converting back to N-(6-(4-phenylbutoxy)hexyl)benzenemethamine by treating with base. The present inventors have found that there is no need to convert N-(6-(4- phenylbutoxy)hexyl) benzenemethamine hydrochloride to N-(6-(4- phenylbutoxy)hexyl)benzenemethamine. The hydrochloride salt with purity greater than 98% can be reacted as such with methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) with purity , greater than 99% to obtain 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV]. This significantly improves the process economics and commercial viability.
V VI
The isolation may include filtration, filtration under vacuum, centrifugation, and decantation. The product obtain may be further or additionally dried to achieve the desired moisture values. For example, the product may be dried in a tray dryer, dried under vacuum and/or in a fluid bed drier.
Accordingly, in one general aspect there is provided process for the preparation of Salmeterol (II). The process includes reacting methyl 2-(benzyloxy)-5-(2- bromoacetyl)benzoate (V) and - N-(6-(4-phenylbutoxy)hexyl)benzenemethamine
hydrochloride (VI) in a suitable polar organic solvent in presence of base to obtain novel intermediate 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)- acetyl)-2-
(benzyloxy)benzoate [IV].
(IV)
The process further includes reacting 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] with suitable reducing agent in a suitable organic solvent followed by treating with suitable base under heating to get 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l -(4-(benzyloxy)-3-
(hydroxymethyl)phenyl)ethanol (III) .
(III)
According to still further aspect, the process further includes hydrogenating 2- (benzyl-(6-(4-phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3- (hydroxymethyl)phenyl)ethanol (III) with suitable catalyst in polar solvent under 5-6 Kg/cm2 pressure and temperature of about 30°C to 80°C to obtain Salmeterol base (II). '
(II)
The process further includes conversion of salmeterol base (II) into its pharmaceutically acceptable salts preferably xinafoate salt by reaction with xinafoic acid in
polar organic solvent with optionally purification in polar organic solvent to get salmeterol xinafoate (I) with high yield and purity.
(I)
Accordingly, in second aspect there is provided an improved process for the preparation of Salmeterol Xinafoate (I). The process includes reacting methyl 2- (benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) in a suitable polar organic solvent in presence of base to obtain 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV]; reducing 5-(2-(benzyl-(6- (4-phenylbutoxy)hexyl)amino)- acetyl)-2-(benzyloxy)benzoate [IV] with suitable reducing agent in a suitable organic solvent followed by treating with suitable base under heating to get 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3 -
(hydroxymethyl)phenyl)ethanol (III); hydro genating 2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)-l-(4-(benzyloxy)-3-(hydroxymethyl) phenyl)ethanol (III) with suitable catalyst in polar solvent under 5-6 Kg/cm2 pressure and temperature of about 30°C to 80°C to obtain Salmeterol base (II); converting salmeterol base (II) into its pharmaceutically acceptable salts preferably Xinafoate salt by reaction with Xinafoic acid in polar organic solvent; optionally purifying Salmeterol Xinafoate (I) with suitable polar solvent; and isolating Salmeterol Xinafoate (I).
The purification of Salmeterol Xinafoate can be done by any process known in the art, which may optionally include crystallization in one or more suitable polar solvents like C]-C4 alcohols, C2-C8 ketones, amides, nitriles etc.
In another general aspect there is provided the process for the preparation of methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V)
(V)
the process includes reacting benzylating methyl-5-acetyl-2-hydroxybenzoate (VIII) with benzyl chloride in presence of base and catalyst in polar solvent followed by bromination of methyl 5-acetyl-2-(benzyloxy)benzoate (VII) with suitable brominating agent in suitable solvent in presence of acid catalyst and optionally purifying thus obtained methyl 2-
(benzyloxy)-5-(2-bromoacetyl)benzoate (V).
In another general aspect there is provided the process for the preparation of N-(6- (4-phenylbutoxy)hexyl)benzenemethamine h drochloride (VI)
(VI)
the process includes reacting 4-phenyl-l-butanol (XII) with 1 ,6-dibromobutane (XI) in presence of base and phase transfer catalyst in a hydrocarbon solvent to obtain (4-(6- bromohexyloxy)butyl)benzene (X); reacting with benzylamine in presence of base and catalyst in polar solvent and optionally purifying the obtained N-(6-(4- phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI).
In another general aspect Salmeterol obtained by the process for the present invention, can be converted into its pharmaceutically acceptable salts like Xinafoate. The Salmeterol Xinafoate obtained by the process of the present invention is crystalline form.
In another general aspect of the present invention, there is provided an novel intermediate 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate (IV)
(IV)
The details of one or more embodiments of the inventions are set forth in the description below. Other features, objects and advantages of the inventions will be apparent from the description and claims.
Brief description of figures: -
A preferred embodiment of the invention will now be described, by way of example only, with reference to the accompaying figures in which:
FIG.l: X-ray diffraction of crystalline Salmeterol Xinafoate (I).
FIG.2: Differential Scanning Calorimetry of Salmeterol Xinafoate (I).
DETAILED DESCRIPTION OF THE INVENTION
In one aspect there is provided an improved process for the preparation of methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V),
(V)
the process includes:
(a) benzylating methyl-5-acetyl-2-hydroxybenzoate (VIII) with benzyl chloride in presence of base and catalyst in a suitable polar solvent to obtain 5-acetyl-2-benzyloxy benzoate (VII);
(VII)
(b) brominating methyl 5-acetyl-2-(benzyloxy)benzoate (VII) with suitable brominating agent in suitable solvent in presence of acid catalyst to obtain methyl 2-(benzyloxy)-5- (2-bromoacetyl)benzoate (V);
(V)
(c) optionally purifying methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzpate (V) in suitable solvent; and
(d) isolating methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V).
Examples of base includes inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc., preferably potassium carbonate.
In general, any catalyst, which promotes the reaction, can be used as catalyst for example potassium iodide.
Benzylation reaction can be carried out in one or more suitable polar solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-Q alcohols, C3-C6 ketones or C3-C6 esters, preferably dimethylformamide.
The bromination of intermediate methyl 5-acetyl-2-(benzyloxy)benzoate (VII) can be carried out in with suitable brominating agent selected from N-bromosuccinimide,
Hydrobromic acid, dibromodimethylhydatoin (DBDMH), Liquid bromine etc, preferably N-bromosuccinimide in presence of acid catalyst.
In general, any acid catalyst, which promotes the reaction, can be used as a catalyst. For example, mineral acids like hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid or lewis acids like aluminum chloride, zinc chloride, ferric chloride, copper chloride or magnesium chloride can be used as catalyst. Preferably the acid catalyst is sulfuric acid or aluminum chloride.
Embodiments of the process may include purification of methyl 2-(benzyloxy)-5- (2-bromoacetyl)benzoate (V) by using suitable solvent selected from Ci-6 alcohols, C3-6 ketones, C3-6 esters, acetonitrile, dimethylformamide etc. The solvents for purification may include methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
In general, the compound methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) can be isolated by filtration, centrifugation, decantation etc, preferably filtration followed drying.
In another aspect there is provided a process for preparation of Salmeterol or its pharmaceutically acceptable salts of formula (I),
the process comprises:
(a) reacting methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4- phenylbutoxy)- hexyl)benzenemethamine hydrochloride (VI) in a suitable polar organic solvent in presence of base to obtain 5-(2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV];
(b) reducing 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] with suitable reducing agent in a suitable organic solvent followed by treating with suitable base . under heating to get 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l- (4-(benzyloxy)-3 -(hydroxy- methyl)phenyl)ethanol (III);
(III)
(c) hydrogenating 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3-
(hydroxymethyl) phenyl)ethanol (III) with suitable catalyst in polar solvent under 5-6 Kg/cm2 pressure and temperature of about 30°C to 80°C to obtain Salmeterol base (II);
(II)
(d) converting salmeterol base (II) into its pharmaceutically acceptable salts in polar organic solvent; .
(e) optinally purifying pharmaceutically acceptable salt of Salmeterol in a polar organic solvent; and
(f) isolating pure pharmaceutically acceptable salt of Salmeterol (I) with high yield.
Embodiments of the process may include condensation of methyl 2-(benzyloxy)-5-(2- bromoacetyl)benzoate (V) and N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) in suitable polar solvent can be selected from one or more solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Cj-C6 alcohols, C3-C6 ketones or C3-C6 esters, preferably dimethylformamide.
Examples of base may include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium carbonate.
> In general, the reduction of novel intermediate (IV) can be done with suitable reducing agent can be selected from lithium aluminum hydride, vitride, sodium borohydride etc, preferably lithium aluminum hydride in suitable organic solvent which includes C]-C5 alcohols, C3-C6 ethers, tetrahydrofuran, 2-methyl tetrahydrofuran etc, preferably tetrahydrofuran.
Embodiments of the process further provides quenching of reaction mixture with suitable base under heating at about 35°C to get 2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)-l-(4-(benzyloxy)-3-(hydroxymethyl)- phenyl)ethanol (III). Base is selected from inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably sodium hydroxide.
Further embodiments of the present invention, includes hydrogenation of 2-(benzyl-(6- (4-phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3-(hydroxymethyl)- phenyl)ethanol (III) in presence of catalyst which includes Pd/C, Pt/C, mixture of Pd/C and ZnCl2, Raney Nickel etc., preferably Pd/C in polar solvents including Ci- alcohols, C3-6 ketones, C3- esters, acetonitrile, dimethylformamide etc.
In general, the polar solvent can be selected from methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethyl formamide etc., preferably acetone or methanol.
The hydrogenation reaction is carried out under 5-6 Kg/cm pressure and temperature of about 30°C to 80°C, preferably at about 40°C to obtain Salmeterol base (II) in suitable polar organic solvent which includes methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone.
The isolation of Salmeterol Xinafoate (I) after hydrogenation reaction is completed is achieved by addition of suitable antisolvent before purification which includes ethyl acetate, n-butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n-hexane, n-heptane etc, preferably methyl tertbutyl ether.
In general, the Salmeterol Xinafoate (I) prepared by the process as described above is required to be purified to obtain desired purity. The process of purification thus includes treatment in suitable solvent selected from Ci-6 alcohols, C3- ketones, C3-6 esters, acetonitrile, dimethylformamide etc.
In general, the solvents for purification includes but not limited to methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n- butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
In yet another aspect there is provided a process for the preparation of N-(6-(4- phenylbutoxy)hexyl)benzenemethamine h drochloride (VI),
(VI)
(a) reacting 4-phenyl-l-butanol (XII) with 1 ,6-dibromobutane (XI) in presence of base and phase transfer catalyst in a hydrocarbon solvent to obtain (4-(6- bromohexyloxy)butyl)benzene (X)
(X)
(b) reacting compound (X) with benzylamine in presence of base and catalyst in polar solvent to obtain N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI);
(c) optionally purifying N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI); and
(d) isolating pure N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI).
Examples of base may include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium hydroxide.
The reaction can be carried out in presence of phase transfer catalyst may include tetrabutyl ammonium bromide (TBAB), benzyltriethyl ammonium chloride (TEBAC), polyethylene Glycol (PEG-200, 400, 600, 800, 1000 etc.), tetrabutylammonium hydrogen sulphate (TBAHS), preferably TBAHS in a hydrocarbon solvent selected from toluene, xylene, ethyl benzene, cyclohexane, pentane, hexane, heptane etc., preferably toluene.
The compound of formula (X) is reacted with benzylamine in presence of base, which include inorganic base like sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate etc, preferably potassium carbonate and in presence of catalyst.
In general, any catalyst, which promotes the reaction, can be used as catalyst for example potassium iodide.
The reaction with benzylamine can be performed in suitable polar solvent may include one or more solvents comprising dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N-methylpyrrolidone, tetrahydrofuran, 2-methyl
tetrahydrofuran, Cj-C6 alcohols, C3-C6 ketones or C3-C6 esters preferably dimethylformamide.
In general, the N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI) prepared by the process as described above is required to be purified to obtain desired purity. The process of purification thus includes treatment in suitable solvent selected from Ci-6 alcohols, C3-6 ketones, C3-6 esters, acetonitrile, dimethylformamide etc. preferably ethyl acetate.
In yet another aspect there is provided a process for preparation of Salmeterol or pharmaceutically acceptable salts thereof, the process includes:
(a) obtaining a mixture Salmeterol and pharmaceutically acceptable acid in suitable solvent;
(b) heating to get solution or suspension;
(c) adding suitable antisolvent at about 15°C to 35°C temperature;
(d) cooling to less than 15°C;
(e) isolating Salmeterol Xinafoate (I) by filtration; and
(f) optionally purifying with suitable solvent.
Embodiments of the process may include one or more of the following features. For example, the solution of suspension may be obtained by dissolving or suspending
Salmeterol and pharmaceutically acceptable acid in suitable solvent. Alternatively, such a solution may be obtained directly from a reaction mixture in a process in which Salmeterol is formed.
Examples of suitable solvent may include methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone and heating the reaction mixture at about 45°C
Further embodiments of the process includes, isolation of Salmeterol and pharmaceutically acceptable salt (I) by addition of suitable antisolvent which may include ethyl acetate, n- butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n- hexane, n-heptane etc, preferably methyl tertbutyl ether.
In general, the Salmeterol and pharmaceutically acceptable salt (I) prepared by the process as described above is required to be purified to obtain desired purity. The process
of purification thus includes treatment in suitable solvent selected from Ci-6 alcohols, C3-6 ketones, C3-6 esters, acetonitrile, dimethylformamide etc.
In general, the solvents for purification includes but not limited to methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, dimethylformamide etc., preferably acetone or methanol.
In yet another aspect of there is provided a novel intermediate compound 5-(2- (benzyl-(6-(4-phenyl
(IV)
Salmeterol Xinafoate (I) according to the process provided herein above is obtained in crystalline form characterized by X-ray diffraction pattern as shown in Figure- 1 having characteristic XRD peaks 4.0, 17.1 , 17.6, 19.4, 21.1 and 22.0±0.2° Θ
Salmeterol Xinafoate (I) according to the process provided herein above is obtained in crystalline form characterized by characterized by DSC as shown in Figure-2 having endotherm peak at about 126°C.
Embodiments of the present invention provides salmeterol Xinafoate (I) in crystalline Form I characterized by XRD as shown in Figure-I and DSC as shown in Figure-2 and according to European Patent 1073429 Bl which is incorporated herein as reference.
In yet another aspect as set forth in the following schemes, the process of the invention for the preparation of 4-hydroxy-a'-[[[6-(4-phenylbutoxy)hexyl)amino]methyl]- 1,3-benzenedimethanol l-hydroxy-2-naphthonate (salmeterol Xinafoate) [I], the preparation of (2-(benzyl(6-(4-phenylbutoxy)hexyl) amino)- l-(4-benzyloxy)-3 - (hydroxymethyl)phenyl)ethanol [III] and the preparation of 5-(2-(benzyl(6-(4- phenylbutoxy)hexyl)amino)acetyl)-2-(benzyloxy)benzoate [IV] is as per the following reaction scheme- 8, 9 and 10.
Having thus described the invention with reference to particular preferred embodiments and illustrative examples, those in the art would appreciate modifications to the invention as described and illustrated that do not depart from the spirit and scope of the invention as disclosed in the specification. The Examples are set forth to aid in understanding the invention but are not intended to, and should not be construed to, limit its scope in any way. The examples do not include detailed descriptions of conventional methods. Such methods are well known to those of ordinary skill in the art and are described in numerous publications. Examples:
Example 1: Preparation of Methyl 5-acetyl-2-benzyloxy benzoate (VII) from methyl- 5-acetyl-2-hydroxybenzoate (VIII)
100 g of methyl-5-acetyl-2-hydroxybenzoate and 2 L of dimethylformamide were taken in a round bottom flask at 25°C and stirred to get a clear solution. 85.28 g potassium carbonate was added and the reaction mixture was heated at 50°C for 30 min. 8.54 g of potassium iodide, 68.44 g of benzyl chloride were added at 50°C and further heated to 65°C and stirred for 2 hours. The reaction mixture was treated with 25% liquor ammonia. The reaction mixture was quenched in 2 L of water at 20°C and the product was filtered, washed with water and dried in hot air oven at 65°C to obtain 144 g (98.5%) of methyl 5- acetyl-2-benzyloxy benzoate (VII)
Example 2: Preparation of methyl 5-(2-bromoacetyl)-2-benzyloxy benzoate (VI) from methyl 5-acetyl-2-benzyloxy benzoate (VII)
100 g methyl 5-acetyl-2-benzyloxy benzoate and 300 mL acetonitrile were taken in round bottom flask at 35°C and stirred for 10 min to get clear solution. 2.0 mL of Cone, sulfuric acid was added dropwise to the reaction mixture and 72 g of N-bromosuccinimide was added lot wise into the reaction mass and heated to 50°C for 1 hour alongwith stirring. After the completion of the reaction on TLC, the reaction mixture was cooled to 0°C to 5°C and stirred for 2 hours. The product was filtered and washed with acetonitrile and water and dried in hot air oven at 65°C to obtain 102.7 g (81%) of methyl 5-(2-bromoacetyi)-2- benzyloxy benzoate (V).
Example 3: Purification of methyl 5-(2-bromoacetyl)-2-benzyloxy benzoate (V)
100 g of methyl 5-(2-bromoacetyl)-2-benzyloxy benzoate (V) and 700 mL of acetone were taken in round bottom flask and heated at 60°C. The reaction mixture was charcoalized, filtered and washed with acetone. The filtrate was distilled to remove upto 3.5 times of the input mass at 60°C and cooled gradually to 5°C. The product was filtered, washed with acetone and dried in hot air oven at 65°C to obtain 85 g (85%) of pure methyl 5-(2-bromoacetyl)-2-benzyloxy benzoate (V) having purity greater than 99% by HPLC.
100 g 4-phenyl-l-butanol, 900 mL toluene, 165.2 g KOH and 2.26 g tetrabutylammonium hydrogen sulphate were taken in round bottom flask at 25°C. 487.5 g of 1 ,6-dibromohexane was added to the reaction mixture within 2 hours and reaction mixture was stirred for 20 hours. After the completion of the process on TLC, 770 mL water was added and stirred for 45 min. The aqueous layer was separated and extracted with 300 mL of toluene at 25°C. The combined toluene layer was washed with 10% brine solution and dried over 60 g of sodium sulphate. Toluene was distilled under vacuum completely at 65°C. 400 g residue was heated up to 90°C under high vacuum fractional distillation. Fraction-I was separated around 40°C-45°C vapor temperature at 90°C to 100°C, Fraction-II (1,6-dibromohexane) was separated around 95°C-1 15°C vapor temperature at 145°C to 200°C and Fraction-Ill (4-(6-bromohexyloxy)butyl)benzene (X)
was separated around 150°C-210°C vapor temperature at 220°C to250°C. Fraction-Ill was again subjected to fractional distillation to remove Fraction-I and Fraction-II to get 160 g (77%) (4-(6-bromohexyloxy)butyl)benzene (X).
171 g benzyl amine, 66.06 g potassium carbonate, 2 g of potassium iodide and 200 mL dimethylformamide were taken under nitrogen atmosphere in round bottom flask at 30°C. The reaction mixture was treated with premixed solution of 100 g (4-(6- bromohexyloxy)butylbenzene in 100 mL dimethylformamide at 0°C. The reaction mixture was stirred for 5-6 hours at 20°C and treated with 1 L of water. The organic layer was separated and washed with 1 L of water. The organic layer was treated with mixture of 300 mL of ethyl acetate and 400 mL 20% w/v brine solution. The separated organic layer was dried over 20 g of anhydrous sodium sulfate. Ethyl acetate was distilled under vacuum at 60°C. The residue and 700 mL dichloromethane were taken in another round bottom flask at 30°C and treated with 16% hydrochloric acid to adjust the pH of about 1.0 to 1.5. The reaction mixture was allowed to settle and the organic layer was separated. The organic layer was washed with water and distilled to remove dichloromethane under vacuum at 45°C. 100 mL ethyl acetate was added to the residue and cooled to 25°C followed by addition of 1.5 L of methyltertbutyl ether and further cooled to 10°C. The reaction mixture was stirred for 1 hour and the product was filtered, washed with 100 mL of methyltertbutyl ether and dried under vacuum at 45°C for 6 hours to obtain 75 g (62%) of 6-(4- phenylbutoxy)]hexyl]benzylamine hydrochloride (VI)
Example 6; Purification of 6-(4-phenylbutoxy)]hexyl]benzylamine hydrochloride (VI)
100 g of 6-(4-phenylbutoxy)]hexyl]benzylamine hydrochloride (VI) and 800 mL ethyl acetate were taken in rcmnd bottom flask at 25°C and heated at 55°C to get clear solution. The reaction mixture was stirred for 30 min and gradually cooled to 10°C. The precipitated product was filtered, washed with chilled 50 mL of ethyl acetate and dried in air oven for 6.0 hrs at 55°C to get 65 g (65%) pure 6-(4-phenylbutoxy)]hexyl]benzylamine hydrochloride (VI) having purity greater than 97% by HPLC.
Example-7: Preparation of 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-acetyl)-2- (benzyloxy)benzoate [IV]
500 mL dimethylformamide and 103.5 g of 6-(4-phenylbutoxy)]hexyl]benzylamine hydrochloride (VI) were taken in round bottom flask and stirred for 20 min to get clear solution at 25°C. 95 g of anhydrous potassium carbonate was added and stirred for 45 min. The reaction mixture was cooled to 10°C and 100 g of methyl 5-(2-bromoacetyl)-2- benzyloxy benzoate (V) was added and stirred for 2 hours. After the completion of the reaction by TLC, the reaction mixture was quenched with a mixture of 2 L of water and 500 mL ethyl acetate at 35°C. The aqueous layer was separated and extracted with 250 mL of ethyl acetate. The combined ethyl acetate layer was dried over 40 g of sodium sulphate and charcoalized. The reaction mixture was stirred for 20 min and filtered. The filtrate was distilled under vacuum at 55°C to 65°C to obtain 170 g (99.5%) residue of 5-(2-(benzyl-(6- (4-phenylbutoxy)hexyl)amino)-acetyl)-2-(benzyloxy)benzoate [IV].
Example-8: Preparation of 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l-(4- (b
500 mL dry tetrahydrofuran was cooled to 0°C in a round bottom flask. 12.20 g of Lithium aluminum hydride and 100 g of residue of example-7 in 200 mL dry tetrahydrofuran was added portion wise. The reaction mixture was stirred for 1.5 hours at
10°C. The reaction mixture was further cooled to 0°C and 12.20 niL of water was slowly added followed by addition of 12.20 mL (15%) sodium hydroxide solution. The reaction mixture was heated to get 35°C and the solid was filtered and washed with 100 mL tetrahydrofuran. The filtrate was distilled under vacuum at 65°C to remove tetrahydrofuran to obtain 90 g (95%) 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l-(4-(benzyloxy)-3- (hydroxymethyl)phenyl)ethanol (III) as residue.
Example-8: Preparation of Salmeterol Base [II]
800 mL methanol and 100 g 2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)-l-(4- (benzyloxy)-3-(hydroxymethyl)phenyl)ethanol (III) were taken in dry hydrogenator at 35°C under' nitrogen atmosphere. 3 g of 10% Pd/C was added into the reaction mixture and hydrogen gas was purged at 5-6 Kg/cm2 hydrogen pressure at 40°C. The reaction mixture was maintained for 2 hour and cooled to 25°C. The reaction mixture was filtered and washed with 100 ml methanol. The filtrate was concentrated under vacuum at 60°C to obtain 68 g (98.5%) Salmeterol base as oily residue.
Example-8: Preparation of Salmeterol Xinafoate [I]
100 g Salmeterol Base and 400 mL acetone were taken in round bottom flask at 25°C. 47.54 g of Xinafoic acid was added and heated to get clear solution at 45°C. The reaction mixture was stirred for 30 min and cooled to 25°C. After stirring for 1 hour, 700 mL of methyltertbutyl ether was added and reaction mixture was further cooled to 10°C and stirred for 2 hours. The product was filtered and washed with 150 mL chilled acetone. The product was dried in vacuum tray dryer for 12 hours at 45°C to obtain 100 g (69%) of Salmeterol Xinafoate (I) with purity greater than 97% by HPLC.
Example-9: Purification of Salmeterol Xinafoate [I]
100 g of Salmeterol Xinafoate prepared as per process of Example-8 and 300 mL methanol were taken in round bottom flask and heated at 50°C to get clear solution. The reaction mixture was cooled 25°C and stirred for 15 min. The precipitated product was filtered and washed with 100 mL of chilled methanol. The solid was dried in vacuum tray drier for 6 hours at 45°C to obtain 83 g (83%) pure Salmeterol Xinafoate (I) having purity greater than 99.0% by HPLC.
Example-10: Purification of Salmeterol Xinafoate [I]
100 g of Salmeterol Xinafoate prepared as per process of Example-8 and 1.5 acetone were taken in round bottom flask and heated at 55°C to get clear solution. The reaction mixture was charcoalized and stirred for 20 min. The reaction mixture was filtered through celite bed and washed with 100 mL acetone. The filtrate was cooled to 20°C and the precipitated product was filtered and washed with 100 mL of chilled acetone. The solid was dried in vacuum tray drier for 8 hours at 45°C to obtain 84 g (84%) pure Salmeterol Xinafoate (I) having purity greater than 99.0% by HPLC.
Advantages of the Invention
1) The present invention provides a novel process for the preparation of Salmeterol Xinafoate (I).
2) The present invention a novel intermediate 5-(2-(benzyl(6-(4- phenylbutoxy)hexyl)amino)- acetyl)-2-(benzyloxy)benzoate (IV).
3) The present invention provides a novel process for preparation of methyl 2- (benzyloxy)-5-(2-bromoacetyl)benzoate (V).
4) The main advantage of the present invention is to provide Salmeterol Xinafoate with high yield and high purity by HPLC greater than 99% with single individual impurity < 0.1%.
5) The process is simple, safe, cost effective and can be employed for commercial production.
Claims
1. A process for the preparation of methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V),
(a) benzylating methyl-5-acetyl-2-hydroxybenzoate (VIII) with benzyl chloride in the presence of a base and a catalyst in a suitable polar solvent to obtain 5-acetyl-2- benzyloxy benzoate (VII);
(VII)
(b) brominating methyl 5-acetyl-2-(benzyloxy)benzoate (VII) with a suitable brominating agent in one or more suitable solvents in the presence of an acid catalyst to obtain methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V);
(V)
(c) optionally, purifying the methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) in a suitable solvent; and
(c) isolating the methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V).
2. The process as claimed in claim 1 , wherein the base comprises one or more of sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate.
3. The process as claimed in claim 1, wherein the catalyst is potassium iodide.
4. The process as claimed in claim 1, wherein the suitable polar solvent comprises one or more of dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, C]-C6 alcohols, C3-C6 ketones, and C3-C6 esters.
5. The process as claimed in claim 1, wherein the brominating agent comprises one or more of N-bromosuccinimide, hydrobromic acid, dibromodimethylhydatoin (DBDMH), and liquid bromine.
6. The process as claimed in claim 1, wherein the acid catalyst comprises one or more of hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid, aluminum chloride, zinc chloride, ferric chloride, copper chloride, and magnesium chloride.
7. The process as claimed in claim 1, wherein the suitable solvent for purification of methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) comprises one or more of Cj-6 alcohols, C3-6 ketones, C3-6 esters, acetonitrile, and dimethylformamide.
8. The process as claimed in claim 1, wherein the isolation comprises one or more of filtration, filtration under vacuum, centrifugation, decantation, distillation, and distillation under vacuum.
9. The process for the preparation of Salmeterol and its pharmaceutically acceptable salts of Formula (I),
comprises;
a) reacting methyl 2-(benzyloxy)-5-(2-bromoacetyl)benzoate (V) and N-(6-(4- phenylbutoxy)- hexyl)benzenemethamine hydrochloride (VI)
in one or more suitable polar organic solvents in the presence of a base to obtain (benzyl-(6-(4-phenylb [IV];
b) reducing the 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)acetyl)-2- (benzyloxy)benzoate [IV] with a suitable reducing agent in one or more suitable organic solvents followed by treating with a suitable base under heating to get 2-(benzyl-(6-(4- phenylbutoxy)hexyl)amino)- 1 -(4-(benzyloxy)-3 -(hydroxy- methyl)phenyl)ethanol (III);
(hydroxy methyl) phenyl )ethanol (III) with a suitable catalyst in one or more polar solvents under 5-6 Kg/cm2 pressure and temperature of about 30°C to about 80°C to obtain salmeterol base (II);
(a) converting the salmeterol base (II) into its pharmaceutically acceptable salts;
(b) optinally, purifying the pharmaceutically acceptable salt of Salmeterol in one or more polar organic solvents; and
(c) isolating the pure pharmaceutically acceptable salt of Salmeterol.
10. The process as claimed in claim 9 (a), wherein the suitable polar solvent comprises one or more of dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane, N- methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Ci-C6 alcohols, C3-C6 ketones, and C3-C6 esters.
1 1. The process as claimed in claim 9 (a), wherein the suitable organic solvent comprises one or more of Ci-C5 alcohols, C3-C6 ethers, tetrahydrofuran, and 2-methyl tetrahydrofuran.
12. The process as claimed in claim 9 (b), wherein the suitable reducing agent comprises one or more of lithium aluminum hydride, vitride, and sodium borohydride.
13. The process as claimed in claim 9(b), wherein the base comprises one or more of sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate.
14. The process as claimed in claim 9 (c), wherein the hydrogenation catalyst comprises one or more of Pd/C, Pt/C, mixture of Pd/C and ZnCl2, and Raney Nickel.
15. The process as claimed in claim 9 (c), wherein the polar solvent comprises one or more of C 1 -6 alcohols, C3-6 ketones, C3-6 esters, acetonitrile, and dimethylformamide.
16. The process as claimed in claim 9 (c), wherein the hydrogenation is carried out at about 30°C to about 80°C.
17. The process as claimed in claim 9 (c), wherein the suitable polar organic solvent comprises one or more of methanol, ethanol, isopropanol, butanol, acetone, methylethylketone, methylisobutyl ketone, ethyl acetate, n-butyl acetate, acetonitrile, and dimethylformamide.
18. The process as claimed in claim 9 (c), wherein the pharmaceutically acceptable salts of salmeterol is isolated by addition of one or more suitable anti-solvents before purification comprising one or more of ethyl acetate, n-butyl acetate, toluene, xylene, diisopropyl ether, methyl tertbutyl ether, cyclohexane, n-hexane, and n-heptane.
19. The process as claimed in claim 9 (c), wherein the suitable solvent for purification of pharmaceutically acceptable salts of salmeterol comprises one or more of C1- alcohols, C3-6 ketones, C3-6 esters, acetonitrile, and dimethylformamide.
20. A process for the preparation of N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI),
(VI) the process comprising:
(a) reacting 4-phenyl-l-butanol (XII) with 1,6-dibromobutane (XI) in the presence of a base and a
phase transfer catalyst in one or more hydrocarbon solvents to obtain (4-(6- bromohexyloxy)butyl)benzene (X);
(b) reacting the compound (X) with benzylamine in the presence of a base and a catalyst in one or more polar solvents to obtain N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI);
(c) optionally, purifying the N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI); and
(d) isolating the pure N-(6-(4-phenylbutoxy)hexyl)benzenemethamine hydrochloride (VI).
21. The process as claimed in claim 20(a), wherein the base comprises one or more of sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate.
22. The process as claimed in claim 20(a), wherein the phase transfer catalyst comprises one or more of tetrabutyl ammonium bromide (TBAB), benzyltriethyl ammonium chloride (TEBAC), polyethylene Glycol (PEG-200, 400, 600, 800, 1000 etc.), and tetrabutyl ammonium hydrogen sulphate (TBAHS).
23. The process as claimed in claim 20 (a), wherein the hydrocarbon solvent comprises one or more of toluene, xylene, ethyl benzene, cyclohexane, pentane, hexane, and heptanes.
24. The process as claimed in claim 20(b), wherein the base comprises one or more of sodium hydroxide, potassium hydroxide, lithium hydroxide, sodium hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate.
25. The process as claimed in claim 20(b), wherein the suitable polar solvent comprises one or more of dimethylformamide, dimethylsulfoxide, dimethylacetamide, sulfolane,
N-methylpyrrolidone, tetrahydrofuran, 2-methyl tetrahydrofuran, Ci-C6 alcohols, C3- C ketones, and C3-C6 esters.
26. The process as claimed in claim 20(c), wherein the suitable solvent for purification of N-(6-(4-phenylbutoxy)hexyl)- benzenemethamine hydrochloride (VI) comprises one or more of Cj- alcohols, C3-6 ketones, C3-6 esters, acetonitrile, and dimethylformamide.
27. A compound 5-(2-(benzyl-(6-(4-phenylbutoxy)hexyl)amino)acetyl)-2- (benzyloxy)benzoate (IV)
28. Salmeterol Xinafoate of formula (I) in crystalline form characterized by X-ray diffraction pattern as shown in Figure- 1 :
29. Salmeterol Xinafoate of formula (I) in crystalline form characterized by DSC as shown in Figure-2.
30. Salmeterol Xinafoate of formula (I) of HPLC purity greater than 99.5% having ' individual impurity less than 0.1%.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN2482MU2010 | 2010-09-08 | ||
| IN2482/MUM/2010 | 2010-09-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2012032546A2 true WO2012032546A2 (en) | 2012-03-15 |
| WO2012032546A3 WO2012032546A3 (en) | 2012-06-28 |
Family
ID=45464038
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2011/000615 WO2012032546A2 (en) | 2010-09-08 | 2011-09-08 | Process for the preparation of salmeterol and its intermediates |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012032546A2 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103864629A (en) * | 2012-12-13 | 2014-06-18 | 天津金耀集团有限公司 | Refining method of Salmeterol xinafoate |
| WO2016142582A1 (en) | 2015-03-11 | 2016-09-15 | Fermion Oy | Process for the preparation of crystalline salmeterol and its xinafoate salt |
| CN106478432A (en) * | 2016-08-29 | 2017-03-08 | 鲁南制药集团股份有限公司 | A kind of preparation method of former times how sour salmaterol |
| CN109232884A (en) * | 2018-07-07 | 2019-01-18 | 盐城师范学院 | A kind of interface preparation method of two dimension organic framework materials |
| WO2019170543A1 (en) | 2018-03-07 | 2019-09-12 | Bayer Aktiengesellschaft | Identification and use of erk5 inhibitors |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES345365A1 (en) | 1967-09-22 | 1969-02-01 | Allen & Hanburys Ltd | A procedure for the preparation of new derivatives of 1-phenyl-2-aminoetanol. (Machine-translation by Google Translate, not legally binding) |
| GB2140800A (en) | 1983-04-18 | 1984-12-05 | Glaxo Group Ltd | Phenethanolamine derivatives |
| ES2065269A1 (en) | 1993-05-11 | 1995-02-01 | S A L V A T Lab Sa | 6-[4-(phenylbutoxy)] hexylaminomethyl-4-hydroxy- [alpha]1,[alpha]3-benzenodimethanol. Procedure for obtaining it and new intermediates used in the preparation thereof |
| US5795594A (en) | 1993-07-01 | 1998-08-18 | Glaxo Group Limited | Salmeterol xinafoate with controlled particle size |
| US6680345B2 (en) | 2001-09-14 | 2004-01-20 | Boehringer Ingelheim Pharma Kg | Salicylic acid salts of salmeterol |
| US6756508B2 (en) | 2002-03-04 | 2004-06-29 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Cinnamic acid salts, processes for their preparation, and their use as medicaments |
| US7112701B2 (en) | 2002-03-05 | 2006-09-26 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Process for the manufacture of 4-(6-bromohexyloxy)-butylbenzene |
| WO2007045857A1 (en) | 2005-10-17 | 2007-04-26 | Generics (Uk) Limited | Novel process |
| EP1073429B1 (en) | 1998-04-24 | 2007-07-11 | Glaxo Group Limited | Aerosol formulations of salmeterol xinafoate |
| ES2288114B1 (en) | 2006-04-26 | 2008-12-01 | Quimica Sintetica S.A. | NEW SYNTHESIS PROCEDURE OF (6- (4-PHENYLBUTOXY) HEXIL) N-SUBSTITUTED AMINES AND ITS USE IN THE SYNTHESIS OF SALMETEROL XINAFOATO. |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1997025311A1 (en) * | 1996-01-10 | 1997-07-17 | Asahi Kasei Kogyo Kabushiki Kaisha | Novel tricyclic compounds and drug compositions containing the same |
| US6541669B1 (en) * | 1998-06-08 | 2003-04-01 | Theravance, Inc. | β2-adrenergic receptor agonists |
| OA11558A (en) * | 1999-12-08 | 2004-06-03 | Advanced Medicine Inc | Beta 2-adrenergic receptor agonists. |
| WO2003099764A1 (en) * | 2002-05-28 | 2003-12-04 | Theravance, Inc. | ALKOXY ARYL β2 ADRENERGIC RECEPTOR AGONISTS |
| PE20040950A1 (en) * | 2003-02-14 | 2005-01-01 | Theravance Inc | BIPHENYL DERIVATIVES AS AGONISTS OF ß2-ADRENERGIC RECEPTORS AND AS ANTAGONISTS OF MUSCARINAL RECEPTORS |
| ES2329586T3 (en) * | 2003-11-21 | 2009-11-27 | Theravance, Inc. | COMPOUNDS THAT HAVE AGONIST ACTIVITY OF THE BETA2 ADRENERGIC RECEIVER AND ANTAGONIST OF THE MUSCARINE RECEIVER. |
| SI2035004T1 (en) * | 2006-06-09 | 2012-12-31 | Parion Sciences, Inc. | Phenyl substituted pyrazinoylguanidine sodium channel blockers possessing beta agonist activity |
| JP2012001454A (en) * | 2010-06-15 | 2012-01-05 | Ohara Yakuhin Kogyo Kk | Simple method for producing salmeterol |
-
2011
- 2011-09-08 WO PCT/IN2011/000615 patent/WO2012032546A2/en active Application Filing
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES345365A1 (en) | 1967-09-22 | 1969-02-01 | Allen & Hanburys Ltd | A procedure for the preparation of new derivatives of 1-phenyl-2-aminoetanol. (Machine-translation by Google Translate, not legally binding) |
| GB2140800A (en) | 1983-04-18 | 1984-12-05 | Glaxo Group Ltd | Phenethanolamine derivatives |
| ES531722A0 (en) | 1983-04-18 | 1985-06-01 | Glaxo Group Ltd | A PROCEDURE FOR THE PREPARATION OF PHENETHANOLAMINE DERIVATIVES. |
| ES539625A0 (en) | 1983-04-18 | 1986-07-16 | Glaxo Group Ltd | A PROCEDURE FOR THE PREPARATION OF FENETHANE DERIVATIVES. |
| GB2176476A (en) | 1983-04-18 | 1986-12-31 | Glaxo Group Ltd | Phenethanolamine derivatives |
| US4992474A (en) | 1983-04-18 | 1991-02-12 | Glaxo Group Ltd. | Phenethanolamine derivatives |
| ES2065269A1 (en) | 1993-05-11 | 1995-02-01 | S A L V A T Lab Sa | 6-[4-(phenylbutoxy)] hexylaminomethyl-4-hydroxy- [alpha]1,[alpha]3-benzenodimethanol. Procedure for obtaining it and new intermediates used in the preparation thereof |
| ES2065269B1 (en) | 1993-05-11 | 1995-10-01 | S A L V A T Lab Sa | 6- (4- (FENILBUTOXI) HEXILAMINOMETIL-4-HIDROXI-A1, A3-BENCENODIMETANOL. PROCEDURE FOR THE OBTAINING OF THE SAME AND NEW INTERMEDIATES USED IN ITS PREPARATION. |
| US5795594A (en) | 1993-07-01 | 1998-08-18 | Glaxo Group Limited | Salmeterol xinafoate with controlled particle size |
| EP1073429B1 (en) | 1998-04-24 | 2007-07-11 | Glaxo Group Limited | Aerosol formulations of salmeterol xinafoate |
| US6680345B2 (en) | 2001-09-14 | 2004-01-20 | Boehringer Ingelheim Pharma Kg | Salicylic acid salts of salmeterol |
| US6756508B2 (en) | 2002-03-04 | 2004-06-29 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Cinnamic acid salts, processes for their preparation, and their use as medicaments |
| US7112701B2 (en) | 2002-03-05 | 2006-09-26 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Process for the manufacture of 4-(6-bromohexyloxy)-butylbenzene |
| WO2007045857A1 (en) | 2005-10-17 | 2007-04-26 | Generics (Uk) Limited | Novel process |
| ES2288114B1 (en) | 2006-04-26 | 2008-12-01 | Quimica Sintetica S.A. | NEW SYNTHESIS PROCEDURE OF (6- (4-PHENYLBUTOXY) HEXIL) N-SUBSTITUTED AMINES AND ITS USE IN THE SYNTHESIS OF SALMETEROL XINAFOATO. |
Non-Patent Citations (6)
| Title |
|---|
| INDIAN JOURNAL OF CHEMISTRY, vol. 34B, 1995, pages 629 - 632 |
| J. LABEL. COMPD RADIOPHARM., vol. 45, no. 9, 2002, pages 755 - 762 |
| SYNTH. COMMUN., vol. 29, no. 12, 1999, pages 2155 - 2162 |
| SYNTHETIC COMMUNICATION, vol. 29, no. 12A, 1999, pages 2155 - 2162 |
| TETRAHEDRON LETT., vol. 35, 1994, pages 9375 - 9378 |
| TETRAHEDRON LETTERS, vol. 35, no. 50, 1994, pages 9375 - 9378 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103864629A (en) * | 2012-12-13 | 2014-06-18 | 天津金耀集团有限公司 | Refining method of Salmeterol xinafoate |
| WO2016142582A1 (en) | 2015-03-11 | 2016-09-15 | Fermion Oy | Process for the preparation of crystalline salmeterol and its xinafoate salt |
| CN106478432A (en) * | 2016-08-29 | 2017-03-08 | 鲁南制药集团股份有限公司 | A kind of preparation method of former times how sour salmaterol |
| WO2019170543A1 (en) | 2018-03-07 | 2019-09-12 | Bayer Aktiengesellschaft | Identification and use of erk5 inhibitors |
| CN109232884A (en) * | 2018-07-07 | 2019-01-18 | 盐城师范学院 | A kind of interface preparation method of two dimension organic framework materials |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2012032546A3 (en) | 2012-06-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8614346B2 (en) | Methods and compositions for preparation of amphetamine conjugates and salts thereof | |
| US20120165548A1 (en) | Process for the preparation of indoline derivatives and their intermediates thereof | |
| EP3287434A1 (en) | Process to prepare treprostinil, the active ingredient in remodulin ® | |
| WO2012032546A2 (en) | Process for the preparation of salmeterol and its intermediates | |
| JP2011508767A (en) | Process for the synthesis of bosentan, its polymorphic forms and its salts | |
| AU2010215520B2 (en) | Process for preparing Cinacalcet hydrochloride | |
| WO2008035380A2 (en) | An improved process for the preparation of high purity formoterol and its pharmaceutically acceptable salts | |
| JP5514218B2 (en) | Process for preparing cinacalcet | |
| KR101308258B1 (en) | A novel method of making Endoxifen | |
| US9447067B2 (en) | Method of preparing intermediate of salmeterol | |
| US7538249B2 (en) | Tolterodine, compositions and uses thereof, and preparation of the same | |
| EP1177166B1 (en) | Process for preparing (1r,2s,4r) -(-)-2- (2'- n,n-dimethylamino -ethoxy) -2- phenyl -1,7,7-tri- methyl -bicyclo 2.2.1 heptane and pharmaceutically acceptable acid addition salts thereof | |
| US20090253918A1 (en) | Novel intermediate for glyt1 inhibitor | |
| WO2009063171A1 (en) | Novel rotigotine salts | |
| CN114105872B (en) | Intermediate for preparing procaterol hydrochloride and preparation method thereof | |
| US10501414B2 (en) | Indole analogs as 5-oxo-ETE receptor antagonists and method of use thereof | |
| KR102350458B1 (en) | A new process for the preparation of (R)-2-((4-Aminophenethyl)amino)-1-phenylethanol | |
| WO2014008639A1 (en) | Method for preparing indacaterol | |
| US20060293530A1 (en) | Process for the manufacture of citalopram hydrobromide | |
| JPH0764843B2 (en) | Novel compound, method for producing the same, and pharmaceutical composition containing the same | |
| CN114213323B (en) | New process for synthesizing procaterol hydrochloride | |
| AU2005209382A1 (en) | Method for producing a 2-(ethoxymethyl)tropane derivative | |
| US8703976B2 (en) | Manufacturing process for 8-aryloctanoic acids such as Aliskiren | |
| WO2014008640A1 (en) | Indacaterol intermediate and method for synthesizing indacaterol | |
| CN116836069A (en) | Preparation method of levosalbutamol hydrochloride |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11805623 Country of ref document: EP Kind code of ref document: A2 |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11805623 Country of ref document: EP Kind code of ref document: A2 |